Levels and Potential Health Hazards of Chlorinated Pesticides in Surface Water Samples of Charsadda Area of Pakistan Using SPME-GC-ECD Technique
Abstract
:1. Introduction
2. Materials and Methods
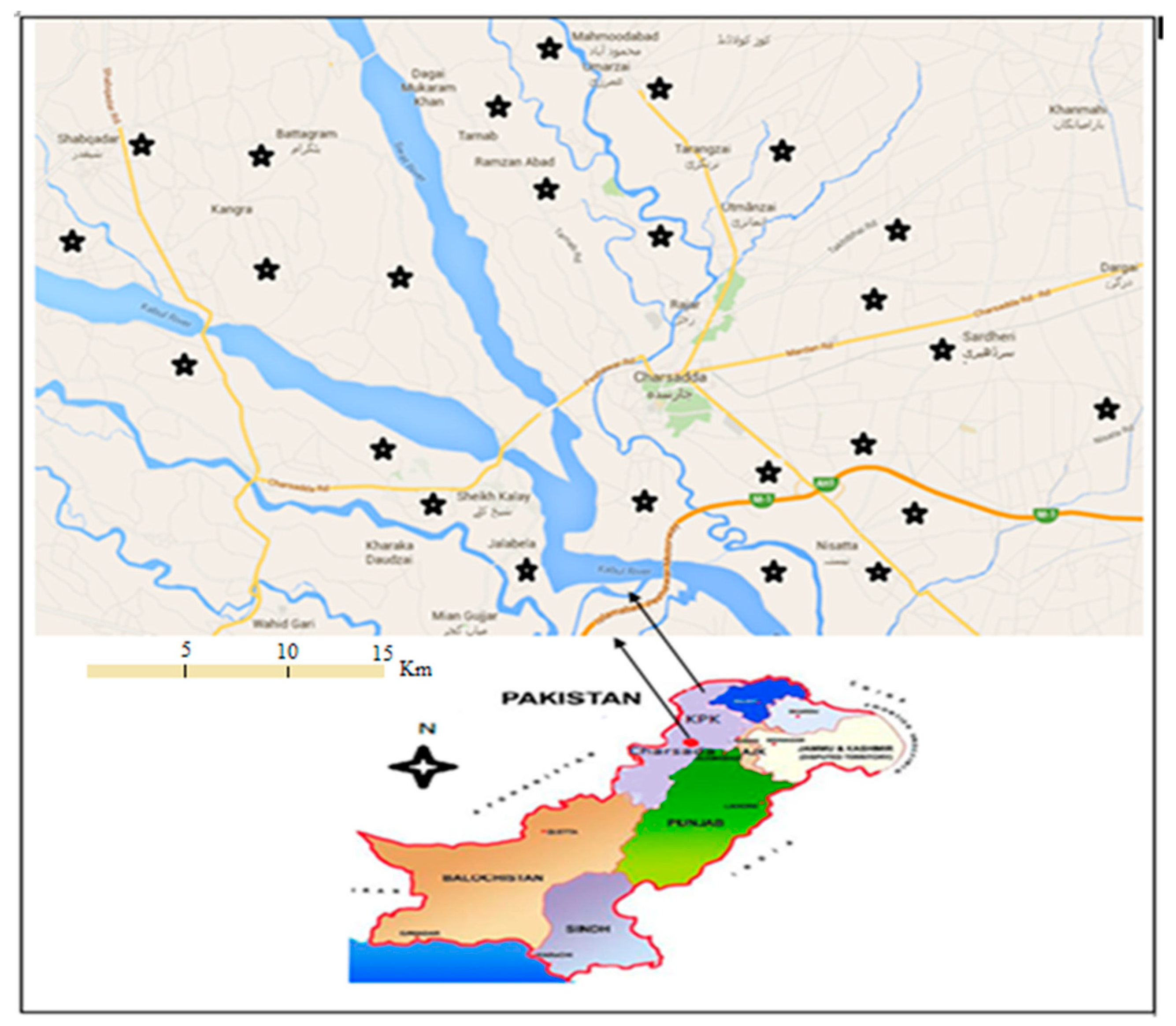
2.1. Sampling Area
2.2. Extraction of Pesticides from Water
2.3. Standards and Reagents
2.4. Solutions Preparation
2.5. Samples Collection
2.6. Analytical Instrumentation and Operating Conditions for GC-ECD
2.7. Health Risk Assessment Model
3. Results and Discussion
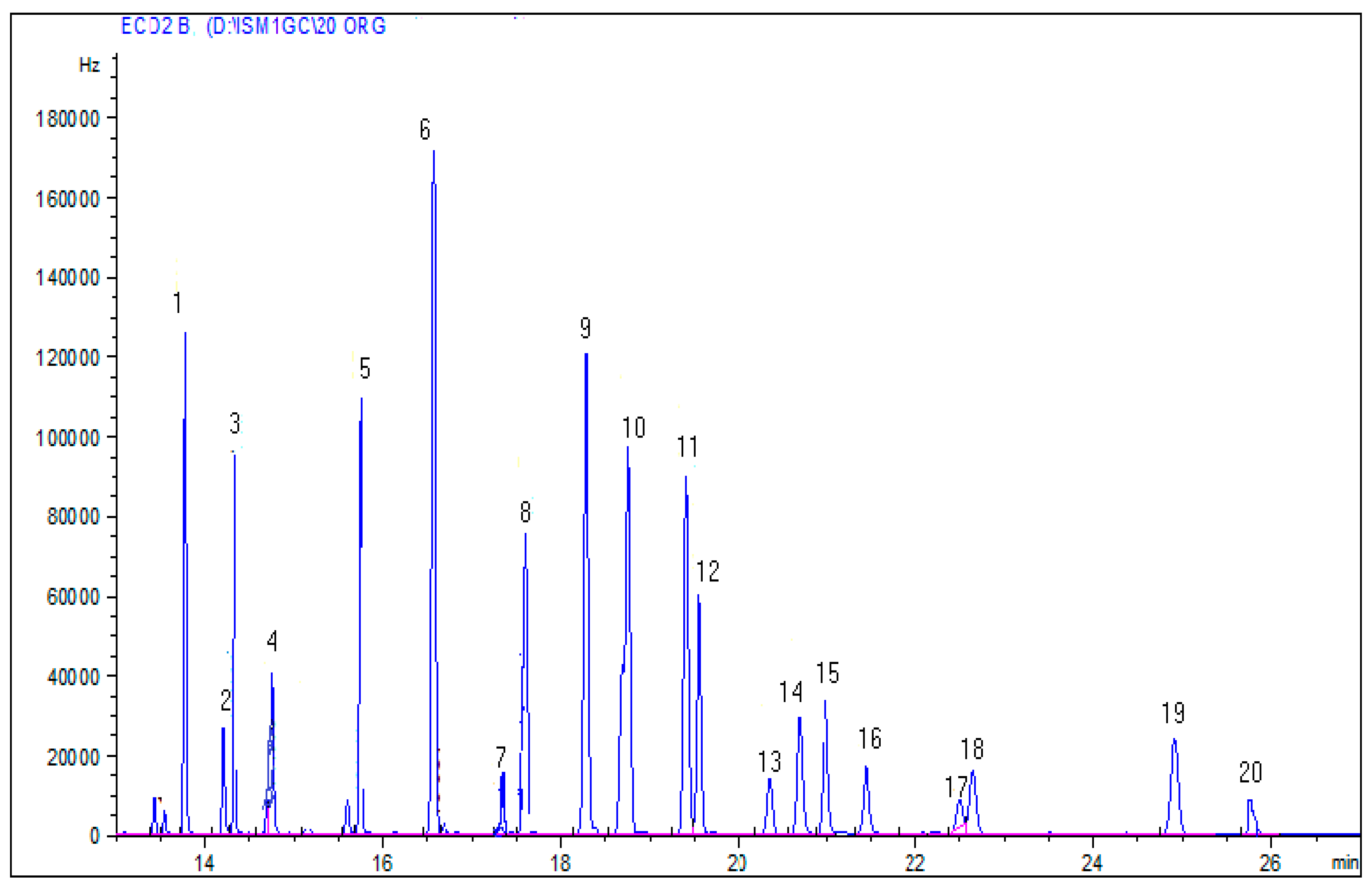
3.1. Optimization of Experimental Conditions
3.2. Method Validation
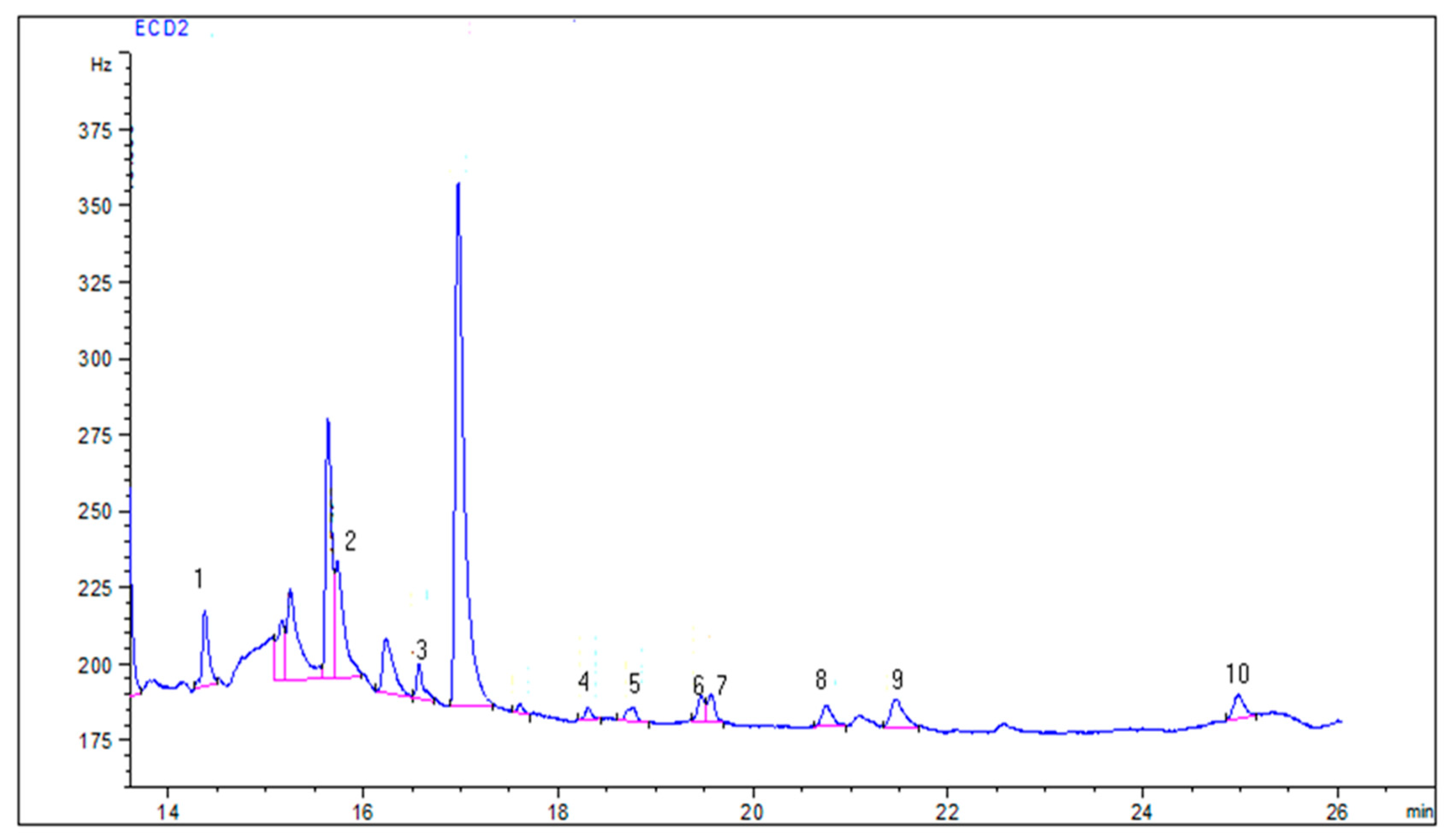
3.3. Pesticide Residues in Water Samples
3.4. Health Risk Assessment of OCPs in Water Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ismail, M.; Khan, H.M.; Sayed, M.; Cooper, W.J. Advanced oxidation for the treatment of chlorpyrifos in aqueous solution. Chemosphere 2013, 93, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, A.; Guillamon, M.; Lacorte, S.; Tauler, R.; Barcelo, D. Impact of pesticides used in agriculture and vineyards to surface and groundwater quality (North Spain). Water Res. 2008, 42, 3315–3326. [Google Scholar] [CrossRef]
- Ali, N.; Ali, L.N.; Eqani, M.A.S.; Ismail, I.M.I.; Malarvannan, G.; Kadi, M.W.; Basahi, J.M.A.; Covaci, A. Organohalogenated contaminants in sediments and bivalves from the Northern Arabian Gulf. Ecotoxicol. Environ. Saf. 2015, 122, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Gao, X.; Cao, Y.; Xu, L.; Jia, L. Influence of Hydroxypropyl-β-cyclodextrin on the Extraction and Biodegradation of p,p′-DDT, o,p′-DDT, p,p′-DDD, and p,p′-DDE in Soils. Water Air Soil Pollut. 2015, 226, 208. [Google Scholar] [CrossRef]
- Guéguen, F.; Stille, P.; Millet, M. Persistent organic pollutants in the atmosphere from urban and industrial environments in the Rhine Valley. Environ. Sci. Pollut. Res. 2013, 20, 3852–3862. [Google Scholar] [CrossRef]
- Bonansea, R.I.; Ame, M.V.; Wunderlin, D.A. Determination of priority pesticides in water samples combining SPE and SPME coupled to GC–MS. A case study:Suquía River basin (Argentina). Chemosphere 2013, 90, 1860–1869. [Google Scholar] [CrossRef]
- Kuranchie-mensah, H.; Atiemo, S.M.; Maud, L.; Palm, L.M.; Blankson-arthur, S.; Tuto, A.O.; Fosu, P. Determination of organochlorine pesticide residue in sediment and water from the Densu river basin, Ghana. Chemosphere 2012, 86, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.X.; He, W.; Qin, N.; Kong, X.; He, Q.; Ouyang, H.; Xu, F. The residues, distribution and partition of organochlorine pesticides in the water, suspended solids, and sediments from a large Chinese lake (Lake Chaohu) during the high water level period. Environ. Sci. Pollut. Res. 2013, 20, 2033–2045. [Google Scholar] [CrossRef]
- Seebunrueng, K.; Santaladchaiyakit, Y.; Srijaranai, S. Vortex-assisted low density solvent based demulsified dispersive liquid–liquid microextraction and high-performance liquid chromatography for the determination of organophosphorus pesticides in water samples. Chemosphere 2014, 103, 51–58. [Google Scholar] [CrossRef]
- Teng, Y.; Li, J.; Wu, J.; Lu, S.; Wang, Y.; Chen, H. Environmental distribution and associated human health risk due to trace elements and organic compounds in soil in Jiangxi province, China. Ecotoxicol. Environ. Saf. 2015, 122, 406–416. [Google Scholar] [CrossRef]
- Tariq, M.I.; Afzal, S.; Hussain, I.; Sultana, N. Pesticides exposure in Pakistan: A review. Environ. Int. 2007, 33, 1107–1122. [Google Scholar] [CrossRef]
- Tindall, J.A.; Chen, A. Variables that Affect Agricultural Chemicals in Groundwater in Nebraska. Water Air Soil Pollut. 2014, 225, 1862. [Google Scholar] [CrossRef]
- Younas, A.; Hilber, I.; Rehman, S. Former DDT factory in Pakistan revisited for remediation: Severe DDT concentrations in soils and plants from within the area. Environ. Sci. Pollut. Res. 2013, 20, 1966–1976. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, J.; Meng, B.; Cheng, J.; Lin, G.; Chen, J.; Zheng, D.; Yu, Y. Distribution and sources of organochlorine pesticides in agricultural soils from central China. Ecotoxicol. Environ. Saf. 2013, 93, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Pitacco, V.; Mistri, M.; Ferrari, C.R.; Sfriso, A.; Sfriso, A.A.; Munari, C. Multiannual Trend of Micro-Pollutants in Sedimentsand Benthic Community Response in a Mediterranean Lagoon (Sacca di Goro, Italy). Water 2020, 12, 1074. [Google Scholar] [CrossRef] [Green Version]
- Curtean-Banaduc, A.; Burcea, A.; Mihu, B.D. The Benthic Trophic Corner StoneCompartment in POPs Transfer fromAbiotic Environment to HigherTrophic Levels—Trichoptera andEphemeroptera Pre-Alert IndicatorRole. Water 2021, 13, 1778. [Google Scholar] [CrossRef]
- Abbasi1, Y.; Mannaerts, C.M.; Makau, W. Modeling Pesticide and Sediment Transport in theMalewa River Basin (Kenya) Using SWAT. Water 2019, 11, 87. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.; Zeng, Z.; Yang, M. Determination of organochlorine pesticides and their derivations in water after HS-SPME using polymethylphenylvinylsiloxane-coated fiber by GC-ECD. Water Res. 2005, 39, 4204–4210. [Google Scholar] [CrossRef] [PubMed]
- Golfinopoulos, S.K.; Nikolaou, A.D.; Kostopoulou, M.N.; Xilourgidis, N.K.; Vagi, M.C.; Lekkas, D.T. Organochlorine pesticides in the surface waters of Northern Greece. Chemosphere 2003, 50, 507–516. [Google Scholar] [CrossRef]
- Fung, C.N.; Zheng, G.J.; Connell, D.W.; Zhang, X.; Wong, H.L.; Giesy, J.P.; Fang, Z.; Lam, P.K. Risks posed by trace organic contaminants in coastal sediments in the Pearl River Delta, China. Mar. Pollut. Bull. 2005, 50, 1036–1049. [Google Scholar] [CrossRef]
- Santhi, V.A.; Hairin, T.; Mustafa, A.M. Simultaneous determination of organochlorine pesticides and bisphenol A in edible marine biota by GC–S. Chemosphere 2012, 86, 1066–1107. [Google Scholar] [CrossRef]
- Sankararamakrishnan, N.; Kumar Sharma, A.; Sanghi, R. Organochlorine and organophosphorous pesticide residues in ground water and surface waters of Kanpur, Uttar Pradesh, India. Environ. Int. 2005, 31, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, G.; Licata, P.; Bruzzese, A.; Naccari, C.; Trombetta, D.; Lo Turco, V.; Dugo, G.; Richetti, A.; Naccari, F. Levels and congener pattern of polychlorinated biphenyl and organochlorine pesticide residues in bluefin tuna (Thunnus thynnus) from the Straits of Messina (Sicily, Italy). Environ. Int. 2006, 32, 705–710. [Google Scholar] [CrossRef]
- PES. Pakistan Economic Survey: 2011–2012; Economic Advisor’s Wing; Finance Division, Government of Pakistan: Islamaabad, Pakistan, 2012.
- ERS Employment & Research Section, Planning & Development Division, Government of Pakistan, Islamabad. Pre-Feasibility Study for Pesticide Industry; International Asset Management Company Ltd.: Karachi, Pakistan, May 2006.
- Khan, M.S. Pakistan crop protection market. PAPA Bull. 1998, 9, 7–9. [Google Scholar]
- Eavy, A.L.; Ahmed, F.; Buriro, A.S. Final report on integrated pest/production/plant management (IPM). Development program ARP-II Sindh submitted by Winrock international institute for agricultural development. Dir. Gen. Agric. Res. 1995, 2, 1–27. [Google Scholar]
- WHO. Public Health Impact of Pesticides Used in Agriculture; WHO: Geneva, Switzerland, 1990. [Google Scholar]
- Tempowski, J. (WHO) Personal Communication; WHO: Geneva, Switzerland, 10 January 2012. [Google Scholar]
- Prüss-Ustün, A.; Vickers, C.; Haefliger, P.; Bertollini, R. Knowns and Unknowns on Burden of Disease due to Chemicals: A Systematic Review. Environ. Health 2011, 10, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. The Global Burden of Disease–2004 Update; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- Ismail, M.; Sayed, M.; Khan, H.M.; Cooper, W.J. Analysis of Pesticides in Water Samples and Removal of Monocrotophos by γ-Irradiation. J. Anal. Bioanal. Tech. 2014, 5, 181–191. [Google Scholar]
- Richardson, S.D.; Ternes, T.A. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 2011, 83, 4614–4648. [Google Scholar] [CrossRef]
- Esther, M.; Padrón, T.; Afonso-olivares, C.; Sosa-ferrera, Z.; Santana-rodríguez, J.J.; Química, D.D. Microextraction Techniques Coupled to Liquid Chromatography with Mass Spectrometry for the Determination of Organic Micropollutants in Environmental Water Samples. Molecule 2014, 19, 10320–10349. [Google Scholar]
- Boussahel, R.; Bouland, S.; Moussaoui, K.M.; Baudu, M.; Montiel, A. Determination of chlorinated pesticides in water by SPME/GC. Water Res. 2002, 36, 1909–1911. [Google Scholar] [CrossRef]
- Perez-Trujillo, J.P.; Frias, S.; Conde, J.E.; Rodriguez-Delgado, M.A. Comparison of different coatings in solid-phase microextraction for the determination of organochlorine pesticides in ground water. J. Chromatogr. A 2002, 963, 95–105. [Google Scholar] [CrossRef]
- Phan, K.; Sthiannopkao, S.; Kim, K.W.; Wong, M.H.; Sao, V.; Hashim, J.H. Health risk assessment of inorganic arsenic intake of Cambodia residents through groundwater drinking pathway. Water Res. 2010, 44, 5777–5788. [Google Scholar] [CrossRef]
- Hu, Y.; Qi, S.H.; Zhang, J.P.; Tan, L.Z.; Zhang, J.Q.; Wang, Y.H. Assessment of organochlorine pesticides contamination in underground rivers in Chongqing, Southwest China. J. Geochem. Explor. 2011, 111, 47–55. [Google Scholar] [CrossRef]
- IRIS (Integrated Risk Information System). US Environmental Protection Agency: Cincinnati, OH, USA, 2005. Available online: http://www.epa.gov/iris (accessed on 1 September 2021).
- US EPA. Risk Assessment Guidance for Superfund. Volume I: Human Health Evaluation Manual (Part A); EPA/540/1–89/002; Office of Emergency and Remedial Response: Washington, DC, USA, 1989.
- US EPA. Guidelines for the Health Risk Assessment of Chemical Mixtures; Office of Emergency and Remedial Response: Washington DC, USA, 1986.
- US EPA. Exposure Factors Handbook; EPA/600/P-95/002Fa; Office of Research and Development National Center for Environmental Assessment: Washington, DC, USA, 1997.
- Cortada, C.; Vidal, L.; Pastor, R.; Santiago, N.; Canals, A. Determination of organochlorine pesticides in water samples by dispersive liquid-liquid microextraction coupled to gas chromatography-mass spectrometry. Anal. Chim. Acta 2009, 649, 218–221. [Google Scholar] [CrossRef]
- U.S. EPA. Method 525.2, Determination of Organic Compounds in Drinking Water by Liquid–Solid Extraction and Capillary Column Gas Chromatography/Mass Spectrometry; U.S. Environmental Protection Agency: Washington, DC, USA, 1995.
- Graña, E.; Fernández-Martínez, G.; Fernández-Villarrenaga, V.; Turnes-Carou, M.I.; Muniategui-Lorenzo, S.; López-Mahía, P. A study of large-volume on-column injection GC-ECD for the ultratrace analysis of organochlorine pesticides in water. Talanta 2009, 78, 764–771. [Google Scholar] [CrossRef]
- Yang, Y.; Yun, X.; Liu, M.; Jiang, Y.; Li, Q.X.; Wang, J. Concentrations, distributions, sources, and risk assessment of organochlorine pesticides in surface water of the East Lake, China. Environ. Sci. Pollut. Res. 2014, 21, 3041–3050. [Google Scholar] [CrossRef] [PubMed]
- Turgut, C. The contamination with organochlorine pesticides and heavy metals in surface water in Kucuk Menderes River in Turkey, 2000–2002. Environ. Int. 2003, 29, 29–32. [Google Scholar] [CrossRef]
| Pesticide | SF/[(mg kg−1 per Day)−1] * | RfD/(mg kg−1 per Day) * | Cancer Risk (10−6) | Hazard Quotient (10−2) | ||
|---|---|---|---|---|---|---|
| (for Children) | (for Adults) | (for Children) | (for Adults) | |||
| α-BHC | 6.3 | 5.0 × 10−4 | 0 | 0 | 0 | 0 |
| β-BHC | 1.8 | 2.0 × 10−4 | 0 | 0 | 0 | 0 |
| γ-BHC | 1.3 | 3.0 × 10−4 | 0–2.05 | 0.96 | 0.52 | 0.24 |
| δ-BHC | / | / | 0 | 0 | ||
| Heptachlor | 4.5 | 5.0 × 10−4 | 0–33.29 | 15.53 | 1.48 | 0.69 |
| Aldrin | 17 | 3.0 × 10−5 | 0–16.30 | 7.61 | 3.20 | 1.49 |
| Heptachlor poxide | 9.1 | 1.3 × 10−5 | 0 | 0 | 0 | 0 |
| γ-Chlordane | / | / | 0 | 0 | 0 | 0 |
| α-Chlordane | / | / | 0 | 0 | 0 | 0 |
| Endosulfan I | / | / | 0 | 0 | 0 | 0 |
| 4,4′-DDE | 0.34 | / | 0.47 | 0.22 | - | - |
| Dieldrin | 16 | 5.0 × 10−5 | 14.25 | 6.65 | 1.78 | 0.83 |
| Endrin | / | 3.0 × 10−4 | 0 | 0 | 0 | 0 |
| Endosulfan II | / | / | 0 | 0 | 0 | 0 |
| 4,4′-DDD | 0.24 | 2.0 × 10−3 | 0 | 0 | 0 | 0 |
| Endrin aldehyde | / | 3.0 × 10−4 | - | - | 0.68 | 0.32 |
| Endrin ketone | / | / | 0 | 0 | 0 | 0 |
| 4,4′-DDT | 0.34 | 5.0 × 10−4 | 0 | 0 | 0 | 0 |
| Endosulfan sulfate | / | / | 0 | 0 | 0 | 0 |
| Methoxychlor | / | / | 0 | 0 | 0 | 0 |
| Compound | Correlation Coefficient (r) a | Lower Limits of Detection (µg L−1) | Guide Line Values (µg L−1) | |||
|---|---|---|---|---|---|---|
| Present Study | Cortada et al., 2009 | Golfinopoulos et al., 2003 | EPA 525.2 b | |||
| α-BHC | 0.9985 | 0.003 | 0.003 | 0.008 | 0.110 | - |
| β-BHC | 0.9996 | 0.006 | 0.005 | 0.020 | 0.085 | - |
| γ-BHC | 0.9998 | 0.002 | 0.008 | 0.008 | 0.084 | 2 c, 0.05 d, 0.2 e |
| δ-BHC | 0.9995 | 0.003 | 0.006 | 0.008 | 0.049 | 0.05 d |
| Heptachlor | 0.9997 | 0.004 | 0.007 | 0.008 | 0.061 | 0.03 c, 0.4 e, 0.05 d |
| Aldrin | 0.9998 | 0.002 | 0.009 | 0.008 | 0.045 | 0.03 c, 0.01 d |
| Heptachlor epoxide | 0.9887 | 0.02 | 0.002 | 0.008 | 0.130 | 0.03 c, 0.2 e |
| γ-Chlordane | 0.9996 | 0.003 | - | 0.008 | - | 0.2 c, 2 e, 0.01 d |
| α-Chlordane | 0.9995 | 0.002 | - | 0.008 | - | 0.2 c, 2 e |
| Endosulfan I | 0.9998 | 0.002 | 0.005 | 0.008 | 0.110 | 0.05 d |
| 4,4′-DDE | 0.9997 | 0.004 | 0.002 | 0.008 | 0.070 | 0.06 d |
| Dieldrin | 0.9998 | 0.002 | 0.004 | 0.008 | 0.150 | 0.03 c, 0.01 d |
| Endrin | 0.9947 | 0.004 | 0.004 | 0.008 | 0.340 | 2 e |
| Endosulfan II | 0.9998 | 0.003 | 0.025 | 0.008 | 0.074 | 0.05 d |
| 4,4′-DDD | 0.9957 | 0.005 | 0.004 | 0.008 | 0.055 | |
| Endrin aldehyde | 0.9976 | 0.004 | 0.001 | 0.008 | 0.150 | |
| Endrin ketone | 0.9968 | 0.003 | 0.004 | 0.008 | - | |
| 4,4′-DDT | 0.9955 | 0.009 | 0.004 | 0.020 | 0.093 | 2 c, 0.06 d |
| Endosulfan sulfate | 0.9999 | 0.002 | 0.003 | 0.008 | 0.039 | 0.05 d |
| Methoxychlor | 0.9899 | 0.03 | 0.001 | 0.008 | 0.130 | 20 c, 0.2 d |
| Compound | 1 µg L−1 (Present Study) | 2 µg L−1 (Present Study) | Golfinopoulos et al., 2003 (0.4 µg L−1) | Concha-Grana et al., 2009 (1 µg L−1) | Cortada et al., 2009 (10 µg L−1) |
|---|---|---|---|---|---|
| α-BHC | 96.2 | 97.4 | 100.4 | 69 | 101 |
| β-BHC | 106.0 | 108.2 | 121.6 | 90 | 96 |
| γ-BHC | 98.2 | 102.9 | 100.2 | 73 | 97 |
| δ-BHC | 97.3 | 98.1 | 104.2 | 97 | 86 |
| Heptachlor | 90.6 | 89.4 | 74.5 | 79 | 85 |
| Aldrin | 98.1 | 96.8 | 49.7 | 98 | 81 |
| Heptachlor epoxide | 89.9 | 91.8 | 67.6 | 102 | 79 |
| γ-Chlordane | 95.4 | 96.0 | - | 99 | - |
| α-Chlordane | 92.8 | 88.5 | - | 100 | - |
| Endosulfan I | 93.5 | 95.1 | 99.7 | 105 | 83 |
| 4,4′-DDE | 91.4 | 90.2 | 57.6 | 107 | 81 |
| Dieldrin | 96.9 | 98.4 | 95.5 | 106 | 82 |
| Endrin | 101.1 | 97.0 | 104.0 | 86 | 81 |
| Endosulfan II | 103.8 | 107.9 | 95.2 | 75 | 85 |
| 4,4′-DDD | 94.8 | 95.7 | 79.9 | 50 | 84 |
| Endrin aldehyde | 98.2 | 99.5 | 101.1 | 100 | 86 |
| Endrin ketone | 103.7 | 109.2 | 96.9 | 100 | 82 |
| 4,4′-DDT | 87.5 | 90.2 | 63.9 | 104 | 75 |
| Endosulfan sulfate | 98.0 | 96.8 | 44.6 | 90 | 82 |
| Methoxychlor | 96.9 | 95.0 | 95.4 | 72 | 75 |
| No. | Compound | Total Samples Tested | Contaminated Samples | Range (µg L−1) |
|---|---|---|---|---|
| 1 | α-BHC | 30 | 0 | nd * |
| 2 | β-BHC | 30 | 0 | nd |
| 3 | γ-BHC | 30 | 16 | nd–0.023 |
| 4 | δ-BHC | 30 | 0 | nd |
| 5 | Heptachlor | 30 | 17 | nd–0.108 |
| 6 | Aldrin | 30 | 14 | nd–0.014 |
| 7 | Heptachlor epoxide | 30 | 0 | nd |
| 8 | γ-Chlordane | 30 | 0 | nd |
| 9 | α-Chlordane | 30 | 0 | nd |
| 10 | Endosulfan I | 30 | 2 | nd–0.005 |
| 11 | 4,4′-DDE | 30 | 5 | nd–0.020 |
| 12 | Dieldrin | 30 | 11 | nd–0.013 |
| 13 | Endrin | 30 | 0 | nd |
| 14 | Endosulfan II | 30 | 0 | nd |
| 15 | 4,4′-DDD | 30 | 0 | nd |
| 16 | Endrin aldehyde | 30 | 2 | 0.030 |
| 17 | Endrin ketone | 30 | 0 | nd |
| 18 | 4,4′-DDT | 30 | 0 | nd |
| 19 | Endosulfan sulfate | 30 | 1 | 0.009 |
| 20 | Methoxychlor | 30 | 0 | nd |
| Compound | Present Study | Kuranchie-Mensah et al., 2012 | Yang et al., 2014 | Liu et al., 2013 | Golfinopoulos et al., 2003 |
|---|---|---|---|---|---|
| α-BHC | nd * | - | nd–0.003 | nd–0.003 | nd–0.131 |
| β-BHC | nd | - | nd–0.006 | nd–0.009 | nd–0.096 |
| γ-BHC | nd–0.023 | 0.02–0.08 | nd–0.003 | nd–0.003 | nd–0.081 |
| δ-BHC | nd | 0.01–0.12 | nd–0.010 | - | nd–0.189 |
| Heptachlor | nd–0.108 | 0.02–0.04 | nd–0.012 | nd–0.001 | nd–0.020 |
| Aldrin | nd–0.014 | 0.01–0.02 | - | nd–0.001 | nd–0.101 |
| γ-Chlordane | nd | 0.01–0.12 | - | nd–0.002 | - |
| α-Chlordane | nd | - | - | nd–0.001 | - |
| Endosulfan I | nd–0.005 | 0.01–0.04 | nd–0.010 | nd–0.004 | nd–0.020 |
| 4,4′-DDE | nd–0.020 | nd | nd–0.005 | nd–0.001 | nd–0.064 |
| Dieldrin | nd–0.013 | nd | - | nd–0.002 | nd–0.039 |
| Endrin | nd | 0.01–0.03 | - | nd–0.004 | nd |
| Endosulfan II | nd | - | nd–0.013 | - | nd–0.022 |
| 4,4′-DDD | nd | - | nd–0.005 | nd–0.001 | nd |
| Endrin aldehyde | 0.030 | 0.05–0.15 | - | - | nd–0.080 |
| Endrin ketone | nd | nd | - | - | nd |
| 4,4′-DDT | nd | 0.01–0.02 | nd–0.014 | nd–0.010 | nd–0.035 |
| Endosulfan sulfate | 0.009 | 0.11–0.26 | - | - | nd–0.058 |
| Methoxychlor | nd | nd | - | nd–0.018 | nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, M.; Alam, S.; Khan, M.S.; Shah, L.A.; Shah, S.M.M.; Wahab, M.; Rukh, G.; Rahman, N.u.; Rehman, N.; Amin, N.u.; et al. Levels and Potential Health Hazards of Chlorinated Pesticides in Surface Water Samples of Charsadda Area of Pakistan Using SPME-GC-ECD Technique. Water 2021, 13, 2468. https://doi.org/10.3390/w13182468
Ismail M, Alam S, Khan MS, Shah LA, Shah SMM, Wahab M, Rukh G, Rahman Nu, Rehman N, Amin Nu, et al. Levels and Potential Health Hazards of Chlorinated Pesticides in Surface Water Samples of Charsadda Area of Pakistan Using SPME-GC-ECD Technique. Water. 2021; 13(18):2468. https://doi.org/10.3390/w13182468
Chicago/Turabian StyleIsmail, Muhammad, Sultan Alam, Muhammad Sufaid Khan, Luqman Ali Shah, S. M. Mukaram Shah, Muhammad Wahab, Gul Rukh, Najeeb ur Rahman, Noor Rehman, Noor ul Amin, and et al. 2021. "Levels and Potential Health Hazards of Chlorinated Pesticides in Surface Water Samples of Charsadda Area of Pakistan Using SPME-GC-ECD Technique" Water 13, no. 18: 2468. https://doi.org/10.3390/w13182468
APA StyleIsmail, M., Alam, S., Khan, M. S., Shah, L. A., Shah, S. M. M., Wahab, M., Rukh, G., Rahman, N. u., Rehman, N., Amin, N. u., Burlakovs, J., Kallistova, A., Pimenov, N., Vincevica-Gaile, Z., Jani, Y., Zahoor, M., & Zekker, I. (2021). Levels and Potential Health Hazards of Chlorinated Pesticides in Surface Water Samples of Charsadda Area of Pakistan Using SPME-GC-ECD Technique. Water, 13(18), 2468. https://doi.org/10.3390/w13182468








