Role of Borate Buffer in Organic Degradation by Peroxymonosulfate in the Presence of Metal Oxides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Experimental Procedure
2.3. Analytical Methods
3. Results and Discussion
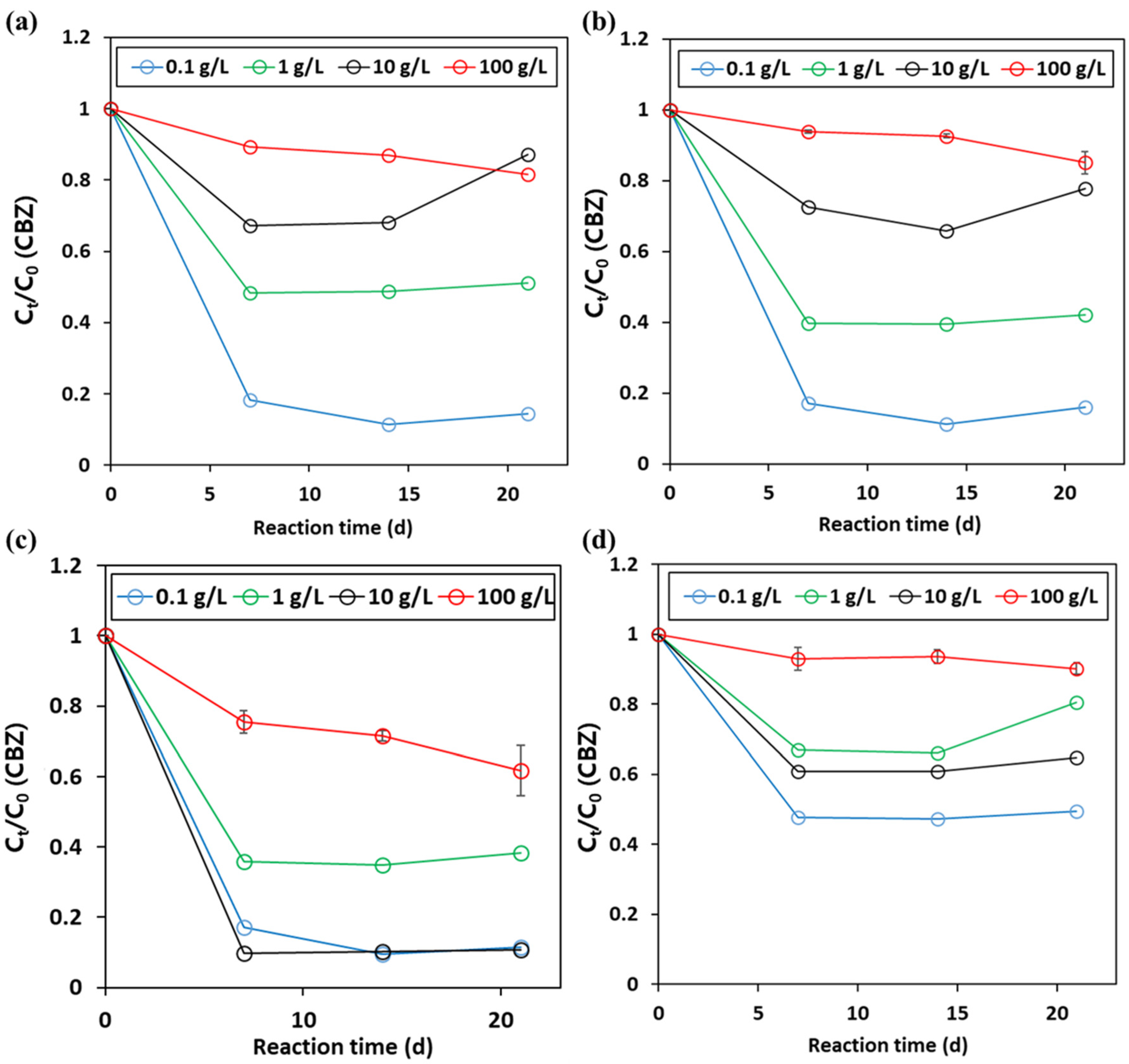
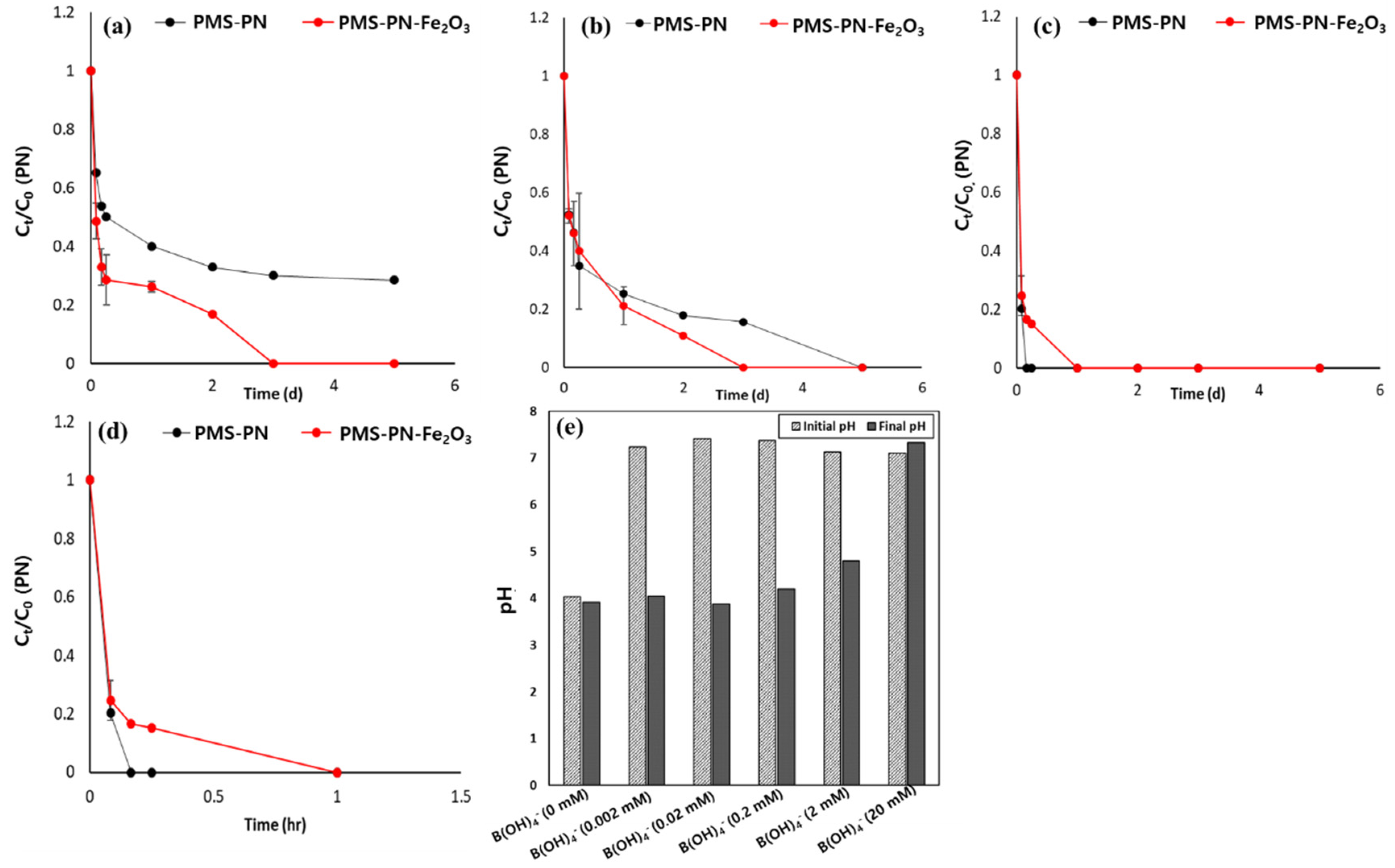
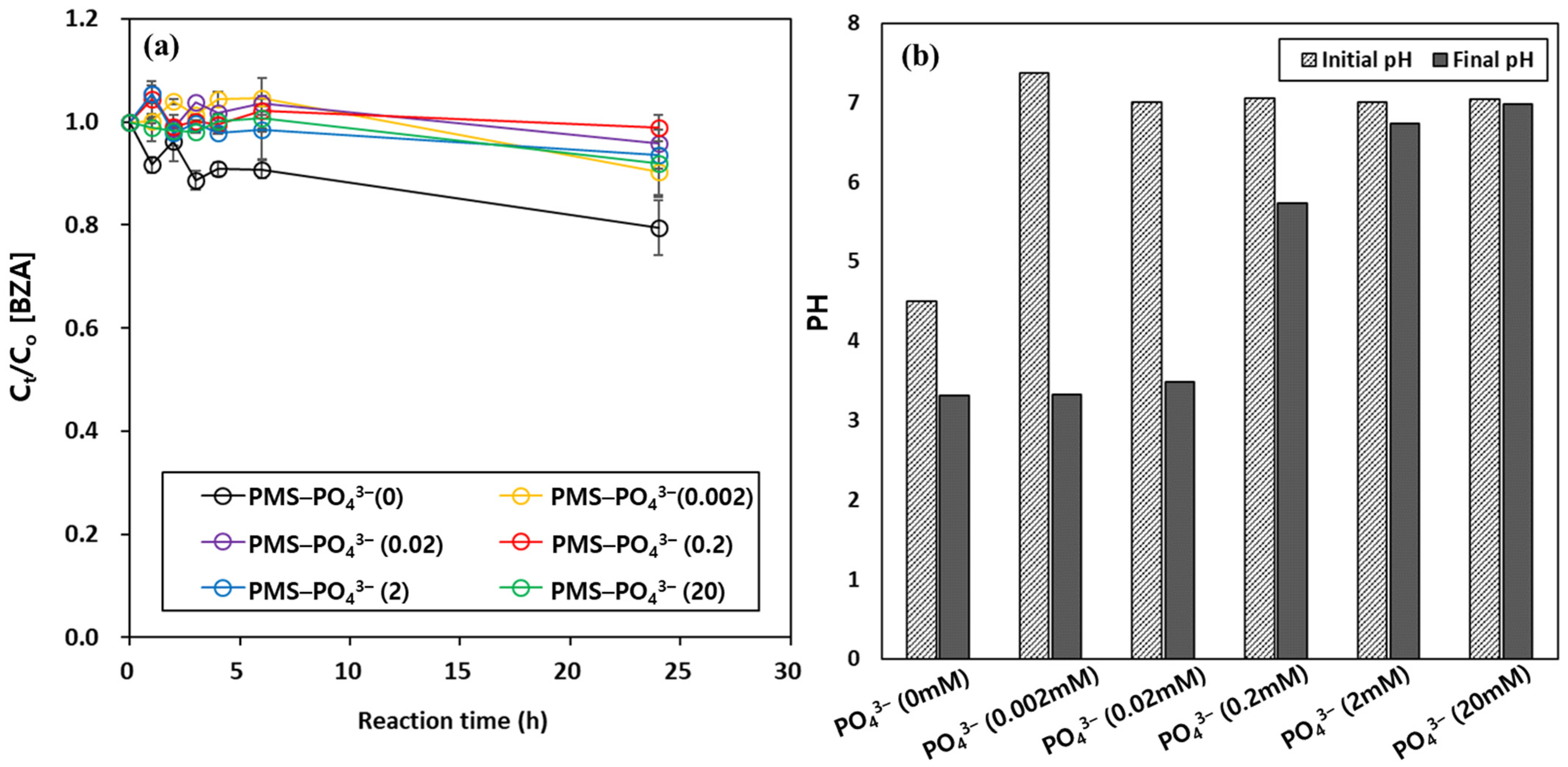
3.1. Organic Degradation by the Peroxymonosulfate (PMS) Oxidant in the Presence of Metal Oxides and Borate Buffer
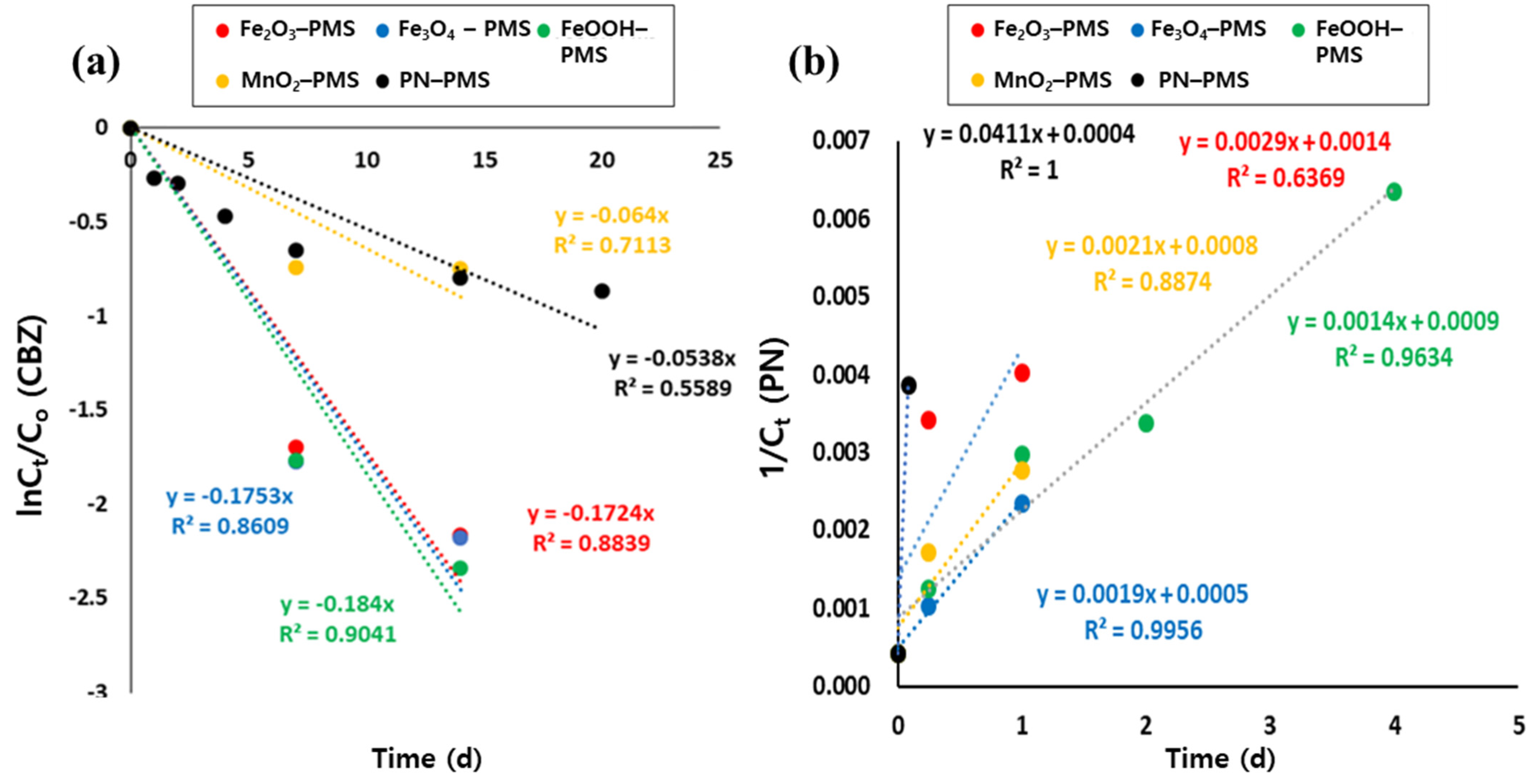
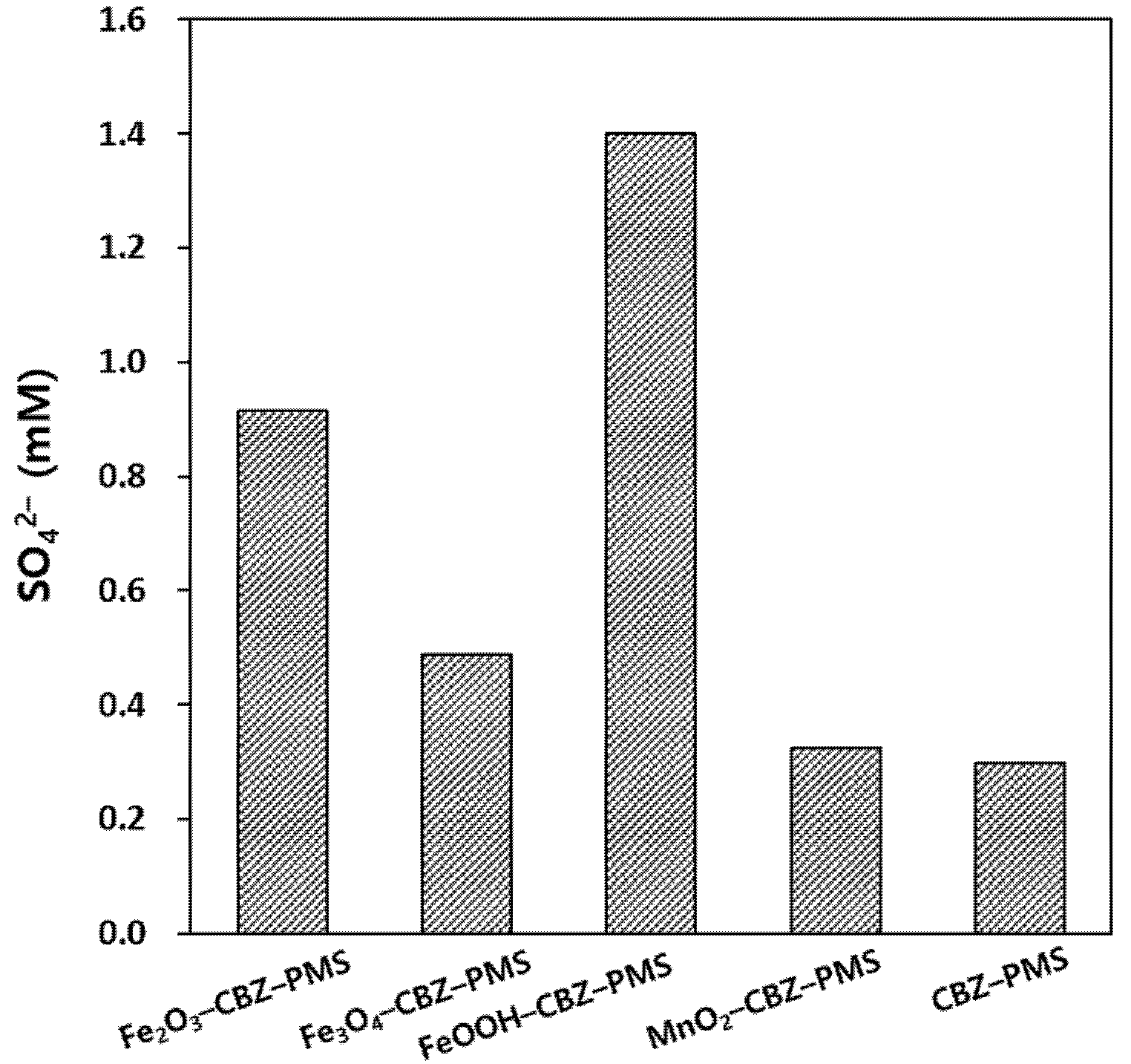
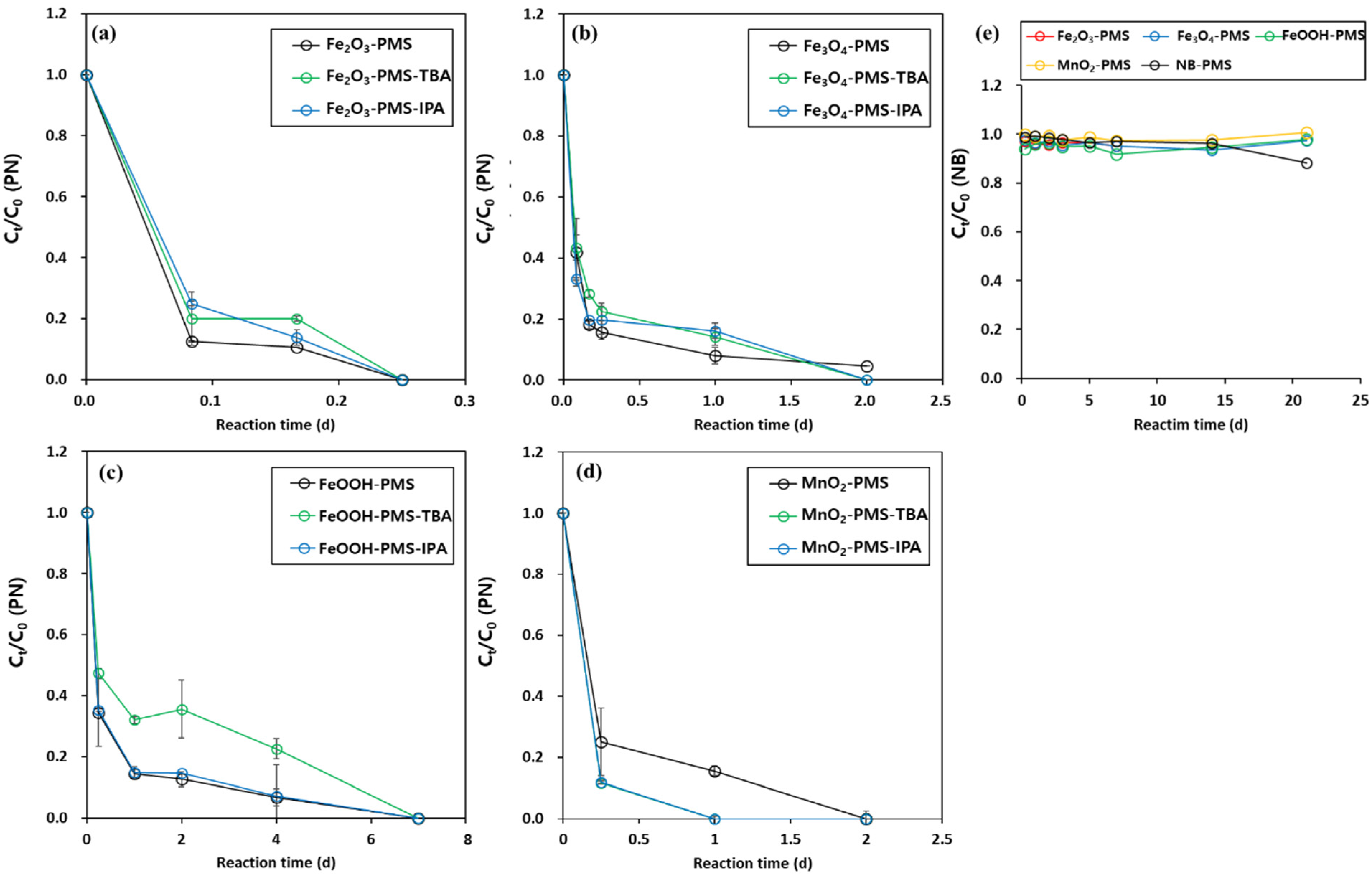
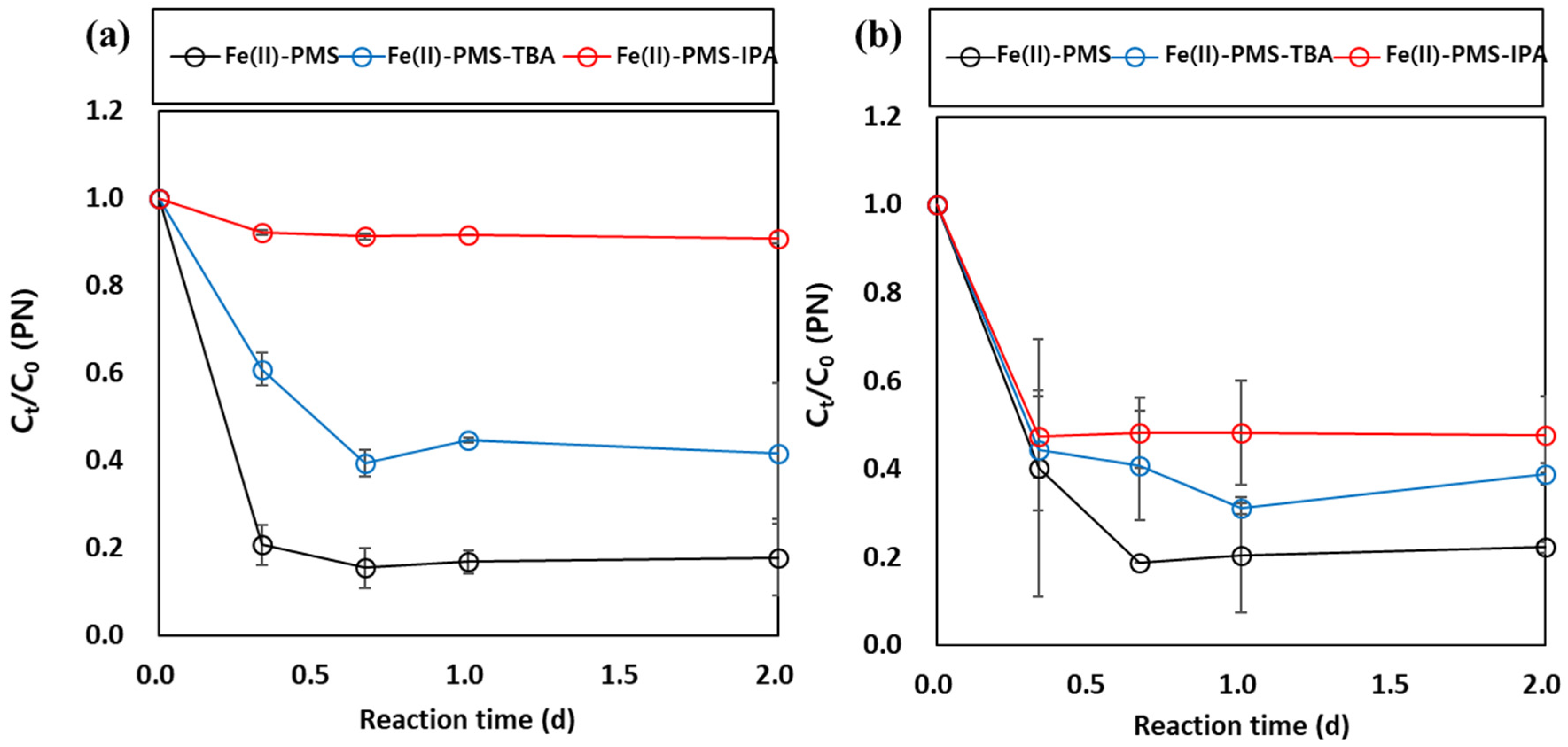
3.2. Identification of the Major Reactive Species in PMS Oxidation Systems in the Presence of Metal Oxides and Borate Buffer
3.3. Optimization of Borate Buffer Concentration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Yu, D.; Wang, D.; Yang, T.; Li, Z.; Wu, M.; Petru, M.; Crittenden, J. Accelerating Fe(III)/Fe(II) via Fe(II) substitution for enhancing Fenton-like performance of Fe-MOFs. Appl. Catal B 2021, 286, 119859. [Google Scholar] [CrossRef]
- Mishra, N.; Reddy, R.; Kuila, A.; Rani, A.; Nawaz, A.; Pichiah, S. A Review on Advanced Oxidation Processes for Effective Water Treatment. Curr. World Environ. 2017, 12, 469–489. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M.; Gohari, F. Degradation of 2,4,6-trichlorophenol in aqueous solutions using peroxymonosulfate/activated carbon/UV process via sulfate and hydroxyl radicals. J. Water Process. Eng. 2016, 9, 22–28. [Google Scholar] [CrossRef]
- Lee, J.; Von Gunten, U.; Kim, J.-H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef]
- Song, W.; Li, J.; Wang, Z.; Zhang, X. A mini review of activated methods to persulfate-based advanced oxidation process. Water Sci. Technol. 2018, 79, 573–579. [Google Scholar] [CrossRef]
- Brienza, M.; Katsoyiannis, I.A. Sulfate Radical Technologies as Tertiary Treatment for the Removal of Emerging Contaminants from Wastewater. Sustainability 2017, 9, 1604. [Google Scholar] [CrossRef] [Green Version]
- Matta, R.; Tlili, S.; Chiron, S.; Barbati, S. Removal of carbamazepine from urban wastewater by sulfate radical oxidation. Environ. Chem. Lett. 2010, 9, 347–353. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Tsitonaki, A.; Smets, B.F.; Bjerg, P.L. Effects of heat-activated persulfate oxidation on soil microorganisms. Water Res. 2008, 42, 1013–1022. [Google Scholar] [CrossRef]
- Kim, E.-J.; Park, S.; Adil, S.; Lee, S.; Cho, K. Biogeochemical Alteration of an Aquifer Soil during In Situ Chemical Oxidation by Hydrogen Peroxide and Peroxymonosulfate. Environ. Sci. Technol. 2021, 55, 5301–5311. [Google Scholar] [CrossRef]
- Zeng, H.; Zhao, X.; Zhao, F.; Park, Y.; Repo, E.; Thangaraj, S.K.; Jänis, J.; Sillanpää, M. Oxidation of 2,4-dichlorophenol in saline water by unactivated peroxymonosulfate: Mechanism, kinetics and implication for in situ chemical oxidation. Sci. Total. Environ. 2020, 728, 138826. [Google Scholar] [CrossRef]
- Oba, B.T.; Zheng, X.; Aborisade, M.A.; Liu, J.; Yohannes, A.; Kavwenje, S.; Sun, P.; Yang, Y.; Zhao, L. Remediation of trichloroethylene contaminated soil by unactivated peroxymonosulfate: Implication on selected soil characteristics. J. Environ. Manag. 2021, 285, 112063. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Gao, J.; Dionysiou, D.D.; Liu, C.; Zhou, D. Activation of Persulfate by Quinones: Free Radical Reactions and Implication for the Degradation of PCBs. Environ. Sci. Technol. 2013, 47, 4605–4611. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jiang, J.; Gao, Y.; Ma, J.; Pang, S.-Y.; Li, J.; Lu, X.-T.; Yuan, L.-P. Activation of Peroxymonosulfate by Benzoquinone: A Novel Nonradical Oxidation Process. Environ. Sci. Technol. 2015, 49, 12941–12950. [Google Scholar] [CrossRef]
- Wen, G.; Qiang, C.; Feng, Y.; Huang, T.; Ma, J. Bromate formation during the oxidation of bromide-containing water by ozone/peroxymonosulfate proess: Influencing factors and mechanisms. Chem. Eng. J. 2018, 352, 316–324. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Oyekunle, D.T.; Zhou, X.; Shahzad, A.; Chen, Z. Review on carbonaceous materials as persulfate activators: Structure-performance relationship, mechanism and future perspectives on water treatment. J. Mater. Chem. A 2021, 9, 8012. [Google Scholar] [CrossRef]
- Zhao, C.; Shao, B.; Yan, M.; Liu, Z.; Liang, Q.; He, Q.; Wu, T.; Liu, Y.; Pan, Y.; Huang, J.; et al. Activation of perxoxymonosulfate by biochar-based catalysts and applications in the degradation of organic contaminants: A review. Chem. Eng. J. 2021, 416, 128829. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate Constants for Reactions of Inorganic Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
- Munter, R. Advanced oxidation processe –Current status and prospects. Proc. Est. Acad. Sci. Chem. 2001, 50, 59–80. [Google Scholar]
- Wang, L.; Jiang, J.; Pang, S.-Y.; Zhou, Y.; Li, J.; Sun, S.; Gao, Y.; Jiang, C. Oxidation of bisphenol A by nonradical activation of peroxymonosulfate in the presence of amorphous manganese dioxide. Chem. Eng. J. 2018, 352, 1004–1013. [Google Scholar] [CrossRef]
- Yang, Y.; Banerjee, G.; Brudvig, G.W.; Kim, J.-H.; Pignatello, J.J. Oxidation of Organic Compounds in Water by Unactivated Peroxymonosulfate. Environ. Sci. Technol. 2018, 52, 5911–5919. [Google Scholar] [CrossRef]
- Nihemaiti, M.; Permala, R.R.; Croué, J.-P. Reactivity of unactivated peroxymonosulfate with nitrogenous compounds. Water Res. 2019, 169, 115221. [Google Scholar] [CrossRef]
- Park, S.; Anggraini, T.M.; Chung, J.; Kang, P.K.; Lee, S. Microfluidic pore model study of precipitates induced by the pore-scale mixing of an iron sulfate solution with simulated groundwater. Chemosphere 2021, 271, 129857. [Google Scholar] [CrossRef] [PubMed]
- DeSimone, L.A.; Pope, J.P.; Ransom, K.M. Machine-learning models to map pH and redox conditions in groundwater in a layered aquifer system, Northern Atlantic Coastal Plain, eastern USA. J. Hydrol. Reg. Stud. 2020, 30, 100697. [Google Scholar] [CrossRef]
- Chen, Z.; Wan, Q.; Wen, G.; Luo, X.; Xu, X.; Wang, J.; Li, K.; Huang, T.; Ma, J. Effect of borate buffer on organics degradation with unactivated peroxymonosulfate: Influencing factors and mechanisms. Sep. Purif. Technol. 2020, 256, 117841. [Google Scholar] [CrossRef]
- Xu, X.; Thomson, N.R. Hydrogen Peroxide Persistence in the Presence of Aquifer Materials. Soil Sediment. Contam. Int. J. 2010, 19, 602–616. [Google Scholar] [CrossRef]
- Murad, E.; Fischer, W.R. The geochemical cycle of iron. In Iron in Soils and Clay Minerals; Stucki, J.W., Goodman, B.A., Schwertmann, U., Reidel, D., Eds.; Springer: Dordrecht, The Netherlands, 1988; pp. 1–18. [Google Scholar]
- Fuller, W.W.; Warrick, A.W. Soils in Waste Treatment and Utilization. Land Treatment I; CR Press, Inc.: Boca Raton, FL, USA, 1985. [Google Scholar]
- Yu, M.; Teel, A.L.; Watts, R.J. Activation of Peroxymonosulfate by Subsurface Minerals. J. Contam. Hydrol. 2016, 191, 33–43. [Google Scholar] [CrossRef]
- Liu, H.; Bruton, T.A.; Li, W.; Buren, J.V.; Prasse, C.; Doyle, F.M.; Sedlak, D.L. Oxidation of benzene by persulfate in the presence of Fe(III)- and Mn(VI)-containing oxides: Stoichiometric efficiency and transformation products. Environ. Sci. Technol. 2016, 50, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical Generation by the Interaction of Transition Metals with Common Oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef]
- Feng, J.; Aki, S.N.V.K.; Chateauneuf, J.E.; Brennecke, J.F. Hydroxyl Radical Reactivity with Nitrobenzene in Subcritical and Supercritical Water. J. Am. Chem. Soc. 2002, 124, 6304–6311. [Google Scholar] [CrossRef] [PubMed]
- Sang, W.; Li, Z.; Huang, M.; Wu, X.; Li, D.; Mei, L.; Cui, J. Enhanced transition metal oxide based peroxymonosulfate activation by hydroxylamine for the degradation of sulfamethoxazole. Chem. Eng. J. 2019, 383, 123057. [Google Scholar] [CrossRef]
- Li, W.; Orozco, R.; Camargos, N.; Liu, H. Mechanisms on the Impacts of Alkalinity, pH, and Chloride on Persulfate-Based Groundwater Remediation. Environ. Sci. Technol. 2017, 51, 3948–3959. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Chen, W.; Xu, H.; Shen, Z.; Lin, T.; Hu, K.; Lu, C.H.; Xie, Z. Novel a-Fe2O3/MXene nanocomposite as heterogenous activator of peroxymonosulfate for the degradation of salicyclic acid. J. Haz. Mater. 2020, 382, 121964. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.-D.; Dong, Z.; Hu, Z.-T.; Lim, T.-T. A novel quasi-cubic CuFe2O4–Fe2O3 catalyst prepared at low temperature for enhanced oxidation of bisphenol A via peroxymonosulfate activation. J. Mater. Chem. A 2015, 3, 22208–22217. [Google Scholar] [CrossRef]
- Ji, F.; Li, C.; Wei, X.; Yu, J. Efficient performance of porous Fe2O3 in heterogeneous activation of peroxymonosulfate for decolorization of Rhodamine B. Chem. Eng. J. 2013, 231, 434–440. [Google Scholar] [CrossRef]
- Gong, C.; Chen, F.; Yang, Q.; Luo, K.; Yao, F.; Wang, S.; Wang, X.; Wu, J.; Li, X.; Wang, D.; et al. Heterogeneous activation of peroxymonosulfate by Fe-Co layered doubled hydroxide for efficient catalytic degradation of Rhoadmine B. Chem. Eng. J. 2017, 321, 222–232. [Google Scholar] [CrossRef]
- Li, L.; Wu, H.; Chen, H.; Zhang, J.; Xu, X.; Wang, S.; Wang, S.; Sun, H. Heterogeneous activation of peroxymonosulfate by hierarchically porous cobalt/iron bimetallic oxide nanosheets for degradation of phenol solutions. Chemosphere 2020, 256, 127160. [Google Scholar] [CrossRef]
- Fan, J.; Zhao, Z.; Ding, Z.; Liu, J. Synthesis of different crystallographic FeOOH catalysts for peroxymonosulfate activation towards organic matter degradation. RSC Adv. 2018, 8, 7269–7279. [Google Scholar] [CrossRef] [Green Version]
- Othman, I.; Zain, J.H.; Abu Haija, M.; Banat, F. Catalytic activation of peroxymonosulfate using CeVO4 for phenol degradation: An insight into the reaction pathway. Appl. Catal. B Environ. 2020, 266, 118601. [Google Scholar] [CrossRef]
- Muhammad, S.; Saputra, E.; Sun, P.H.; Izidoro, J.D.C.; Fungaro, D.A.; Ang, H.M.; Tade, M.; Wang, S. Coal fly ash supported Co3O4 catalysts for phenol degradation using peroxymonosulfate. RSC Adv. 2012, 2, 5645–5650. [Google Scholar] [CrossRef]
- Saputra, E.; Muhammad, S.; Sun, P.H.; Ang, H.-M.; Tade, M.; Wang, S. Manganese oxides at different oxidation states for heterogeneous activation of peroxymonosulfate for phenol degradation in aqueous solutions. Appl. Catal. B Environ. 2013, 142–143, 729–735. [Google Scholar] [CrossRef] [Green Version]
- Metiu, H.; Chrétien, S.; Hu, Z.; Li, B.; Sun, X.Y. Chemistry of Lewis Acid-Base paris on oxide surfaces. J. Phys. Chem. C. 2012, 116, 10439–10450. [Google Scholar] [CrossRef]
- Lou, X.; Wu, L.; Guo, Y.; Chen, C.; Wang, Z.; Xiao, D.; Fang, C.; Liu, J.; Zhao, J.; Lu, S. Peroxymonosulfate activation by phosphate anion for organics degradation in water. Chemosphere 2014, 117, 582–585. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Oh, S.; Kim, I. Role of Borate Buffer in Organic Degradation by Peroxymonosulfate in the Presence of Metal Oxides. Water 2021, 13, 2698. https://doi.org/10.3390/w13192698
Park S, Oh S, Kim I. Role of Borate Buffer in Organic Degradation by Peroxymonosulfate in the Presence of Metal Oxides. Water. 2021; 13(19):2698. https://doi.org/10.3390/w13192698
Chicago/Turabian StylePark, Saerom, Sungjik Oh, and Ilho Kim. 2021. "Role of Borate Buffer in Organic Degradation by Peroxymonosulfate in the Presence of Metal Oxides" Water 13, no. 19: 2698. https://doi.org/10.3390/w13192698
APA StylePark, S., Oh, S., & Kim, I. (2021). Role of Borate Buffer in Organic Degradation by Peroxymonosulfate in the Presence of Metal Oxides. Water, 13(19), 2698. https://doi.org/10.3390/w13192698




