Comparing Trace Elements (As, Cu, Ni, Pb, and Zn) in Soils and Surface Waters among Montane, Upland Watersheds and Lowland, Urban Watersheds in New England, USA
Abstract
1. Introduction
2. Material and Methods
2.1. Watersheds
2.2. Geology and Soils
2.3. Soil Sampling and Processing
2.4. River Water Sampling and Analyses
2.5. Soil and Water Elemental Analyses
2.6. Dissolved Export Quantification
2.7. Statistical Analyses
3. Results
3.1. Watershed Soils
3.2. River Water Concentrations and Export
3.3. Linear Regressions and Principal Component Analysis
4. Discussion
4.1. Soil–River Water Linkage
4.2. Land-Use Impacts on Soil–River Linkages
4.3. Geologic Controls on Soil–River Linkage
5. Conclusions and Implications
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bricker, S.B. The history of Cu, Pb, and Zn inputs to Narragansett Bay, Rhode Island as recorded by salt-marsh sediments. Estuaries 1993, 16, 589–607. [Google Scholar] [CrossRef]
- Davis, A.; Kempton, J.H.; Nicholson, A.; Yare, B. Groundwater transport of arsenic and chromium at a historical tannery, Woburn, Massachusetts, USA. Appl. Geochem. 1994, 9, 569–582. [Google Scholar] [CrossRef]
- Aurilio, A.C.; Durant, J.L.; Hemond, H.F.; Knox, M.L. Sources and distribution of arsenic in the Aberjona watershed, eastern Massachusetts. Water Air Soil Pollut. 1995, 81, 265–282. [Google Scholar] [CrossRef]
- Breault, R.F.; Harris, S.L. Geographical Distribution and Potential for Adverse Biological Effects of Selected Trace Elements and Organic Compounds in Streambed Sediment in the Connecticut, Housatonic, and Thames River Basins, 1992–1994; No. 4169; US Department of the Interior, US Geological Survey: Reston, VA, USA, 1997; Volume 97. [Google Scholar]
- Adriano, D.C. Arsenic. In Trace Elements in Terrestrial Environments; Springer: New York, NY, USA, 2001; pp. 219–261. [Google Scholar]
- Richardson, J.B.; King, E.K. Regolith weathering and sorption influences molybdenum, vanadium, and chromium export via stream water at four granitoid Critical Zone Observatories. Front. Earth Sci. 2018, 6, 193. [Google Scholar] [CrossRef]
- Ravera, O.; Cenci, R.; Beone, G.M.; Dantas, M.; Lodigiani, P. Trace element concentrations in freshwater mussels and macrophytes as related to those in their environment. J. Limnol. 2003, 62, 61–70. [Google Scholar] [CrossRef]
- Vardy, D.W.; Oellers, J.; Doering, J.A.; Hollert, H.; Giesy, J.P.; Hecker, M. Sensitivity of early life stages of white sturgeon, rainbow trout, and fathead minnow to copper. Ecotoxicology 2013, 22, 139–147. [Google Scholar] [CrossRef]
- Jović, M.; Stanković, S. Human exposure to trace metals and possible public health risks via consumption of mussels Mytilus galloprovincialis from the Adriatic coastal area. Food Chem. Toxicol. 2014, 70, 241–251. [Google Scholar] [CrossRef]
- Ibemenuga, K.N. Bioaccumulation and toxic effects of some heavy metals in freshwater fishes. Anim. Res. Int. 2013, 10, 1792–1798. [Google Scholar]
- Varekamp, J.C.; McElroy, A.E.; Mullaney, J.R.; Breslin, V.T. Metals, organic compounds, and nutrients in Long Island Sound: Sources, magnitudes, trends, and impacts. In Long Island Sound; Springer: New York, NY, USA, 2014; pp. 203–283. [Google Scholar]
- Connecticut Department of Energy and Environmental Protection [CT DEEP]. Long Island Sound Blue Plant. 2019. Available online: https://portal.ct.gov/DEEP/Coastal-Resources/LIS-Blue-Plan/Blue-Plan-Basic-Background (accessed on 1 October 2020).
- Berggren, D.; Bergkvist, B.; Falkengren-Grerup, U.; Folkeson, L.; Tyler, G. Metal solubility and pathways in acidified forest ecosystems of south Sweden. Sci. Total Environ. 1990, 96, 103–114. [Google Scholar] [CrossRef]
- Ge, Y.; Murray, P.; Hendershot, W.H. Trace metal speciation and bioavailability in urban soils. Environ. Pollut. 2000, 107, 137–144. [Google Scholar] [CrossRef]
- Gandois, L.; Probst, A.; Dumat, C. Modelling trace metal extractability and solubility in French forest soils by using soil properties. Eur. J. Soil Sci. 2010, 61, 271–286. [Google Scholar] [CrossRef]
- Adamo, P.; Zampella, M.; Gianfreda, L.; Renella, G.; Rutigliano, F.A.; Terribile, F. Impact of river overflowing on trace element contamination of volcanic soils in south Italy: Part I. Trace element speciation in relation to soil properties. Environ. Pollut. 2006, 144, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Nordin, R.; Mazumder, A. Watershed land use as a determinant of metal concentrations in freshwater systems. Environ. Geochem. Health 2009, 31, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Stolpe, B.; Guo, L.; Shiller, A.M.; Aiken, G.R. Abundance, size distributions and trace-element binding of organic and iron-rich nanocolloids in Alaskan rivers, as revealed by field-flow fractionation and ICP-MS. Geochim. Cosmochim. Acta 2013, 105, 221–239. [Google Scholar] [CrossRef]
- Tamrat, W.Z.; Rose, J.; Grauby, O.; Doelsch, E.; Levard, C.; Chaurand, P.; Basile-Doelsch, I. Soil organo-mineral associations formed by co-precipitation of Fe, Si and Al in presence of organic ligands. Geochim. Cosmochim. Acta 2019, 260, 15–28. [Google Scholar] [CrossRef]
- Pédrot, M.; Dia, A.; Davranche, M.; Bouhnik-Le Coz, M.; Henin, O.; Gruau, G. Insights into colloid-mediated trace element release at the soil/water interface. J. Colloid Interface Sci. 2008, 325, 187–197. [Google Scholar] [CrossRef]
- Mistikawy, J.A.; Mackowiak, T.J.; Butler, M.J.; Mischenko, I.C.; Cernak, R.S.; Richardson, J.B. Chromium, manganese, nickel, and cobalt mobility and bioavailability from mafic-to-ultramafic mine spoil weathering in western Massachusetts, USA. Environ. Geochem. Health 2020, 42, 3263–3279. [Google Scholar] [CrossRef]
- Wilhelm, J.F.; Bain, D.J.; Green, M.B.; Bush, K.F.; McDowell, W.H. Trace metals in Northern New England streams: Evaluating the role of road salt across broad spatial scales with synoptic snapshots. PLoS ONE 2019, 14, e0212011. [Google Scholar] [CrossRef]
- Chrysochoou, M.; Ferreira, D.R.; Johnston, C.P. Calcium polysulfide treatment of Cr (VI)-contaminated soil. J. Hazard Mater. 2010, 179, 650–657. [Google Scholar] [CrossRef]
- Lindbo, D.L.; Veneman, P.L.M. Morphological and physical properties of selected fragipan soils in Massachusetts. Soil Sci. Soc. Am. J. 1993, 57, 429–436. [Google Scholar] [CrossRef]
- Metzler, K.J.; Tiner, R.W. Wetlands of Connecticut; State Geological and Natural History Survey of Connecticut, Department of Environmental Protection: Hartford, CT, USA, 1992.
- Lindbo, D.L.; Brigham-Grette, J.; Veneman, P.L. Depositional and Post-Depositional Features in the Late Illinoian and Late Wisconsinan Tills of Massachusetts. Whole Regolith Pedol. 1994, 34, 75–95. [Google Scholar]
- Friesz, P.J. Geohydrology of Stratified Drift and Streamflow in the Deerfield River Basin, northwestern Massachusetts; No. 4115; US Department of the Interior, US Geological Survey: Reston, VA, USA, 1996; Volume 96. [Google Scholar]
- Jin, S.; Homer, C.; Yang, L.; Danielson, P.; Dewitz, J.; Li, C.; Zhu, Z.; Xian, G.; Howard, D. Overall methodology design for the United States national land cover database 2016 products. Remote Sens. 2019, 11, 2971. [Google Scholar] [CrossRef]
- Zen, E.A.; Goldsmith, R.; Ratcliffe, N.M.; Robinson, P.; Stanley, R.S. Bedrock geologic map of Massachusetts: US Geological Survey and the Commonwealth of Massachusetts. Department of Public Works, scale, 1(2), 1983.
- Marek, R.S.; Richardson, J.B. Investigating surficial geologic controls on soil properties, inorganic nutrient uptake, and northern hardwood growth in Western Massachusetts, USA. J. Soil Sci. Plant Nutr. 2020, 20, 901–911. [Google Scholar] [CrossRef]
- Rodgers, J. Bedrock geological map of Connecticut: Hartford. Connecticut, Connecticut Geological and Natural History Survey, Connecticut Natural Resources Atlas Series, scale, 1(250,000), 1985.
- Chen, M.; Ma, L.Q. Comparison of four USEPA digestion methods for trace metal analysis using certified and Florida soils. J. Environ. Qual. 1998, 27, 1294–1300. [Google Scholar] [CrossRef]
- U.S. Geological Survey (USGS). Collection of Water Samples (ver. 2.0): U.S Geological Survey Techniques of Water-Resources Investigations. 2006. Available online: http://pubs.water.usgs.gov/twri9A4/ (accessed on 17 September 2020).
- Marsh, A.S.; Siccama, T.G. Use of formerly plowed land in New England to monitor the vertical distribution of lead, zinc and copper in mineral soil. Water Air Soil Pollut. 1997, 95, 75–85. [Google Scholar] [CrossRef]
- Richardson, J.B.; Friedland, A.J.; Kaste, J.M.; Jackson, B.P. Forest floor lead changes from 1980 to 2011 and subsequent accumulation in the mineral soil across the northeastern United States. J. Environ. Qual. 2014, 43, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Tiefenthaler, L.L.; Stein, E.D.; Schiff, K.C. Watershed and land use–based sources of trace metals in urban storm water. Environ. Toxicol. Chem. Int. J. 2008, 27, 277–287. [Google Scholar] [CrossRef]
- Zeng, Y.; Bi, C.; Jia, J.; Deng, L.; Chen, Z. Impact of intensive land use on heavy metal concentrations and ecological risks in an urbanized river network of Shanghai. Ecol. Indic. 2020, 116, 106501. [Google Scholar] [CrossRef]
- Karouna-Renier, N.K.; Sparling, D.W. Relationships between ambient geochemistry, watershed land-use and trace metal concentrations in aquatic invertebrates living in stormwater treatment ponds. Environ. Pollut. 2001, 112, 183–192. [Google Scholar] [CrossRef]
- Zhang, C.; Qiao, Q.; Piper, J.D.; Huang, B. Assessment of heavy metal pollution from a Fe-smelting plant in urban river sediments using environmental magnetic and geochemical methods. Environ. Pollut. 2011, 159, 3057–3070. [Google Scholar] [CrossRef]
- Chen, Q.; Mei, K.; Dahlgren, R.A.; Wang, T.; Gong, J.; Zhang, M. Impacts of land use and population density on seasonal surface water quality using a modified geographically weighted regression. Sci. Total Environ. 2016, 572, 450–466. [Google Scholar] [CrossRef] [PubMed]
- Davenport, J.R.; Peryea, F.J. Phosphate fertilizers influence leaching of lead and arsenic in a soil contaminated with lead arsenate. Water Air Soil Pollut. 1991, 57, 101–110. [Google Scholar] [CrossRef]
- Puckett, L.J.; Woodside, M.D.; Libby, B.; Schening, M.R. Sinks for trace metals, nutrients, and sediments in wetlands of the Chickahominy River near Richmond, Virginia. Wetlands 1993, 13, 105–114. [Google Scholar] [CrossRef]
- Weis, J.S.; Weis, P. Metal uptake, transport and release by wetland plants: Implications for phytoremediation and restoration. Environ. Int. 2004, 30, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Allinson, G.; Zhang, P.; Bui, A.; Allinson, M.; Rose, G.; Marshall, S.; Pettigrove, V. Pesticide and trace metal occurrence and aquatic benchmark exceedances in surface waters and sediments of urban wetlands and retention ponds in Melbourne, Australia. Environ. Sci. Pollut. Res. 2015, 22, 10214–10226. [Google Scholar] [CrossRef]
- Monnin, L.; Ciffroy, P.; Garnier, J.M.; Ambrosi, J.P.; Radakovitch, O. Remobilization of trace metals during laboratory resuspension of contaminated sediments from a dam reservoir. J. Soils Sediments 2018, 18, 2596–2613. [Google Scholar] [CrossRef]
- Carey, J.C.; Fulweiler, R.W. Human activities directly alter watershed dissolved silica fluxes. Biogeochemistry 2012, 111, 125–138. [Google Scholar] [CrossRef]
- Anderson, S.P.; Dietrich, W.E. Chemical weathering and runoff chemistry in a steep headwater catchment. Hydrol. Process. 2001, 15, 1791–1815. [Google Scholar] [CrossRef]
- Wilson, H.F.; Raymond, P.A.; Saiers, J.E.; Sobczak, W.V.; Xu, N. Increases in humic and bioavailable dissolved organic matter in a forested New England headwater stream with increasing discharge. Mar. Freshw. Res. 2016, 67, 1279–1292. [Google Scholar] [CrossRef]
- Augusto, L.; Turpault, M.P.; Ranger, J. Impact of forest tree species on feldspar weathering rates. Geoderma 2000, 96, 215–237. [Google Scholar] [CrossRef]
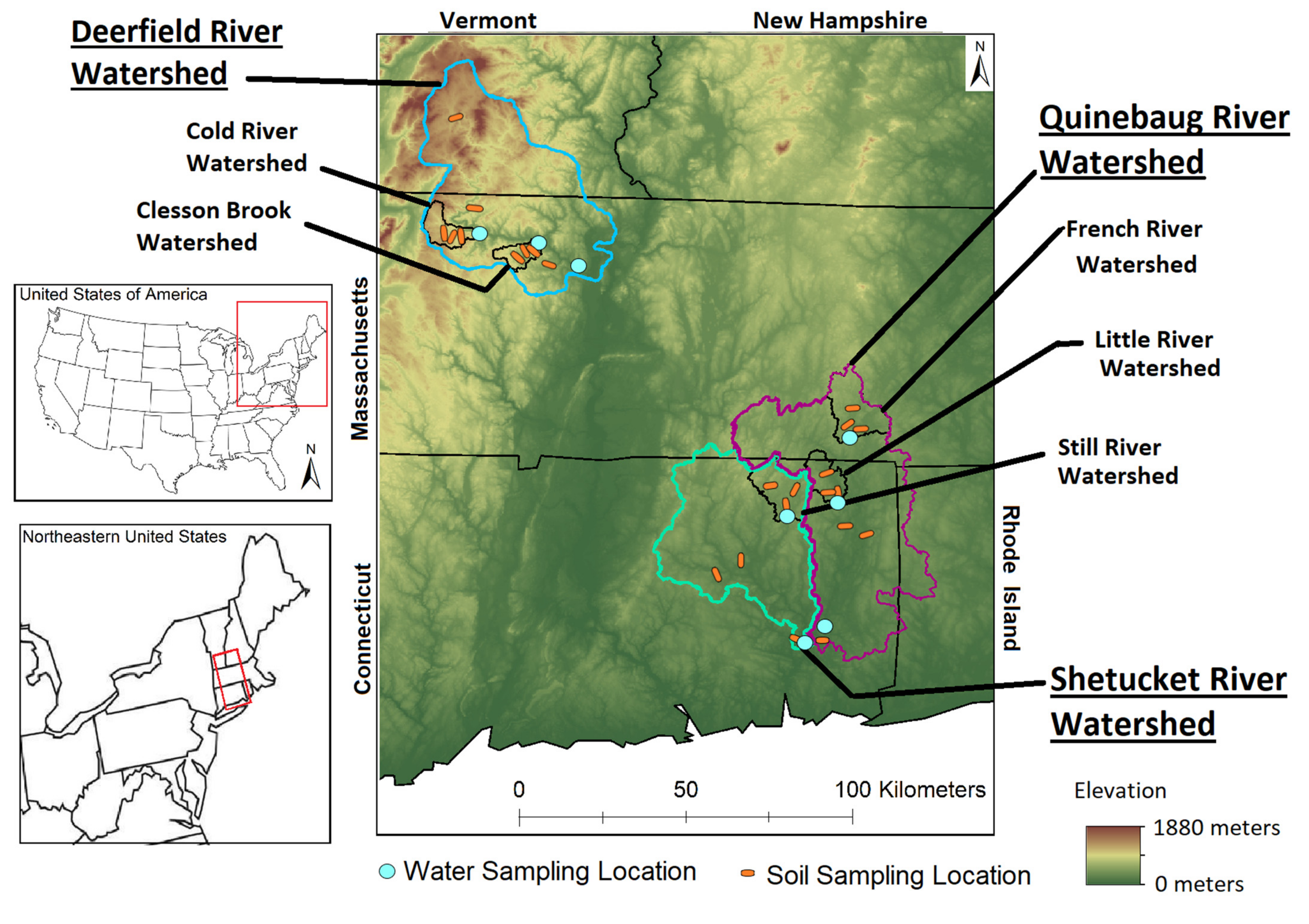
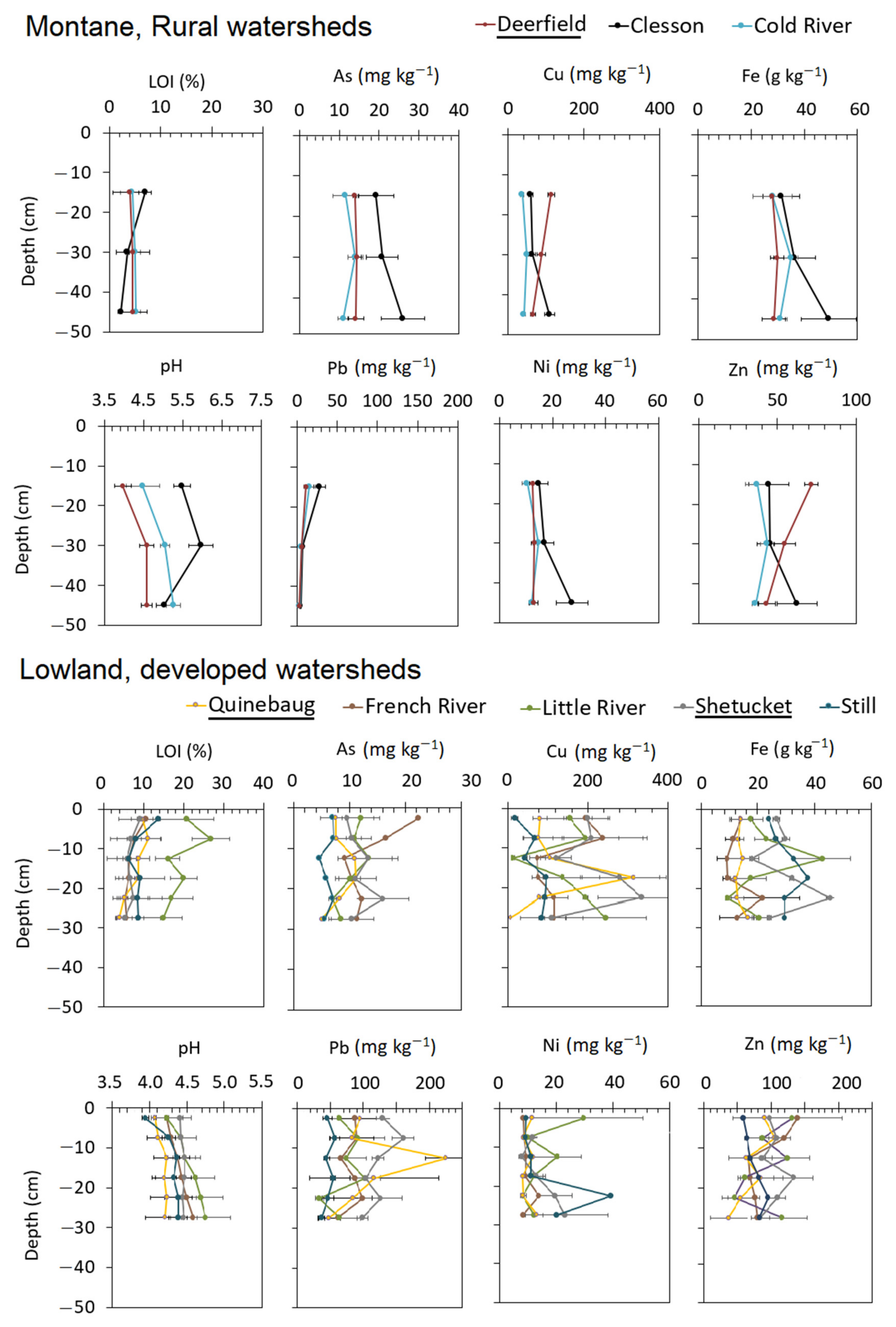
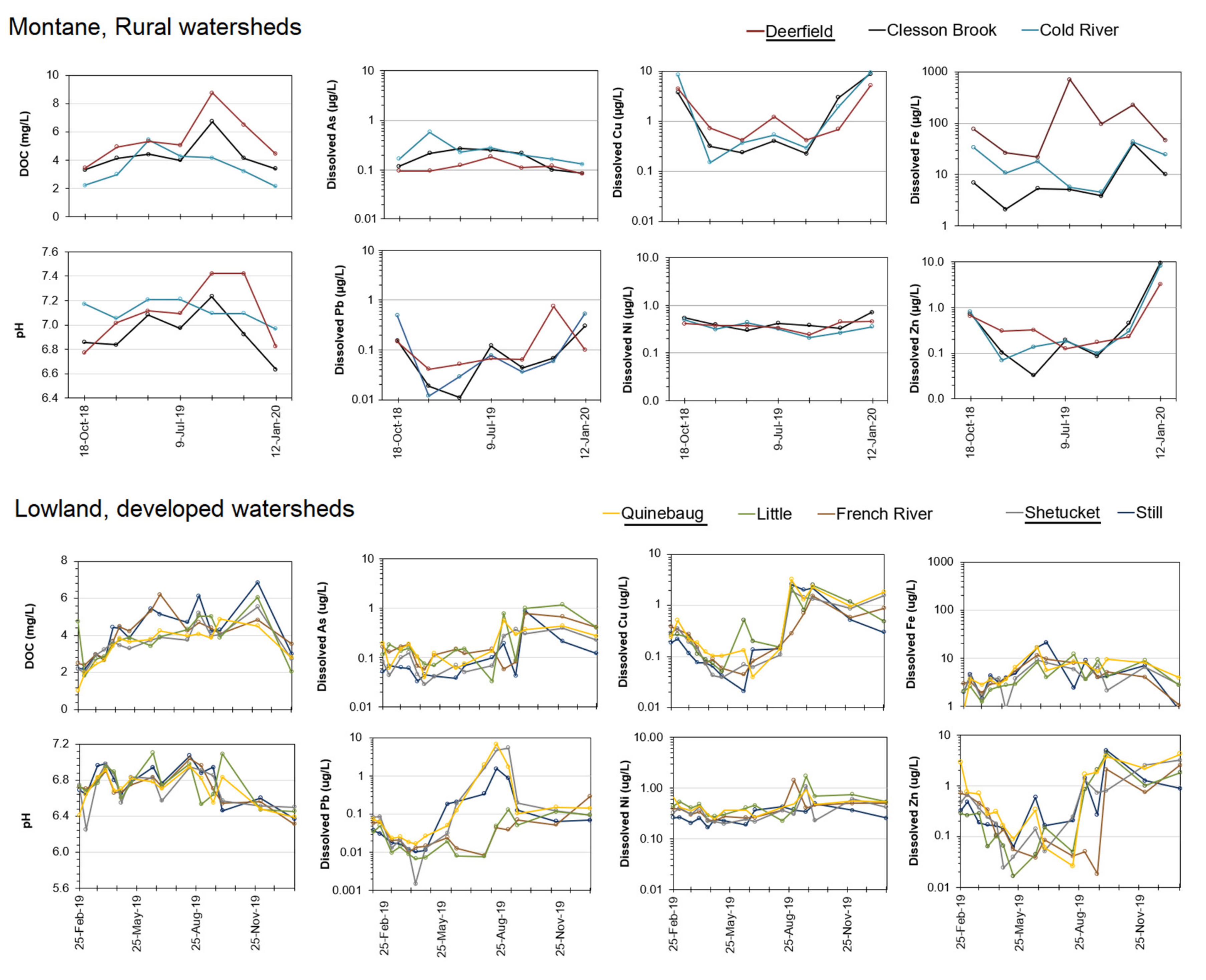
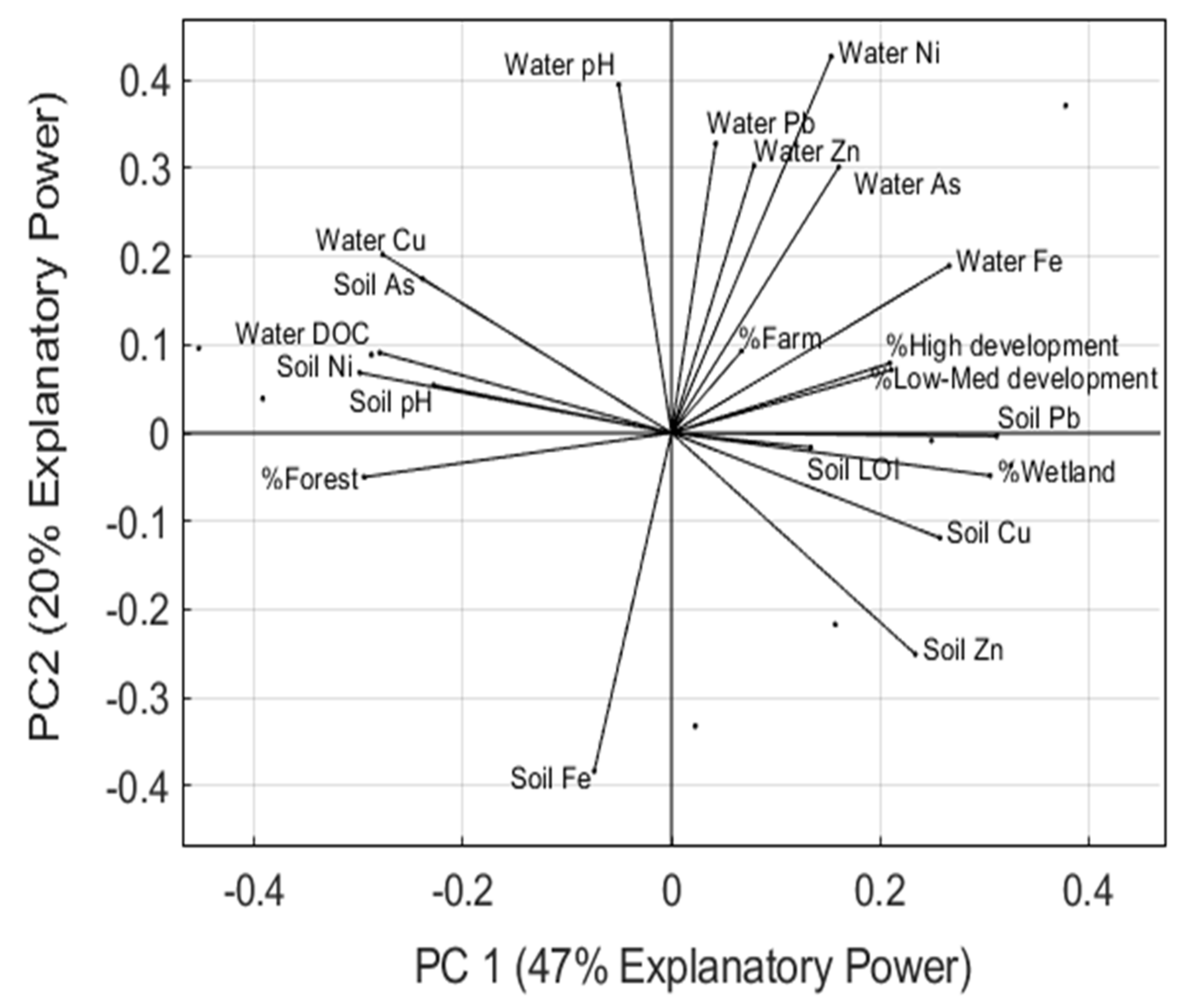
| River | Watershed Area | Discharge † | Forest Area | Farmland | Wetland | Low–Medium Development | High Development |
|---|---|---|---|---|---|---|---|
| km2 | km3/yr | % | % | % | % | % | |
| Deerfield | 1722 | 1.29 | 82 | 5 | 4 | 2 | 0.080 |
| Clesson | 55.6 | 0.04 | 81 | 11 | 2 | 1 | 0.007 |
| Cold | 81.9 | 0.06 | 90 | 1 | 4 | 1 | 0.001 |
| Quinebaug | 1032 | 1.39 | 61 | 7 | 14 | 5 | 0.450 |
| French | 261 | 0.20 | 58 | 4 | 11 | 10 | 0.985 |
| Little | 78 | 0.06 | 60 | 17 | 13 | 2 | 0.091 |
| Shetucket | 1362 | 1.05 | 71 | 6 | 10 | 4 | 0.258 |
| Still | 143 | 0.03 | 76 | 4 | 11 | 1 | 0.004 |
| Arsenic | Copper | Iron | Lead | Nickel | Zinc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| kg/yr | kg/yr/km2 | kg/yr | kg/yr/km2 | kg/yr | kg/yr/km2 | kg/yr | kg/yr/km2 | kg/yr | kg/yr/km2 | kg/yr | kg/yr/km2 | |
| Deerfield | 151 | 0.09 | 2416 | 1.4 | 58 | 0.03 | 331 | 0.19 | 492 | 0.29 | 942 | 0.55 |
| Clesson | 7 | 0.12 | 90 | 1.6 | 0.5 | 0.01 | 4 | 0.07 | 17 | 0.30 | 59 | 1.07 |
| Cold | 14 | 0.17 | 168 | 2.1 | 1.4 | 0.02 | 13 | 0.16 | 19 | 0.23 | 79 | 0.96 |
| Quinebaug | 285 | 0.28 | 561 | 0.5 | 190 | 0.18 | 382 | 0.37 | 603 | 0.58 | 1563 | 1.51 |
| French | 46 | 0.18 | 50 | 0.2 | 22 | 0.08 | 9 | 0.03 | 82 | 0.31 | 71 | 0.27 |
| Little | 24 | 0.31 | 26 | 0.3 | 5.8 | 0.07 | 2 | 0.03 | 28 | 0.35 | 29 | 0.37 |
| Shetucket | 145 | 0.11 | 321 | 0.2 | 100 | 0.07 | 187 | 0.14 | 342 | 0.25 | 811 | 0.60 |
| Still | 6 | 0.04 | 18 | 0.1 | 10 | 0.07 | 11 | 0.08 | 21 | 0.14 | 35 | 0.24 |
| Aluminum | Potassium | Silicon | DOC | |||||
|---|---|---|---|---|---|---|---|---|
| Mg/yr | Mg/yr/km2 | Gg/yr | Mg/yr/km2 | Gg/yr | Mg/yr/km2 | Mg/yr | Mg/yr/km2 | |
| Deerfield | 92.9 | 0.05 | 10.24 | 6.0 | 3.4 | 2.0 | 8360 | 4.9 |
| Clesson | 4.8 | 0.09 | 0.97 | 17.4 | 0.4 | 7.2 | 137 | 2.5 |
| Cold | 6.2 | 0.08 | 0.56 | 6.8 | 0.2 | 2.4 | 187 | 2.3 |
| Quinebaug | 61 | 0.06 | 2.47 | 2.4 | 2.3 | 2.3 | 4389 | 4.3 |
| French | 10 | 0.04 | 0.31 | 1.2 | 0.2 | 0.9 | 772 | 2.9 |
| Little | 4.4 | 0.06 | 0.16 | 2.0 | 0.1 | 1.5 | 306 | 3.9 |
| Shetucket | 48 | 0.04 | 2.25 | 1.7 | 1.7 | 1.3 | 3648 | 2.7 |
| Still | 3.6 | 0.02 | 0.05 | 0.4 | 0.2 | 1.1 | 292 | 2.0 |
| Soil Cu | Soil Fe | Soil Ni | Soil Pb | Soil Zn | Soil pH | Soil LOI | Water As | Water Cu | Water Fe | Water Ni | Water Pb | Water Zn | Water pH | Water DOC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil As | −0.6 | −0.3 | 0.9 | −0.7 | −0.7 | 0.9 | −0.4 | −0.2 | 0.7 | −0.6 | −0.1 | −0.2 | 0.3 | 0.6 | 0.0 |
| Soil Cu | 0.1 | −0.7 | 0.8 | 0.7 | −0.4 | 0.6 | 0.5 | −0.8 | 0.8 | 0.2 | −0.2 | −0.4 | −0.8 | 0.1 | |
| Soil Fe | 0.1 | −0.2 | 0.3 | 0.0 | 0.0 | −0.6 | −0.1 | 0.5 | −0.7 | −0.3 | −0.3 | 0.0 | −0.5 | ||
| Soil Ni | −0.9 | −0.8 | 0.8 | −0.3 | −0.4 | 0.8 | −0.3 | −0.3 | −0.2 | 0.2 | 0.8 | −0.1 | |||
| Soil Pb | 0.7 | −0.7 | 0.3 | 0.5 | −0.9 | 0.8 | 0.5 | 0.1 | −0.1 | −0.9 | 0.1 | ||||
| Soil Zn | −0.6 | 0.0 | −0.1 | −0.9 | 0.5 | −0.1 | −0.1 | −0.4 | −0.8 | −0.1 | |||||
| Soil pH | −0.2 | −0.1 | 0.6 | −0.7 | −0.2 | −0.4 | 0.2 | 0.5 | −0.3 | ||||||
| Soil LOI | 0.6 | −0.4 | 0.2 | 0.2 | −0.3 | −0.3 | −0.3 | 0.3 | |||||||
| Water As | −0.1 | 0.5 | 0.8 | 0.2 | 0.3 | −0.3 | 0.4 | ||||||||
| Water Cu | 0.6 | 0.7 | 0.3 | 0.5 | 0.9 | 0.0 | |||||||||
| Water Fe | 0.8 | 0.6 | 0.3 | −0.7 | 0.4 | ||||||||||
| Water Ni | 0.7 | 0.7 | −0.3 | 0.6 | |||||||||||
| Water Pb | 0.8 | 0.0 | 0.5 | ||||||||||||
| Water Zn | 0.1 | 0.2 | |||||||||||||
| Water pH | 0.1 | ||||||||||||||
| Water DOC |
| Watershed Land Use | |||||
|---|---|---|---|---|---|
| Wetland | Forest | Farm | Low/Med Develop. | High Develop. | |
| Soil As | −0.4 | 0.5 | 0.1 | −0.2 | −0.2 |
| Soil Cu | 0.3 | −0.8 | 0.5 | 0.4 | 0.4 |
| Soil Fe | 0.0 | 0.6 | −0.1 | −0.6 | −0.6 |
| Soil Ni | −0.5 | 0.8 | 0.1 | −0.6 | −0.6 |
| Soil Pb | 0.5 | −0.9 | 0.2 | 0.7 | 0.7 |
| Soil Zn | 0.6 | −0.6 | −0.1 | 0.5 | 0.5 |
| Soil pH | −0.5 | −0.3 | −0.4 | −0.6 | −0.5 |
| Soil LOI | −0.3 | −0.3 | −0.2 | −0.3 | −0.3 |
| Water As | 0.1 | −0.6 | 0.6 | 0.3 | 0.3 |
| Water Cu | −0.3 | 0.8 | −0.2 | −0.2 | −0.2 |
| Water Fe | 0.7 | −0.7 | 0.1 | 0.3 | 0.3 |
| Water Ni | 0.5 | −0.5 | 0.3 | 0.3 | 0.3 |
| Water Pb | 0.7 | −0.5 | −0.3 | 0.0 | 0.0 |
| Water Zn | 0.4 | 0.2 | 0.0 | −0.1 | −0.1 |
| Water pH | 0.4 | 0.1 | 0.3 | 0.4 | 0.3 |
| Water DOC | −0.4 | 0.2 | −0.1 | −0.6 | −0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richardson, J.B. Comparing Trace Elements (As, Cu, Ni, Pb, and Zn) in Soils and Surface Waters among Montane, Upland Watersheds and Lowland, Urban Watersheds in New England, USA. Water 2021, 13, 59. https://doi.org/10.3390/w13010059
Richardson JB. Comparing Trace Elements (As, Cu, Ni, Pb, and Zn) in Soils and Surface Waters among Montane, Upland Watersheds and Lowland, Urban Watersheds in New England, USA. Water. 2021; 13(1):59. https://doi.org/10.3390/w13010059
Chicago/Turabian StyleRichardson, Justin B. 2021. "Comparing Trace Elements (As, Cu, Ni, Pb, and Zn) in Soils and Surface Waters among Montane, Upland Watersheds and Lowland, Urban Watersheds in New England, USA" Water 13, no. 1: 59. https://doi.org/10.3390/w13010059
APA StyleRichardson, J. B. (2021). Comparing Trace Elements (As, Cu, Ni, Pb, and Zn) in Soils and Surface Waters among Montane, Upland Watersheds and Lowland, Urban Watersheds in New England, USA. Water, 13(1), 59. https://doi.org/10.3390/w13010059





