Influence of Environmental Factors on Occurrence of Cyanobacteria and Abundance of Saxitoxin-Producing Cyanobacteria in a Subtropical Drinking Water Reservoir in Brazil
Abstract
:1. Introduction
2. Materials and Methods
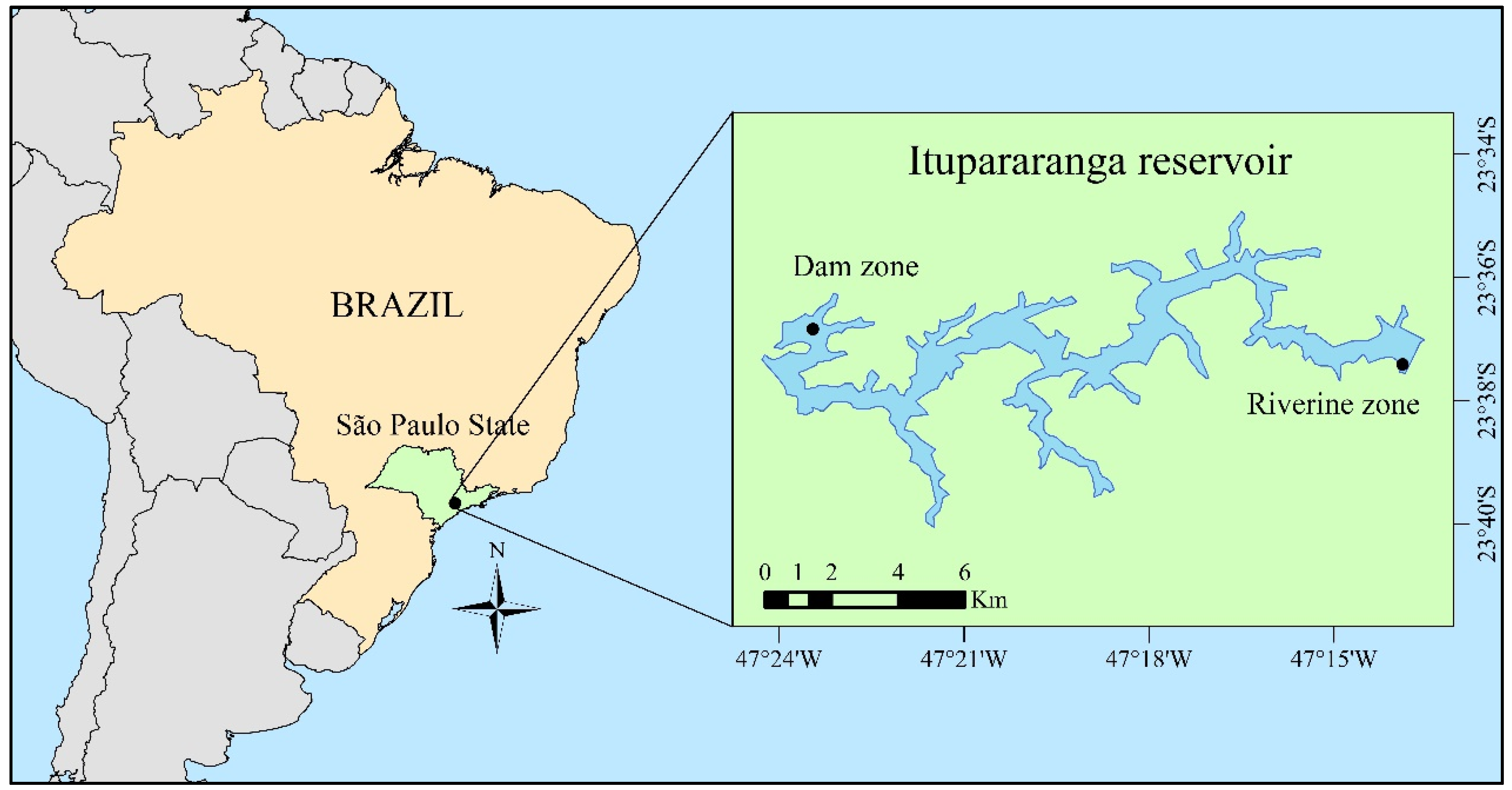
2.1. Study Area and Sampling
2.2. Environmental Variables
2.3. Phytoplankton Community
2.4. Cyanotoxins
2.5. DNA Extraction, Primer Design and PCR of the sxtA Gene
2.6. Data Analysis
3. Results
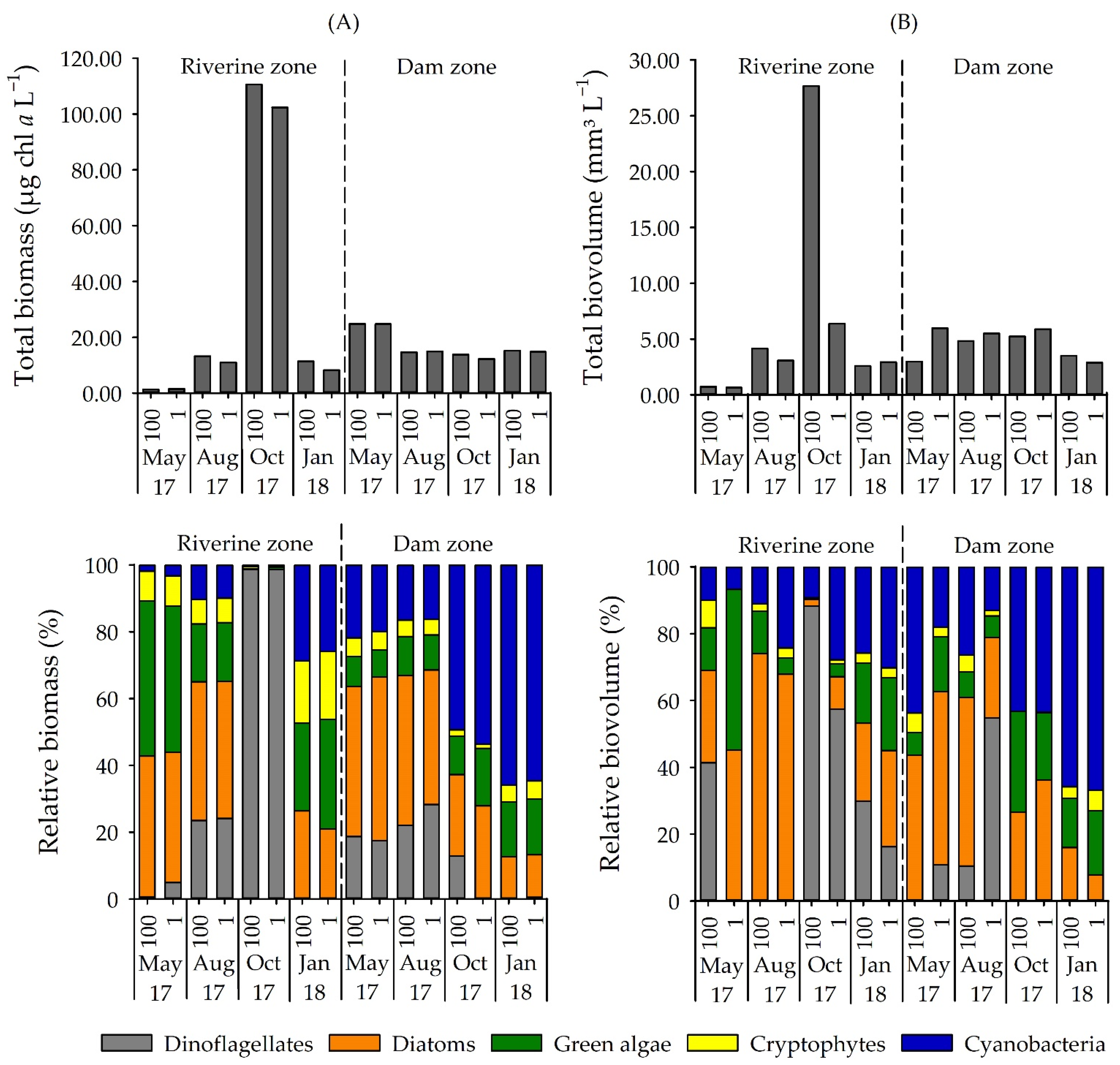
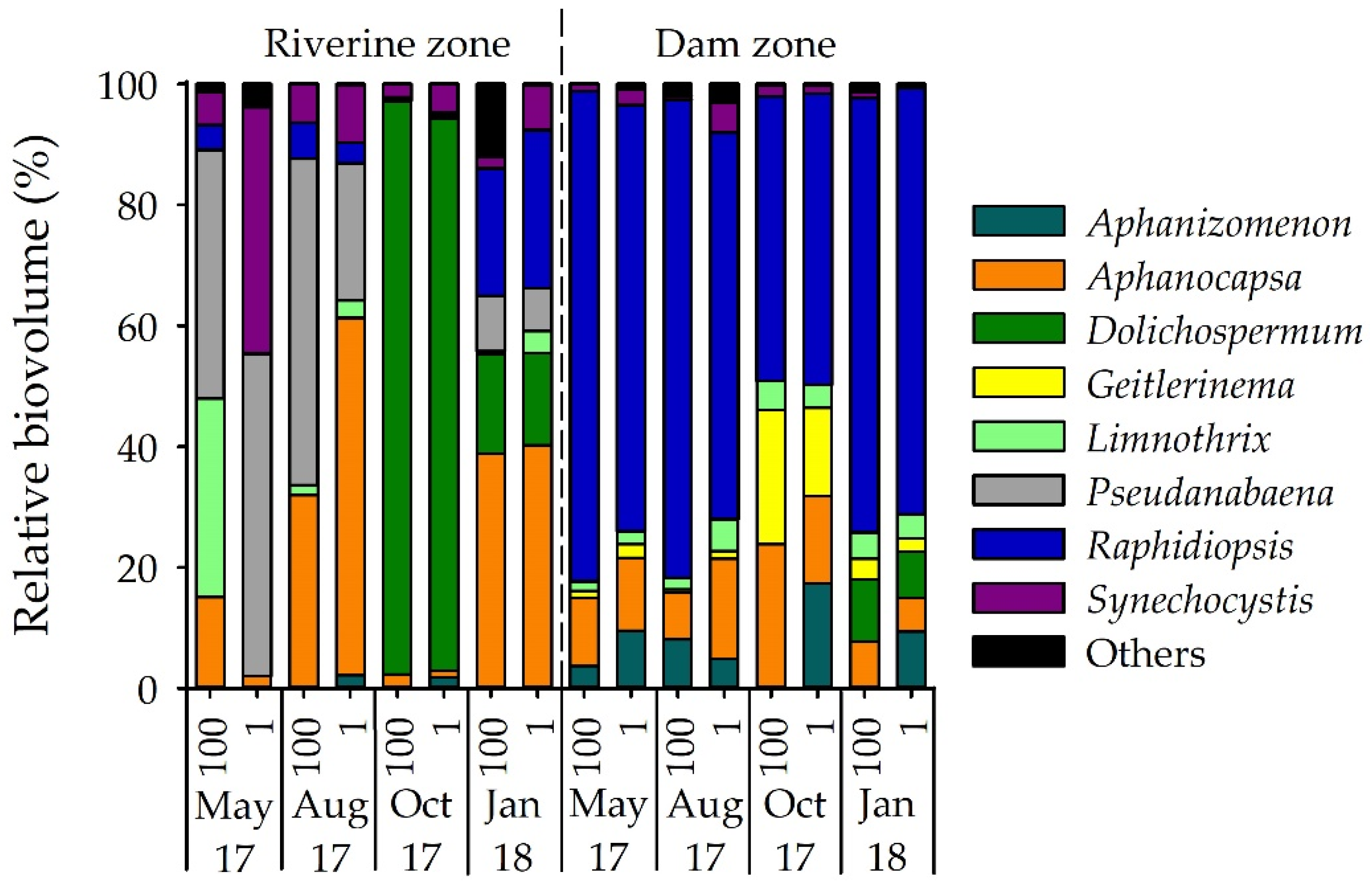
3.1. Phytoplankton Composition
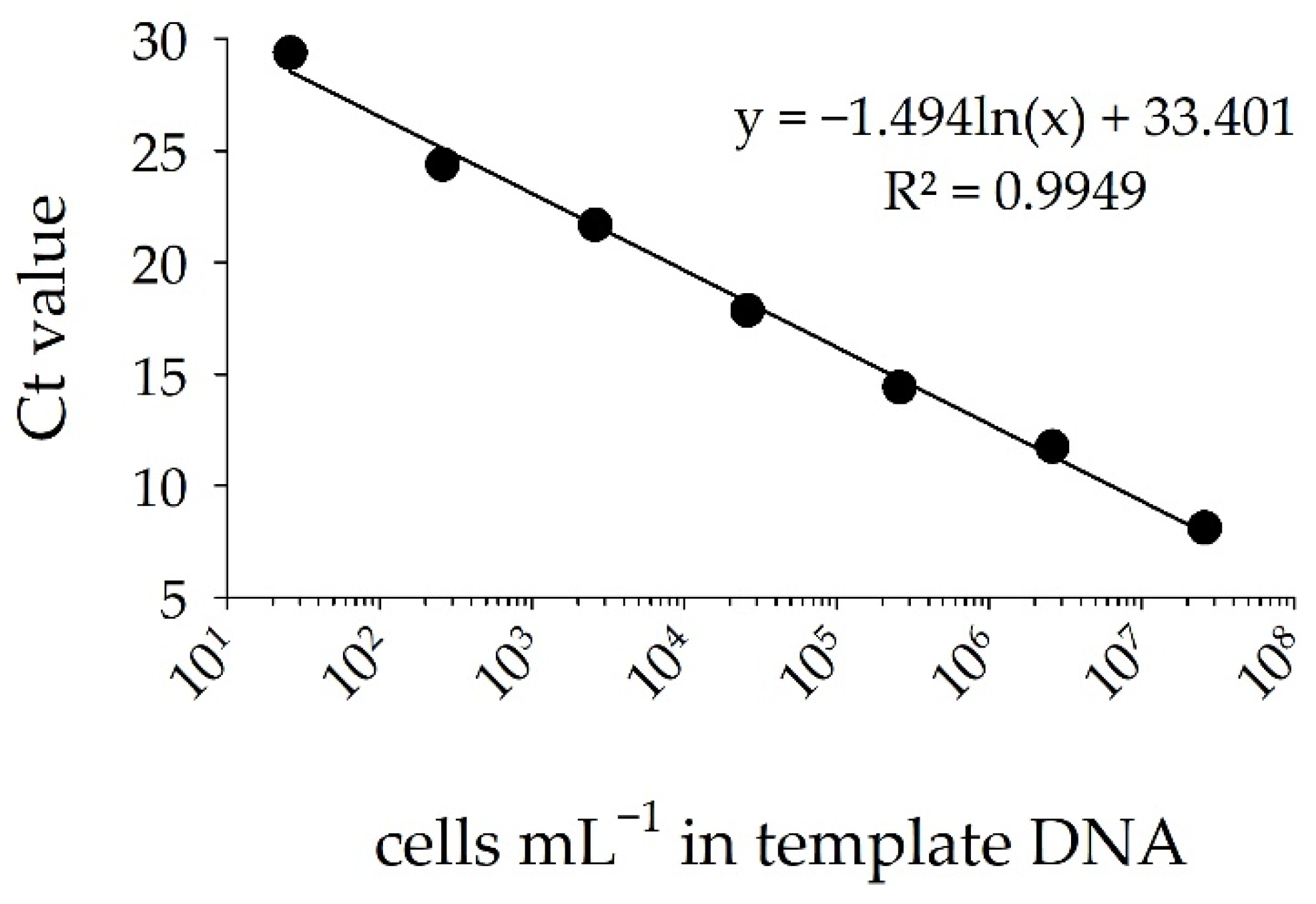
3.2. Detection of STX Producers: Validation of the sxtA-Cyano Primer Set and Standard Curve
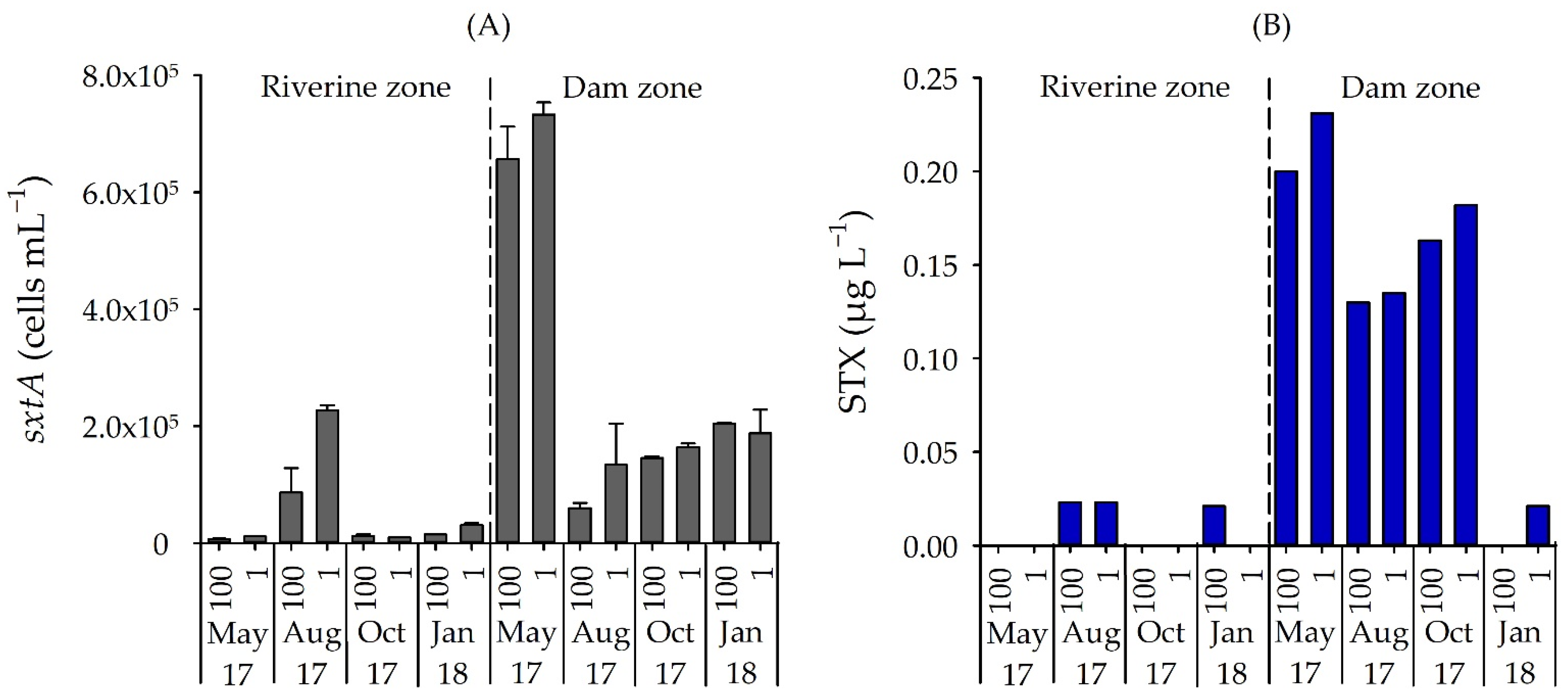
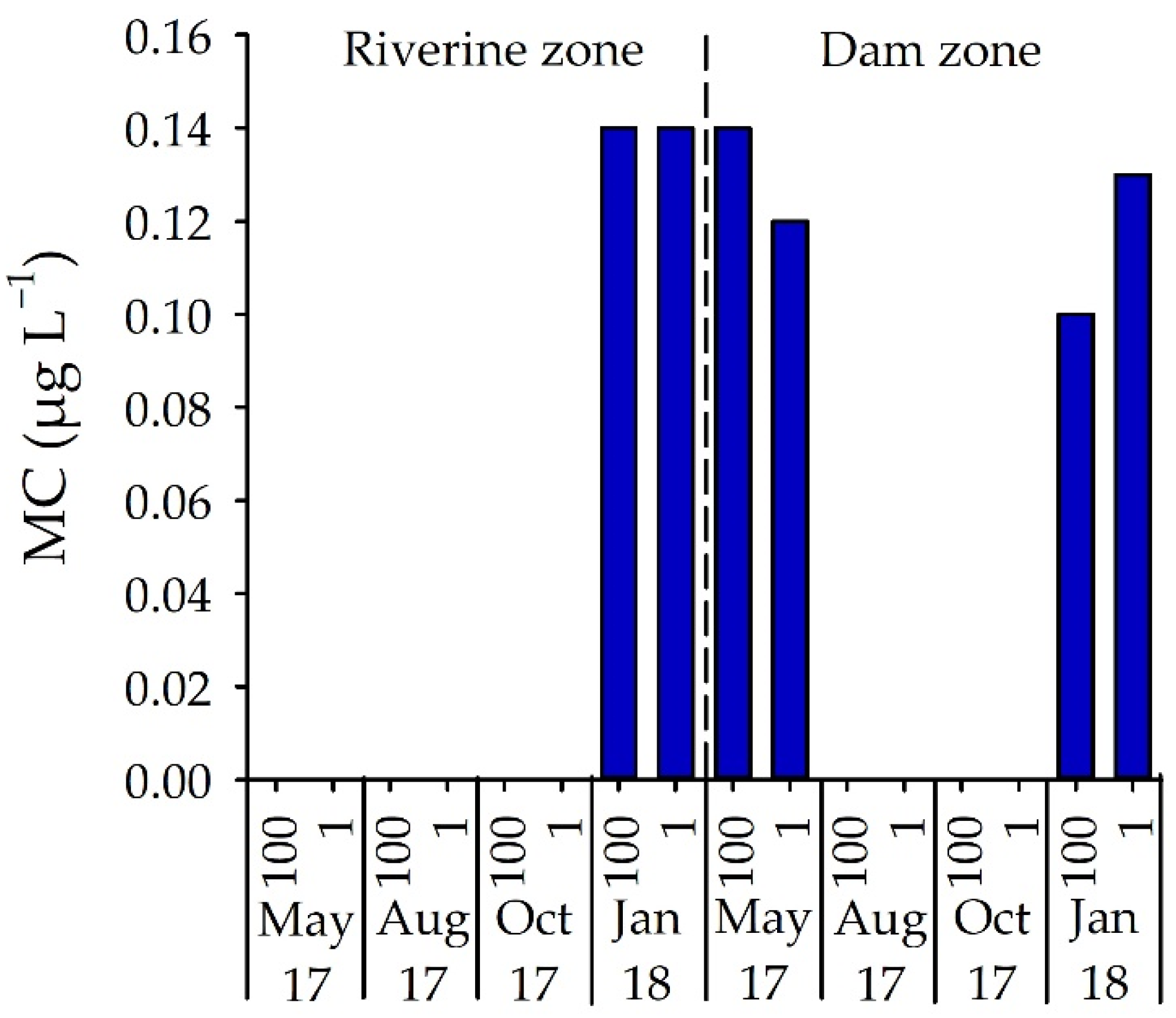
3.3. Number of STX-Producing Cells and Concentrations of STX and MC
3.4. Linkage between Environmental Variables, Cyanobacteria and Cyanotoxins
4. Discussion
4.1. Environmental Variables and Cyanobacteria
4.2. qPCR Assay, Cyanotoxin and Cyanobacteria
4.3. Saxitoxin and Environmental Variables
4.4. Microcystin and Environmental Variables
4.5. Pigment Analysis and qPCR Assay as Tools for Monitoring of Toxic Cyanobacteria
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sabart, M.; Crenn, K.; Perrière, F.; Abila, A.; Leremboure, M.; Colombet, J.; Jousse, C.; Latour, D. Co-occurrence of microcystin and anatoxin-a in the freshwater lake Aydat (France): Analytical and molecular approaches during a three-year survey. Harmful Algae 2015, 48, 12–20. [Google Scholar] [CrossRef]
- Spoof, L.; Catherine, A. Appendix 3: Tables of Microcystins and Nodularins. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 526–537. [Google Scholar] [CrossRef]
- Bouaïcha, N.; Miles, C.O.; Beach, D.G.; Labidi, Z.; Djabri, A.; Benayache, N.Y.; Nguyen-Quang, T. Structural diversity, characterization and toxicology of microcystins. Toxins 2019, 11, 714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wacklin, P.; Hoffmann, L.; Komárek, J. Nomenclatural validation of the genetically revised cyanobacterial genus Dolichospermum (Ralfs ex Bornet et Flahault) comb, nova. Fottea 2009, 9, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilera, A.; Gómez, E.B.; Kaštovský, J.; Echenique, R.O.; Salerno, G.L. The polyphasic analysis of two native Raphidiopsis isolates supports the unification of the genera Raphidiopsis and Cylindrospermopsis (Nostocales, Cyanobacteria). Phycologia 2018, 57, 130–146. [Google Scholar] [CrossRef]
- Valério, E.; Chaves, S.; Tenreiro, R. Diversity and impact of prokaryotic toxins on aquatic environments: A review. Toxins 2010, 2, 2359–2410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holland, A.; Kinnear, S. Interpreting the Possible Ecological Role(s) of Cyanotoxins: Compounds for Competitive Advantage and/or Physiological Aide? Mar. Drugs 2013, 11, 2239–2258. [Google Scholar] [CrossRef] [Green Version]
- Calijuri, M.C.; Alves, M.S.A.; dos Santos, A.C.A. Cianobactérias e Cianotoxinas em Águas Continentais; Rima: São Carlos, Brazil, 2006. [Google Scholar]
- Ferrão-Filho, A.D.S.; Kozlowsky-Suzuki, B. Cyanotoxins: Bioaccumulation and effects on aquatic animals. Mar. Drugs 2011, 9, 2729–2772. [Google Scholar] [CrossRef]
- Pimentel, J.S.M.; Giani, A. Estimating toxic cyanobacteria in a Brazilian reservoir by quantitative real-time PCR, based on the microcystin synthetase D gene. J. Appl. Phycol. 2013, 25, 1545–1554. [Google Scholar] [CrossRef]
- Ribeiro, M.S.F.; Tucci, A.; Matarazzo, M.P.; Viana-niero, C.; Nordi, C.S.F. Detection of Cyanotoxin-Producing Genes in a Eutrophic Reservoir (Billings Reservoir, São Paulo, Brazil). Water 2020, 12, 1–13. [Google Scholar] [CrossRef] [Green Version]
- McKindles, K.M.; Zimba, P.V.; Chiu, A.S.; Watson, S.B.; Gutierrez, D.B.; Westrick, J.; Kling, H.; Davis, T.W. A multiplex analysis of potentially toxic cyanobacteria in Lake Winnipeg during the 2013 bloom season. Toxins 2019, 11, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Tebrineh, J.; Merrick, C.; Ryan, D.; Humpage, A.; Bowling, L.; Neilan, B.A. Community composition, toxigenicity, and environmental conditions during a cyanobacterial bloom occurring along 1,100 kilometers of the Murray River. Appl. Environ. Microbiol. 2012, 78, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Al-Tebrineh, J.; Mihali, T.K.; Pomati, F.; Neilan, B.A. Detection of saxitoxin-producing cyanobacteria and Anabaena circinalis in environmental water blooms by quantitative PCR. Appl. Environ. Microbiol. 2010, 76, 7836–7842. [Google Scholar] [CrossRef] [Green Version]
- Fortin, N.; Munoz-Ramos, V.; Bird, D.; Lévesque, B.; Whyte, L.G.; Greer, C.W. Toxic cyanobacterial bloom triggers in Missisquoi Bay, Lake Champlain, as determined by next-generation sequencing and quantitative PCR. Life 2015, 5, 1346–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savela, H.; Spoof, L.; Perälä, N.; Preede, M.; Lamminmäki, U.; Nybom, S.; Häggqvist, K.; Meriluoto, J.; Vehniäinen, M. Detection of cyanobacterial sxt genes and paralytic shellfish toxins in freshwater lakes and brackish waters on Åland Islands, Finland. Harmful Algae 2015, 46, 1–10. [Google Scholar] [CrossRef]
- dos Anjos, F.M.; do Bittencourt-Oliveira, M.C.; Zajac, M.P.; Hiller, S.; Christian, B.; Erler, K.; Luckas, B.; Pinto, E. Detection of harmful cyanobacteria and their toxins by both PCR amplification and LC-MS during a bloom event. Toxicon 2006, 48, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, A.S. Implementação da Técnica de qPCR para o Monitoramento de Microcystis e Genótipos Potencialmente Produtores de Microcistinas; Universidade de São Paulo: São Paulo, Brazil, 2008. [Google Scholar]
- Bittencourt-Oliveira, M.; Santos, D.; Moura, N. Toxic cyanobacteria in reservoirs in northeastern Brazil: Detection using a molecular method. Brazilian J. Biol. 2010, 70, 1005–1010. [Google Scholar] [CrossRef] [Green Version]
- Lorenzi, A.S.; Chia, M.A.; Piccin-Santos, V.; Bittencourt-Oliveira, M.D.C. Microcystins and cylindrospermopsins molecular markers for the detection of toxic cyanobacteria: A case study of northeastern Brazilian reservoirs. Limnetica 2015, 34, 269–282. [Google Scholar] [CrossRef]
- Guedes, I.A.; da Costa Leite, D.M.; Manhães, L.A.; Bisch, P.M.; Azevedo, S.M.F.O.; Pacheco, A.B.F. Fluctuations in microcystin concentrations, potentially toxic Microcystis and genotype diversity in a cyanobacterial community from a tropical reservoir. Harmful Algae 2014, 39, 303–309. [Google Scholar] [CrossRef]
- Da Conceição, F.T.; de Godoy, D.S.S.L.H.; Pedrazzi; Fernandes, A.M.; de Pedrazzi, F.J.M. Influência sazonal no transporte específico de metais totais e dissolvidos nas águas fluviais da bacia do Alto Sorocaba (SP). Geochim. Bras. 2015, 29, 23–34. [Google Scholar] [CrossRef]
- Manfré, L.A.; Da Silva, A.M.; Urban, R.C. Atributos de qualidade de solos sob dois diferentes tipos de manejo no município de Ibiúna/SP, Brazil. Interciencia 2011, 36, 757–763. [Google Scholar]
- de Beghelli, F.G.S.; dos Santos, A.C.A.; Urso-Guimarães, M.V.; do Calijuri, M.C. Relationship between space distribution of the benthic macroinvertebrates community and trophic state in a Neotropical reservoir (Itupararanga, Brazil). Biota Neotrop. 2012, 12, 114–124. [Google Scholar] [CrossRef]
- Taniwaki, R.H.; Rosa, A.H.; De Lima, R.; Rodrigues Maruyama, C.; Ferrari Secchin, L.; do Calijuri, M.C.; Moschini Carlos, V. A influência do uso e ocupação do solo na qualidade e genotoxicidade da água no reservatório de itupararanga, São Paulo, Brasil. Interciencia 2013, 38, 164–170. [Google Scholar]
- de Pedrazzi, F.J.M.; da Conceição, F.T.; de Sardinha, D.S.; Moschini-Carlos, V.; Pompêo, M. Spatial and Temporal Quality of Water in the Itupararanga Reservoir, Alto Sorocaba Basin (SP), Brazil. J. Water Resour. Prot. 2013, 5, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, E.H.C.; Vicentin, A.M.; dos Machado, L.S.; Pompêo, M.L.M.; Moschini-Carlos, V. Phytoplankton, Trophic State and Ecological Potential in reservoirs in the State of São Paulo, Brazil. Rev. Ambient. Agua 2019, 14, 1–12. [Google Scholar] [CrossRef]
- Cunha, D.G.F.; Calijuri, M.D.C. Variação sazonal dos grupos funcionais fitoplanctônicos em braços de um reservatório tropical de usos múltiplos no estado de são paulo (Brasil). Acta Bot. Brasilica 2011, 25, 822–831. [Google Scholar] [CrossRef]
- De Souza Beghelli, F.G.; Frascareli, D.; Pompêo, M.L.M.; Moschini-Carlos, V. Trophic State Evolution over 15 Years in a Tropical Reservoir with Low Nitrogen Concentrations and Cyanobacteria Predominance. Water. Air. Soil Pollut. 2016, 227, 95. [Google Scholar] [CrossRef] [Green Version]
- Casali, S.P.; Dos Santos, A.C.A.; De Falco, P.B.; Do Carmo Calijuri, M. Influence of environmental variables on saxitoxin yields by Cylindrospermopsis raciborskii in a mesotrophic subtropical reservoir. J. Water Health 2017, 15, 509–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nusch, E.A. Comparison of different methods for chlorophyll and pheopigment determination. Arch. Hydrobiol. 1980, 14, 14–36. [Google Scholar]
- APHA; AWWA; WPCF. Standard Methods for the Examination of Water and Wastewater, 25th ed.; American Public Health Association; American Water Works Association; Water Pollution Control Federation: Washington, DC, USA, 2005. [Google Scholar]
- Cunha, D.G.F.; do Calijuri, M.C.; Lamparelli, M.C. A trophic state index for tropical/subtropical reservoirs (TSItsr). Ecol. Eng. 2013, 60, 126–134. [Google Scholar] [CrossRef]
- Utermöhl, H. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Int. Vereinigung Theor. Angew. Limnol. Mitteilungen 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Lund, J.W.G.; Kipling, C.; Le Cren, E.D. The inverted microscope method of estimating algal numbers and the statistical basis of estimations by counting. Hydrobiologia 1958, 11, 143–170. [Google Scholar] [CrossRef]
- Hillebrand, H.; Dürselen, C.D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Sun, J.; Liu, D. Geometric models for calculating cell biovolume and surface area for phytoplankton. J. Plankton Res. 2003, 25, 1331–1346. [Google Scholar] [CrossRef] [Green Version]
- Schlüter, L.; Behl, S.; Striebel, M.; Stibor, H. Comparing microscopic counts and pigment analyses in 46 phytoplankton communities from lakes of different trophic state. Freshw. Biol. 2016, 61, 1627–1639. [Google Scholar] [CrossRef]
- Schlüter, L.; David, G.S.; Jørgensen, N.O.G.; Podduturi, R.; Tucci, A.; Dias, A.S.; da Silva, R.J. Characterization of phytoplankton by pigment analysis and the detection of toxic cyanobacteria in reservoirs with aquaculture production. Aquac. Environ. Interact. 2018, 10, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Van Heukelem, L.; Thomas, C.S. Computer-assisted high-performance liquid chromatography method development with applications to the isolation and analysis of phytoplankton pigments. J. Chromatogr. A 2001, 910, 31–49. [Google Scholar] [CrossRef]
- Mackey, M.D.; Mackey, D.J.; Higgins, H.W.; Wright, S.W. CHEMTAX—A program for estimating class abundances from chemical markers: Application to HPLC measurements of phytoplankton. Mar. Ecol. Prog. Ser. 1996, 144, 265–283. [Google Scholar] [CrossRef] [Green Version]
- Schlüter, L.; Lauridsen, T.L.; Krogh, G.; Jørgensen, T. Identification and quantification of phytoplankton groups in lakes using new pigment ratios—A comparison between pigment analysis by HPLC and microscopy. Freshw. Biol. 2006, 51, 1474–1485. [Google Scholar] [CrossRef]
- Desjardins, P.; Conklin, D. NanoDrop microvolume quantitation of nucleic acids. J. Vis. Exp. 2010, 45, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [Green Version]
- Untergasser, A.; Nijveen, H.; Rao, X.; Bisseling, T.; Geurts, R.; Leunissen, J.A.M. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007, 35, 71–74. [Google Scholar] [CrossRef] [Green Version]
- Gorham, P.R.; McLachlan, J.; Hammer, U.T.; Kim, W.K. Isolation and culture of toxic strains of Anabaena flos-aquae (Lyngb.) de Bréb. In SIL Proceedings, 1922–2010; Taylor & Francis: London, UK, 1964; Volume 15, pp. 796–804. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software package for education. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Rantala, A.; Rajaniemi-Wacklin, P.; Lyra, C.; Lepistö, L.; Rintala, J.; Mankiewicz-Boczek, J.; Sivonen, K. Detection of microcystin-producing cyanobacteria in Finnish lakes with genus-specific microcystin synthetase gene E (mcyE) PCR and associations with environmental factors. Appl. Environ. Microbiol. 2006, 72, 6101–6110. [Google Scholar] [CrossRef] [Green Version]
- Fortin, N.; Aranda-Rodriguez, R.; Jing, H.; Pick, F.; Bird, D.; Greer, C.W. Detection of microcystin-producing cyanobacteria in Missisquoi Bay, Quebec, Canada, using quantitative PCR. Appl. Environ. Microbiol. 2010, 76, 5105–5112. [Google Scholar] [CrossRef] [Green Version]
- Vargas, S.R.; dos Santos, P.V.; Bottino, F.; do Carmo Calijuri, M. Effect of nutrient concentration on growth and saxitoxin production of Raphidiopsis raciborskii (Cyanophyta) interacting with Monoraphidium contortum (Chlorophyceae). J. Appl. Phycol. 2020, 32, 421–430. [Google Scholar] [CrossRef]
- Kenesi, G.; Shafik, H.M.; Kovács, A.W.; Herodek, S.; Présing, M. Effect of nitrogen forms on growth, cell composition and N2 fixation of Cylindrospermopsis raciborskii in phosphorus-limited chemostat cultures. Hydrobiologia 2009, 623, 191–202. [Google Scholar] [CrossRef]
- Li, R.; Watanabe, M. Physiological properties of planktic species of Anabaena (Cyanobacteria) and their taxonomic value at species level. Algol. Stud. Hydrobiol. Suppl. Vol. 2001, 103, 31–45. [Google Scholar] [CrossRef]
- Nalewajko, C.; Murphy, T.P. Effects of temperature, and availability of nitrogen and phosphorus on the abundance of Anabaena and Microcystis in Lake Biwa, Japan: An experimental approach. Limnology 2001, 2, 45–48. [Google Scholar] [CrossRef]
- Qian, K.; Dokulil, M.; Chen, Y. Do the regular annual extreme water level changes affect the seasonal appearance of Anabaena in Poyang Lake? PeerJ 2019, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, K.P.; Zanotelli, J.C.; Müller, C.C.; Scherer, K.D.; Frizzo, J.K.; Ludwig, T.A.V.; Cardo, L.D.S. First record of expansive Ceratium Schrank, 1793 species (Dinophyceae) in Southern Brazil, with notes on their dispersive patterns in Brazilian environments. Check List 2013, 9, 862–866. [Google Scholar] [CrossRef] [Green Version]
- Jati, S.; Rodrigues, L.C.; Bortolini, J.C.; Paula, A.C.M.; Moresco, G.A.; Reis, L.M.; Zanco, B.F.; Train, S. First record of the occurrence of Ceratium furcoides (Levander) Langhans (Dinophyceae) in the Upper Paraná River floodplain (PR/MS), Brazil. Brazilian J. Biol. 2014, 74, 235–236. [Google Scholar] [CrossRef]
- Moreira, R.; Rocha, O.; Santos, R.; Laudares-Silva, R.; Dias, E.; Eskinazi-Sant’Anna, E. First record of Ceratium furcoides (Dinophyta), an invasive species, in a temporary high-altitude lake in the Iron Quadrangle (MG, Southeast Brazil). Brazilian J. Biol. 2015, 75, 98–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campanelli, L.C.; Tundisi, J.G.; Abe, D.S.; Sidagis-Galli, C.; Matsumura-Tundisi, T. Record of the occurrence of dinoflagellate Ceratium furcoides in a fish farming lake located in the countryside of São Carlos (SP, Brazil). Brazilian J. Biol. 2017, 77, 426–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CETESB—Companhia Ambiental do Estado de São Paulo. Qualidade das Águas Superficiais no Estado de São Paulo 2010; CETESB: São Paulo, Brazil, 2011. [Google Scholar]
- Crossetti, L.O.; de Bicudo, D.C.; Bini, L.M.; Dala-Corte, R.B.; Ferragut, C.; de Mattos Bicudo, C.E. Phytoplankton species interactions and invasion by Ceratium furcoides are influenced by extreme drought and water-hyacinth removal in a shallow tropical reservoir. Hydrobiologia 2019, 831, 71–85. [Google Scholar] [CrossRef]
- Da Silva, L.N.; De Medeiros, C.M.; Cavalcante, K.P.; Cardoso, L.D.S. Invasion and establishment of Ceratium furcoides (Dinophyceae) in an urban lake in Porto Alegre, RS, Brazil. Acta Bot. Brasilica 2019, 33, 654–663. [Google Scholar] [CrossRef]
- Hackett, J.D.; Wisecaver, J.H.; Brosnahan, M.L.; Kulis, D.M.; Anderson, D.M.; Bhattacharya, D.; Gerald Plumley, F.; Erdner, D.L. Evolution of saxitoxin synthesis in cyanobacteria and dinoflagellates. Mol. Biol. Evol. 2013, 30, 70–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca, J.R.; Vieira, P.C.S.; Kujbida, P.; Costa, I.A.S.D. Cyanobacterial occurrence and detection of microcystins and saxitoxins in reservoirs of the Brazilian semi-arid. Acta Limnol. Bras. 2015, 27, 78–92. [Google Scholar] [CrossRef] [Green Version]
- Lopes, I.K.C.; Barros, M.U.G.; Pestana, C.J.; Capelo Neto, J. Prevalence of paralytic shellfish poison-producing Planktothrix agardhii and Cylindrospermopsis raciborskii in a Brazilian semi-arid reservoir. Acta Limnol. Bras. 2015, 27, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Zhu, B.; Struewing, I.; Xu, N.; Duan, S. Nitrogen–phosphorus-associated metabolic activities during the development of a cyanobacterial bloom revealed by metatranscriptomics. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zupancic, M.; Kogovšek, P.; Šter, T.; Remec Rekar, Š.; Cerasino, L.; Baebler, Š.; Krivograd Klemencic, A.; Eleršek, T. Potentially Toxic Planktic and Benthic Cyanobacteria in Slovenian Freshwater Bodies: Detection by Quantitative PCR. Toxins 2021, 13, 133. [Google Scholar] [CrossRef] [PubMed]
- Kramer, B.J.; Davis, T.W.; Meyer, K.A.; Rosen, B.H.; Goleski, J.A.; Dick, G.J.; Oh, G.; Gobler, C.J. Nitrogen limitation, toxin synthesis potential, and toxicity of cyanobacterial populations in Lake Okeechobee and the St. Lucie River Estuary, Florida, during the 2016 state of emergency event. PLoS ONE 2018, 13, e0196278. [Google Scholar] [CrossRef] [Green Version]
- Chaffin, J.D.; Mishra, S.; Kane, D.D.; Bade, D.L.; Stanislawczyk, K.; Slodysko, K.N.; Jones, K.W.; Parker, E.M.; Fox, E.L. Cyanobacterial blooms in the central basin of Lake Erie: Potentials for cyanotoxins and environmental drivers. J. Great Lakes Res. 2019, 45, 277–289. [Google Scholar] [CrossRef]
- Podduturi, R.; Schlüter, L.; Liu, T.; Osti, J.A.S.; de Moraes, M.A.B.; Jørgensen, N.O.G. Monitoring of saxitoxin production in lakes in Denmark by molecular, chromatographic and microscopic approaches. Harmful Algae 2021, 101, 101966. [Google Scholar] [CrossRef]
- Ballot, A.; Fastner, J.; Wiedner, C. Paralytic shellfish poisoning toxin-producing cyanobacterium Aphanizomenon gracile in Northeast Germany. Appl. Environ. Microbiol. 2010, 76, 1173–1180. [Google Scholar] [CrossRef] [Green Version]
- Casero, M.C.; Ballot, A.; Agha, R.; Quesada, A.; Cirés, S. Characterization of saxitoxin production and release and phylogeny of sxt genes in paralytic shellfish poisoning toxin-producing Aphanizomenon gracile. Harmful Algae 2014, 37, 28–37. [Google Scholar] [CrossRef]
- Cirés, S.; Wörmer, L.; Ballot, A.; Agha, R.; Wiedner, C.; Velázquez, D.; Casero, M.C.; Quesada, A. Phylogeography of cylindrospermopsin and paralytic shellfish toxin-producing Nostocales cyanobacteria from Mediterranean Europe (Spain). Appl. Environ. Microbiol. 2014, 80, 1359–1370. [Google Scholar] [CrossRef] [Green Version]
- Moreira, C.; Ramos, V.; Azevedo, J.; Vasconcelos, V. Methods to detect cyanobacteria and their toxins in the environment. Appl. Microbiol. Biotechnol. 2014, 98, 8073–8082. [Google Scholar] [CrossRef]
- Brentano, D.M.; Giehl, E.L.H.; Petrucio, M.M. Abiotic variables affect STX concentration in a meso-oligotrophic subtropical coastal lake dominated by Cylindrospermopsis raciborskii (Cyanophyceae). Harmful Algae 2016, 56, 22–28. [Google Scholar] [CrossRef]
- Yunes, J.S.; De La Rocha, S.; Giroldo, D.; Da Silveira, S.B.; Comin, R.; Bicho, M.D.S.; Melcher, S.S.; Sant’Anna, C.L.; Vieira, A.A.H. Release of carbohydrates and proteins by a subtropical strain of Raphidiopsis brookii (Cyanobacteria) able to produce saxitoxin at three nitrate concentrations. J. Phycol. 2009, 45, 585–591. [Google Scholar] [CrossRef]
- Granéli, E.; Flynn, K. Chemical and Physical Factors Influencing Toxin Content. Ecol. Harmful Algae 2006, 189, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Frangópulos, M.; Guisande, C.; DeBlas, E.; Maneiro, I. Toxin production and competitive abilities under phosphorus limitation of Alexandrium species. Harmful Algae 2004, 3, 131–139. [Google Scholar] [CrossRef]
- Pomati, F.; Rossetti, C.; Manarolla, G.; Burns, B.P.; Neilan, B.A. Interactions between intracellular Na+ levels and saxitoxin production in Cylindrospermopsis raciborskii T3. Microbiology 2004, 150, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Graham, J.L.; Jones, J.R.; Jones, S.B.; Downing, J.A.; Clevenger, T.E. Environmental factors influencing microcystin distribution and concentration in the Midwestern United States. Water Res. 2004, 38, 4395–4404. [Google Scholar] [CrossRef]
- Te, S.H.; Gin, K.Y.H. The dynamics of cyanobacteria and microcystin production in a tropical reservoir of Singapore. Harmful Algae 2011, 10, 319–329. [Google Scholar] [CrossRef]
- Cunha, D.G.F.; Dodds, W.K.; Loiselle, S.A. Factors related to water quality and thresholds for microcystin concentrations in subtropical Brazilian reservoirs. Inland Waters 2018, 8, 368–380. [Google Scholar] [CrossRef]
- Christensen, V.G.; Maki, R.P.; Stelzer, E.A.; Norland, J.E.; Khan, E. Phytoplankton community and algal toxicity at a recurring bloom in Sullivan Bay, Kabetogama Lake, Minnesota, USA. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, S.; Karnjanapiboonwong, A.; Maul, J.D.; Wang, D.; Anderson, T.A. Monitoring cyanobacterial toxins in a large reservoir: Relationships with water quality parameters. PeerJ 2019, 7, e7305. [Google Scholar] [CrossRef]
- Clemente, Z.; Busato, R.H.; Oliveira Ribeiro, C.A.; Cestari, M.M.; Ramsdorf, W.A.; Magalhães, V.F.; Wosiack, A.C.; Silva de Assis, H.C. Analyses of paralytic shellfish toxins and biomarkers in a southern Brazilian reservoir. Toxicon 2010, 55, 396–406. [Google Scholar] [CrossRef]
- de Calado, S.L.M.; Wojciechowski, J.; Santos, G.S.; de Magalhães, V.F.; Padial, A.A.; Cestari, M.M.; de Assis, H.C.D.S. Neurotoxins in a water supply reservoir: An alert to environmental and human health. Toxicon 2017, 126, 12–22. [Google Scholar] [CrossRef]
- Miller, T.R.; Beversdorf, L.J.; Weirich, C.A.; Bartlett, S.L. Cyanobacterial toxins of the Laurentian great lakes, their toxicological effects, and numerical limits in drinking water. Mar. Drugs 2017, 15, 160. [Google Scholar] [CrossRef] [Green Version]
- WHO—World Health Organization. Cyanobacterial Toxins: Saxitoxins. Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Brasil Portaria MS 2914 de 12 de Dezembro de 2011. Dispõe Sobre os Procedimentos de Controle e de Vigilância da Qualidade da Água para Consumo Humano e seu Padrão de Potabilidade; Ministério da Saúde: Brasília, Brazil, 2011; 38p.
- WHO—World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Jia, J.; Chen, Q.; Wang, M.; Zhang, J.; Yi, Q.; Hu, L. The production and release of microcystin related to phytoplankton biodiversity and water salinity in two cyanobacteria blooming lakes. Environ. Toxicol. Chem. 2018, 37, 2312–2322. [Google Scholar] [CrossRef] [PubMed]
- Vergalli, J.; Fayolle, S.; Combes, A.; Franquet, E.; Comte, K. Persistence of microcystin production by Planktothrix agardhii (Cyanobacteria) exposed to different salinities. Phycologia 2020, 59, 24–34. [Google Scholar] [CrossRef]
- Bui, T.; Dao, T.S.; Vo, T.G.; Lürling, M. Warming affects growth rates and microcystin production in tropical bloom-forming microcystis strains. Toxins 2018, 10, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walls, J.T.; Wyatt, K.H.; Doll, J.C.; Rubenstein, E.M.; Rober, A.R. Hot and toxic: Temperature regulates microcystin release from cyanobacteria. Sci. Total Environ. 2018, 610–611, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Rediske, R.R.; Gillett, N.D.; O’Keefe, J.P.; Scull, B.; Xue, Q. The impact of environmental parameters on microcystin production in dialysis bag experiments. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Y.; Huang, S.; Peng, C.; Hao, Z.; Li, D. Nitrogen limitation significantly reduces the competitive advantage of toxic Microcystis at high light conditions. Chemosphere 2019, 237, 124508. [Google Scholar] [CrossRef]
- Francy, D.S.; Brady, A.M.G.; Ecker, C.D.; Graham, J.L.; Stelzer, E.A.; Struffolino, P.; Dwyer, D.F.; Loftin, K.A. Estimating microcystin levels at recreational sites in western Lake Erie and Ohio. Harmful Algae 2016, 58, 23–34. [Google Scholar] [CrossRef]
- Huang, Y.; Pan, H.; Liu, H.; Xi, Y.; Ren, D. Characteristics of growth and microcystin production of Microcystis aeruginosa exposed to low concentrations of naphthalene and phenanthrene under different pH values. Toxicon 2019, 169, 103–108. [Google Scholar] [CrossRef]
- Barros, M.U.G.; Wilson, A.E.; Leitão, J.I.R.; Pereira, S.P.; Buley, R.P.; Fernandez-Figueroa, E.G.; Capelo-Neto, J. Environmental factors associated with toxic cyanobacterial blooms across 20 drinking water reservoirs in a semi-arid region of Brazil. Harmful Algae 2019, 86, 128–137. [Google Scholar] [CrossRef]
- Brandenburg, K.; Siebers, L.; Keuskamp, J.; Jephcott, T.G.; van de Waal, D.B. Effects of nutrient limitation on the synthesis of N-rich phytoplankton toxins: A meta-analysis. Toxins 2020, 12, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, V.G.; Khan, E. Freshwater neurotoxins and concerns for human, animal, and ecosystem health: A review of anatoxin-a and saxitoxin. Sci. Total Environ. 2020, 736, 139515. [Google Scholar] [CrossRef] [PubMed]
- Zapata, M. Recent advances in pigment analysis as applied to picophytoplankton. Vie Milieu 2005, 55, 233–248. [Google Scholar]
- Cunha, D.G.F. Heterogeneidade Espacial e Variabilidade Temporal no Reservatório de Itupararanga: Uma Condição ao Manejo Sustentável dos Recursos Hídricos da Bacia do Rio Sorocaba (SP); Universidade de São Paulo: São Paulo, Brazil, 2012. [Google Scholar]

| Variables | Raphidiopsis | Geitlerinema | Aphanizomenon | Dolichospermum |
|---|---|---|---|---|
| sxtA | 0.71 | 0.72 | 0.51 | −0.27 |
| STX | 0.50 | 0.63 | 0.62 | −0.65 |
| Variables | pH | Turbidity | TP | SRP | NO3−-N | TN:TP | Biomass_cyano |
|---|---|---|---|---|---|---|---|
| sxtA | 0.39 | −0.63 | −0.55 | −0.61 | −0.60 | 0.59 | 0.72 |
| STX | 0.57 | −0.74 | −0.58 | −0.58 | −0.46 | 0.62 | 0.51 |
| MC | 0.20 | −0.06 | 0.19 | −0.04 | 0.09 | −0.15 | 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moraes, M.A.B.; Rodrigues, R.A.M.; Schlüter, L.; Podduturi, R.; Jørgensen, N.O.G.; Calijuri, M.C. Influence of Environmental Factors on Occurrence of Cyanobacteria and Abundance of Saxitoxin-Producing Cyanobacteria in a Subtropical Drinking Water Reservoir in Brazil. Water 2021, 13, 1716. https://doi.org/10.3390/w13121716
Moraes MAB, Rodrigues RAM, Schlüter L, Podduturi R, Jørgensen NOG, Calijuri MC. Influence of Environmental Factors on Occurrence of Cyanobacteria and Abundance of Saxitoxin-Producing Cyanobacteria in a Subtropical Drinking Water Reservoir in Brazil. Water. 2021; 13(12):1716. https://doi.org/10.3390/w13121716
Chicago/Turabian StyleMoraes, Munique A. B., Raphaella A. M. Rodrigues, Louise Schlüter, Raju Podduturi, Niels O. G. Jørgensen, and Maria C. Calijuri. 2021. "Influence of Environmental Factors on Occurrence of Cyanobacteria and Abundance of Saxitoxin-Producing Cyanobacteria in a Subtropical Drinking Water Reservoir in Brazil" Water 13, no. 12: 1716. https://doi.org/10.3390/w13121716
APA StyleMoraes, M. A. B., Rodrigues, R. A. M., Schlüter, L., Podduturi, R., Jørgensen, N. O. G., & Calijuri, M. C. (2021). Influence of Environmental Factors on Occurrence of Cyanobacteria and Abundance of Saxitoxin-Producing Cyanobacteria in a Subtropical Drinking Water Reservoir in Brazil. Water, 13(12), 1716. https://doi.org/10.3390/w13121716






