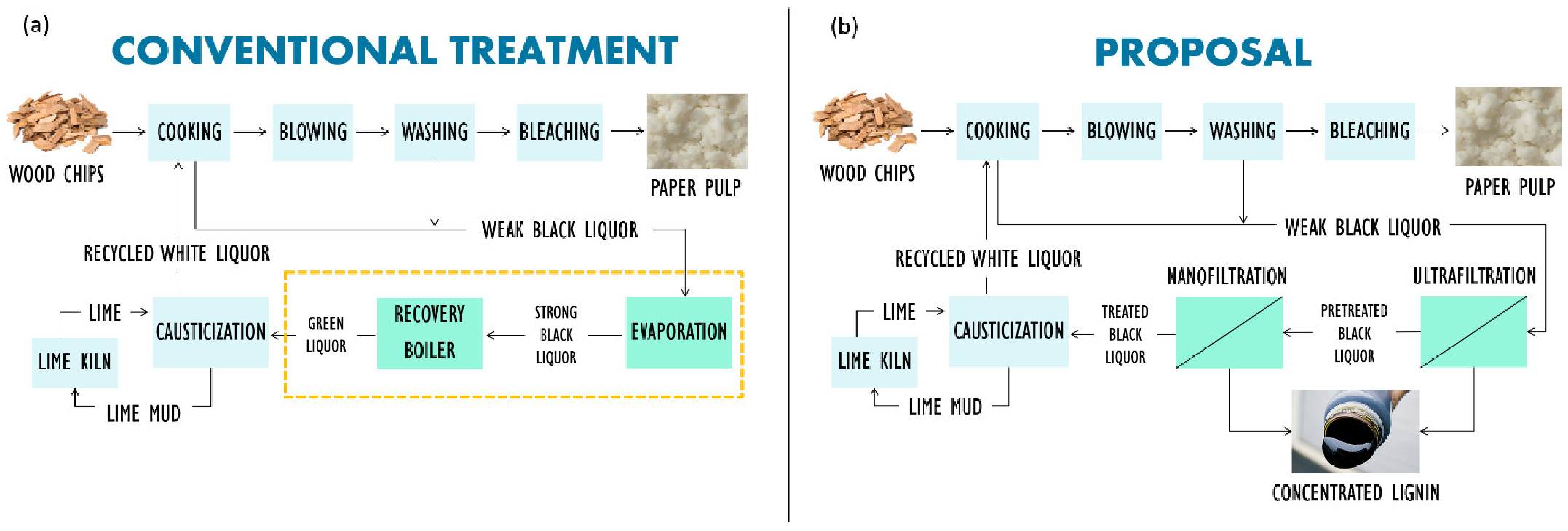
3.3. Rejection
The permeate samples were characterized to determine the contents of organic carbon (TOC), CO
32−, SO
42−, magnesium, sodium and OH
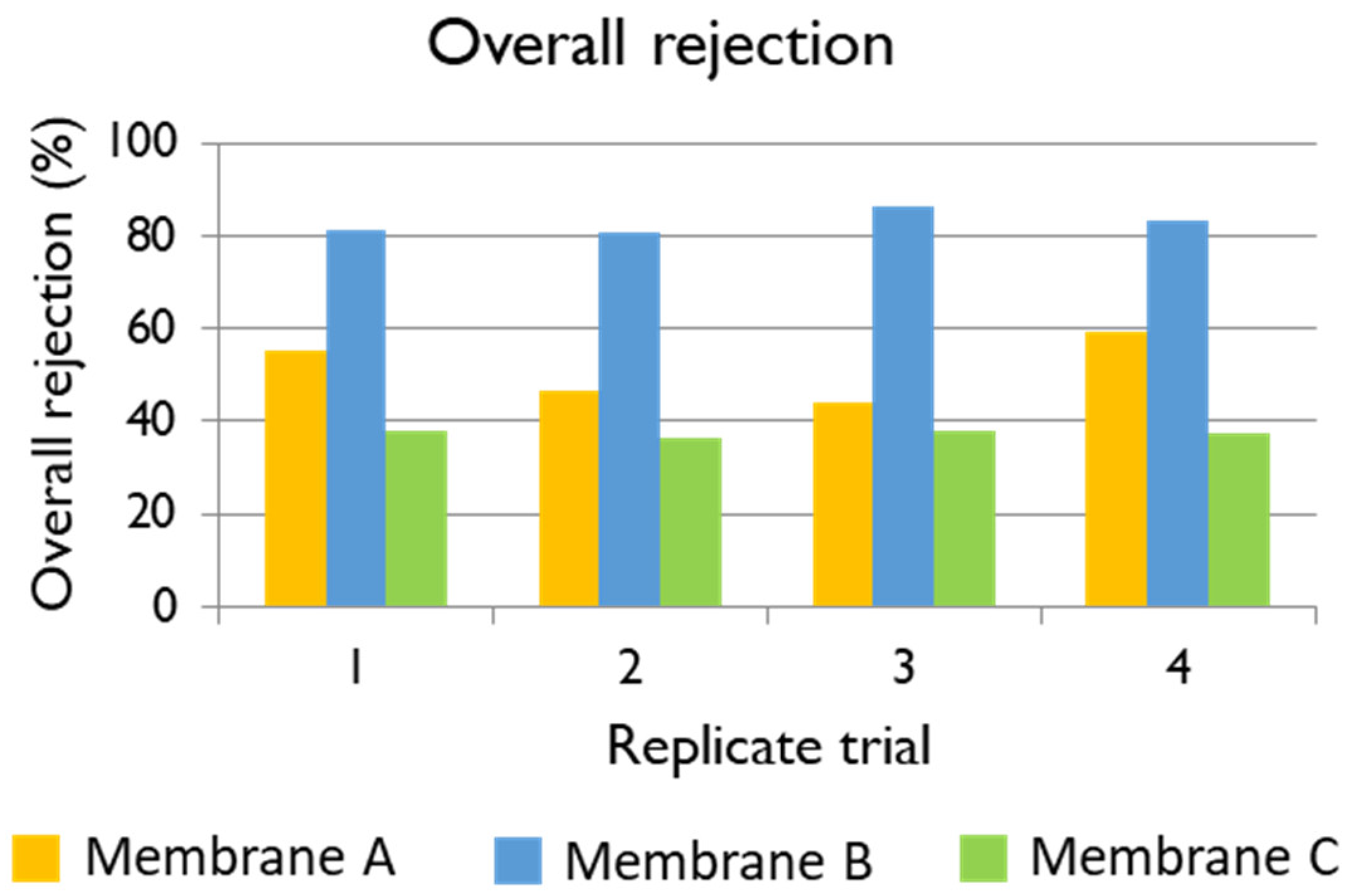
−. The average rejections of Membranes A, B, and C are shown in
Table 6.
TOC: Taking into account that the feed TOC concentration was in average 4226.67 mg L
−1, the average rejections of Membranes A, B, and C are shown in
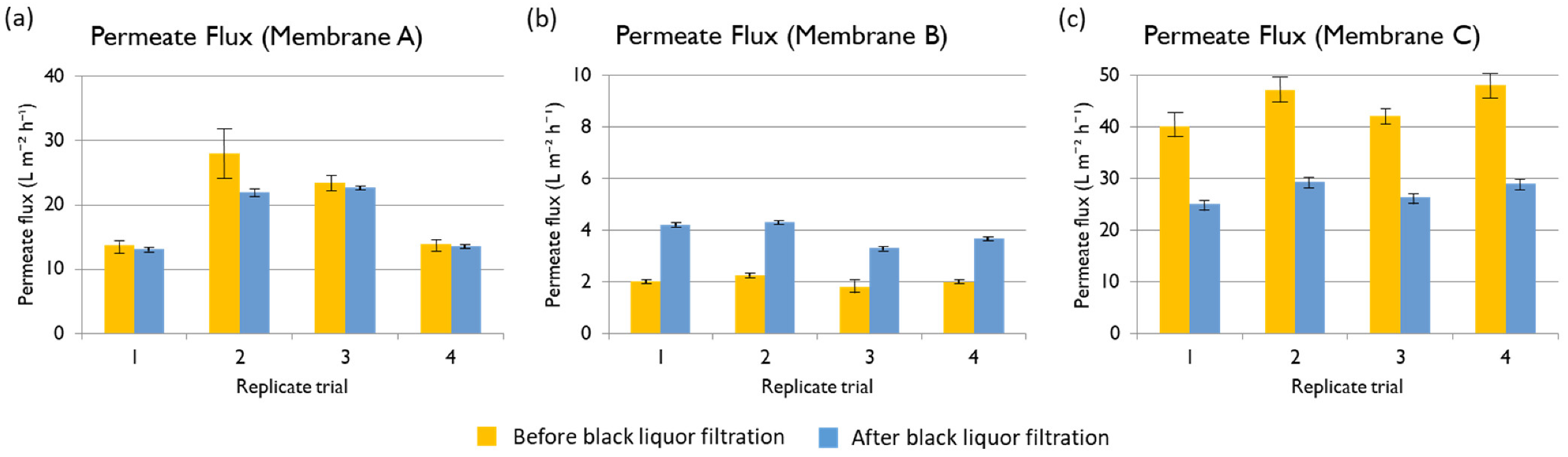
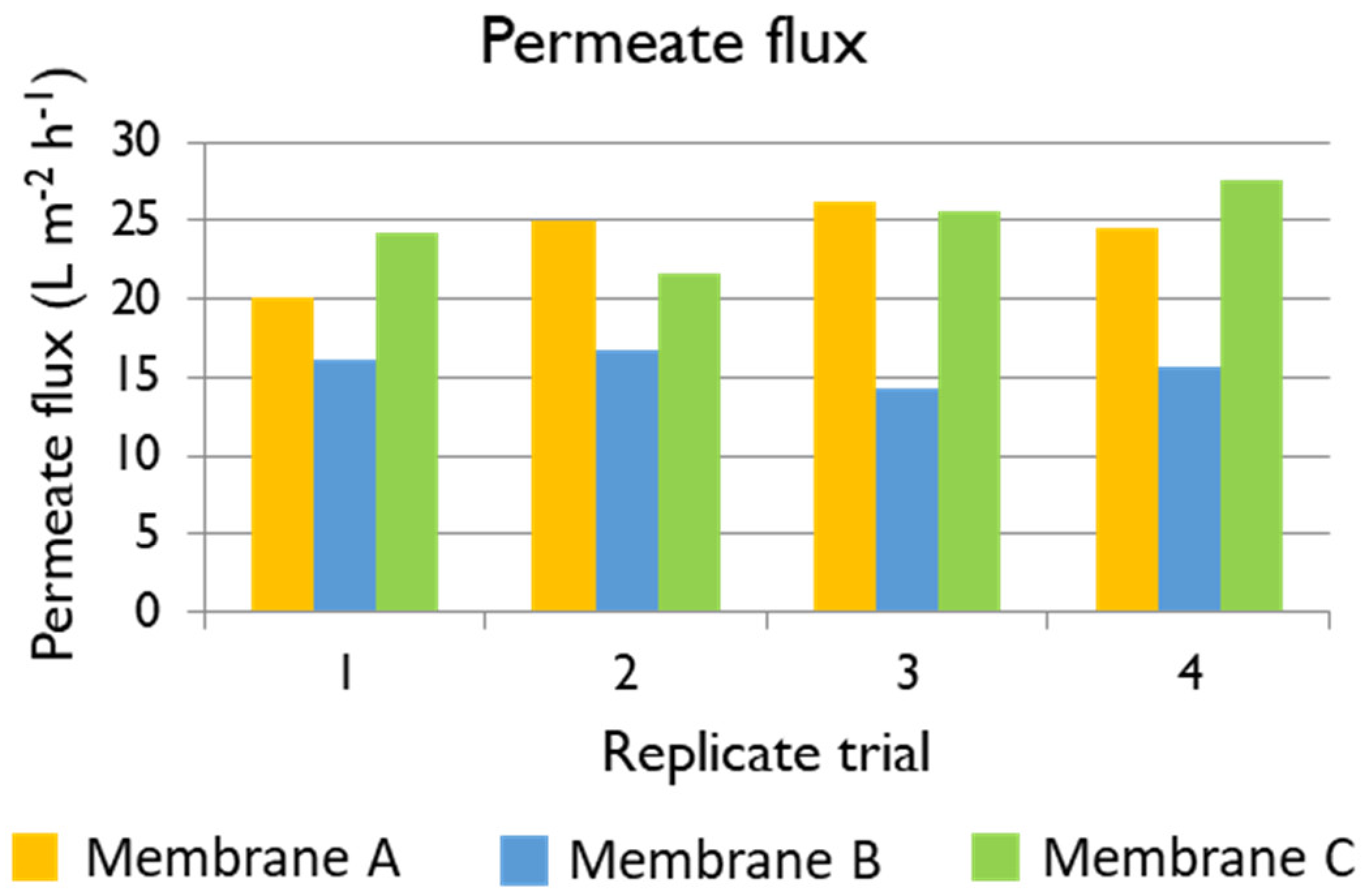
Table 6. The best membrane for the treatment of black liquor at an industrial scale would be such providing high permeate flux, which would result in more recycled white liquor, albeit containing a low TOC concentration. The three membranes under evaluation showed very good performance in terms of TOC rejection, higher than 90% in all the cases. Membrane B was the one that provided the permeate with the lowest TOC concentration, but the permeate flux was compromised. In contrast, the permeate flux using Membrane A at 35 bars was 23.93 L m
−2 h
−1 on average (only 0.82 L m
−2 h
−1 less than the flux with the Membrane C), while the TOC rejection was 92.5% on average. Therefore, Membrane A would be the most suitable for the process in terms of TOC rejection. The main contributors to the TOC in the black liquor are the lignin and hemicelluloses. Even though the exact concentration of each component was not accessible due to its wide MW distribution, it is known that xylans have a higher MW than other hemicelluloses by virtue of their higher resistance to degradation [
4]. For this reason, they are generally easier to remove through NF than lignin, leading to the conclusion that most of the TOC detected the NF permeate is lignin. The obtained results are competitive compared to those reported by other authors [
13,
16,
21].
CO
32−: Taking into consideration that the feed CO
32− concentration is 4120 mg/L, the average rejections of Membranes A, B, and C are shown in
Table 6. The best membrane for the treatment of black liquor at industrial scale would be a membrane with high permeate flux and low CO
32− concentration. Membrane B would not be a good choice given its low permeate flux, while Membrane C was the one with the lower CO
32− rejection. Therefore, Membrane A would be the best option.
Sulphates: With a feed sulphate concentration of 5272 mg L
−1. the average rejections of Membranes A, B, and C are shown in
Table 6. The best membrane for the treatment of black liquor at industrial scale would be a membrane with high permeate flux and low sulphate concentration in the permeate. Membrane B would not be a good choice despite its higher rejection given its low permeate flux, while Membrane C was the one with the lower sulphate rejection. In contrast, Membrane A provided high flux with 88.7% rejection of sulphate. Membrane A would be the best option, having very high sulphate rejections in comparison to other studies [
10].
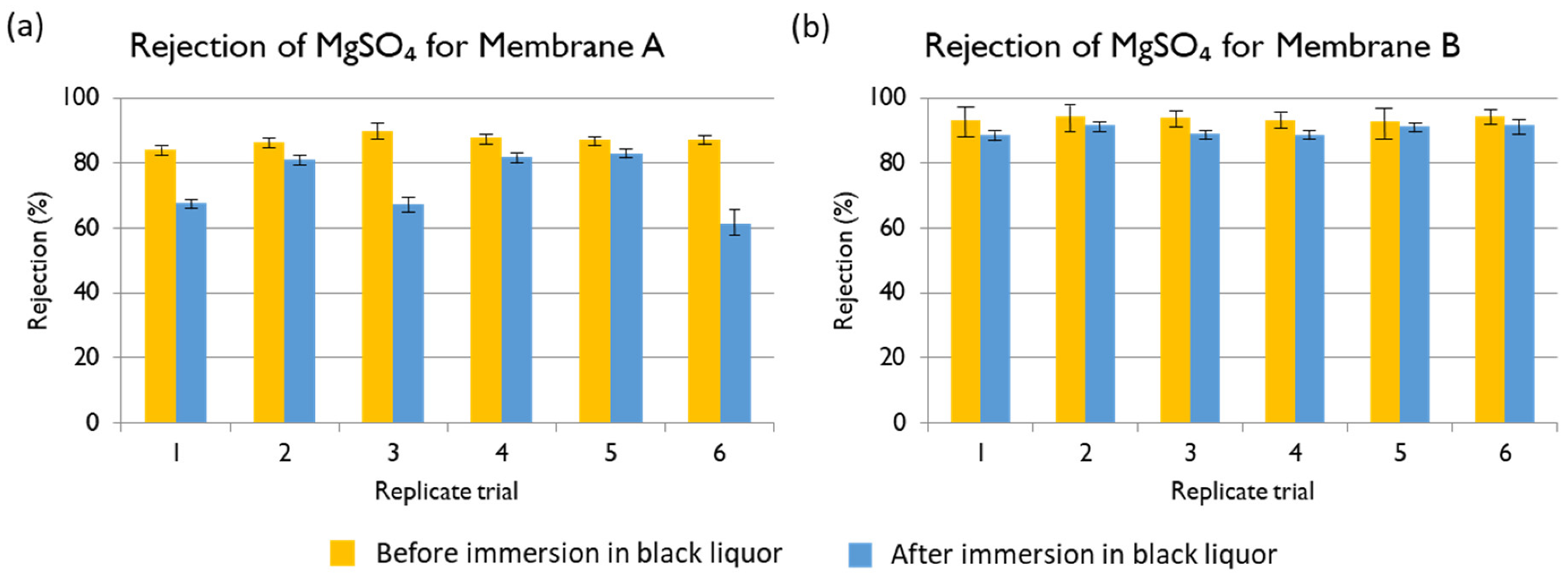
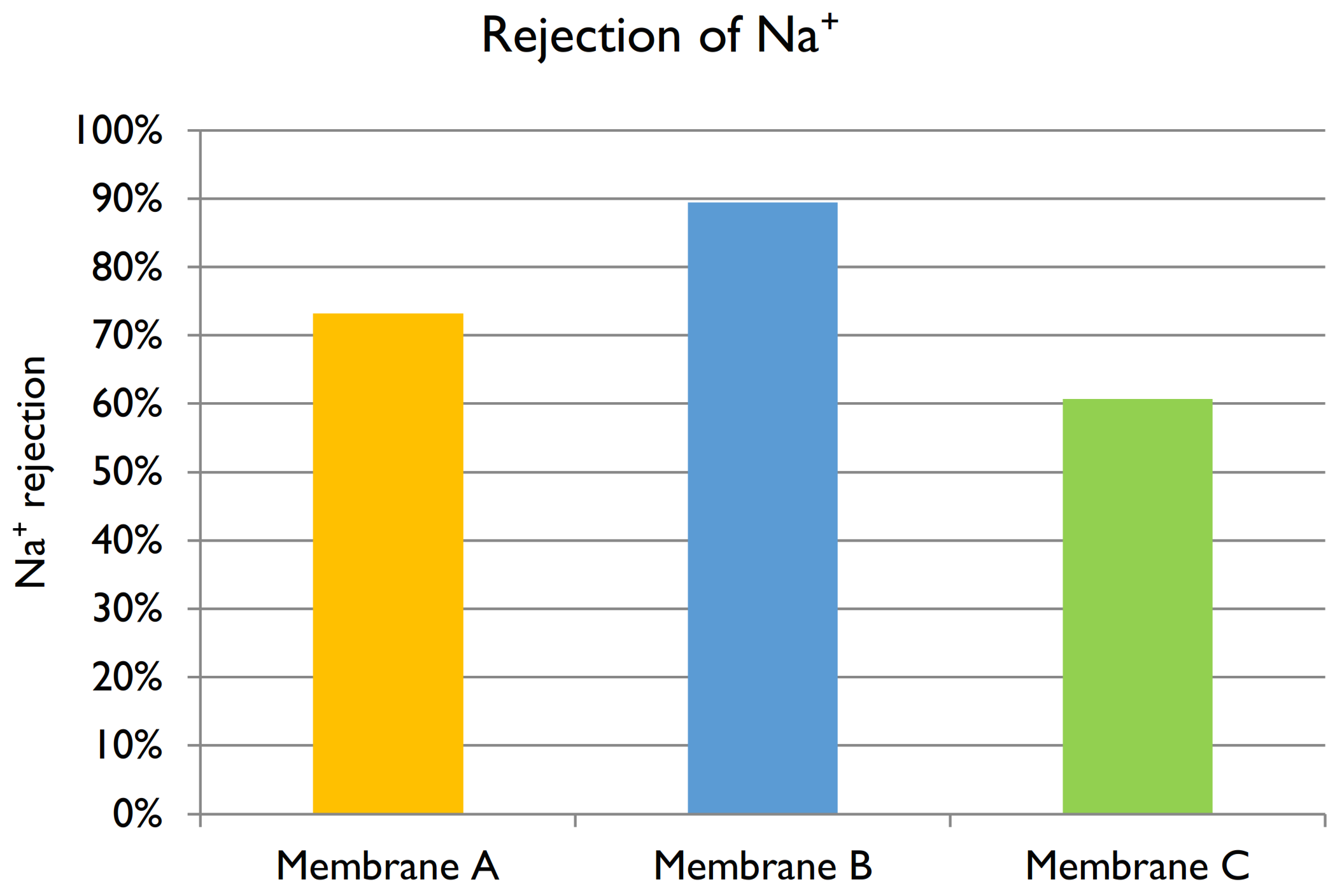
Sodium ion: Given a feed Na
+ concentration of 5600 mg L
−1, the rejections of Membranes A, B, and C are shown in
Figure 12. The best membrane for the treatment of black liquor at industrial scale would be one with high permeate flux and that allows the permeation of Na
+ to form NaOH. The membranes used for the filtration of black liquor were negatively charged, so the positive ions (including Na
+) accumulated in the surface. However, due to their relatively small size and positive charge, the permeation of Na
+ ions through the NF membrane is preferred in comparison to other larger counter-ions. This is discussed in more details in Section Charge Balance. Membrane C showed 60.7% of Na
+ rejection, which was the lowest value observed. Membrane B provided the highest rejection, but at the cost of a lower permeate flux. Hence, Membrane A would be the best option given its high flux and relatively good rejection (73.2%). The obtained results agree with those found in the literature in regards to high sodium hydroxide permeation [
38].
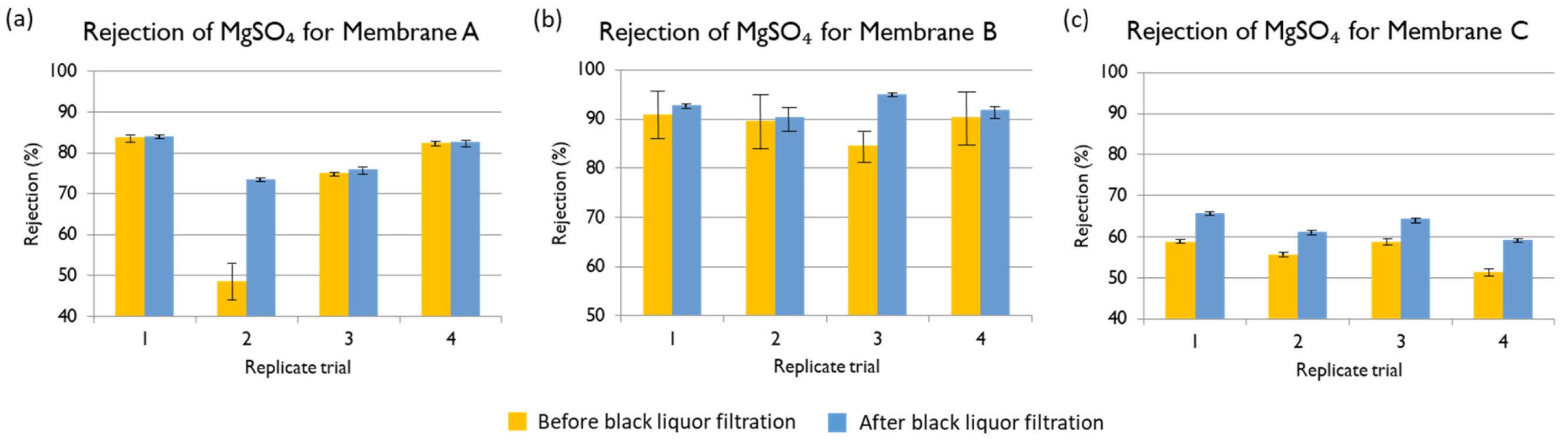
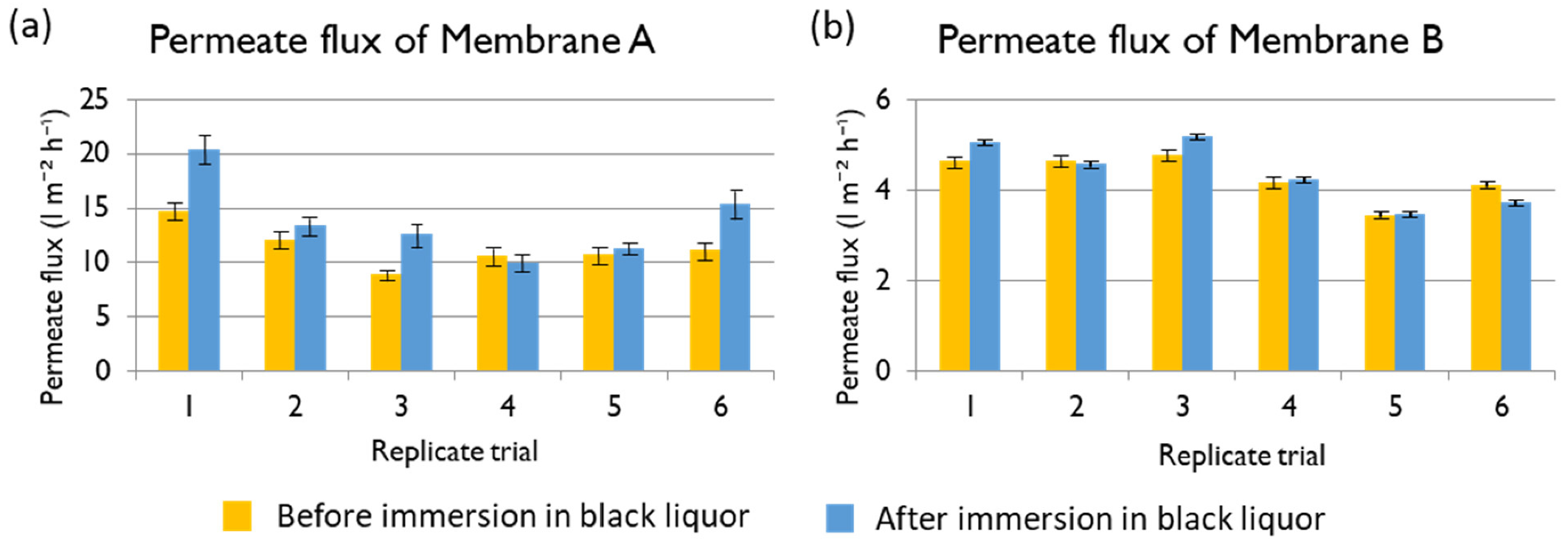
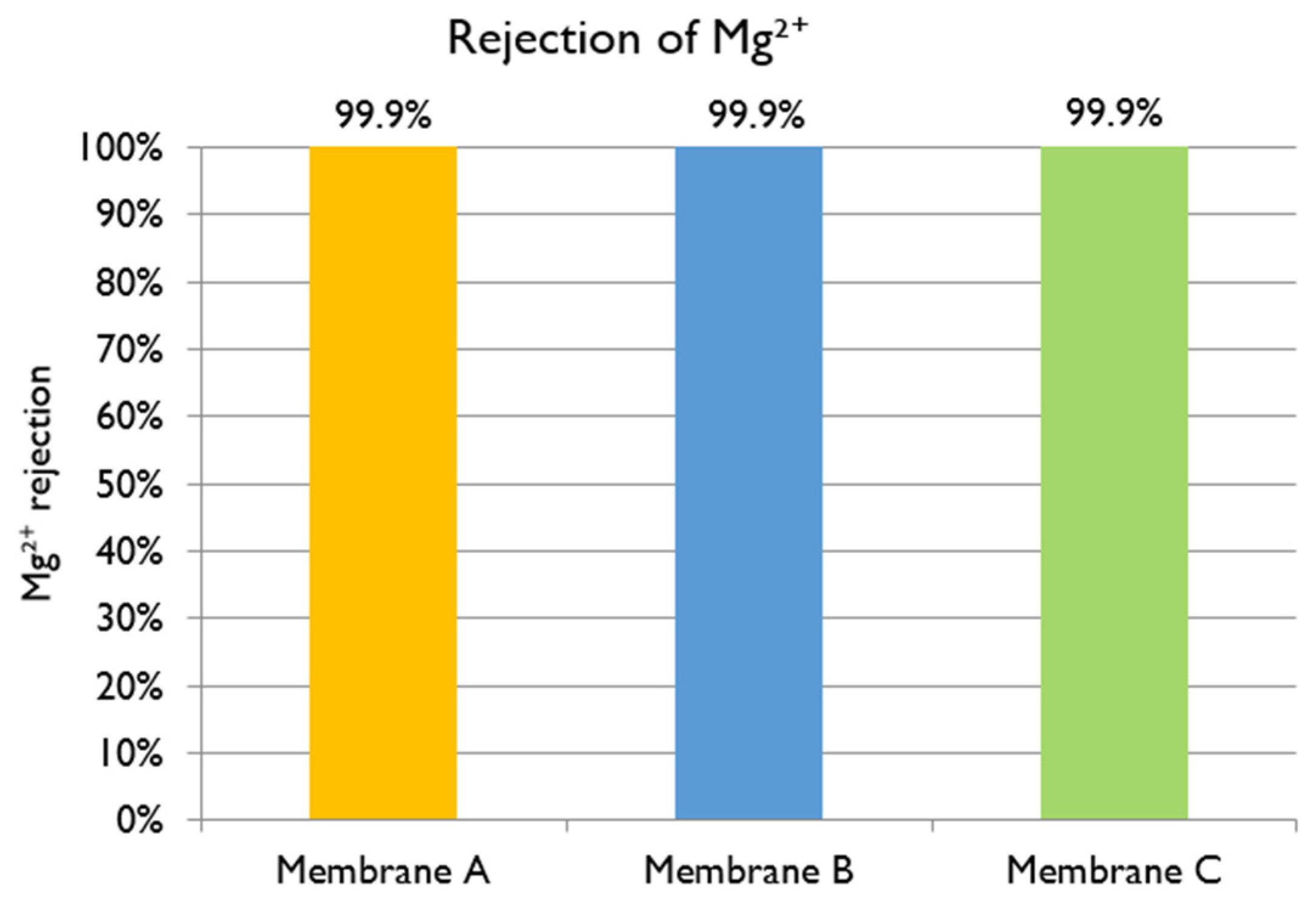
Magnesium ion: With a feed Mg
2+ concentration of 1300 mg L
−1, the rejections of Membranes A, B, and C are shown in
Figure 13. The best membrane for the treatment of black liquor at industrial scale would be one with high permeate flux and low Mg
2+ concentration in the permeate. The three membranes in evaluation offered very high rejection of Mg
2+ (higher than 99.9% in all the cases), offering results that agree with those already reported in the literature [
10]. The main criteriium to select the best membrane was the obtained permeate flux, so Membranes A and C would both be good options.
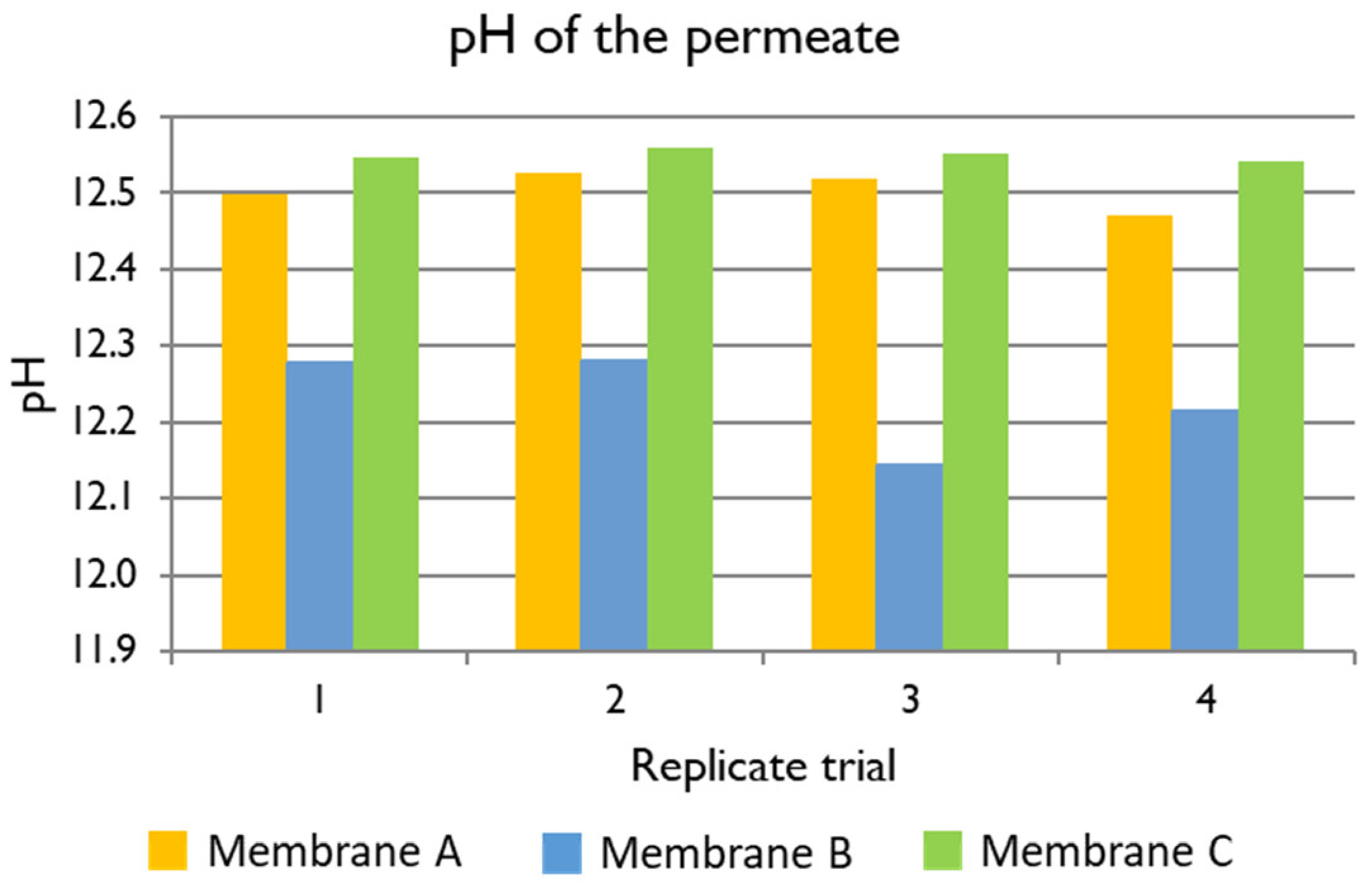
Hydroxide ion: The content of OH
− in the feed and the permeate was measured taking into account the pH of the samples and a feed pH of 11.857. The average rejections of Membranes A, B, and C are shown in
Table 7. In the case of OH
−, a negative rejection of hydroxide was observed. This point is explained in more deeply in
Section 3.4, where the charge balance is discussed. The best membrane for the treatment of black liquor at industrial scale would be a membrane with high permeate flux and high OH
− concentration in the permeate, as OH
− is one of the required components of white liquor. Membrane B showed a high negative rejection, but it was the lowest among the three membranes and it had the lowest permeate flux. Membranes A and C had high flux and high negative rejection to OH
− (−317.9% and −365.2% in average, respectively), so they could both have a good performance in terms of providing OH
− permeability.
3.4. Charge Balance
At the high pH of the black liquor, the surfaces of Membranes A, B, and C were negatively charged. Given the presence of charged species in the feed, the mechanisms of solute rejection that occurred at the membrane-solution interface were not limited to steric exclusion. The repulsion or attraction forces between the membrane and the solutes due to their electric charges, caused by the Donnan effect, were also be taken into account for the analysis. As a negatively charged membrane was used for the filtration of the simulated black liquor, the counter-ions (in this case, the positive ions) tended to accumulate in the surface of the membrane while co-ions (negative ions) did not. As a result, a Donnan potential was created. The Mg
2+ and Na
+ ions were attracted to the surface of the membrane due to their charges, but the permeation of Na
+ was preferred due to its smaller size. For this reason, the rejection of Na
+ shown in
Figure 7 was lower than the rejection of Mg
2+, which was higher than 99.99%. In order to maintain the electroneutrality, the permeation of co-ions was necessary to balance the positive charges in the permeate. In the presence of two different co-ions with the same charge, there was a preferred rejection of divalent to monovalent ions. Therefore, the rejection of CO
32− and SO
42− was greater than the rejection of OH
− due to the strong electrostatic repulsion caused by the higher valency. The passage of OH
− was attributed to the presence of Na
+, which shielded the negative charge of the membrane causing a negative rejection of OH
−. As a result of high negative rejection OH
−, the pH of the permeate was higher than the pH of the feed. This was a very positive result since the recovered permeate would be more concentrated in the cooking liquors required for the pulping process.
Table 7 presents the results of the charge balance developed for Membranes A, B, and C. As seen in the table, the charge contributions of the cations and anions analyzed did not completely satisfy the balance. In all of the cases, there was an unknown contribution that could be attributed to the organics that were not measured with the methods used or the analytical error during the measurements. This analytical error was higher when the concentration of ions is too low, as in the permeate samples. The unknown contribution was higher for the feed, which was coherent because the feed had higher concentrations of organic compounds that were the potential contributors of the unknown charges.
As a compliment, the calculations of the osmotic pressures and transmembrane pressures (TMP) are presented as
Supplementary Materials. For the evaluations performed in the following economical assessment, the energy requirements to operate Membrane A were considered. The calculated TMP for Membrane A was 21.5 bars, which is in the reported range for the NF of black liquor [
4,
13].
3.5. Economic Assessment
Our economic analysis had the goal of assessing the economic viability of both proposals in order to find out which of them was more profitable. This study included information on the capital expenditures (CAPEX), operating expenditures (OPEX) and revenues or savings. According to data provided by a Swedish paper and pulp industry, the recovery boiler cost represents approximately half of the total capital expenditures. The capacity of a pulp manufacturer is then limited by the amount of black liquor that can be burned in the recovery boiler. For this reason, reducing the load of the recovery boiler using other alternatives is an attractive option. As mentioned in previous sections, the lignin is normally burned to obtain energy that is used in the paper making process. However, lignin itself is potentially valuable as a specialty chemical and its recovery can provide an additional income to the company. Lignin can be sold as low-quality lignin or high-quality lignin. Low-quality lignin may be used, for instance, to manufacture phenolic resin substitutes or asphalt conditioner. Digitalization is reducing the dependency on paper, which is decreasing its demand. Therefore, the paper and pulp industries need to diversify their product offering in order to make their business more profitable in the following years.
The implementation of the proposal considered in this study was a capital project that required an initial investment, and its capacity to earn net benefits over time was evaluated in this section. For Scenario 1, which consisted in treating 100% of the black liquor using the conventional process, no capital cost was required. For Scenario 2, the investment was destined to the portion of the capacity that would be replaced by membrane technologies. The estimations of the capital costs in machinery, equipment, and installment took into the account the required capacity and the assumption that the investment was three times higher when resistance to extreme pH was necessary. The total CAPEX for the implementation of the membrane system with 15 m
3 h
−1 of capacity is 842,048.20 EUR. Additionally, considering that the membrane life is assumed to be 1.5 years [
4], the capital cost would be periodically increased during operation to replace the UF and NF membranes. Taking into account the capacity, the number of modules required are one for ultrafiltration and twenty-two for nanofiltration. Assuming that the price per module is 1200.00 EUR, the additional cost is 26,400.00 EUR.
The operational costs considered in this study corresponded to the consumption of chemicals and energy during for the production and the maintenance. As in other big industries, it was assumed that the maintenance would be scheduled once a year and that the energy consumption was 10% of the one in regular operating conditions.
For Scenario 1, the calculation of the total energy consumption considered that the conventional process (which includes the evaporators, recovery boilers, and causticizers) demanded 13 kWh m−3 to operate. With a feed of 150 m3 h−1 and with 8760 h of operation per year, the total energy cost was 1,205,989.20 EUR per year. The maintenance cost was 10,963.54 EUR per year. Finally, the cost of the chemicals was 7851.00 EUR per year. These costs were considered starting from the first year of operation. The calculation of the indirect manufacturing costs took into account the office supplies and communications, which was assumed as a fixed cost of 20,000.00 EUR per year, and the salaries and wages, which sum 1,397,155.70 EUR per year.
For Scenario 2, the calculation of the total energy consumption considered that the conventional process demanded 13 kWh m−3 to process 135 m3 of black liquor per hour and the membrane process required 1.763 kWh m−3 to process 15 m3 of black liquor per hour. In this case, it was also assumed that the pulp producer would operate 8760 h in a year. As a result, the total energy cost was 1,101,748.60 EUR per year. The maintenance cost was 10,015.90 EUR per year. Finally, the cost of the chemicals was 19,452.30 EUR per year. These costs were considered since the first year of operation. Therefore, the total direct manufacturing costs of Scenario 2 is 1,131,216.80 EUR per year. The calculation of the indirect manufacturing cost took into account the office supplies and communications, which was assumed as a fixed cost of 20,000.00 EUR per year, and the salaries and wages, which sum 947,312.20 EUR per year.
To conclude with the input data for the economic assessment, the income from both scenarios was quantified. For Scenario 1, the source of income was in the savings of electricity generated by the black liquor processed by the conventional wastewater treatment. This amount of energy could be calculated taking into account that 1500 tons of black liquor solids generate 25 to 35 MW of electricity [
5]. For this study, 30 MW were considered. The amount of electricity produced was then multiplied by the cost of electricity for industrial customers in Sweden, which is 0.0706 EUR/kWh. The assumption is that all the black liquor solids were burned. If the energy of the 100% of the black liquor solids was recovered, that amount would be 9,184,860 kWh per year. Considering this very optimistic assumption, the total savings would be 648,451.10 EUR per year. For Scenario 2, the source of income was the sale of lignin recovered in the retentate of the membrane processes, assuming that the lignin rejection of the UF process using a 10 kDa membrane is 84.80% [
33]. The prices of lignin obtained from the kraft process varies between 260 and 500 EUR/ton. For this study, the price was assumed to be 380 EUR/ton [
34]. The annual benefits were calculated with the income in each of the studied scenarios and the operational costs.
Using all of the data, the profit after taxes in both scenarios could be calculated and it is shown in
Table 8. In Scenario 1, the profit was negative because the savings in electricity costs generated from the energy recovery were not higher than the operational costs. However, the savings covered more than half of the total electricity consumption cost of the process (53.77%, to be more specific), which was compatible to the reality of paper and pulp industries.
In Scenario 2, UF and NF would allow a recovery of lignin that can be sold to other industries. This additional income generated an attractive profit of 106,580.80 after taxes. In
Table 9 the project financial indicators are presented. The conclusion from these results was that the project is also feasible from a financial point of view given the considered assumptions for the study. The NPV value was >0; this number represented that the company’s value would increase 51,033.80 EUR in 10 years as a result from the investment. The IRR of the investment was higher than the opportunity cost of capital considered for the calculation, so it is recommended to invest in the project. Finally, the cumulative cash flow from the economic evaluation indicated that the payback period was of five years, assuming that the 100% of the recovered lignin can be sold in the market. It is important to mention that these calculations did not take into account the potential penalties that companies may be forced to pay if their toxic emissions are over the maximum permissible limit. In addition to this technical and economic study, a preliminary simplified life cycle assessment was performed to highlight the potential relevance of this proposal from an environmental point of view (in
Supplementary Materials).