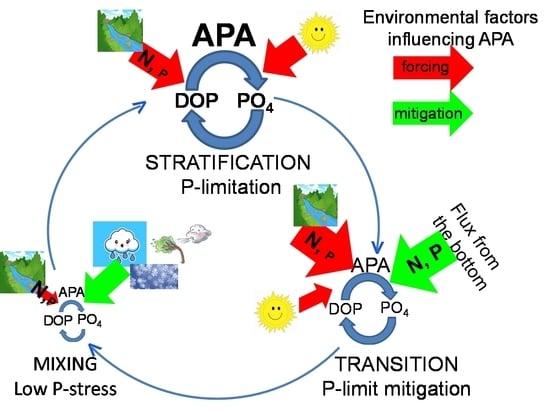
Ecological Importance of Alkaline Phosphatase Activity in Changing Marine Environmental Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Area Description and Main Case Study
2.2. Sampling Strategy
2.3. Analytical Protocol
2.4. Definition of Water Types
2.5. Data Processing
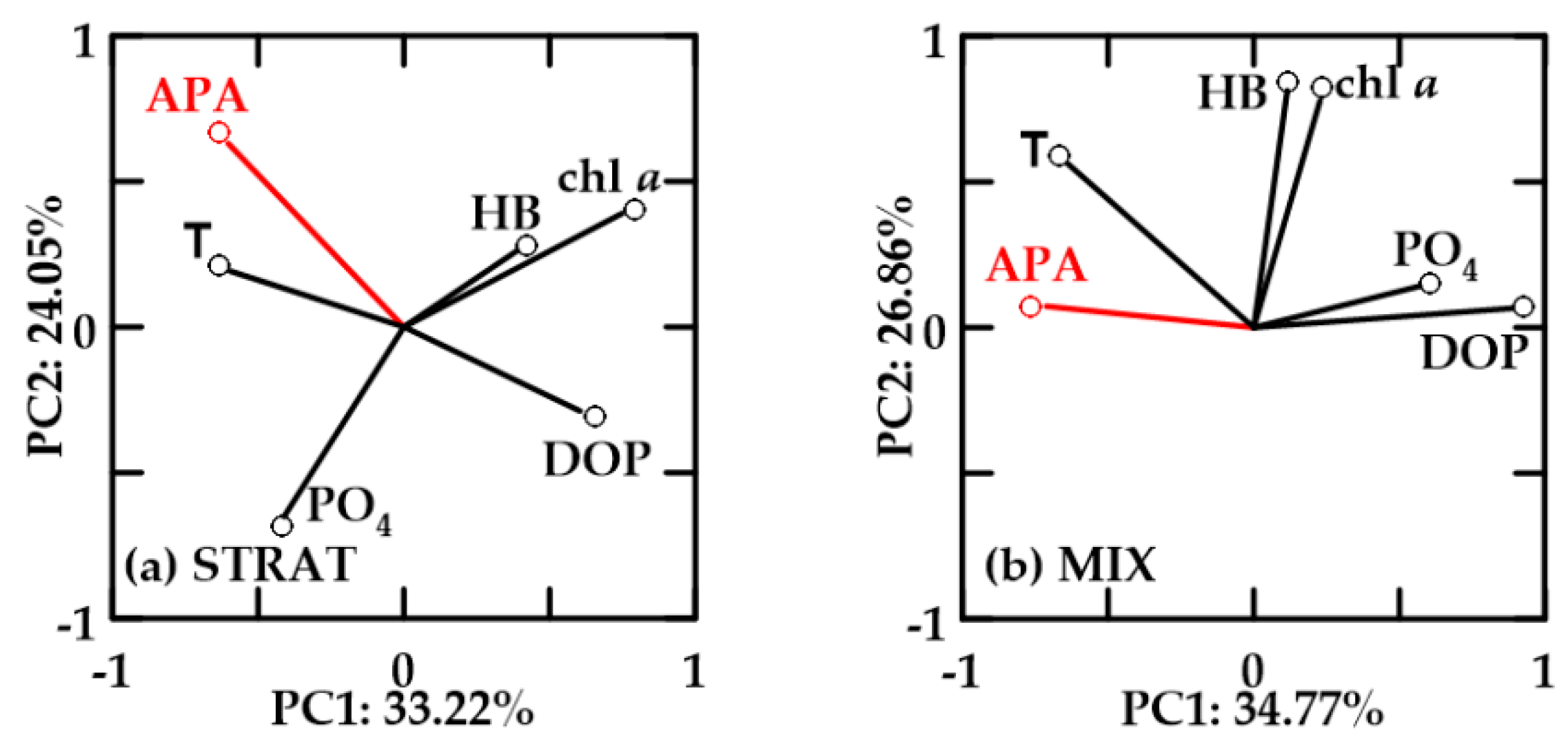
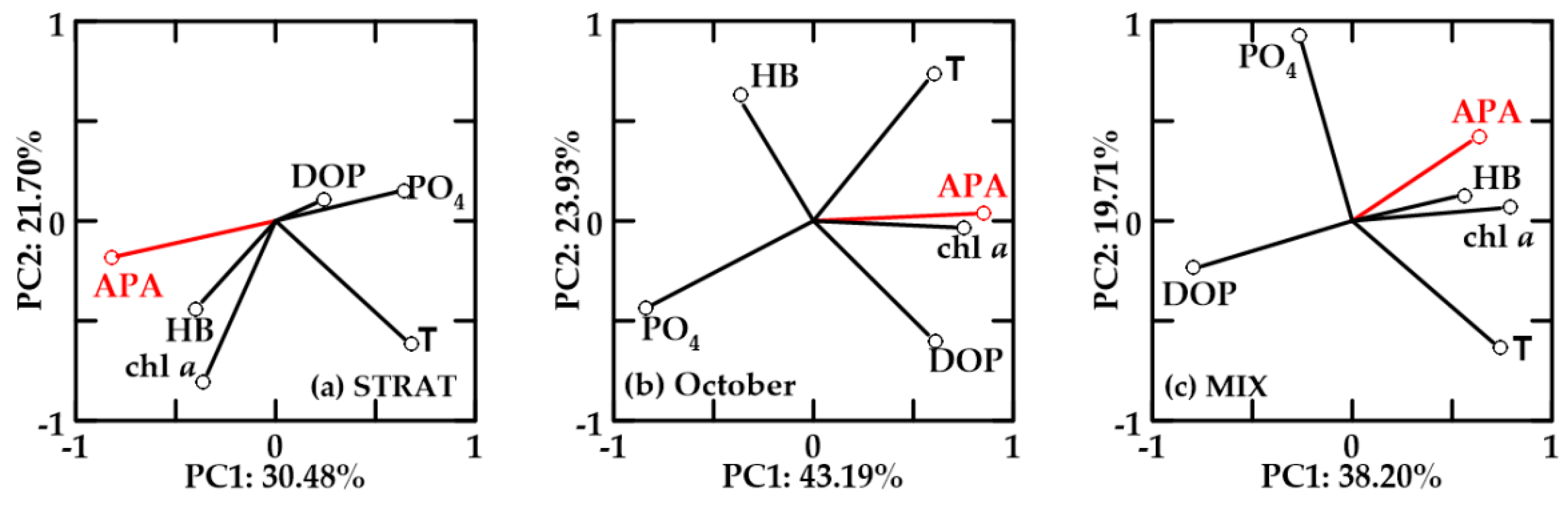
3. Results
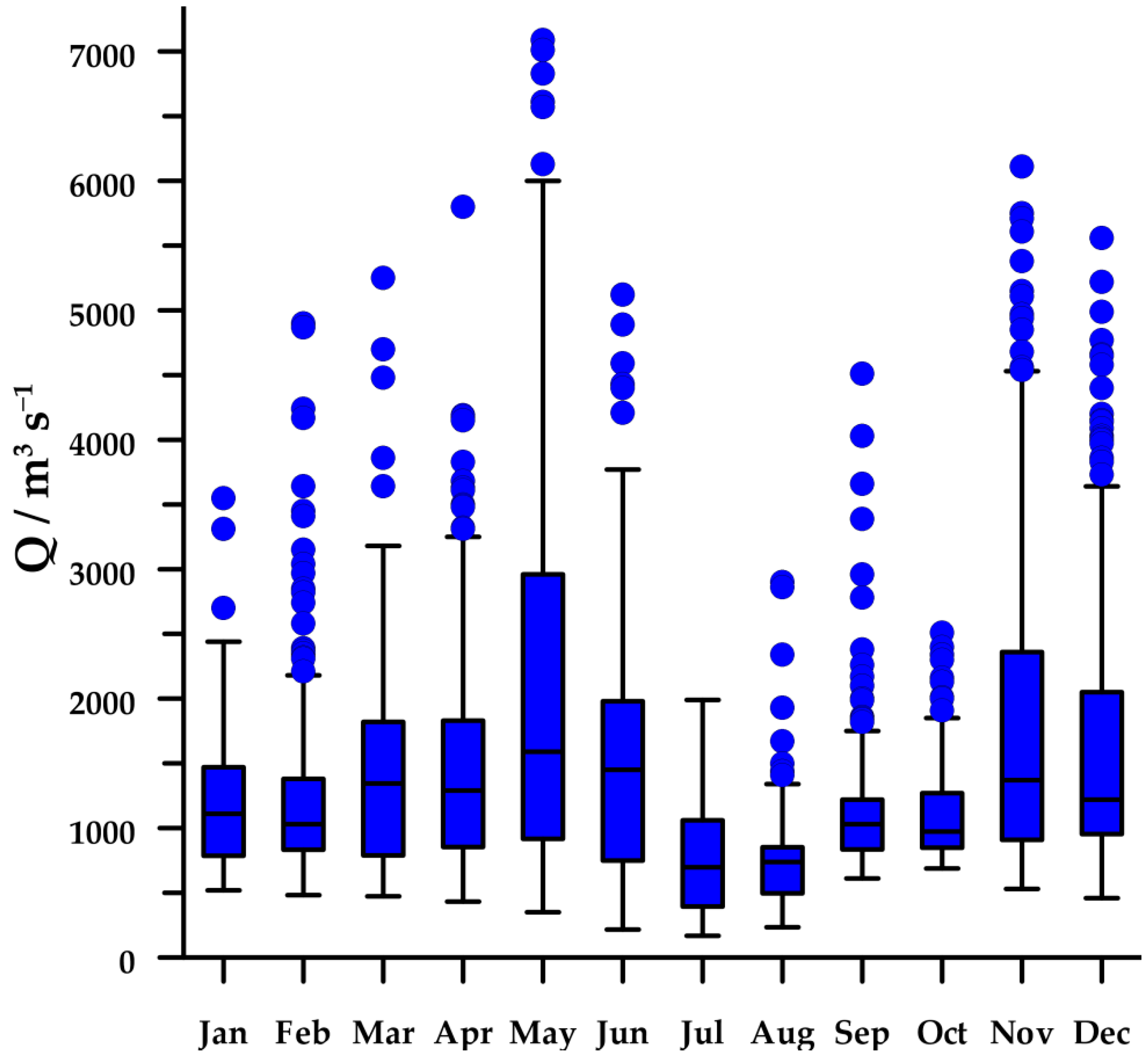
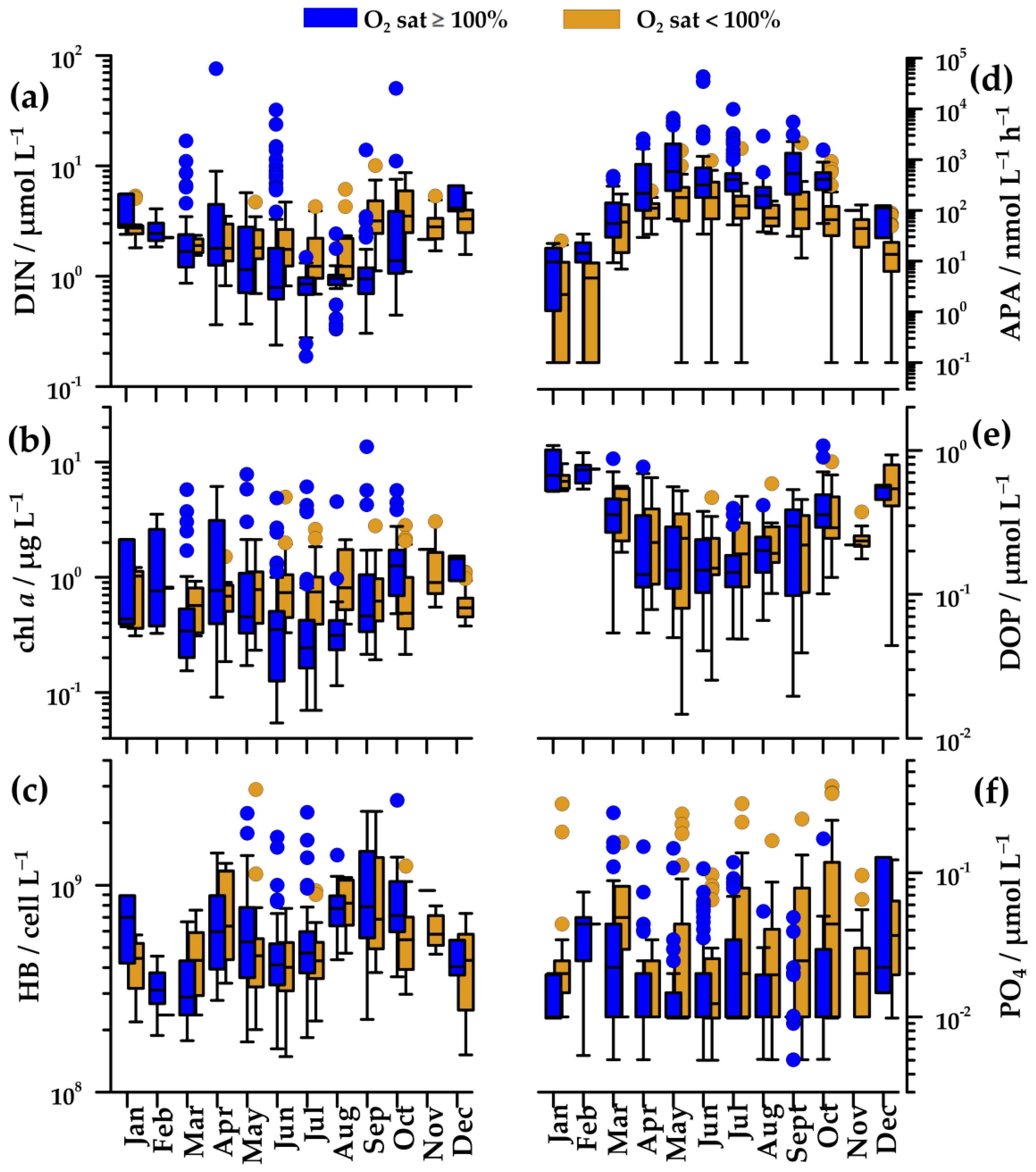
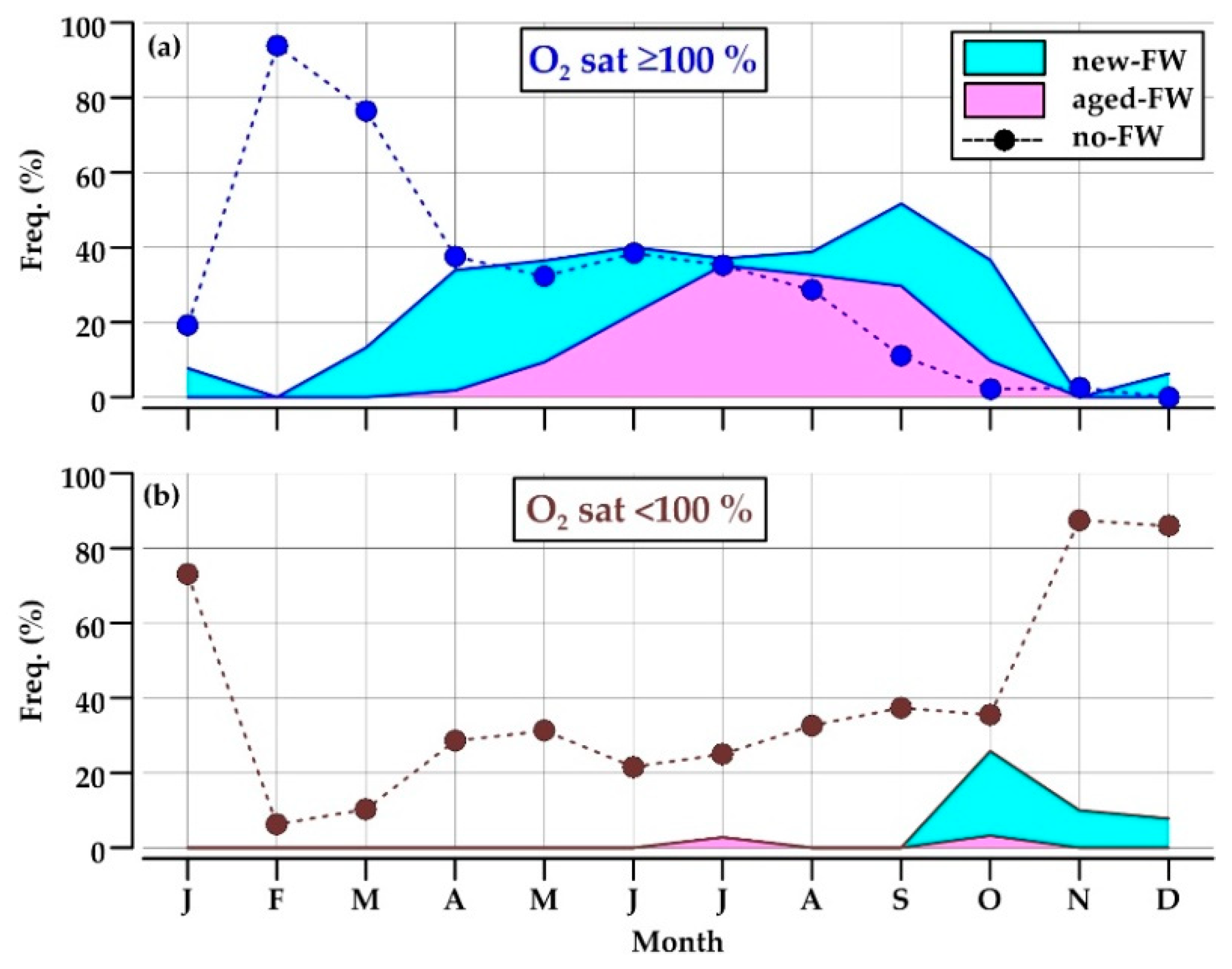
3.1. Annual Cycles of the Po River Flow, Environmental Conditions, Microbial Biomass, and APA
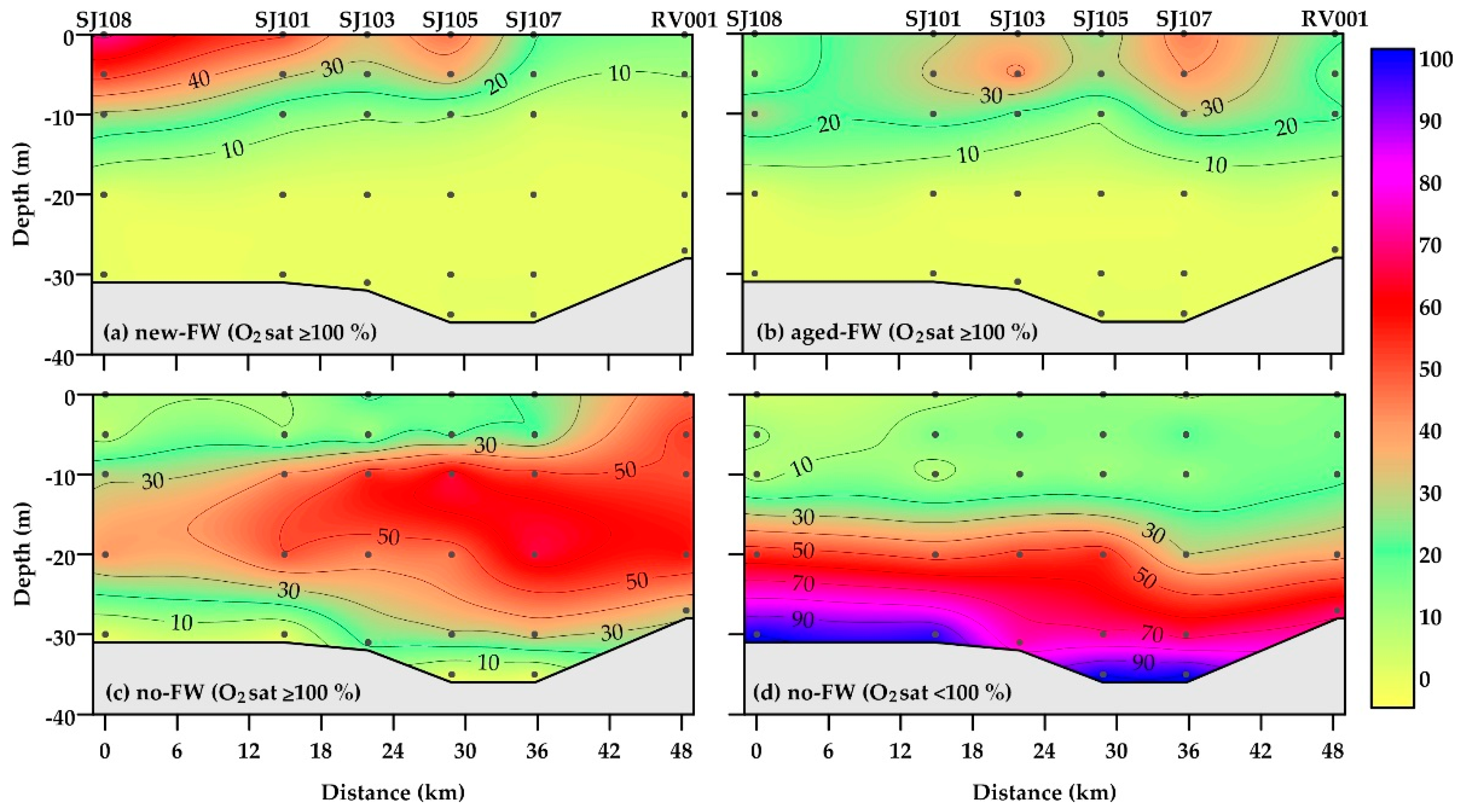
3.2. Spatial and Temporal Patterns of Water Types
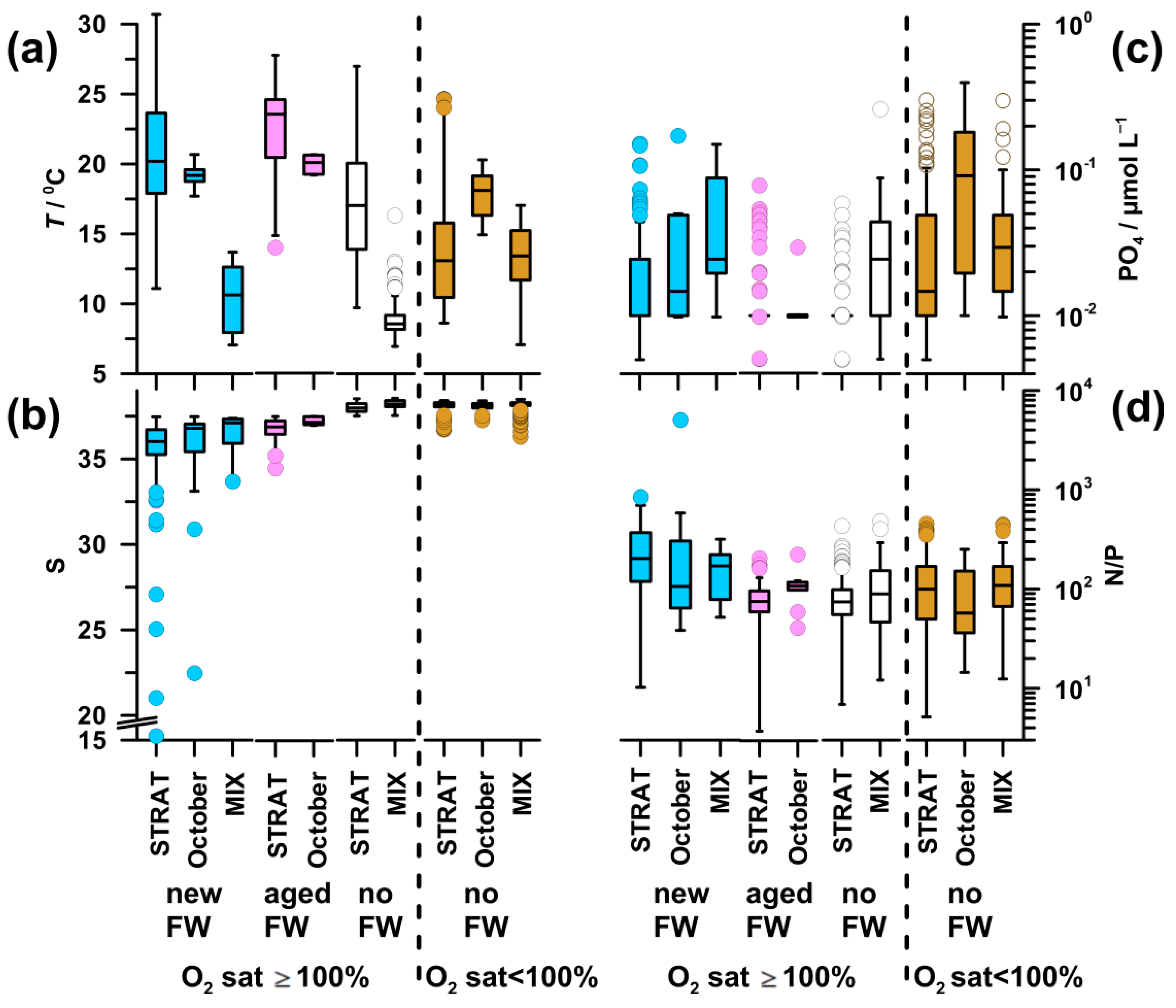
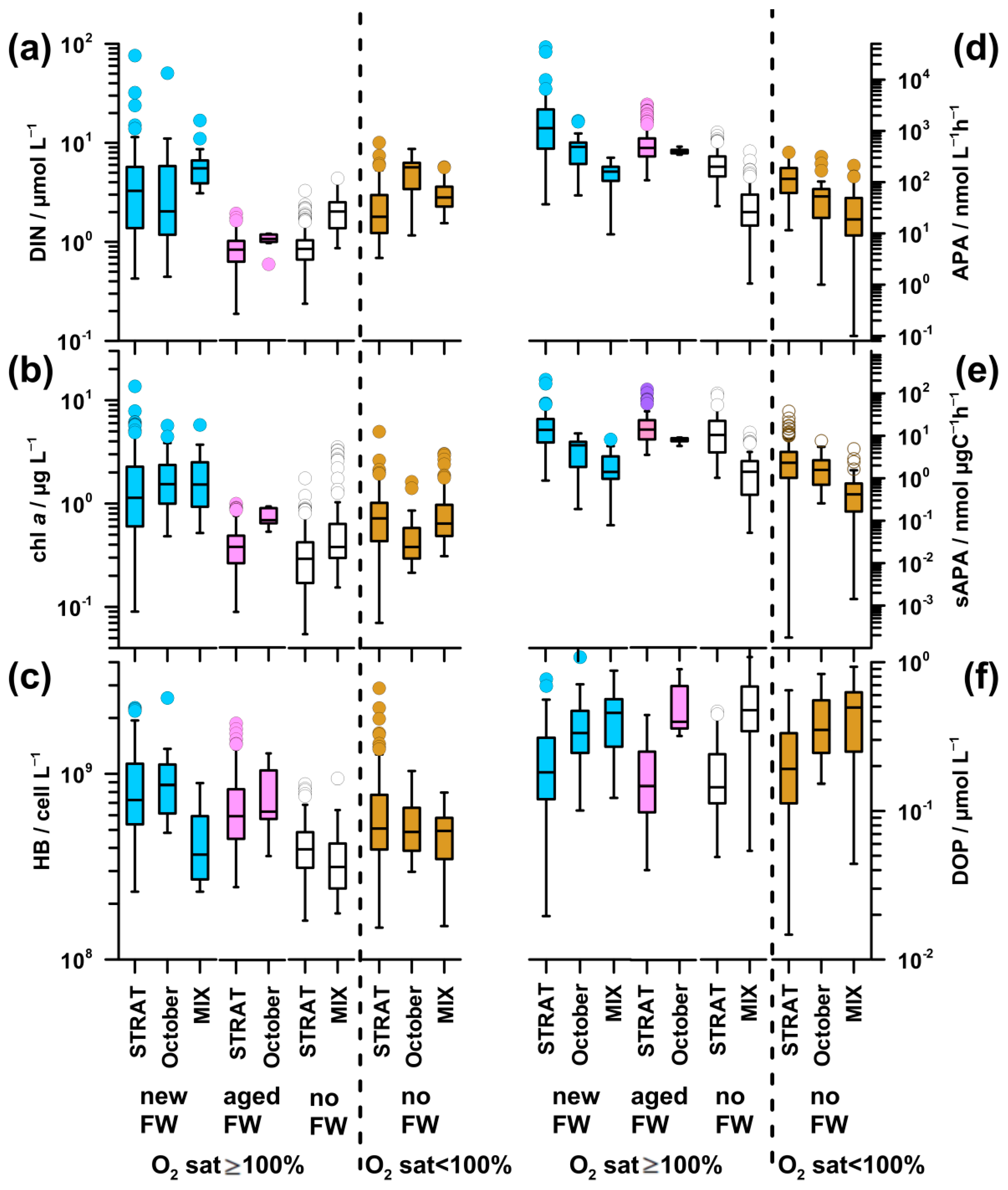
3.3. Environmental and Trophic Characteristics of Water Types
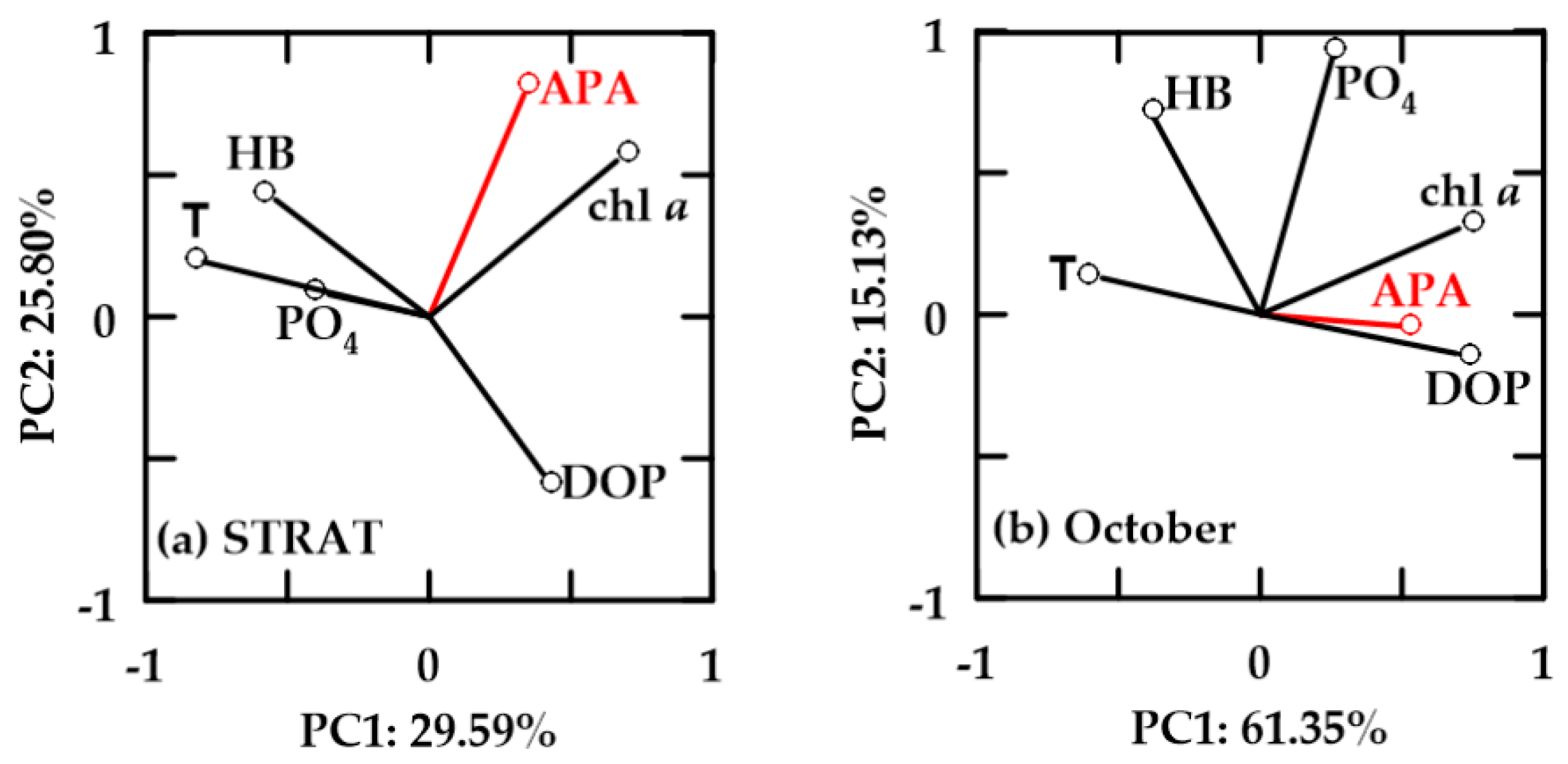
3.4. Relationships between APA and Relevant Parameters
4. Discussion
4.1. Eutrophic Events
4.2. Post-Eutrophic Conditions
4.3. Oligotrophic Conditions
4.4. Undersaturated Waters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| (a) | New-FW | Aged-FW | No-FW | No-FW | |
| O2 Sat ≥ 100% | ≥100% | ≥100% | <100% | ||
| Parameter *** | Strat, Oct, Mix | Strat, Oct | Strat, Mix | Strat, Oct, Mix | Fig. |
| T °C | 37.2 | 154.5 | 8.7 | 61.3 | Figure A1a |
| S | 10.3 | 36.4 | 5.9 | 7.0 | Figure A1b |
| DIN µmol L−1 | 9.4 | 114.2 | 6.9 | 56.1 | Figure 7a |
| PO4 µmol L−1 | 6.3 | 26.5 | 1.8 | 30.0 | Figure A1c |
| N/P | 6.4 | 4.7 | 5.1 | 8.9 | Figure A1d |
| Chl a µg L−1 | 2.0 | 21.3 | 16.9 | 21.2 | Figure 7b |
| HB cell L−1 | 18.1 | 8.0 | 0.4 | 4.1 | Figure 7c |
| APA nmol L−1h−1 | 40.2 | 130.5 | 0.9 | 122.4 | Figure 7d |
| sAPA nmol µC−1h−1 | 45.1 | 72.8 | 8.4 | 80.7 | Figure 7e |
| DOP µmol L−1 | 26.0 | 115.7 | 20.9 | 92.0 | Figure 7f |
| n **** | 15, 92, 35 | 119, 9 | 93, 158 | 118, 144, 33 | |
| Mix | Strat | Oct | Oct | ||
| (b) | O2 Sat ≥ 100% | ≥100% | ≥100% | <100% | |
| New-, No-FW | New-, Aged-, No-FW | New-, Aged-FW | New-, No-FW | Fig. | |
| T °C | 6.4 | 92.9 | 6.7 | 8.4 | Figure A1a |
| S | 38.1 | 284.8 | 9.6 | 36.7 | Figure A1b |
| DIN µmol L−1 | 34.2 | 135.9 | 7.4 | 6.4 | Figure 7a |
| PO4 µmol L−1 | 2.4 | 14.1 | 5.3 | 16.3 | Figure A1c |
| N/P | 7.8 | 96.7 | 0.3 | 18.5 | Figure A1d |
| Chl a µmol L−1 | 19.8 | 105.1 | 7.2 | 17.3 | Figure 7b |
| HB cell L−1 | 1.5 | 84.5 | 2.1 | 1.7 | Figure 7c |
| APA nmol L−1 h−1 | 17.1 | 132.2 | 0.3 | 5.6 | Figure 7d |
| sAPA nmol µgC−1 h−1 | 0.9 | 8.2 | 4.4 | 0.0 | Figure 7e |
| DOP µmol L−1 | 0.9 | 4.7 | 4.0 | 0.6 | Figure 7f |
| n ***** | 15, 93 | 92, 119, 158 | 35, 9 | 21, 33 |
References
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [Green Version]
- Ly, J.; Philippart, C.J.M.; Kromkampa, J.C. Phosphorus limitation during a phytoplankton spring bloom in the western Dutch Wadden Sea. J. Sea Res. 2014, 88, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Boge, G.; Lespilette, M.; Jamet, D.; Jamet, J.-L. Role of DOP on the alkaline phosphatase activity of size fractionated plankton in coastal waters in the NW Mediterranean Sea (Toulon Bay, France). Mar. Pollut. Bull. 2017, 117, 264–273. [Google Scholar] [CrossRef]
- Decembrini, F.; Caroppo, C.; Caruso, G.; Bergamasco, A. Linking Microbial Functioning and Trophic Pathways to Ecological Status in a Coastal Mediterranean Ecosystem. Water 2021, 13, 1325. [Google Scholar] [CrossRef]
- Krom, M.D.; Emeis, K.-C.; Van Cappellen, P. Why is the eastern Mediterranean phosphorus limited? Prog. Oceanogr. 2010, 85, 236–244. [Google Scholar] [CrossRef]
- Powley, H.R.; Krom, M.D.; Van Cappellen, P. Understanding the unique biogeochemistry of the Mediterranean Sea: Insights from a coupled phosphorus and nitrogen model, Glob. Biogeochem. Cycles 2017, 31, 1010–1031. [Google Scholar] [CrossRef]
- Lomas, M.W.; Burke, A.L.; Lomas, D.A.; Bell, D.W.; Shen, C.; Dyhrman, S.T.; Ammerman, J.W. Sargasso Sea phosphorus biogeochemistry: An important role for dissolved organic phosphorus (DOP). Biogeosciences 2010, 7, 695–710. [Google Scholar] [CrossRef] [Green Version]
- Girault, M.; Arakawa, H.; Hashihama, F. Phosphorus stress of microphytoplankton community in the western subtropical North Pacific. J. Plankton Res. 2012, 35, 146–157. [Google Scholar] [CrossRef] [Green Version]
- Moore, C.M.; Mills, M.M.; Arrigo, K.R.; Berman-Frank, I.; Bopp, L.; Boyd, P.W.; Galbraith, E.D.; Geider, R.J.; Guieu, C.; Jaccard, S.L.; et al. Processes and patterns of oceanic nutrient limitation. Nat. Geosci. 2013, 6, 701–710. [Google Scholar] [CrossRef] [Green Version]
- Ramos, J.B.E.; Schulz, K.G.; Voss, M.; Narciso, Á.; Müller, M.N.; Reis, F.V.; Cachão, M.; Azevedo, E.B. Nutrient-specific responses of a phytoplankton community: A case study of the North Atlantic Gyre, Azores. J. Plankton Res. 2017, 39, 744–761. [Google Scholar] [CrossRef] [Green Version]
- Tyrrell, T. The relative influences of nitrogen and phosphorus on oceanic primary production. Nature 1999, 400, 525–531. [Google Scholar] [CrossRef]
- Karl, D.M. The marine phosphorus cycle. In Manual of Environmental Microbiology, 3rd ed.; Hurst, C.J., Ed.; ASM Press: Washington, DC, USA, 2007; pp. 523–539. [Google Scholar]
- Hoppe, H.-G. Phosphatase activity in the sea. Hydrobiologia 2003, 493, 187–200. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Adachi, M. The utilization of organic phosphorus by eukaryotic phytoplankton in marine environments (review). Bull. Plankton Soc. Jpn. 2010, 57, 1–12. [Google Scholar]
- Lin, S.; Litaker, R.W.; Sunda, W.G. Phosphorus physiological ecology and molecular mechanisms in marine phytoplankton. J. Phycol. 2016, 52, 10–36. [Google Scholar] [CrossRef]
- Dell’Aquila, G.; Maier, U.G. Specific acclimations to phosphorus limitation in the marine diatom Phaeodactylum tricornutum. Biol. Chem. 2020, 401, 1495–1501. [Google Scholar] [CrossRef]
- Doney, S.C. The Growing Human Footprint on Coastal and Open-Ocean Biogeochemistry. Science 2010, 328, 1512–1516. [Google Scholar] [CrossRef] [Green Version]
- Peñuelas, J.; Poulter, B.; Sardans, J.; Ciais, P.; van der Velde, M.; Bopp, L.; Boucher, O.; Godderis, Y.; Hinsinger, P.; Llusia, J.; et al. Human-induced nitrogen–phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. 2013, 4, 2934. [Google Scholar] [CrossRef] [Green Version]
- Smith, V.H. Eutrophication of freshwater and coastal marine ecosystems. A global problem. Environ. Sci. Pollut. Res. 2003, 10, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Howarth, R.; Chan, F.; Conley, D.J.; Garnier, J.; Doney, S.; Marino, R.; Billen, G. Coupled biogeochemical cycles: Eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems. Front. Ecol. Environ. 2011, 9, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Cozzi, S.; Giani, M. River water and nutrient discharges in the Northern Adriatic Sea: Current importance and long term changes. Cont. Shelf Res. 2011, 31, 1881–1893. [Google Scholar] [CrossRef]
- Volf, G.; Atanasova, N.; Kompare, B.; Ožanić, N. Modeling nutrient loads to the northern Adriatic. J. Hydrol. 2013, 504, 182–193. [Google Scholar] [CrossRef]
- Viaroli, P.; Soana, E.; Pecora, S.; Laini, A.; Naldi, M.; Fano, E.A.; Nizzoli, D. Space and time variations of watershed N and P budgets and their relationships with reactive N and P loadings in a heavily impacted river basin (Po river, Northern Italy). Sci. Total Environ. 2018, 639, 1574–1587. [Google Scholar] [CrossRef]
- Bastari, A.; Micheli, F.; Ferretti, F.; Pusceddu, A.; Cerrano, C. Large marine protected areas (LMPAs) in the Mediterranean Sea: The opportunity of the Adriatic Sea. Mar. Policy 2016, 68, 165–177. [Google Scholar] [CrossRef]
- Furlan, E.; Torresan, S.; Critto, A.; Lovato, T.; Solidoro, C.; Lazzari, P.; Marcomini, A. Cumulative Impact Index for the Adriatic Sea: Accounting for interactions among climate and anthropogenic pressures. Sci. Total Environ. 2019, 670, 379–397. [Google Scholar] [CrossRef]
- Giani, M.; Djakovac, T.; Degobbis, D.; Cozzi, S.; Solidoro, C.; Umani, S.F. Recent changes in the marine ecosystems of the northern Adriatic Sea. Estuar. Coast. Shelf Sci. 2012, 115, 1–13. [Google Scholar] [CrossRef]
- Cozzi, S.; Ibáñez, C.; Lazar, L.; Raimbault, P.; Giani, M. Flow Regime and Nutrient-Loading Trends from the Largest South European Watersheds: Implications for the Productivity of Mediterranean and Black Sea’s Coastal Areas. Water 2019, 11, 1. [Google Scholar] [CrossRef] [Green Version]
- Grilli, F.; Accoroni, S.; Acri, F.; Aubry Bernardi, F.; Bergami, C.; Cabrini, M.; Campanelli, A.; Giani, M.; Guicciardi, S.; Marini, M.; et al. Seasonal and Interannual Trends of Oceanographic Parameters over 40 Years in the Northern Adriatic Sea in Relation to Nutrient Loadings Using the EMODnet Chemistry Data Portal. Water 2020, 12, 2280. [Google Scholar] [CrossRef]
- Geider, R.J.; La Roche, J. Redfield revisited: Variability of C:N:P in marine microalgae and its biochemical basis. Eur. J. Phycol. 2002, 37, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Maestrini, S.Y.; Berland, B.R.; Bréret, M.; Béchemin, C.; Poletti, R.; Rinaldi, A. Nutrients Limiting the Algal Growth Potential (AGP) in the Po River Plume and an Adjacent Area, Northwest Adriatic Sea: Enrichment Bioassays with the Test Algae Nitzschia closterium and Thalassiosira pseudonana. Estuaries 1997, 20, 416–429. [Google Scholar] [CrossRef]
- Ivančić, I.; Degobbis, D. Mechanisms of production and fate of organic phosphorus in the northern Adriatic Sea. Mar. Biol. 1987, 94, 117–125. [Google Scholar] [CrossRef]
- Cozzi, S.; Lipizer, M.; Cantoni, C.; Catalano, G. Nutrient balance in the ecosystem of the North Western Adriatic Sea. Chem. Ecol. 2002, 18, 1–12. [Google Scholar] [CrossRef]
- Ivančić, I.; Pfannkuchen, M.; Godrijan, J.; Djakovac, T.; Pfannkuchen, D.M.; Korlević, M.; Gašparović, B.; Najdek, M. Alkaline phosphatase activity related to phosphorus stress of microphytoplankton in different trophic conditions. Progr. Oceanogr. 2016, 146, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Malfatti, F.; Turk, V.; Tinta, T.; Mozetič, P.; Manganelli, M.; Samo, T.J.; Ugalde, J.A.; Kovač, N.; Stefanelli, M.; Antonioli, M.; et al. Microbial mechanisms coupling carbon and phosphorus cycles in phosphorus-limited northern Adriatic Sea. Sci. Total Environ. 2014, 470–471, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Socal, G.; Acri, F.; Bastianini, M.; Bernardi Aubry, F.; Bianchi, F.; Cassin, D.; Coppola, J.; De Lazzari, A.; Bandelj, V.; Cossarini, G.; et al. Hydrological and biogeochemical features of the Northern Adriatic Sea in the period 2003–2006. Mar. Ecol. 2008, 29, 449–468. [Google Scholar] [CrossRef]
- Kraus, R.; Supić, N. Sea dynamics impacts on the macroaggregates: A case study of the 1997 mucilage event in the northern Adriatic. Prog. Oceanogr. 2015, 138, 249–267. [Google Scholar] [CrossRef] [Green Version]
- Ciglenečki, I.; Paliaga, P.; Budiša, A.; Čanković, M.; Dautović, J.; Djakovac, T.; Dutour-Sikirić, M.; Kraus, R.; Kužat, N.; Lučić, D.; et al. Dissolved organic carbon accumulation during a bloom of invasive gelatinous zooplankton Mnemiopsis leidyi in the northern Adriatic Sea; case of the anomalous summer in 2017. J. Mar. Syst. 2021, 222, 103599. [Google Scholar] [CrossRef]
- Solidoro, C.; Bastianini, M.; Bandelj, V.; Codermatz, R.; Cossarini, G.; Melakucanu, D.; Ravagnan, E.; Salon, S.; Trevisani, S. Current state, scales of variability, and trends of biogeochemical properties in the northern Adriatic Sea. J. Geophys. Res. 2009, 114, C07S91. [Google Scholar] [CrossRef]
- Raicich, F.; Colucci, R.R. A near-surface sea temperature time series from Trieste, northern Adriatic Sea (1899–2015). Earth Syst. Sci. Data 2019, 11, 761–768. [Google Scholar] [CrossRef] [Green Version]
- Supić, N.; Orlić, M.; Degobbis, D. Istrian coastal counter current and its year-to year variability. Estuar. Coast. Shelf Sci. 2000, 51, 385–397. [Google Scholar] [CrossRef]
- Zavatarelli, M.; Baretta, J.W.; Baretta-Bekker, J.G.; Pinardi, N. The dynamics of the Adriatic Sea ecosystem: An idealized model study. Deep Sea Res. Part I Oceanogr. Res. Pap. 2000, 47, 937–970. [Google Scholar] [CrossRef] [Green Version]
- Zavatarelli, M.; Raicich, F.; Bregant, D.; Russo, A.; Artegiani, A. Climatological biogeochemical characteristics of the Adriatic Sea. J. Mar. Syst. 1998, 18, 227–263. [Google Scholar] [CrossRef]
- Viličić, D.; Kuzmić, M.; Bosak, S.; Šilović, T.; Hrustić, E.; Burić, Z. Distribution of phytoplankton along the thermohaline gradient in the northeastern Adriatic channel; winter aspect. Oceanologia 2009, 51, 495–513. [Google Scholar] [CrossRef] [Green Version]
- Kraus, R.; Supić, N.; Precali, R. Factors favoring phytoplankton blooms in the northern Adriatic: Towards the northern Adriatic empirical ecological model. Ocean Sci. 2016, 12, 19–37. [Google Scholar] [CrossRef] [Green Version]
- Artegiani, A.; Paschini, E.; Russo, A.; Bregant, D.; Raicich, F.; Pinardi, N. The Adriatic Sea general circulation. Part II: Baroclinic circulation structure. J. Phys. Oceanogr. 1997, 27, 1515–1532. [Google Scholar] [CrossRef]
- Kuzmić, M.; Janeković, I.; Book, J.W.; Martin, P.J.; Doyle, J.D. Modeling the northern Adriatic double-gyre response to intense bora wind: A revisit. J. Geophys. Res. 2006, 111, C03S13. [Google Scholar] [CrossRef] [Green Version]
- Supić, N.; Kraus, R.; Kuzmić, M.; Paschini, E.; Precali, R.; Russo, A.; Vilibić, I. Predictability of northern Adriatic winter conditions. J. Mar. Syst. 2012, 90, 42–57. [Google Scholar] [CrossRef]
- Kraus, R.; Supić, N.; Lučić, D.; Njire, J. Impact of winter oceanographic conditions on zooplankton abundance in northern Adriatic with implications on Adriatic anchovy stock prognosis. Estuar. Coast. Shelf Sci. 2015, 167, 56–66. [Google Scholar] [CrossRef]
- Russo, A.; Maccaferri, S.; Djakovac, T.; Precali, R.; Degobbis, D.; Deserti, M.; Paschini, E.; Lyons, D.M. Meteorological and oceanographic conditions in the northern Adriatic Sea during the period June 1999–July 2002: Influence on the mucilage phenomenon. Sci. Total Environ. 2005, 353, 24–38. [Google Scholar] [CrossRef]
- Lyons, D.M.; Supić, N.; Smodlaka, N. Geostrophic circulation patterns in the Northeastern Adriatic Sea and the effects of air-sea coupling: May–September 2003. J. Geophys. Res. 2007, 112, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Fuks, D.; Ivančić, I.; Najdek, M.; Lučić, D.; Njire, J.; Godrijan, J.; Marić, D.; Šilović, T.; Paliaga, P.; Blažina, M.; et al. Changes in the planktonic community structure related to trophic conditions: The case study of the northern Adriatic Sea. J. Mar. Syst. 2012, 96–97, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Ciglenečki, I.; Vilibić, I.; Dautović, J.; Vojvodić, V.; Ćosović, B.; Zemunik, P.; Dunić, N.; Mihanović, H. Dissolved organic carbon and surface active substances in the northern Adriatic Sea: Long-term trends, variability and drivers. Sci. Total Environ. 2020, 730, 139104. [Google Scholar] [CrossRef]
- Marić, D.; Frka, S.; Godrijan, J.; Tomažić, I.; Penezić, A.; Djakovac, T.; Vojvodić, V.; Precali, R.; Gašparović, B. Organic matter production during late summer–winter period in a temperate sea. Cont. Shelf Res. 2013, 55, 52–65. [Google Scholar] [CrossRef]
- Kraus, R.; Supić, N. Impact of circulation on high phytoplankton blooms and fish catch in the northern Adriatic (1990–2004). Estuar. Coast. Shelf Sci. 2011, 91, 198–210. [Google Scholar] [CrossRef]
- Celussi, M.; Del Negro, P. Microbial degradation at a shallow coastal site: Long- term spectra and rates of exoenzymatic activities in the NE Adriatic Sea. Estuar. Coast Shelf Sci. 2012, 115, 75–86. [Google Scholar] [CrossRef]
- Ivančić, I.; Degobbis, D. An optimal manual procedure for ammonia analysis in natural waters by the indophenol blue method. Water Res. 1984, 18, 1143–1147. [Google Scholar] [CrossRef]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Seawater Analysis; Pergamon Press: Oxford, UK; New York, NY, USA; Toronto, ON, Canada; Sydney, Australia; Frankfurt, Germany, 1984. [Google Scholar] [CrossRef]
- Menzel, D.W.; Corwin, N. The measurement of total phosphorus in seawater based on the liberation of organically bound fractions by persulfate oxidation. Limnol. Oceanogr. 1965, 10, 280–282. [Google Scholar] [CrossRef]
- Porter, K.G.; Feig, Y.S. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 1980, 25, 943–948. [Google Scholar] [CrossRef]
- Hoppe, H.-G. Significance of exoenzymatic activities in the ecology of brackish water: Measurements by means of methylumbelliferyl-substrates. Mar. Ecol. Progr. Ser. 1983, 11, 299–308. [Google Scholar] [CrossRef]
- Ivančić, I.; Radić, T.; Lyons, D.M.; Fuks, D.; Precali, R.; Kraus, R. Alkaline phosphatase activity in relation to nutrient status in the northern Adriatic Sea. Mar. Ecol. Progr. Ser. 2009, 378, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Fuhrman, J.A. Relationships between biovolume and biomass of naturally derived marine bacterioplankton. Appl. Environ. Microbiol. 1987, 53, 1298–1303. [Google Scholar] [CrossRef] [Green Version]
- Antia, N.J.; McAllister, C.D.; Parsons, T.R.; Stephens, K.; Strickland, J.D.H. Further measurements of primary production using a large-volume plastic sphere. Limnol. Oceanogr. 1963, 8, 166–183. [Google Scholar] [CrossRef]
- Conover, W.J. Practical Nonparametric Statistics, 3rd ed.; John Wiley and Sons: New York, NY, USA, 1999; p. 608. ISBN 9780471160687. [Google Scholar]
- Gilmartin, M.; Degobbis, D.; Revelante, N.; Smodlaka, N. The mechanism controlling plant nutrient concentrations in the northern Adriatic Sea. Int. Rev. Hydrobiol. Hydrogr. 1990, 75, 425–445. [Google Scholar] [CrossRef]
- Brush, M.J.; Giani, M.; Totti, C.; Testa, J.M.; Faganeli, J.; Ogrinc, N.; Kemp, W.M.; Umani, S.F. Eutrophication, Harmful Algae, Oxygen Depletion, and Acidification. In Coastal Ecosystems in Transition: A Comparative Analysis of the Northern Adriatic and Chesapeake Bay, 1st ed.; Malone, T.C., Malej, A., Faganeli, J., Eds.; (Geophysical Monograph Series 256); American Geophysical Union. John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2021; pp. 75–104. [Google Scholar] [CrossRef]
- Silkin, V.A.; Pautova, L.A.; Lifanchuk, A.V. Physiological regulatory mechanisms of the marine phytoplankton community structure. Russ. J. Plant Physiol. 2013, 60, 541–548. [Google Scholar] [CrossRef]
- Wasmund, N.; Nausch, G.; Hansen, A. Phytoplankton succession in an isolated upwelled Benguela water body in relation to different initial nutrient conditions. J. Mar. Syst. 2014, 140, 163–174. [Google Scholar] [CrossRef]
- Zaccone, R.; Caruso, G. Microbial enzymes in the Mediterranean Sea: Relationship with climate changes. AIMS Microbiol. 2019, 5, 251–271. [Google Scholar] [CrossRef] [PubMed]
- Labry, C.; Delmas, D.; Herbland, A. Phytoplankton and bacterial alkaline phosphatase activities in relation to phosphate and DOP availability within the Gironde plume waters (Bay of Biscay). J. Exp. Mar. Biol. Ecol. 2005, 318, 213–225. [Google Scholar] [CrossRef] [Green Version]
- Quigg, A.; Sylvan, J.B.; Gustafson, A.B.; Fisher, T.R.; Oliver, R.L.; Tozzi, S.; Ammerman, J.W. Going West: Nutrient Limitation of Primary Production in the Northern Gulf of Mexico and the Importance of the Atchafalaya River. Aquat. Geochem. 2011, 17, 519–544. [Google Scholar] [CrossRef]
- Nausch, M. Alkaline phosphatase activities and the relationship to inorganic phosphate in the Pomeranian Bight (Southern Baltic Sea). Aquat. Microb. Ecol. 1998, 16, 87–94. [Google Scholar] [CrossRef]
- Kuss, J.; Nausch, G.; Engelke, C.; von Weber, M.; Lutterbeck, H.; Naumann, M.; Waniek, J.J.; Schulz-Bull, D.E. Changes of Nutrient Concentrations in the Western Baltic Sea in the Transition Between Inner Coastal Waters and the Central Basins: Time Series from 1995 to 2016 with source analysis. Front. Earth Sci. 2020, 8, 1–13. [Google Scholar] [CrossRef]
- Wambeke, F.; Christaki, U.; Giannakourou, A.; Moutin, T.; Souvemerzoglou, K. Longitudinal and Vertical Trends of Bacterial Limitation by Phosphorus and Carbon in the Mediterranean Sea. Microb. Ecol. 2002, 43, 119–133. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Song, C.; Zhou, Y. Limitations of using extracellular alkaline phosphatase activities as a general indicator for describing P deficiency of phytoplankton in Chinese shallow lakes. J. Appl. Phycol. 2010, 22, 33–41. [Google Scholar] [CrossRef]
- Zaccone, R.; Caruso, G.; Calì, C. Heterotrophic bacteria in the northern Adriatic Sea: Seasonal changes and ectoenzyme profile. Mar. Environ. Res. 2002, 54, 1–19. [Google Scholar] [CrossRef]
- Ivančić, I.; Fuks, D.; Radić, T.; Lyons, D.M.; Šilović, T.; Kraus, R.; Precali, R. Phytoplankton and bacterial alkaline phosphatase activity in the northern Adriatic Sea. Mar. Environ. Res. 2010, 69, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thingstad, T.F.; Skjoldal, E.F.; Bohne, R.A. Phosphorus cycling and algal-bacterial competition in Sandsfjord, western Norway. Mar. Ecol. Progr. Ser. 1993, 99, 239–259. [Google Scholar] [CrossRef]
- Joint, I.; Henriksen, P.; Fonnes, G.A.; Bourne, D.; Thingstad, T.F.; Riemann, B. Competition for inorganic nutrients between phytoplankton and bacterioplankton in nutrient manipulated mesocosms. Aquat. Microb. Ecol. 2002, 29, 145–159. [Google Scholar] [CrossRef]
- Tanaka, T.; Henriksen, P.; Lignell, R.; Olli, K.; Seppälä, J.; Tamminen, T.; Thingstad, T.F. Specific affinity for phosphate uptake and specific alkaline phosphatase activity as diagnostic tools for detecting phosphorus-limited phytoplankton and bacteria. Estuar. Coast. 2006, 29, 1226–1241. [Google Scholar] [CrossRef]
- Xu, J.; Yin, K.; He, L.; Yuan, X.; Ho, A.Y.T.; Harrison, P.J. Phosphorus limitation in the northern South China Sea during late summer: Influence of the Pearl River. Deep Sea Res. Part I Oceanogr. Res. Pap. 2008, 55, 1330–1342. [Google Scholar] [CrossRef]
- Degobbis, D. A stoichiometric model of nutrient cycling in the northern Adriatic Sea and its relation to regeneration processes. Mar. Chem. 1990, 29, 235–253. [Google Scholar] [CrossRef]
- Ivančić, I.; Degobbis, D.; Pečar, O.; Fuks, D.; Manganelli, M.; Kraus, R.; Djakovac, T.; Precali, R.; Scenati, R. Northern Adriatic mesocosm experiment Rovinj 2003: Nutrient dynamics. Period. Biolog. 2004, 106, 17–22. [Google Scholar]
- Orlić, S.; Najdek, M.; Supić, N.; Ivančić, I.; Fuks, D.; Blažina, M.; Šilović, T.; Paliaga, P.; Godrijan, J.; Marić, D. Structure and variability of a microbial community at a transect crossing a double gyre structure in the northeastern Adriatic Sea. Aquat. Microb. Ecol. 2013, 69, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Smodlaka, N. Primary production of the organic matter as an indicator of the eutrophication in the northern Adriatic Sea. Sci. Total Environ. 1986, 56, 211–220. [Google Scholar] [CrossRef]
- Kršinić, F.; Bojanić, D.; Precali, R.; Kraus, R. Quantitative variability of the copepod assemblages in the northern Adriatic Sea from 1993 to 1997. Estuar. Coast. Shelf Sci. 2007, 74, 528–538. [Google Scholar] [CrossRef]
- Pugnetti, A.; Armeni, M.; Camatti, E.; Crevatin, E.; Dell’Anno, A.; Del Negro, P.; Milandri, A.; Socal, G.; Umani, S.F.; Danovaro, R. Imbalance between phytoplankton production and bacterial carbon demand in relation with mucilage formation in the Northern Adriatic Sea. Sci. Total Environ. 2005, 353, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Umani, S.F.; Del Negro, P.; Larato, C.; De Vittor, C.; Cabrini, M.; Celio, M.; Falconi, C.; Tamberlich, F.; Azam, F. Major inter-annual variations in microbial dynamics in the Gulf of Trieste (northern Adriatic Sea) and their ecosystem implications. Aquat. Microb. Ecol. 2007, 46, 163–175. [Google Scholar] [CrossRef]
- Zaccone, R.; Azzaro, M.; Caruso, G.; Crisafi, E.; Decembrini, F.; Leonardi, M.; Maimone, G.; Monticelli, L.; La Ferla, R. Effects of climate changes on the microbial activities and prokaryotic abundances in the euphotic layer of the Central Mediterranean Sea. Hydrobiologia 2019, 842, 5–30. [Google Scholar] [CrossRef]
- Gotschalk, C.C.; Alldredge, A.L. Enhanced primary production and nutrient regeneration within aggregated marine diatoms. Mar. Biol. 1989, 103, 119–129. [Google Scholar] [CrossRef]
- Grossart, H.-P.; Ploug, H. Microbial degradation of organic carbon and nitrogen on diatom aggregates. Limnol. Oceanogr. 2001, 46, 267–277. [Google Scholar] [CrossRef]
- Ivančić, I.; Najdek, M.; Kraus, R. Importance of Phytoplankton and Bacterial Alkaline Phosphatase Activity in Different Trophic Conditions: The Case Study of the Northern Adriatic Sea; Institute Ruđer Bošković: Rovinj, Croatia, 2022; manuscript in preparation. [Google Scholar]
- Siokou-Frangou, I.; Christaki, U.; Mazzochi, M.G.; Montresor, M.; Ribera d’Alcala, M.; Vaqué, D.; Zingone, A. Plankton in the open Mediterranean Sea: A review. Biogeosciences 2010, 7, 1543–1586. [Google Scholar] [CrossRef] [Green Version]
- Spillman, C.M.; Imberger, J.; Hamilton, D.P.; Hipsey, M.R.; Romero, J.R. Modeling the effects of Po River discharge, internal nutrient cycling and hydrodynamics on biogeochemistry of the northern Adriatic Sea. J. Mar. Syst. 2007, 68, 167–200. [Google Scholar] [CrossRef]
- Ivančić, I.; Godrijan, J.; Pfannkuchen, M.; Marić, D.; Gašparović, B.; Ðakovac, T.; Najdek, M. Survival mechanisms of phytoplankton in conditions of stratification induced deprivation of orthophosphate: Northern Adriatic case study. Limnol. Oceanogr. 2012, 57, 1721–1731. [Google Scholar] [CrossRef] [Green Version]
- Najdek, M.; Paliaga, P.; Šilović, T.; Batistić, M.; Garić, R.; Supić, N.; Ivančić, I.; Ljubimir, S.; Korlević, M.; Jasprica, N.; et al. Picoplankton community structure before, during and after convection event in the offshore waters of the Southern Adriatic Sea. Biogeosciences 2014, 11, 2645–2659. [Google Scholar] [CrossRef] [Green Version]
- Riemann, B.; Simonsen, P.; Stensgaard, L. The carbon and chlorophyll content of phytoplankton from various nutrient regimes. J. Plankton Res. 1989, 11, 1037–1045. [Google Scholar] [CrossRef] [Green Version]
- Felip, M.; Catalan, J. The relationship between phytoplankton biovolume and chlorophyll in a deep oligotrophic lake: Decoupling in their spatial and temporal maxima. J. Plankton Res. 2000, 22, 91–105. [Google Scholar] [CrossRef]
- Pugnetti, A.; Camatti, E.; Mangoni, O.; Morabito, G.; Oggioni, A.; Saggiomo, V. Phytoplankton production in Italian freshwater and marine ecosystems: State of the art and perspectives. Chem. Ecol. 2006, 22, S49–S69. [Google Scholar] [CrossRef]
- Celussi, M.; Zoccarato, L.; Bernardi Aubry, F.; Bastianini, M.; Casotti, R.; Balestra, C.; Giani, M.; Del Negro, P. Links between microbial processing of organic matter and the thermohaline and productivity features of a temperate river-influenced Mediterranean coastal area. Estuar. Coast. Shelf Sci. 2019, 228, 106378. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivančić, I.; Kraus, R.; Najdek, M.; Cozzi, S. Ecological Importance of Alkaline Phosphatase Activity in Changing Marine Environmental Conditions. Water 2021, 13, 2750. https://doi.org/10.3390/w13192750
Ivančić I, Kraus R, Najdek M, Cozzi S. Ecological Importance of Alkaline Phosphatase Activity in Changing Marine Environmental Conditions. Water. 2021; 13(19):2750. https://doi.org/10.3390/w13192750
Chicago/Turabian StyleIvančić, Ingrid, Romina Kraus, Mirjana Najdek, and Stefano Cozzi. 2021. "Ecological Importance of Alkaline Phosphatase Activity in Changing Marine Environmental Conditions" Water 13, no. 19: 2750. https://doi.org/10.3390/w13192750