Novel AOPs-Based Dual-Environmental Digestion Method for Determination of Total Dissolved Nitrogen in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
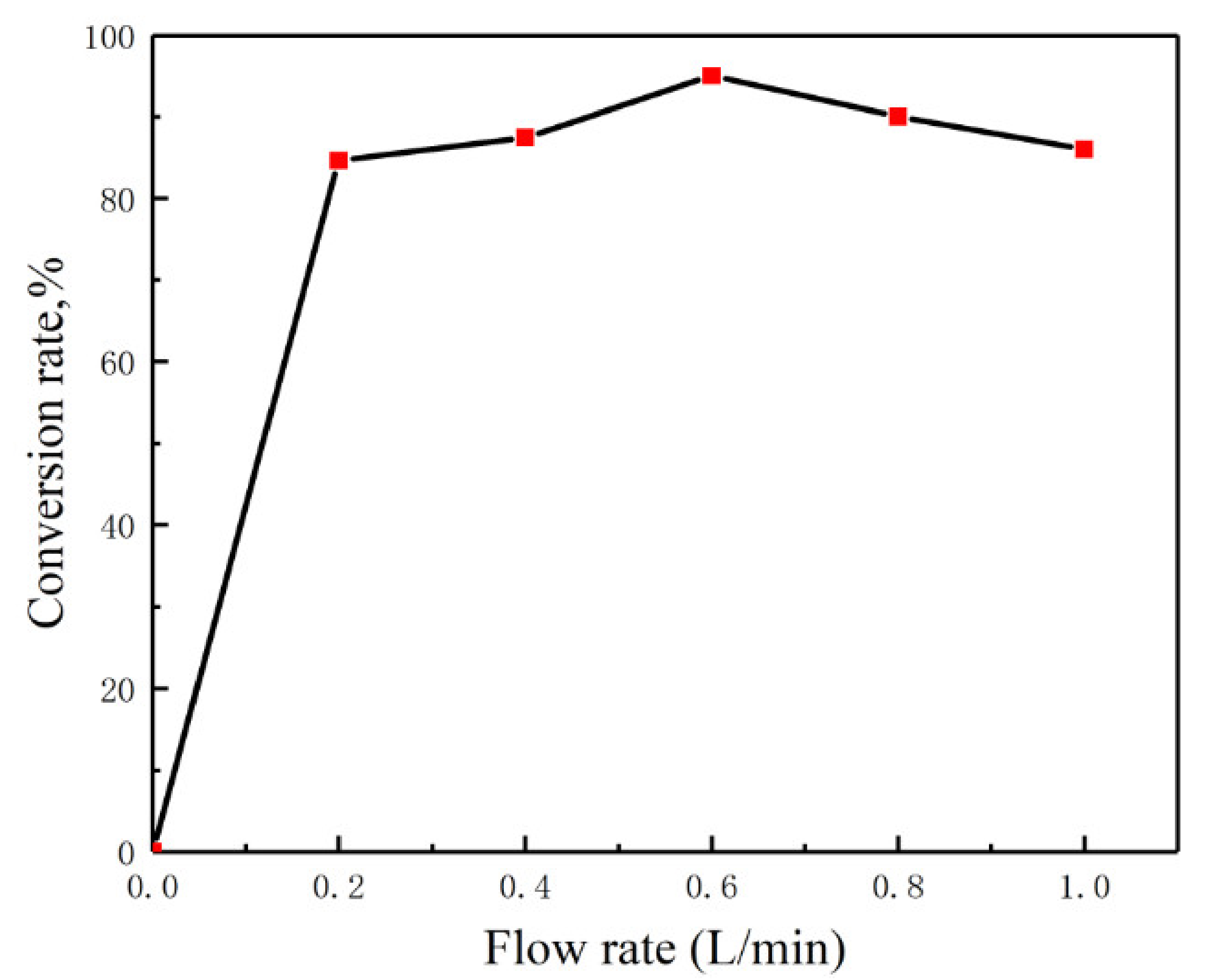
2.2. Apparatus and Devices
2.3. Procedure and Methods
- (1)
- First, clean the reactor three times with distilled water to remove impurities, and adjust the gas flow rate to 0.6 L/min;
- (2)
- Then, turn on the thermostatic magnetic agitator and set the temperature to 40 °C for the reaction, and then turn on the water pump to cycle water and control the digestion reaction temperature;
- (3)
- Meanwhile, transfer 16 mL of working standard solution or water sample to the reactor using an adjustable pipette, and then add 18 mL of distilled water and 2 mL of sulfuric acid to adjust the pH of the solution;
- (4)
- When the water temperature is constant, turn on the UV lamp and ozone generator for the reaction, and then close the liquid inlet and open the check valve at the bottom of the reactor at the same time;
- (5)
- After 10 min of reaction under acidic conditions, add 3 mL of sodium hydroxide solution to adjust the pH to 11, and continue the reaction for 10 min under alkaline conditions;
- (6)
- After reaction for 20 min, turn off the UV lamp and the ozone generator, and continue to pass oxygen to discharge the remaining ozone in the solution or water sample;
- (7)
- After another 5 min, add 1 mL of sulfuric acid to adjust the pH of the solution or water sample to neutral or weakly acidic, and then stop supplying the oxygen and transfer 3.5 mL of digested water samples to the quartz colorimetric cell for the detection of absorbance at 220 nm and 275 nm, respectively;
- (8)
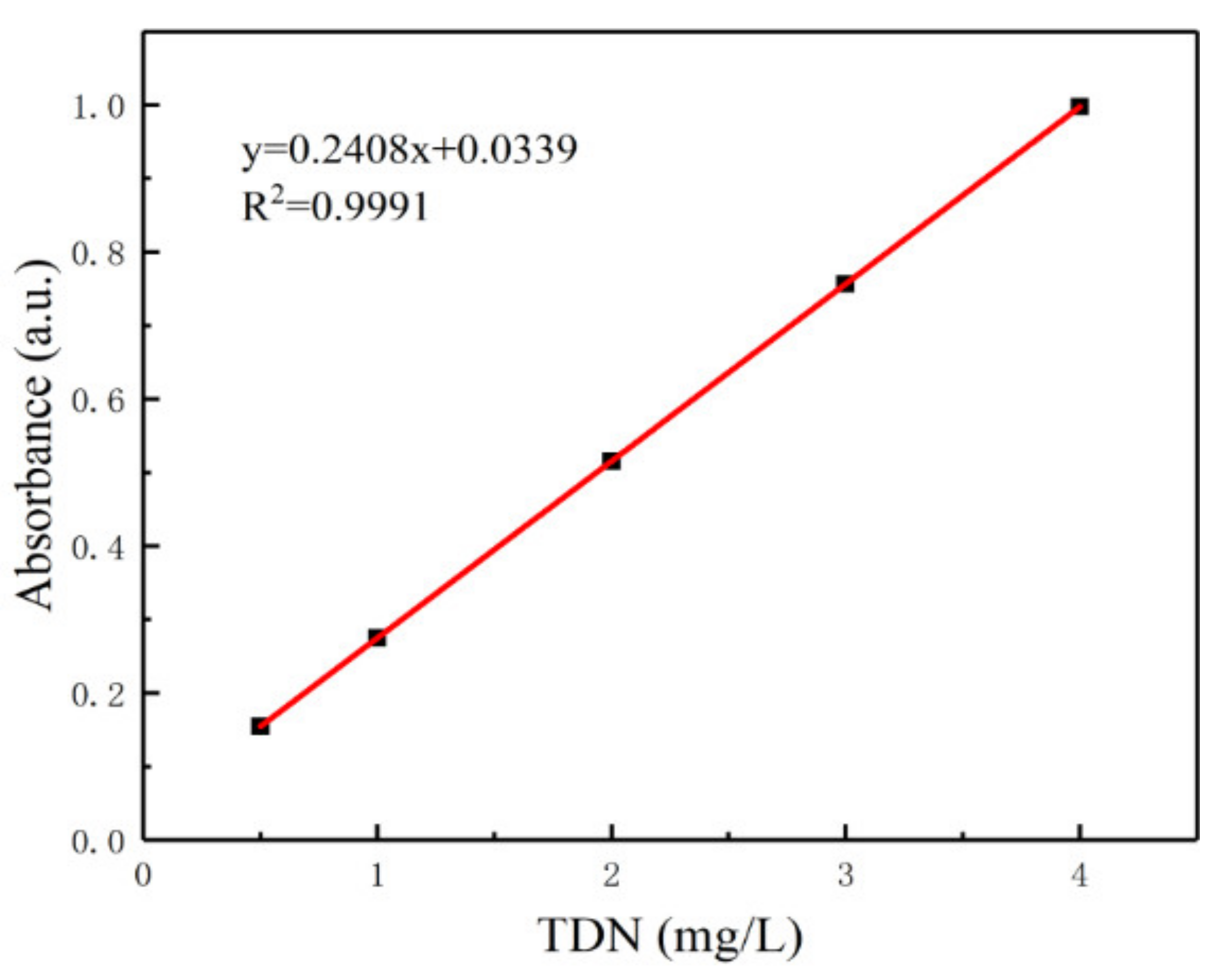
- Finally, calculate the concentrations of the water samples based on the net absorbances and the prepared calibration curve of TDN.
3. Results and Discussion
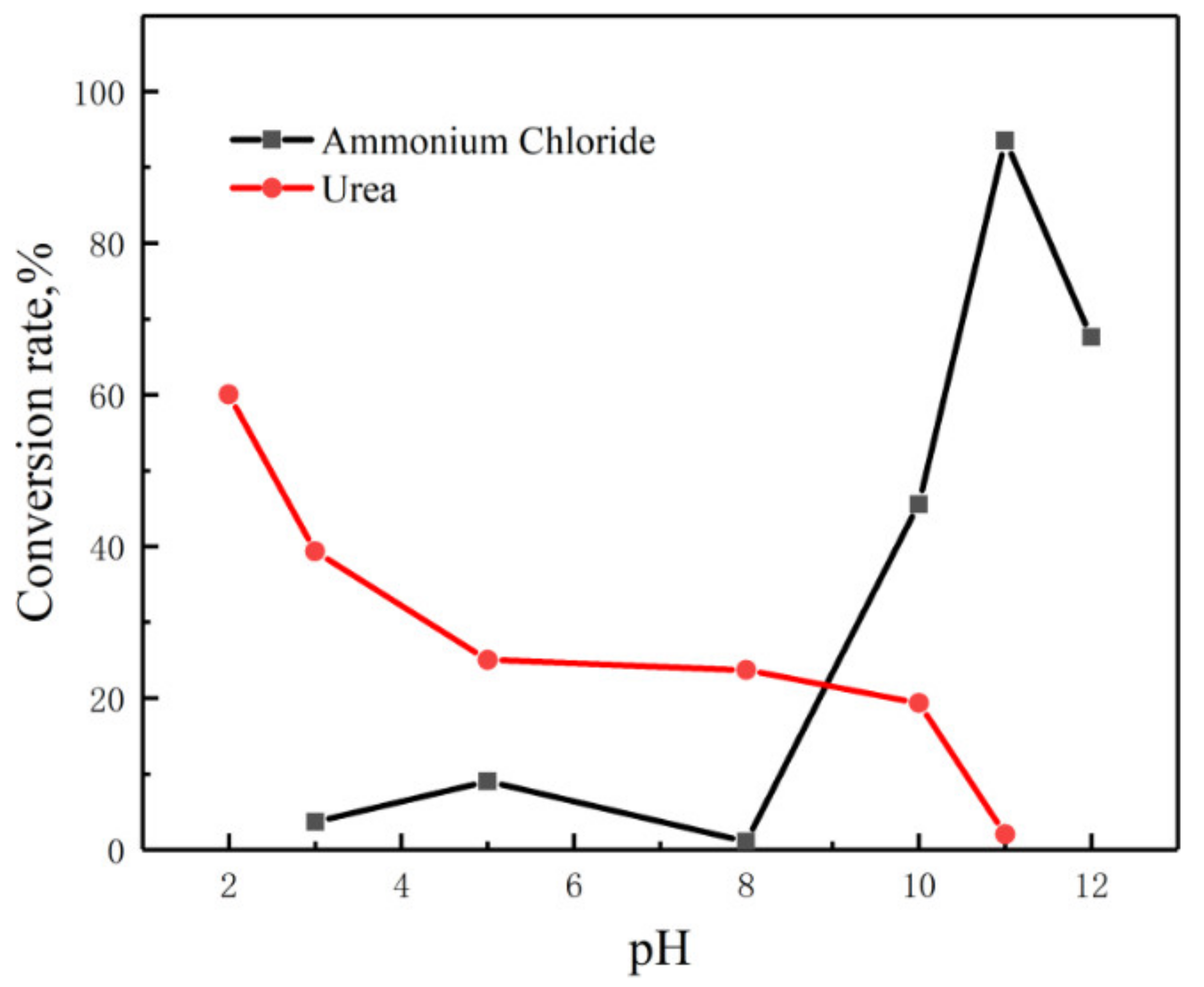
3.1. Effect of pH on Digestion Efficiency
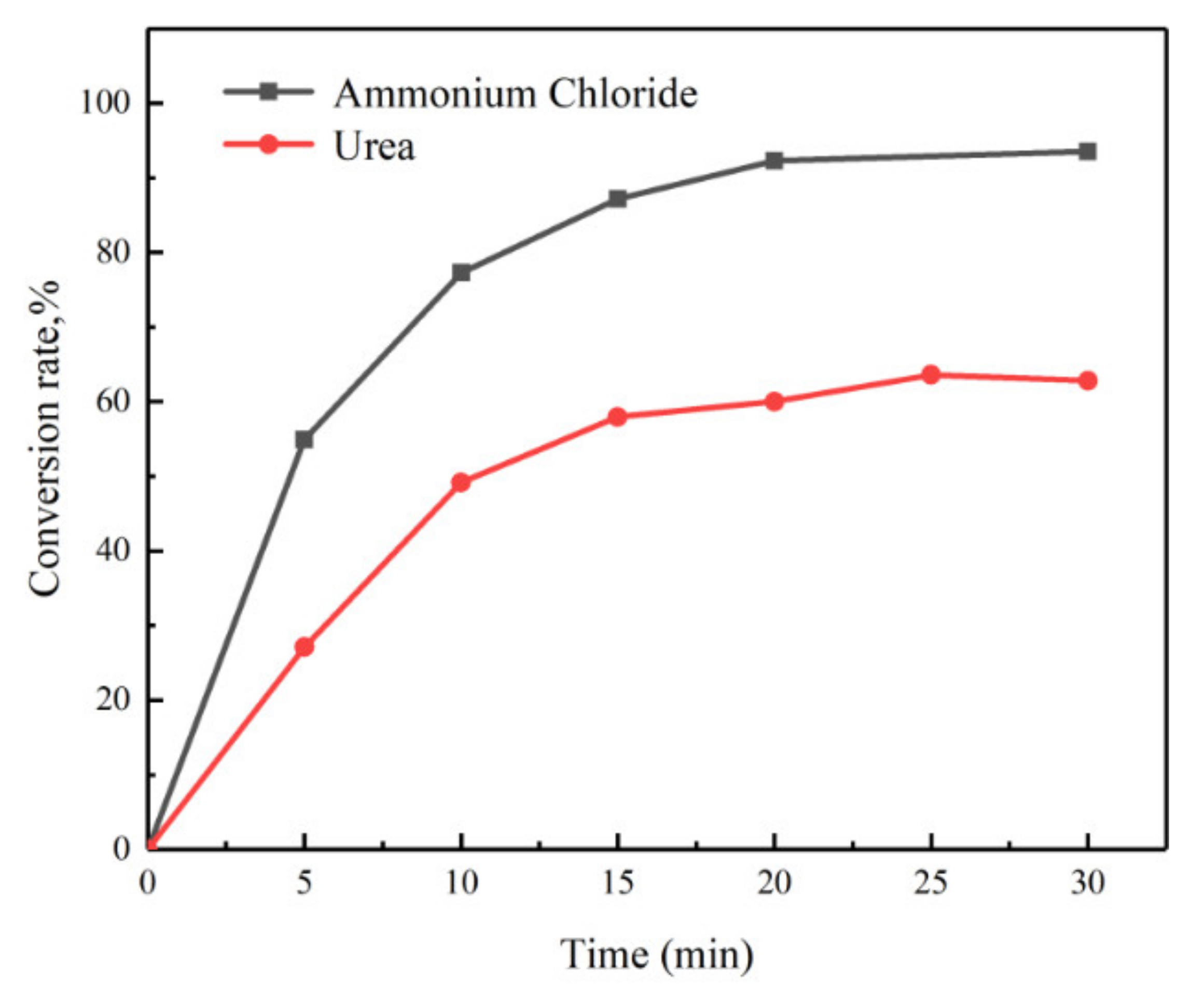
3.2. Effect of Reaction Time on Digestion Efficiency
3.3. Effect of Cl− on Digestion Efficiency
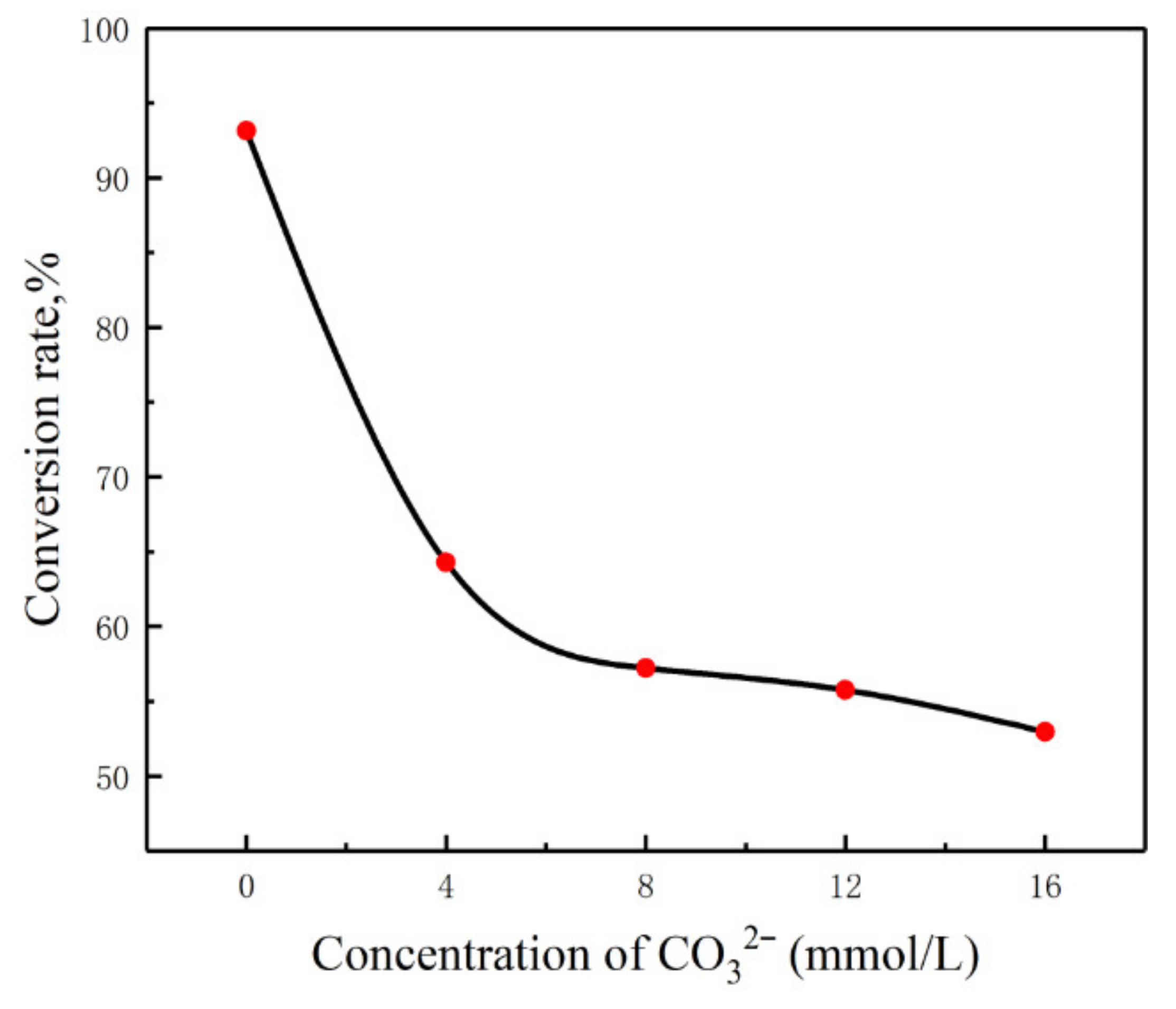
3.4. Effects of CO32− and HCO3− on Digestion Efficiency
3.5. Validation of the Dual-Environmental Digestion Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, J.; Yuan, D.X.; Lin, K.N.; Feng, S.C.; Zhou, T.J.; Li, Q.L. Applications of flow techniques in seawater analysis: A review. Trends Environ. Anal. Chem 2016, 10, 1–10. [Google Scholar] [CrossRef]
- Fan, C.L.; Patricia, M.G.; Jo, A.M.B. Characterization of the affinity for nitrogen, uptake kinetics, and environmental relationships for prorocentrum minimum in natural blooms and laboratory cultures. Harmful Algae 2003, 4, 283–299. [Google Scholar] [CrossRef]
- Chen, D.X.; Jie, D. The application in water determination of we-Tn-Gc2007 total nitrogen on-site or on-line automatic monitoring instrument. In Proceedings of the 2nd International Conference on Bioinformatics and Biomedical Engineering, Tianjin, China, 19–21 September 2008. [Google Scholar]
- Sun, X.F.; Chen, H.X.; Liu, Z.Y.; Zhou, M.F.; Cai, Y.J.; Pan, H.T.; Xia, L.Y. Investigations on ozone-based and uv/us-assisted synergistic digestion methods for the determination of total dissolved nitrogen in waters. Processes 2020, 4, 490. [Google Scholar] [CrossRef] [Green Version]
- Worsfold, P.; Ian, M.; Phil, M. Determination of phosphorus in natural waters: A historical review. Anal. Chim. Acta 2016, 918, 8–20. [Google Scholar] [CrossRef] [Green Version]
- Maher, W.; Woo, L. Procedures for the storage and digestion of natural waters for the determination of filterable reactive phosphorus, total filterable phosphorus and total phosphorus. Anal. Chim. Acta 1998, 375, 5–47. [Google Scholar] [CrossRef]
- Kroon, H. Determination of nitrogen in water: Comparison of a continuous-flow method with on-line uv digestion with the original kjeldahl method. Anal. Chim. Acta 1993, 276, 287–293. [Google Scholar] [CrossRef]
- Chromy, V.; Bára, V.; Luděk, P.; Miroslava, B. The kjeldahl method as a primary reference procedure for total protein in certified reference materials used in clinical chemistry. I. a review of kjeldahl methods adopted by laboratory medicine. Crit. Rev. Anal. Chem. 2015, 45, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Rogora, M.; Marco, M.; Arianna, O.; Gabriele, A.T. A comparison between high-temperature catalytic oxidation and persulphate oxidation for the determination of total nitrogen in freshwater. Int. J. Environ. Anal. Chem 2006, 86, 1065–1078. [Google Scholar] [CrossRef]
- Yang, J.E.; Kim, J.J.; Skogley, E.O.; Schaff, B.E. A simple spectrophotometric determination of nitrate in water, resin, and soil extracts. Soil Sci. Soc. Am. J. 1998, 62, 368–375. [Google Scholar] [CrossRef]
- Salvestrini, S.; Fenti, A.; Chianese, S.; Iovino, P.; Musmarra, D. Electro-oxidation of humic acids using platinum electrodes: Anexperimental approach and kinetic modelling. Water 2020, 12, 2250. [Google Scholar] [CrossRef]
- Xiong, Z.K.; Yuan, Y.; Lai, B.; Yang, P.; Zhou, Y.X. Mineralization of ammunition wastewater by a micron-size Fe0/O3 process (Mfe0/O3). RSC Adv. 2016, 61, 55726–55735. [Google Scholar] [CrossRef]
- Jaafarzadeh, N.; Farshid, G.; Mehdi, A. Efficient degradation of 2,4-dichlorophenoxyacetic acid by peroxymonosulfate/ magnetic copper ferrite nanoparticles/ozone: A novel combination of advanced oxidation processes. Chem. Eng. J. 2017, 320, 436–447. [Google Scholar] [CrossRef]
- Barik, A.J.; Parag, R.G. Degradation of 4-chloro 2-aminophenol using a novel combined process based on hydrodynamic cavitation, uv photolysis and ozone. Ultrason. Sonochem. 2016, 30, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Nihemaiti, M.; Miklos, D.B.; Hübner, U.; Linden, K.G.; Drewes, J.E.; Croué, J.P. Removal of trace organic chemicals in wastewater effluent by uv/H2O2 and uv/Pds. Water Res. 2018, 145, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Rehman, F.; Murtaza, S.; Javed, A.K.; Noor, S.S.; Hasan, M.K.; Dionysios, D.D. Oxidative removal of brilliant green by uv/S2O82‒, uv/HsO5‒ and uv/H2O2 processes in aqueous media: A comparative study. J. Hazard. Mater. 2018, 357, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Lu, X.L.; Jiang, J.; Ma, J.; Liu, G.Q.; Cao, Y.; Liu, W.L.; Li, J.; Pang, S.Y.; Kong, X.J.; et al. Degradation of sulfamethoxazole by uv, uv/H2O2 and uv/persulfate (pds): Formation of oxidation products and effect of bicarbonate. Water Res. 2017, 118, 196–207. [Google Scholar]
- Luo, C.W. Factors affecting formation of deethyl and deisopropyl products from atrazine degradation in uv/H2O2 and uv/pds. RSC Adv. 2017, 7, 29255–29262. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Liu, Y.Q.; Fu, Y.S. Degradation kinetics and mechanism of diclofenac by uv/peracetic acid. RSC Adv. 2020, 10, 9907–9916. [Google Scholar]
- Liu, N.; Feng, D.; Chih, H.W.; Chi, C.H.; Yao, T.L. Effective degradation of primary color direct azo dyes using FeO aggregates-activated persulfate process. J. Environ. Manag. 2018, 206, 565–576. [Google Scholar] [CrossRef]
- Nidheesh, P.V. Heterogeneous fenton catalysts for the abatement of organic pollutants from aqueous solution: A review. RSC Adv. 2015, 51, 40552–40577. [Google Scholar] [CrossRef]
- Wei, Q.; Qiao, S.F.; Sun, B.C.; Zou, H.K.; Chen, J.F.; Shao, L. Study on the treatment of simulated coking wastewater by O3 and O3/fenton processes in a rotating packed bed. RSC Adv. 2015, 5, 93386–93393. [Google Scholar] [CrossRef]
- Ajmal, A.; Imran, M.; Riffat, N.M.; Hicham, I.; Muhammad, A.N. Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: A comparative overview. RSC Adv. 2014, 70, 37003–37026. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Fu, C.C.; Juang, R.S. Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: Efficiency and degradation pathways. J. Clean. Prod. 2018, 202, 413–427. [Google Scholar] [CrossRef]
- Ji, Y.F.; Shi, Y.Y.; Kong, D.Y.; Lu, J.H. Degradation of roxarsone in a sulfate radical mediated oxidation process and formation of polynitrated by-products. RSC Adv. 2016, 85, 82040–82048. [Google Scholar] [CrossRef]
- Shi, H.L.; Zhou, G.F.; Liu, Y.Q.; Fu, Y.S.; Wang, H.B.; Wu, P. Kinetics and pathways of diclofenac degradation by heat-activated persulfate. RSC Adv. 2019, 54, 31370–31377. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Fang, J.Y.; Fan, C.H.; Shang, C. Oxidative degradation of n-nitrosopyrrolidine by the ozone/uv process: Kinetics and pathways. Chemosphere 2016, 150, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Water Quality-Determination of Total Nitrogen-Alkaline Potassium Persulfate Digestion UV Spectrophoto-Metric Method. Available online: http://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffffbz/201203/t20120307_224383.shtml (accessed on 7 March 2012).
- Tong, Q. Study on Key Technologies of On-Line Detection of Total Nitrogen in Water Quality Based on Oxidation Digestion Method. Master’s Thesis, Zhejiang University of Technology, Hangzhou, China, 2019. [Google Scholar]
- Ince, N.H. Light-enhanced chemical oxidation for tertiary treatment of municipal landfill leachate. Water Environ. Res. 1998, 70, 1107–1231. [Google Scholar] [CrossRef]
- Neta, P.; Maruthamuthu, P.; Carton, P.M.; Fessenden, R.W. Formation and reactivity of the amino radical. J. Phys. Chem. 1978, 82, 1875–1878. [Google Scholar] [CrossRef]
- Sonntag, C.V.; Gunten, U.V. Chemistry of Ozone in Water and Wastewater Treatment; IWA Publishing: London, UK, 2012; pp. 300–310. [Google Scholar]
- Huang, C.R.; Shu, H.Y. The reaction-kinetics, decomposition pathways and intermediate formations of phenol in ozonation, uv/O3 and uv/H2O2 processes. J. Hazard. Mater. 1995, 41, 47–64. [Google Scholar] [CrossRef]
- Mare, M.; Waldner, G.; Bauer, R.; Jacobs, H.; Broekaert, J.A.C. Degradation of nitrogen containing organic compounds by combined photocatalysis and ozonation. Chemosphere 1999, 38, 2013–2027. [Google Scholar] [CrossRef]
- Beduk, F.; Aydin, M.E.; Ozcan, S. Degradation of malathion and parathion by ozonation, photolytic ozonation, and heterogeneous catalytic ozonation processes. CLEAN–Soil Air Water 2011, 40, 179–187. [Google Scholar] [CrossRef]
- Cernigoj, U.; Stangar, U.L.; Trebse, P. Degradation of neonicotinoid insecticides by different advanced oxidation processes and studying the effect of ozone on TiO2 photocatalysis. Appl. Catal. B Environ. 2007, 75, 229–238. [Google Scholar] [CrossRef]
- Wang, T.J.; Zhou, P.H. Study on the rule of the rate of hydrolysis of urea at low ph. J. N. China Inst. Sci. Technol. 1994, 17, 94–97. [Google Scholar]
- Vera, G.D.; Gernjak, W.; Weinberg, H.; Farre, M.J.; Keller, J.; Gunten, U.V. Kinetics and mechanisms of nitrate and ammonium formation during ozonation of dissolved organic nitrogen. Water Res. 2017, 108, 451–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klare, M.; Scheen, J.; Vogelsang, K.; Jacobs, H.; Broekaert, J.A.C. Degradation of short-chain alkyl- and alkanolamines by TiO2- and Pt/TiO2-assisted photocatalysis. Chemosphere 2000, 41, 353–362. [Google Scholar] [CrossRef]
- Ao, X.W.; Liu, W.J. Degradation of sulfamethoxazole by medium pressure uv and oxidants: Peroxymonosulfate, persulfate, and hydrogen peroxide. Chem. Eng. J. 2017, 313, 629–637. [Google Scholar] [CrossRef]
- Berger, P.; Leitner, N.; Dore, M. Ozone and hydroxyl radicals induced oxidation of glycine. Water Res. 1999, 33, 433–441. [Google Scholar] [CrossRef]
- Wei, C.H.; Zhang, F.Z.; Hu, Y.; Feng, C.H.; Wu, H.Z. Ozonation in water treatment: The generation, basic properties of ozone and its practical application. Rev. Chem. Eng. 2016, 33, 1–41. [Google Scholar] [CrossRef]




| Experiment No. | Environment | Acid (mL) | Alkali (mL) | Cl-(mL) | NO3−(%) |
|---|---|---|---|---|---|
| 1 | Acidic | 1 | 0 | 0 | 8.89 |
| 2 | Alkaline → Acidic | 1 | 5 | 0 | 7.49 |
| 3 | Alkaline → Acidic | 1 | 5 | 10 | 63.24 |
| 4 | Acidic → Alkaline | 1 + 1 | 12 | 0 | 18.08 |
| 5 | Acidic → Alkaline | 1 + 1 | 12 | 10 | 98.95 |
| Experiment No. | Concentration of CO32−(mmol/L) | Initial pH | Ultimate pH | OH−/CR |
|---|---|---|---|---|
| 1 | 0 | 10.10 | 6.50 | 1.3 × 10−6 |
| 2 | 4 | 10.79 | 10.62 | 3.1 × 10−6 |
| 3 | 8 | 10.93 | 10.79 | 4.1 × 10−6 |
| 4 | 12 | 11.03 | 10.89 | 5.3 × 10−6 |
| 5 | 16 | 11.12 | 10.96 | 7.7 × 10−6 |
| No. | Urea (mL) | Ammonium Chloride (mL) | Glycine (mL) | TDN (mg/L) | Error (mg/L) | CR (%) |
|---|---|---|---|---|---|---|
| 1 | 8 | 8 | 0 | 3.9456 | 0.0544 | 98.64 |
| 2 | 4 | 4 | 8 | 3.7960 | 0.2040 | 94.90 |
| 3 | 2 | 6 | 8 | 3.7292 | 0.2708 | 93.23 |
| 4 | 6 | 2 | 8 | 3.8060 | 0.1940 | 95.15 |
| 5 | 8 | 4 | 4 | 3.7824 | 0.2176 | 94.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, R.; Shou, W.; Hu, X.; Xia, L.; Zhou, M.; Liu, Z.; Sun, X. Novel AOPs-Based Dual-Environmental Digestion Method for Determination of Total Dissolved Nitrogen in Water. Water 2021, 13, 2751. https://doi.org/10.3390/w13192751
Cai R, Shou W, Hu X, Xia L, Zhou M, Liu Z, Sun X. Novel AOPs-Based Dual-Environmental Digestion Method for Determination of Total Dissolved Nitrogen in Water. Water. 2021; 13(19):2751. https://doi.org/10.3390/w13192751
Chicago/Turabian StyleCai, Rongyao, Weiqiang Shou, Xiaochun Hu, Luyue Xia, Mengfei Zhou, Zhengyu Liu, and Xiaofang Sun. 2021. "Novel AOPs-Based Dual-Environmental Digestion Method for Determination of Total Dissolved Nitrogen in Water" Water 13, no. 19: 2751. https://doi.org/10.3390/w13192751
APA StyleCai, R., Shou, W., Hu, X., Xia, L., Zhou, M., Liu, Z., & Sun, X. (2021). Novel AOPs-Based Dual-Environmental Digestion Method for Determination of Total Dissolved Nitrogen in Water. Water, 13(19), 2751. https://doi.org/10.3390/w13192751






