Ecosystem Organic Carbon Stock Estimations in the Sile River, North Eastern Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
- Storga: a resurgent station with spring characteristics and clear shallow water (ca. 7.0 m transept and 0.5 m depth). Storga is located inside the park “Parco dello Storga” of about 58 ha. The park is characterized by riparian formations of willows (Salix sp.), poplars (Populus sp.) and alder (Alnus glutinosa L.) and by the presence of fountains, streams, and flooded lowlands and hygrophilous woods [30]. The mean water column temperature is 13.7 °C [20];
- Morgano: station before Treviso city centres and located inside the oasis of Santa Cristina. The oasis represents a great marsh of Sile River and a biotope of high naturalistic value with numerous springs. The reserve is part of the Natura 2000 Network as a Site of Community Interest (SCI) and a Special Protection Area (SPA). The station is characterized by ca. 10.0 m transept with maximum 2.5 m water depth. The mean water column temperature is 13.7 °C [20];
- Treviso: is located within the town of Treviso (ca. 25.0 m transepts and ca. 2.5 m depth) with anthropogenic impacts from densely populated neighbourhoods and commercial activities and near a hydroelectric plant. The mean water column temperature is 13.9 °C [20].
2.2. SAM Classification and Carbon Quantification
2.3. Sediments Analyses
2.4. Water Environmental Parameters
3. Results
3.1. SAMs Community Composition and Biomass
3.2. Water and Sediment Parameter Variations
3.3. Sediment Carbon Determination
3.4. SAMs Carbon Determination
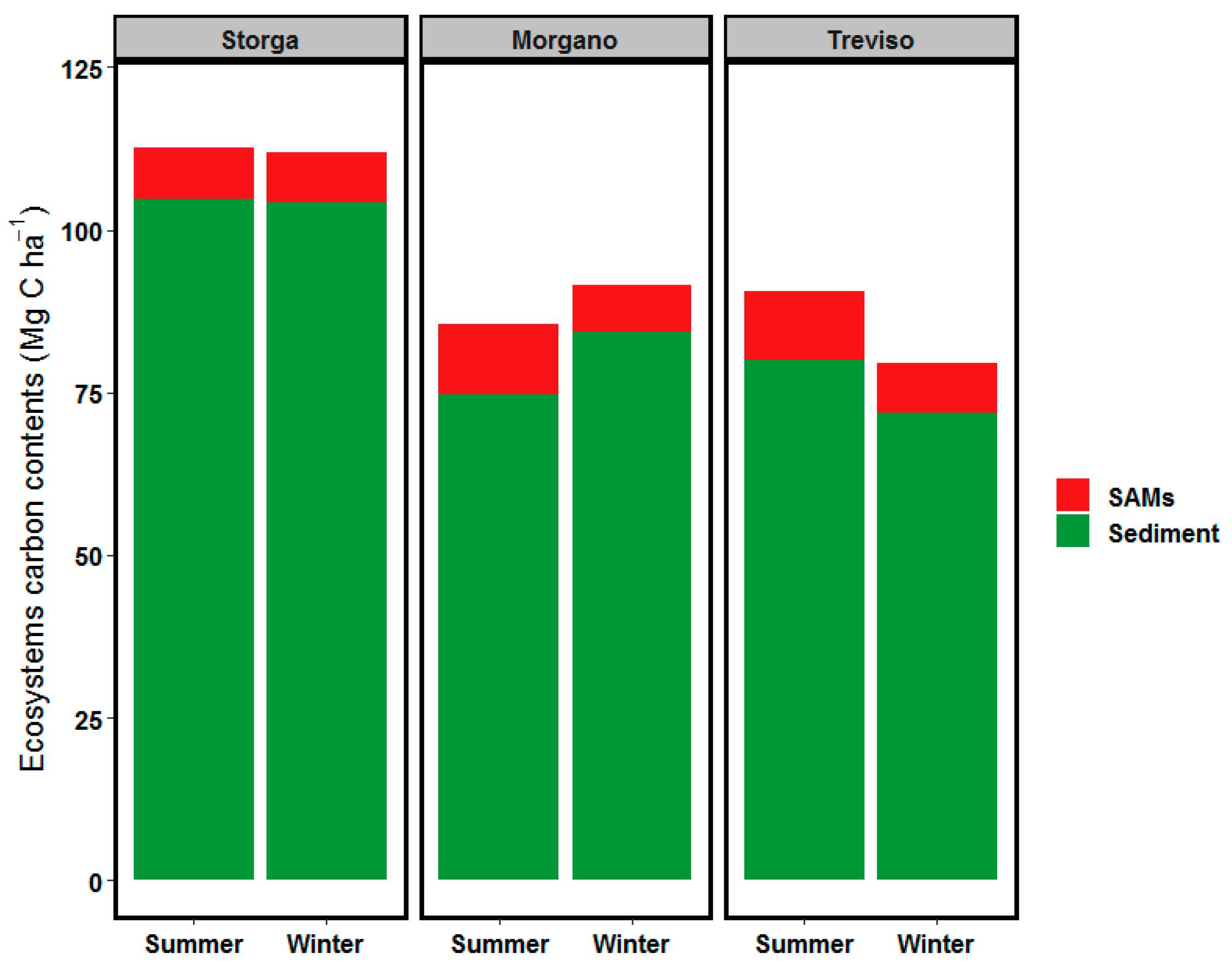
3.5. Ecosystem OC Stock
4. Discussion
4.1. SAM Carbon Estimation and Environmental Considerations
4.2. Ecosystems Carbon Stock Comparison
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cole, J.J.; Prairie, Y.T.; Caraco, N.F.; McDowell, W.H.; Tranvik, L.J.; Striegl, R.G.; Duarte, C.M.; Kortelainen, P.; Downing, J.A.; Middelburg, J.J.; et al. Plumbing the Global Carbon Cycle: Integrating Inland Waters into the Terrestrial Carbon Budget. Ecosystems 2007, 10, 172–185. [Google Scholar] [CrossRef] [Green Version]
- Sutfin, N.A.; Wohl, E.E.; Dwire, K.A. Banking carbon: A review of organic carbon storage and physical factors influencing retention in floodplains and riparian ecosystems. Earth Surf. Process. Landforms 2015, 41, 38–60. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Cummings, D.L.; Ellingson, L.J. Biomass, Carbon, and Nitrogen Pools in Mexican Tropical Dry Forest Landscapes. Ecosystems 2003, 6, 609–629. [Google Scholar] [CrossRef]
- Hoffmann, U.; Hoffmann, T.; Johnson, E.; Kuhn, N.J. Assessment of variability and uncertainty of soil organic carbon in a mountainous boreal forest (Canadian Rocky Mountains, Alberta). Catena 2014, 113, 107–121. [Google Scholar] [CrossRef]
- Aufdenkampe, A.K.; Mayorga, E.; Raymond, P.A.; Melack, J.M.; Doney, S.C.; Alin, S.R.; Aalto, R.E.; Yoo, K. Riverine coupling of biogeochemical cycles between land, oceans, and atmosphere. Front. Ecol. Environ. 2011, 9, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Gregory, J.M.; Jones, C.D.; Cadule, P.; Friedlingstein, P. Quantifying Carbon Cycle Feedbacks. J. Clim. 2009, 22, 5232–5250. [Google Scholar] [CrossRef]
- Tranvik, L.J.; Downing, J.A.; Cotner, J.B.; Loiselle, S.A.; Striegl, R.G.; Ballatore, T.J.; Dillon, P.; Finlay, K.; Fortino, K.; Knoll, L.B.; et al. Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanogr. 2009, 54, 2298–2314. [Google Scholar] [CrossRef] [Green Version]
- Battin, T.J.; Luyssaert, S.; Kaplan, L.A.; Aufdenkampe, A.K.; Richter, A.; Tranvik, L.J. The boundless carbon cycle. Nat. Geosci. 2009, 2, 598–600. [Google Scholar] [CrossRef]
- Hossain, K.; Yadav, S.; Quaik, S.; Pant, G.; Maruthi, A.Y.; Ismail, N. Vulnerabilities of macrophytes distribution due to climate change. Theor. Appl. Clim. 2016, 129, 1123–1132. [Google Scholar] [CrossRef]
- Pletterbauer, F.; Melcher, A.; Graf, W. Climate Change Impacts in Riverine Ecosystems; Schmutz, S., Sendzimir, J., Eds.; Riverine Ecosystem Management; Aquatic Ecology Series; Springer: Cham, Switzerland, 2018; Volume 8. [Google Scholar] [CrossRef]
- Evans, C.D.; Monteith, D.; Cooper, D. Long-term increases in surface water dissolved organic carbon: Observations, possible causes and environmental impacts. Environ. Pollut. 2005, 137, 55–71. [Google Scholar] [CrossRef]
- Hasler, C.T.; Butman, D.; Jeffrey, J.D.; Suski, C.D. Freshwater biota and rising pCO2? Ecol. Lett. 2015, 19, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.J.; Frost, P.C.; Morales-Williams, A.M.; Larson, J.H.; Richardson, W.B.; Chiandet, A.S.; Xenopoulos, M.A. Human activities cause distinct dissolved organic matter composition across freshwater ecosystems. Glob. Chang. Biol. 2015, 22, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Doney, S.C.; Fabry, V.J.; Feely, R.A.; Kleypas, J.A. Ocean Acidification: The Other CO2Problem. Annu. Rev. Mar. Sci. 2009, 1, 169–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobek, S.; Algesten, G.; Bergström, A.-K.; Jansson, M.; Tranvik, L.J. The catchment and climate regulation of pCO2 in boreal lakes. Glob. Chang. Biol. 2003, 9, 630–641. [Google Scholar] [CrossRef]
- Steinberg, C.E.W.; Kamara, S.; Prokhotskaya, V.Y.; Manusadzianas, L.; Karasyova, T.A.; Timofeyev, M.A.; Jie, Z.; Paul, A.; Meinelt, T.; Farjalla, V.F.; et al. Dissolved humic substances—Ecological driving forces from the individual to the ecosystem level? Freshw. Biol. 2006, 51, 1189–1210. [Google Scholar] [CrossRef]
- Song, C.; Ballantyne, F.; Smith, V.H.; Iv, F.B. Enhanced dissolved organic carbon production in aquatic ecosystems in response to elevated atmospheric CO2. Biogeochemistry 2013, 118, 49–60. [Google Scholar] [CrossRef]
- Bondesan, A.; Caniato, G.; Vallerani, F.; Zanetti, M. (Eds.) Il Sile; Cierre Edizioni: San Casciano Val di Pesa, Italy, 1998; p. 369. [Google Scholar]
- De Stefani, G.; Tocchetto, D.; Salvato, M.; Borin, M. Performance of a floating treatment wetland for in-stream water amelioration in NE Italy. Hydrobiologia 2011, 674, 157–167. [Google Scholar] [CrossRef]
- Ferraio, M.E.; Rossa, A.M.; Sansone, M.; della Valle, A. Rapporto Sulla Qualità Delle Acque in Provincia di Treviso; Agenzia Regionale per la Prevenzione e Protezione Ambientale del Veneto (ARPAV): Milan, Italy, 2019; 125p. [Google Scholar]
- Zanandrea, G. Ulteriori Rilievi Biometrici SuLampetra ZanandreaiVladykov. Bolletino Zoologia 1961, 28, 703–715. [Google Scholar] [CrossRef]
- Degobbis, D.; Gilmartin, M. Nitrogen, phosphorus, and biogenic silicon budgets for the northern Adriatic Sea. Oceanol. Acta 1990, 13, 31–45. [Google Scholar]
- Zanetti, M.; Piccolo, D.; Macor, P.; Galante, D.; Vanin, B.G.; Causin, L.; Siligardi, M.; Malavasi, D.; Bedin, L.; Busatto, T.; et al. SilIFFE—LIFE 14/NAT/IT/000809—River Functionality Index as Planning Instrument for a Good Governance of Sile’s Ecosystem; European Commission: Brussels, Belgium, 2018; p. 100. [Google Scholar]
- Autorità di Bacino dell’Adige e dell’Alto Adriatico. Piano di Gestione dei Bacini Idrografici Delle Alpi Orientali; Bacino del Fiume Sile: Treviso, Italy, 2010; p. 202. [Google Scholar]
- Taffarello, A. Turismo Fluviale e Coscienza Ambientale Lungo il Basso Sile: Il Ruolo del Viaggiatore Consapevole. Bachelor’s Thesis, Università Ca’Foscari Venezia, Venice, Italy, 2016. [Google Scholar]
- Calfapietra, C.; Barbati, A.; Perugini, L.; Ferrari, B.; Guidolotti, G.; Quatrini, A.; Corona, P. Carbon mitigation potential of different forest ecosystems under climate change and various managements in Italy. Ecosyst. Health Sustain. 2015, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Grüneberg, E.; Schöning, I.; Riek, W.; Ziche, D.; Evers, J. Carbon Stocks and Carbon Stock Changes in German Forest Soils. In Ecological Studies Status and Dynamics of Forests in Germany; Springer: Cham, Switzerland, 2019; pp. 167–198. [Google Scholar] [CrossRef] [Green Version]
- Penfound, W.T. Primary Production of Vascular Aquatic Plants. Limnol. Oceanogr. 2010, 1, 92–101. [Google Scholar] [CrossRef]
- Feng, W.; Mariotte, P.; Xu, L.; Buttler, A.; Bragazza, L.; Jiang, J.; Santonja, M. Seasonal variability of groundwater level effects on the growth of Carex cinerascens in lake wetlands. Ecol. Evol. 2020, 10, 517–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezzavilla, F. Madonnetta e Storga, 1st ed.; Carpené, B., Mezzavilla, F., Silvestri, S., Simionato, G., Eds.; Comune di Treviso: Treviso, Italy, 1988; pp. 15–41. [Google Scholar]
- Madsen, J.D.; Wersal, R.M. A review of aquatic plant monitoring and assessment methods. J. Aquat. Plant Manag. 2017, 55, 1–12. [Google Scholar]
- Johnson, J.A.; Newman, R.A. Comparison of two methods for sampling biomass of aquatic plants. J. Aquat. Plant Manag. 2011, 49, 1–8. [Google Scholar]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora D’italia, 2nd ed.; Edagricole: Milano, Italy, 2017; p. 1191. [Google Scholar]
- McGeehan, S.; Naylor, D. Automated instrumental analysis of carbon and nitrogen in plant and soil samples. Commun. Soil Sci. Plant Anal. 1988, 19, 493–505. [Google Scholar] [CrossRef]
- Webster, E.A.; Chudek, J.A.; Hopkins, D.W. Carbon transformations during decomposition of different components of plant leaves in soil. Soil Biol. Biochem. 2000, 32, 301–314. [Google Scholar] [CrossRef]
- Kristensen, E.; Andersen, F.Ø. Determination of organic carbon in marine sediments: A comparison of two CHN-analyzer methods. J. Exp. Mar. Biol. Ecol. 1987, 109, 15–23. [Google Scholar] [CrossRef]
- Adriano, S.; Massimiliano, F.; Sonia, C.; Chiara, F.; Antonio, M. Organic carbon changes in the surface sediments of the Venice lagoon. Environ. Int. 2005, 31, 1002–1010. [Google Scholar] [CrossRef]
- Adriano, S.; Chiara, F.; Antonio, M. Sedimentation rates and erosion processes in the lagoon of Venice. Environ. Int. 2005, 31, 983–992. [Google Scholar] [CrossRef]
- Strickland, J.D.; Parsons, T.R. A Practical Handbook of Seawater Analysis, 2nd ed.; Fisheries Research Board of Canada: Ottawa, ON, Canada, 1972. [Google Scholar]
- Lorenzen, C.J. Determination of Chlorophyll and Pheo-Pigments: Spectrophotometric Equations1. Limnol. Oceanogr. 1967, 12, 343–346. [Google Scholar] [CrossRef]
- Sfriso, A.; Facca, C.; Bonometto, A.; Boscolo Brusà, R. Compliance of the macrophyte quality index (MaQI) with the WFD (2000/60/EC) and ecological status assessment in transitional areas: The Venice lagoon as study case. Ecol. Indic. 2014, 46, 536–547. [Google Scholar] [CrossRef]
- Fourqurean, J.W.; Duarte, C.M.; Kennedy, H.; Marbà, N.; Holmer, M.; Mateo, M.A.; Apostolaki, E.T.; Kendrick, G.A.; Krause-Jensen, D.; McGlathery, K.J.; et al. Seagrass ecosystems as a globally significant carbon stock. Nat. Geosci. 2012, 5, 505–509. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Bernardino, A.F.; Ferreira, T.O.; Giovannoni, L.R.; de Gomes, L.E.O.; Romero, D.J.; Jimenez, L.C.Z.; Ruiz, F. Carbon stocks of mangroves and salt marshes of the Amazon region, Brazil. Biol. Lett. 2018, 14, 20180208. [Google Scholar] [CrossRef] [PubMed]
- Adame, M.F.; Kauffman, J.B.; Medina, I.; Gamboa, J.N.; Torres, O.; Caamal, J.P.; Reza, M.; Herrera-Silveira, J.A. Carbon Stocks of Tropical Coastal Wetlands within the Karstic Landscape of the Mexican Caribbean. PLoS ONE 2013, 8, e56569. [Google Scholar] [CrossRef] [Green Version]
- Carnell, P.E.; Windecker, S.M.; Brenker, M.; Baldock, J.; Masqué, P.; Brunt, K.; Macreadie, P.I. Carbon stocks, sequestration, and emissions of wetlands in south eastern Australia. Glob. Chang. Biol. 2018, 24, 4173–4184. [Google Scholar] [CrossRef]
- Chen, R.; Twilley, R.R. Patterns of Mangrove Forest Structure and Soil Nutrient Dynamics along the Shark River Estuary, Florida. Estuaries 1999, 22, 955–970. [Google Scholar] [CrossRef]
- Frodge, J.D.; Thomas, G.L.; Pauley, G.B. Effects of canopy formation by floating and submergent aquatic macrophytes on the water quality of two shallow Pacific Northwest lakes. Aquat. Bot. 1990, 38, 231–248. [Google Scholar] [CrossRef]
- Chambers, P.A.; Prepas, E.E.; Hamilton, H.R.; Bothwell, M.L. Current Velocity and Its Effect on Aquatic Macrophytes in Flowing Waters. Ecol. Appl. 1991, 1, 249–257. [Google Scholar] [CrossRef]
- Cooke, S.S. A Field Guide to the Common Wetland Plants of Western Washington and Northwestern Oregon; Seattle Audubon Society and Washington Native Plant Society, Seattle Audubon Society: Seattle, WA, USA, 1997; 471p. [Google Scholar]
- Cao, J.; Ruan, H. Responses of the submerged macrophyte Vallisneria natans to elevated CO2 and temperature. Aquat. Biol. 2015, 23, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Buosi, A.; Juhmani, A.; Tomio, Y.; Sfriso, A.A.; Frassetto, M.; Caminiti, V.; Sfriso, A. Long-term changes of carbon, phosphorus and nitrogen concentration in the surface sediment of the venice lagoon. In Proceedings of the EuroLag 9 Venice 2020, Venice, Italy, 20–24 January 2020; p. 104. [Google Scholar]
- Sfriso, A.; Buosi, A.; Tomio, Y.; Juhmani, A.-S.; Facca, C.; Sfriso, A.A.; Franzoi, P.; Scapin, L.; Bonometto, A.; Ponis, E.; et al. Aquatic Angiosperm Transplantation: A Tool for Environmental Management and Restoring in Transitional Water Systems. Water 2019, 11, 2135. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, H.; Beggins, J.; Duarte, C.M.; Fourqurean, J.W.; Holmer, M.; Marbà, N.; Middelburg, J.J. Seagrass sediments as a global carbon sink: Isotopic constraints. Glob. Biogeochem. Cycles 2010, 24, 4026. [Google Scholar] [CrossRef] [Green Version]
- IPCC. National Greenhouse Gas Inventory Guidelines; Eggelston, S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; Institute of Global Environmental Strategies (IGES): Kanagawa, Japan, 2006; 20p. [Google Scholar]
- Stringer, C.E.; Trettin, C.C.; Zarnoch, S.J.; Tang, W. Carbon stocks of mangroves within the Zambezi River Delta, Mozambique. For. Ecol. Manag. 2015, 354, 139–148. [Google Scholar] [CrossRef]
- Adame, M.F.; Santini, N.S.; Tovilla, C.; Vázquez-Lule, A.; Castro, L.; Guevara, M. Carbon stocks and soil sequestration rates of tropical riverine wetlands. Biogeosciences 2015, 12, 3805–3818. [Google Scholar] [CrossRef] [Green Version]



| Summer | Winter | |||||
|---|---|---|---|---|---|---|
| Storga | Morgano | Treviso | Storga | Morgano | Treviso | |
| Species | kg/m2 w.w. | |||||
| Berula erecta | 2.53 | 2.27 | 1.27 | 1.15 | ||
| Callitriche brutia | 3.81 | 3.60 | 4.87 | 2.08 | ||
| Callitriche stagnalis | 5.39 | 4.60 | ||||
| Ceratophyllum demersum | 3.95 | 3.85 | 3.19 | 3.91 | 2.75 | 2.58 |
| Elodea canadiensis | 0.54 | 0.49 | 0.56 | 0.43 | ||
| Myosotis scorpioides | 4.34 | 4.17 | ||||
| Myriophyllum spicatum | 1.18 | 1.12 | ||||
| Stuckenia pectinata | 5.12 | 2.45 | 1.41 | 4.91 | ||
| Vallisneria spiralis | 5.56 | 5.27 | 1.43 | 1.04 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buosi, A.; Tomio, Y.; Juhmani, A.-S.; Sfriso, A. Ecosystem Organic Carbon Stock Estimations in the Sile River, North Eastern Italy. Water 2021, 13, 80. https://doi.org/10.3390/w13010080
Buosi A, Tomio Y, Juhmani A-S, Sfriso A. Ecosystem Organic Carbon Stock Estimations in the Sile River, North Eastern Italy. Water. 2021; 13(1):80. https://doi.org/10.3390/w13010080
Chicago/Turabian StyleBuosi, Alessandro, Yari Tomio, Abdul-Salam Juhmani, and Adriano Sfriso. 2021. "Ecosystem Organic Carbon Stock Estimations in the Sile River, North Eastern Italy" Water 13, no. 1: 80. https://doi.org/10.3390/w13010080





