Quantitative Evaluation of the Trade-Off Growth Strategies of Maize Leaves under Different Drought Severities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Descriptions
2.2. Experimental Design
2.3. Measurements
2.3.1. Leaf Traits
- Effective leaf number: The total number of leaves in the plant that were visible and not completely dried and shed (hereinafter referred to as the leaf number).
- Leaf area (LA): The length (Li) and width (the widest part of the leaf, Di) of every fully expanded leaf of the sample plants were measured. LA (m2) of an individual maize plant was calculated with Equation (1):
- Leaf biomass and leaf water content: The leaf fresh biomass was weighed. Then, leaves were placed in paper bags and dried in an oven at 80 °C for more than 24 h until their weights were constant. Then, leaf dry biomass was weighed. Leaf water content and specific leaf weight were calculated with the following formulas [22]:
2.3.2. Soil Water Content
2.4. Quantified Expressions of Drought Intensity and Drought Severity
2.5. Interpolation of Soil Water Content
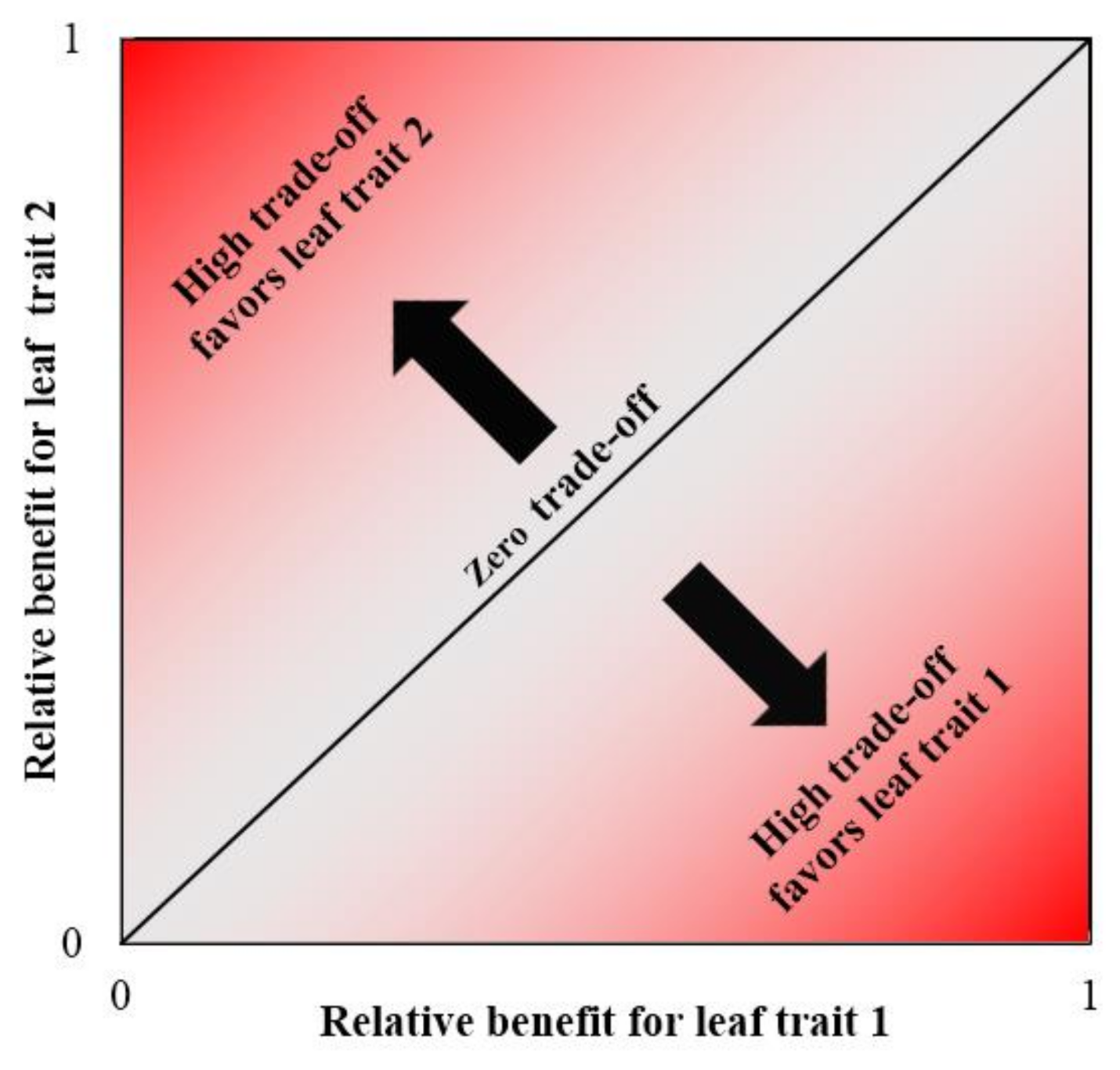
2.6. Calculation of Trade-Off Values
3. Results
3.1. Influence of Drought Severity on Maize Leaf Traits
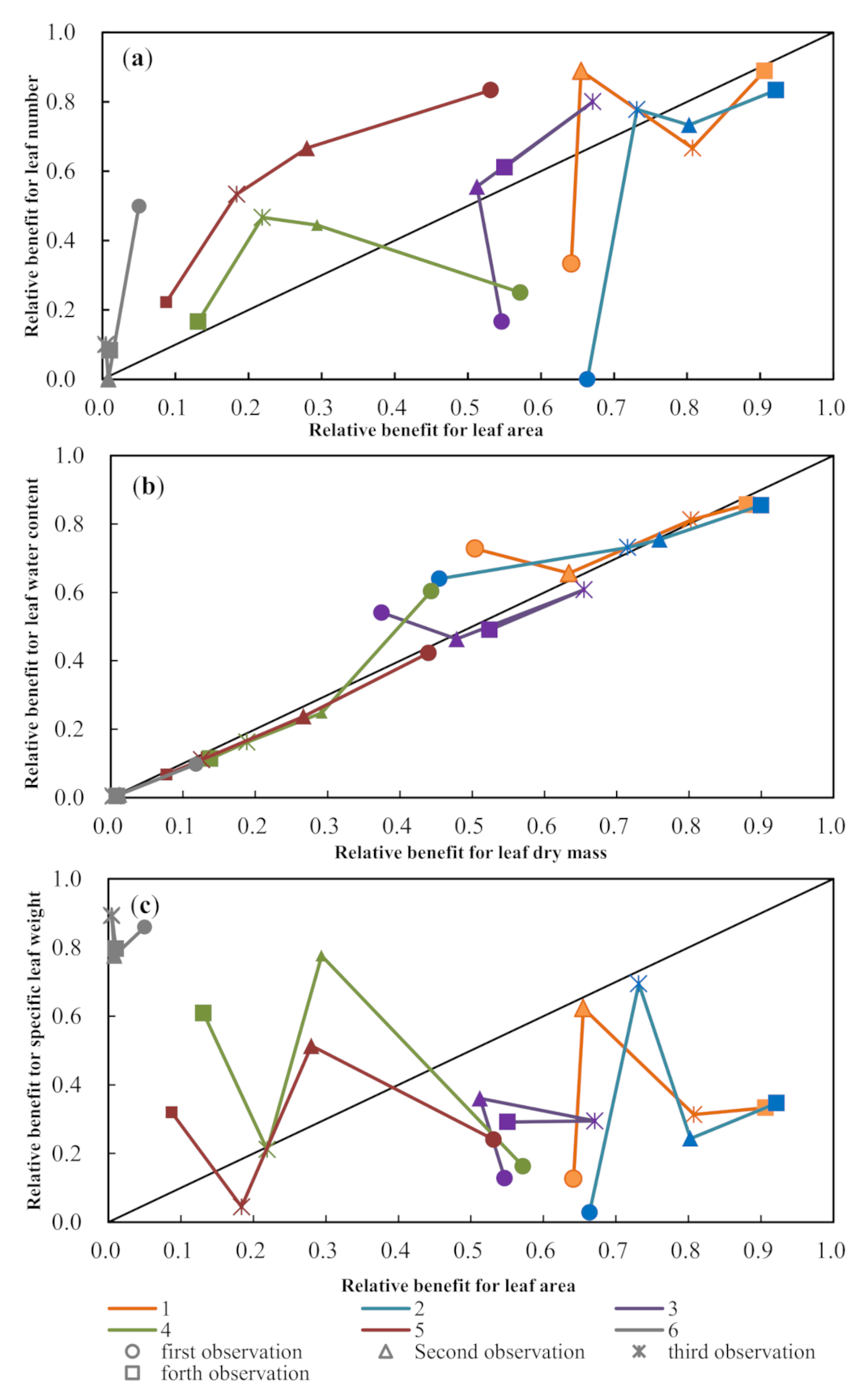
3.2. Dynamics of Paired Maize Leaf Traits
3.3. Trade-Off Growth of Paired Leaf Traits to Different Drought Severity Conditions
4. Discussion
4.1. Quantitative Expression of Drought Intensity and Drought Severity
4.2. Effects of Drought on Maize Leaf Traits
4.3. Trade-Off Strategies of Maize Leaf Traits during Drought Conditions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neumann, P.M. Coping mechanisms for crop plants in drought—Prone environments. Ann. Bot. 2008, 101, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.R.; Nakasathien, S.; Sarobol, E.; Vichukit, V. Responses of physiological traits of maize to water deficit induced at different phonological stages. Kasetsart J. Nat. Sci. 2014, 48, 183–196. [Google Scholar]
- Costa, L.C.; Cunha, A.P.; Anderson, L.O.; Cunningham, C. New approach for drought assessment: A case study in the northern region of Minas Gerais. Int. J. Disaster Risk Reduct. 2021, 102019. [Google Scholar] [CrossRef]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Chang. 2013, 3, 52–58. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis; Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Olesen, J.E.; Trnka, M.; Kersebaum, K.C.; Skjelvåg, A.O.; Seguin, B.; Peltonen-Sainio, P.; Rossi, F.; Kozyra, J.; Micale, F. Impacts and adaptation of European crop production systems to climate change. Eur. J. Agron. 2011, 34, 96–112. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Huang, J. Policy support, social capital, and farmers’ adaptation to drought in China. Glob. Environ. Chang. 2014, 24, 193–202. [Google Scholar] [CrossRef]
- Jiang, P.; Cai, F.; Zhao, Z.-Q.; Meng, Y.; Gao, L.-Y.; Zhao, T.-H. Physiological and Dry Matter Characteristics of Spring Maize in Northeast China under Drought Stress. Water 2018, 10, 1561. [Google Scholar] [CrossRef] [Green Version]
- Saglam, A.; Kadioğlu, A.; Demiralay, M.; Terzi, R. Leaf Rolling Reduces Photosynthetic Loss in Maize Under Severe Drought. Acta Bot. Croat. 2014, 73, 315–332. [Google Scholar] [CrossRef]
- Farooq, M.; Kobayashi, N.; Ito, O.; Wahid, A.; Serraj, R. Broader leaves result in better performance of indica rice under drought stress. J. Plant Physiol. 2010, 167, 1066–1075. [Google Scholar] [CrossRef]
- Poorter, L.; Bongers, F. Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 2006, 87, 1733–1743. [Google Scholar] [CrossRef]
- Freschet, G.T.; Cornelissen, J.H.C.; Van Logtestijn, R.S.P.; Aerts, R. Evidence of the ‘plant economics spectrum’ in a subarctic flora. J. Ecol. 2010, 98, 362–373. [Google Scholar] [CrossRef] [Green Version]
- Pan, S. Variation in Leaf Metabolic Ecological Exponent and Leaf Traits along Environmental Gradients; Zhejiang University: Hangzhou, China, 2014. [Google Scholar]
- Zhu, J.; Zhu, H.; Cao, Y.; Li, J.; Zhu, Q.; Yao, J.; Xu, C. Effect of simulated warming on leaf functional traits of urban greening plants. BMC Plant Biol. 2020, 20, 139. [Google Scholar] [CrossRef]
- Qiu, R.; Katul, G.G.; Wang, J.; Xu, J.; Kang, S.; Liu, C.; Zhang, B.; Li, L.; Cajucom, E.P. Differential response of rice evapotranspiration to varying patterns of warming. Agric. For. Meteorol. 2021, 298–299, 108293. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Bosabalidis, A.M.; Kofidis, G. Comparative effects of drought stress on leaf anatomy of two olive cultivars. Plant Sci. 2002, 163, 375–379. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. Die and let live: Leaf senescence contributes to plant survival under drought stress. Funct. Plant Biol. 2004, 31, 203–216. [Google Scholar] [CrossRef]
- Lefi, E.; GULÍAS, J.; Cifre, J.; Ben Younes, M.; Medrano, H. Drought effects on the dynamics of leaf production and senescence in field-grown Medicago arborea and Medicago citrina. Ann. Appl. Biol. 2004, 144, 169–176. [Google Scholar] [CrossRef]
- Francis, C.A.; Rutger, J.N.; Palmer, A.F.E. A Rapid Method for Plant Leaf Area Estimation in Maize (Zea mays L.). Crop Sci. 1969, 9, 537–539. [Google Scholar] [CrossRef]
- Peuke, A.D.; Schraml, C.; Hartung, W.; Rennenberg, H. Identification of drought-sensitive beech ecotypes by physiological parameters. New Phytol. 2002, 154, 373–387. [Google Scholar] [CrossRef]
- Chen, J.Z.; Wang, S.; Zhang, L.L.; Guo-An, L. Response of Maize to Progressive Drought and Red Soil’s Drought Threshold. Sci. Agric. Sin. 2007, 40, 532–539. [Google Scholar]
- Allen, R.; Pereira, L.; Raes, D.; Smith, M. Crop Evapotranspiration. Guidelines for Computing Crop Water Requirements; FAO Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998. [Google Scholar]
- Bradford, J.B.; D’Amato, A.W. Recognizing trade-offs in multi-objective land management. Front. Ecol. Environ. 2012, 10, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Porporato, A.; Laio, F.; Ridolfi, L.; Rodriguez-Iturbe, I. Plants in water-controlled ecosystems: Active role in hydrologic processes and response to water stress: III. Vegetation water stress. Adv. Water Resour. 2001, 24, 725–744. [Google Scholar] [CrossRef]
- Zhang, Y.; Na, M.; Cheng, P.; Ji, R. Influences of Soil Water Stress on Growth and Development of Maize. Chin. Agric. Sci. Bull. 2012, 28, 1–7. [Google Scholar]
- Elhaak, M.A. Response of Plantago albicans leaves of environmental drought. Feddes Repert. 1990, 101, 645–650. [Google Scholar] [CrossRef]
- Ye, L.; Huang, X.; Li, X. Effects of Drought on Leaf Traits and Drought-resistant Physiology of Trees. World For. Res. 2014, 27, 29–34. [Google Scholar]
- Shi, Y.H.; Zhou, G.S.; Jiang, Y.L.; Ma, X.Y. Thresholds of Stipa baicalensis sensitive indicators response to precipitation change. Acta Ecol. Sin. 2017, 37, 2620–2630. [Google Scholar]
- Aarssen, L.W. Reducing size to increase number: A hypothesis for compound leaves. Ideas Ecol. Evol. 2012, 5, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Sterck, F.J.; Poorter, L.; Schieving, F. Leaf traits determine the growth—Survival trade—Off across rain forest tree species. Am. Nat. 2006, 167, 758–765. [Google Scholar] [CrossRef]
- Yang, D.M.; Feng, Z.; Zhang, H.W. Trade-off between leaf size and number in current-year twigs of deciduous broad-leaved woody species at different altitudes on Qingliang Mountain, southeastern China. Chin. J. Plant Ecol. 2012, 36, 281–291. [Google Scholar] [CrossRef]
- Kleimn, D.; Aarssen, L.W. The leaf size/number trade-off in trees. J. Ecol. 2007, 95, 376–382. [Google Scholar] [CrossRef]
- Qiu, R.; Liu, C.; Cui, N.; Wu, Y.; Wang, Z.; Li, G. Evapotranspiration estimation using a modified Priestley-Taylor model in a rice-wheat rotation system. Agric. Water Manag. 2019, 224, 105755. [Google Scholar] [CrossRef]
- Whitman, T.; Aarssen, L.W. The leaf size/number trade-off in herbaceous angiosperms. J. Plant Ecol. 2009, 3, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Wang, S.; Liu, Z.; Nijs, I.; Ma, K.; Li, Z. Effects of resource availability on the trade-off between seed and vegetative reproduction. J. Plant Ecol. 2010, 3, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Zheng, G.Q.; Duan, S.F.; Yan, S.B.; Lu, B.Q. Simulation Models of the Development of Leaf Age and Organs in Maize. J. Maize Sci. 2003, 11, 63–66. [Google Scholar]
- Ocheltree, T.W.; Nippert, J.B.; Prasad, P.V.V. A safety vs. efficiency trade-off identified in the hydraulic pathway of grass leaves is decoupled from photosynthesis, stomatal conductance and precipitation. New Phytol. 2016, 210, 97–107. [Google Scholar] [CrossRef] [Green Version]

| Treatments | 10 July | 18 July | 31 July | 7 August | ||||
|---|---|---|---|---|---|---|---|---|
| Soil Moisture Content (%) | Drought Severity | Soil Moisture Content (%) | Drought Severity | Soil Moisture Content (%) | Drought Severity | Soil Moisture Content (%) | Drought Severity | |
| 1 | 96.5 ± 1.0 a | 0.00 c | 69.1 ± 3.9 a | 0.000 d | 56.5 ± 6.7 a | 0.26 d | 49.3 ± 2.1 a | 0.44 e |
| 2 | 90.8 ± 1.4 b | 0.00 c | 68.3 ± 1.8 a | 0.02 d | 52.7 ± 3.7 ab | 0.46 c | 44.1 ± 3.3 a | 0.64 d |
| 3 | 83.1 ± 4.7 c | 0.00 c | 63.0 ± 5.2 a | 0.10 d | 47.7 ± 2.5 bc | 0.54 c | 45.1 ± 7.8 a | 0.69 cd |
| 4 | 69.1 ± 2.6 d | 0.04 c | 54.6 ± 2.3 b | 0.48 c | 47.6 ± 1.5 bc | 0.74 b | 42.3 ± 4.9 a | 0.81 bc |
| 5 | 61.3 ± 4.5 e | 0.23 b | 48.0 ± 2.9 b | 0.72 b | 43.7 ± 4.9 c | 0.87 ab | 41.7 ± 4.8 a | 0.90 ab |
| 6 | 45.3 ± 1.1 f | 0.87 a | 41.0 ± 2.2 c | 0.95 a | 31.8 ± 1.4 d | 0.98 a | 32.8 ± 0.7 a | 0.98 a |
| Observation Date | Treatments | Effective Leaf Number | Leaf Area (cm2) | Leaf Dry Mass (g) | Leaf Water Content (g) | Specific Leaf Weight (g.m−2) |
|---|---|---|---|---|---|---|
| 10 July | 1 | 4.3 ± 0.6 | 121.2 ± 24.6 a | 0.31 ± 0.07 | 1.76 ± 0.37 a | 26.02 ± 0.55 a |
| 2 | 4.0 ± 0.0 | 122.0 ± 16.1 a | 0.30 ± 0.05 | 1.64 ± 0.26 a | 25.06 ± 0.39 a | |
| 3 | 4.2 ± 0.3 | 107.9 ± 3.2 a | 0.29 ± 0.04 | 1.51 ± 0.19 a | 26.04 ± 1.03 a | |
| 4 | 4.3 ± 0.4 | 114.1 ± 2.4 a | 0.30 ± 0.01 | 1.59 ± 0.14 a | 26.39 ± 0.01 a | |
| 5 | 4.8 ± 0.3 | 107.6 ± 5.6 a | 0.30 ± 0.01 | 1.36 ± 0.10 ab | 27.15 ± 2.13 b | |
| 6 | 4.5 ± 0.5 | 76.2 ± 3.8 b | 0.25 ± 0.02 | 0.93 ± 0.17 b | 33.26 ± 1.69b | |
| 18 July | 1 | 6.7 ± 0.6 a | 425.3 ± 47.9 a | 1.77 ± 0.23 a | 6.65 ± 1.09 ab | 41.58 ± 0.74 |
| 2 | 6.3 ± 0.6 ab | 466.0 ± 130.1 a | 1.96 ± 0.58 a | 7.33 ± 2.13 a | 41.92 ± 1.56 | |
| 3 | 5.7 ± 0.6 bc | 349.1 ± 58.4 ab | 1.41 ± 0.28 ab | 4.94 ± 0.98 bc | 40.34 ± 1.57 | |
| 4 | 5.3 ± 0.6 c | 232.5 ± 51.9 b | 0.98 ± 0.22 b | 3.01 ± 0.74 cd | 42.31 ± 0.94 | |
| 5 | 6.0 ± 0.0 abc | 225.0 ± 29.0 b | 0.93 ± 0.15 b | 2.92 ± 0.44 cd | 41.06 ± 2.22 | |
| 6 | 4.0 ± 0.0 d | 79.9 ± 6.3 c | 0.34 ± 0.04 c | 0.88 ± 0.11 d | 42.31 ± 1.36 | |
| 31 July | 1 | 7.3 ± 0.6 ab | 1101.1 ± 218.6a | 5.57 ± 1.12 a | 19.62 ± 3.73 a | 50.54 ± 0.75 a |
| 2 | 7.7 ± 0.6 ab | 1095.0 ± 173.8 a | 5.29 ± 1.01 a | 18.30 ± 3.47 a | 48.20 ± 2.67 a | |
| 3 | 8.0 ± 1.0 a | 924.5 ± 341.5 a | 4.62 ± 1.73 a | 14.97 ± 5.40 a | 49.92 ± 2.60 a | |
| 4 | 6.3 ± 0.6 b | 339.4 ± 136.4 b | 1.62 ± 0.72 b | 4.81 ± 2.20 b | 47.13 ± 2.40 a | |
| 5 | 6.7 ± 0.6 ab | 293.8 ± 68.3 b | 1.22 ± 0.29 b | 3.65 ± 1.05 b | 41.54 ± 1.32 b | |
| 6 | 4.5 ± 0.7 c | 62.1 ± 8.8 b | 0.43 ± 0.03 b | 1.22 ± 0.15 b | 69.94 ± 5.08 b | |
| 7 August | 1 | 9.0 ± 0.0 a | 1688.5 ± 143.2 a | 9.28 ± 0.79 a | 28.70 ± 3.67 a | 54.94 ± 1.26 a |
| 2 | 9.7 ± 0.6 a | 1716.3 ± 168.4 a | 9.47 ± 1.06 a | 28.64 ± 4.68 a | 55.24 ± 4.04 a | |
| 3 | 8.3 ± 0.6 a | 1048.1 ± 54.5 b | 5.69 ± 1.00 b | 16.92 ± 3.65 b | 54.03 ± 7.02 a | |
| 4 | 5.3 ± 0.6 b | 293.4 ± 116.4 c | 1.79 ± 0.72 c | 4.78 ± 1.88 c | 61.02 ± 4.04 ab | |
| 5 | 5.7 ± 1.2 b | 215.0 ± 52.9 c | 1.18 ± 0.31 c | 3.29 ± 0.91c | 54.64 ± 4.25 a | |
| 6 | 4.5 ± 0.7 b | 76.1 ± 26.2 c | 0.49 ± 0.12 c | 1.27 ± 0.21c | 65.15 ± 6.33 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Zhou, G.; Li, G.; Wang, Q. Quantitative Evaluation of the Trade-Off Growth Strategies of Maize Leaves under Different Drought Severities. Water 2021, 13, 1852. https://doi.org/10.3390/w13131852
Ma X, Zhou G, Li G, Wang Q. Quantitative Evaluation of the Trade-Off Growth Strategies of Maize Leaves under Different Drought Severities. Water. 2021; 13(13):1852. https://doi.org/10.3390/w13131852
Chicago/Turabian StyleMa, Xueyan, Guangsheng Zhou, Gen Li, and Qiuling Wang. 2021. "Quantitative Evaluation of the Trade-Off Growth Strategies of Maize Leaves under Different Drought Severities" Water 13, no. 13: 1852. https://doi.org/10.3390/w13131852
APA StyleMa, X., Zhou, G., Li, G., & Wang, Q. (2021). Quantitative Evaluation of the Trade-Off Growth Strategies of Maize Leaves under Different Drought Severities. Water, 13(13), 1852. https://doi.org/10.3390/w13131852







