Dynamic Hydraulics in a Drinking Water Distribution System Influence Suspended Particles and Turbidity, But Not Microbiology
Abstract
1. Introduction
2. Materials and Methods
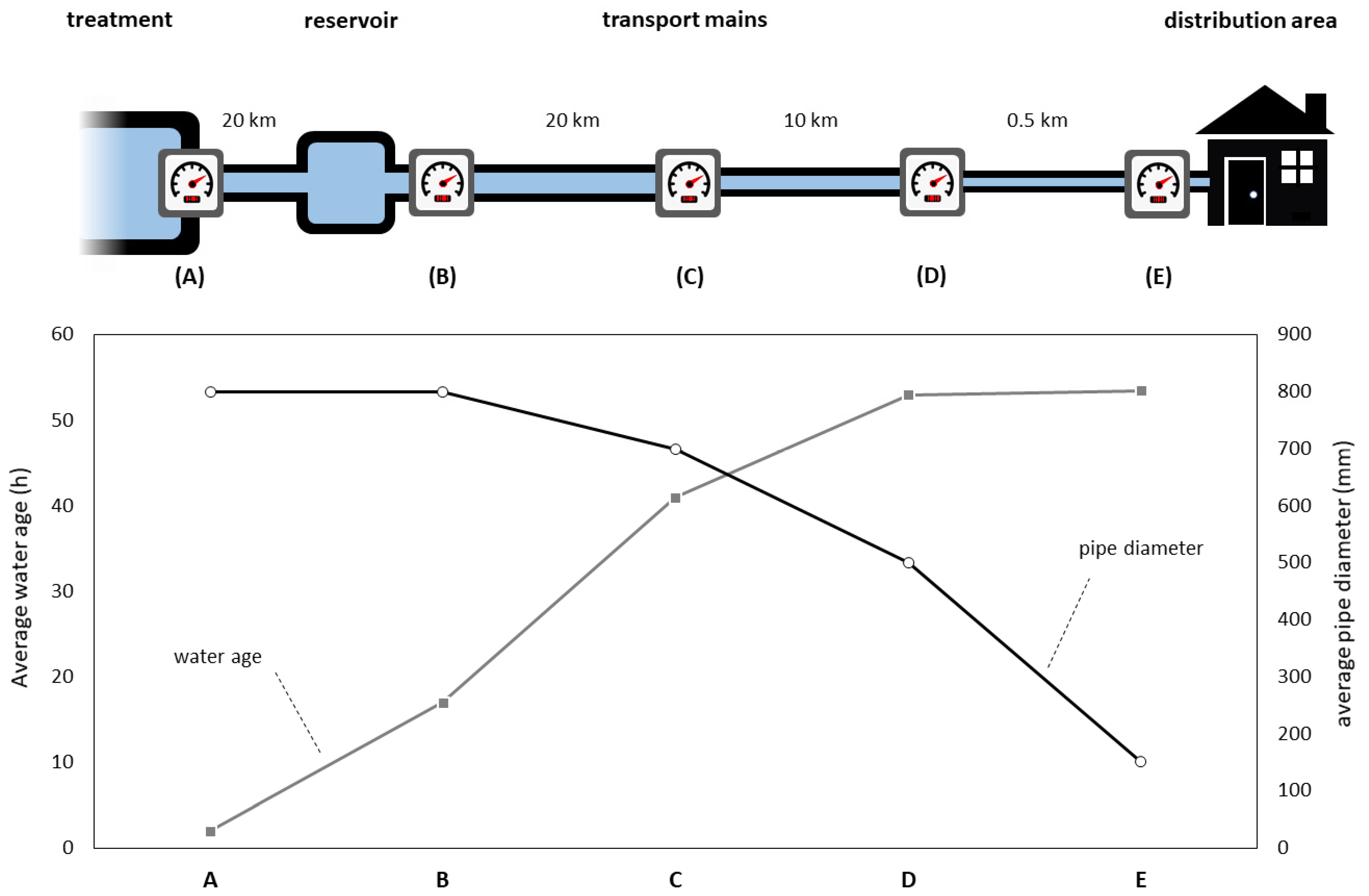
2.1. Study Locations
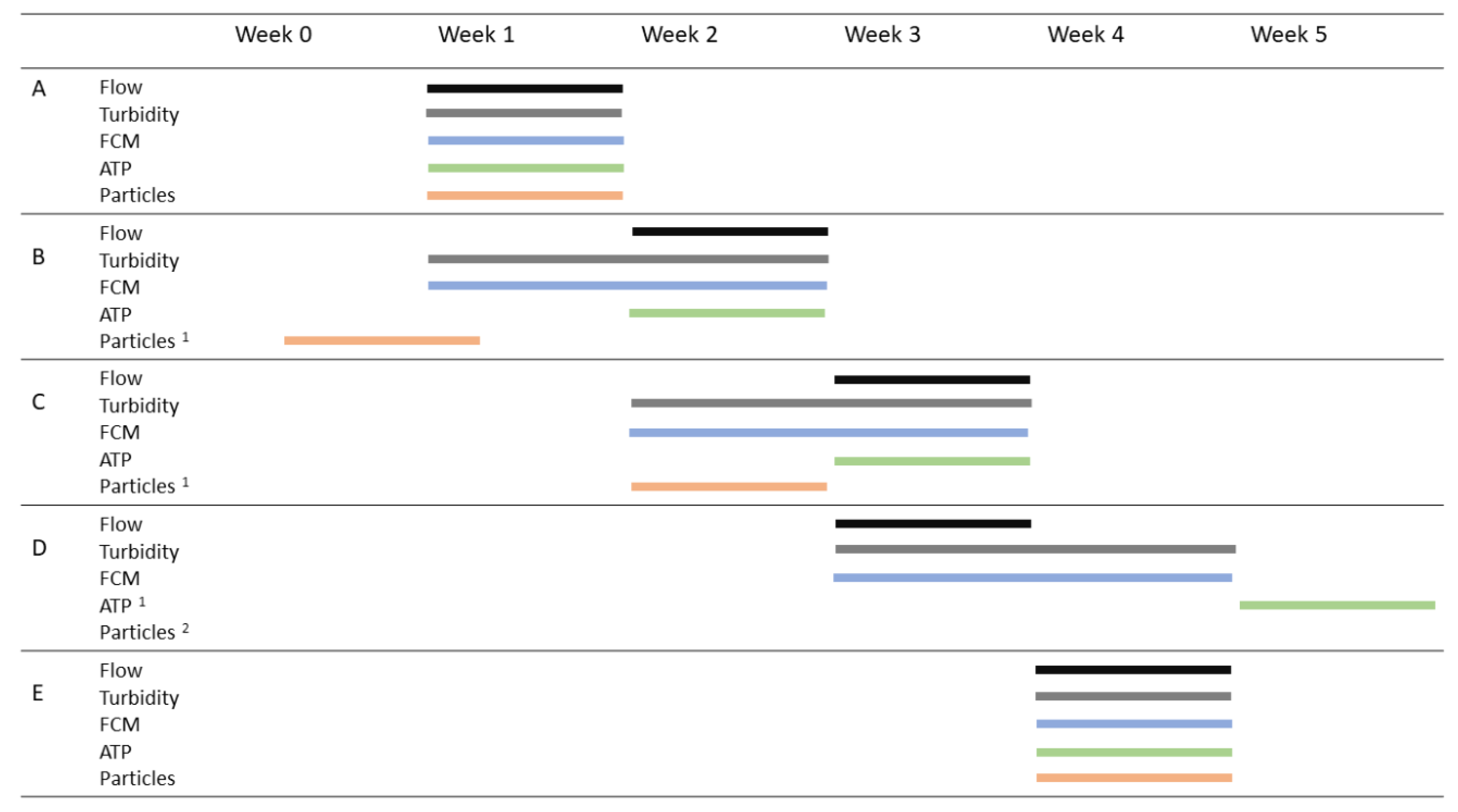
2.2. Measurement Scheme
2.3. Online Turbidity, Particle Counts, and Flow Measurements
2.4. Online Flow Cytometry (FCM) Measurements
2.5. Online ATP Measurements
3. Results
3.1. Spatial Changes in Water Quality
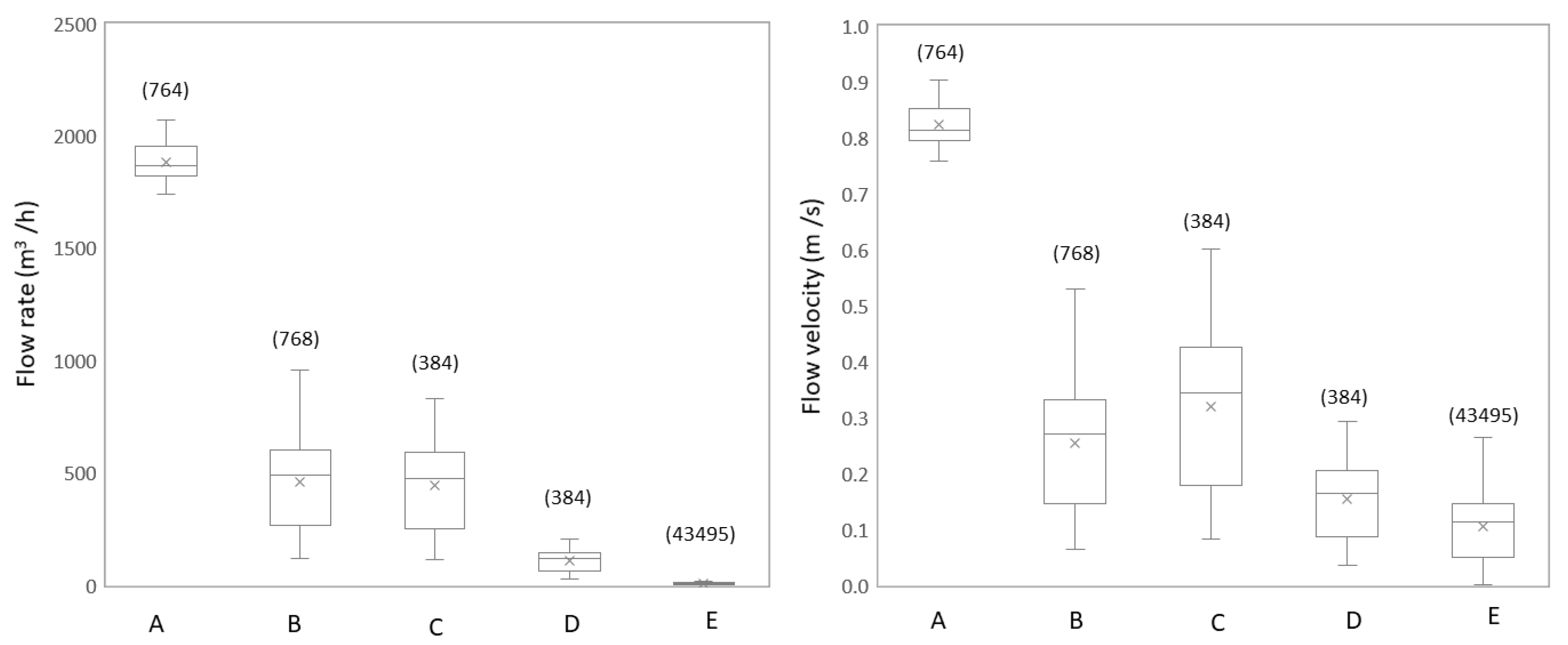
3.1.1. Flow Rate and Flow Velocity Change Due to Network Configuration
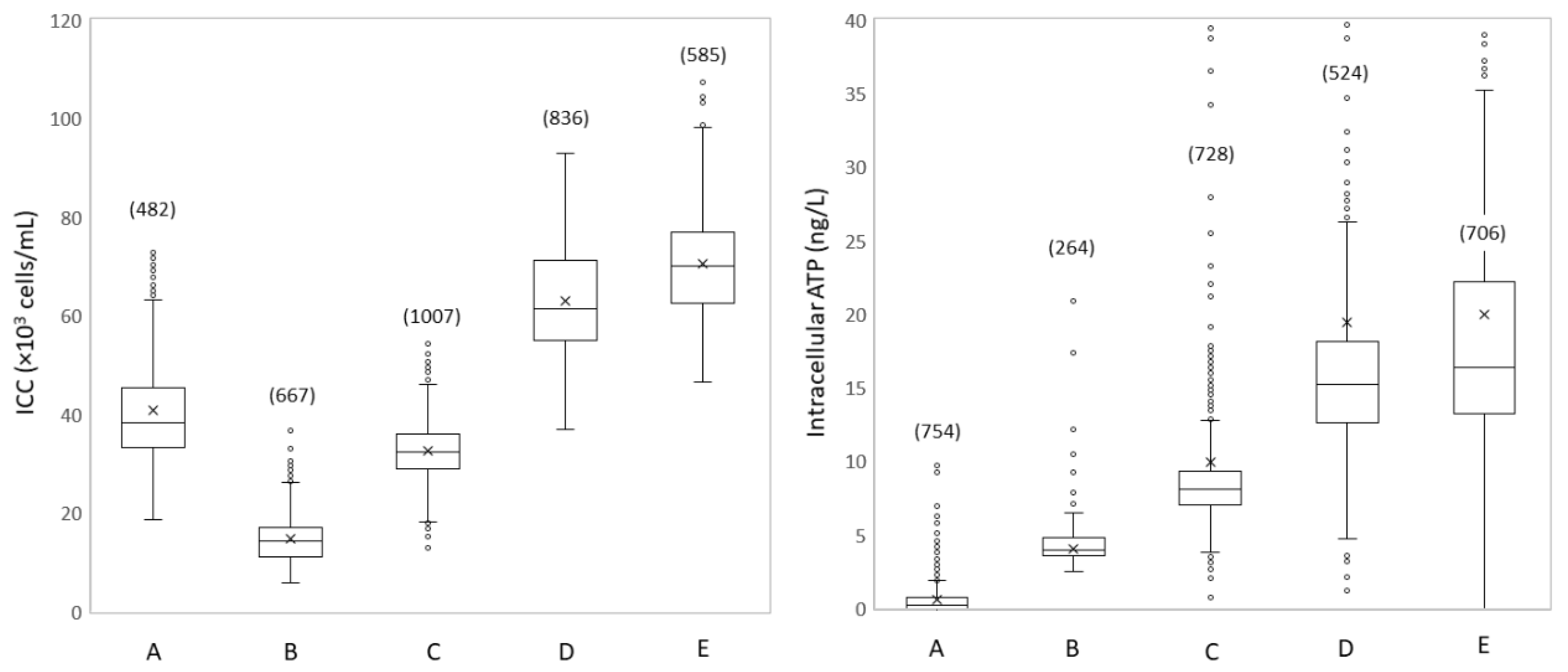
3.1.2. Spatial Microbial Dynamics Show Disinfection and Growth
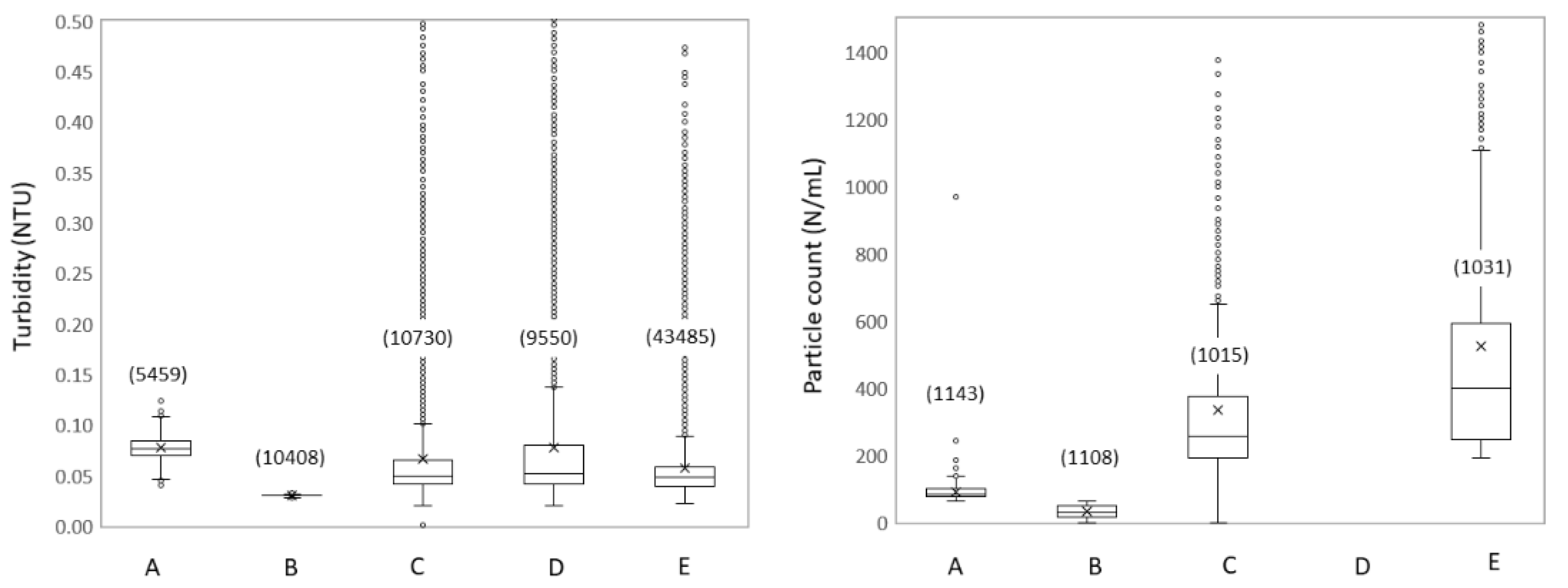
3.1.3. Spatial Dynamics Show Increased Turbidity and Particles
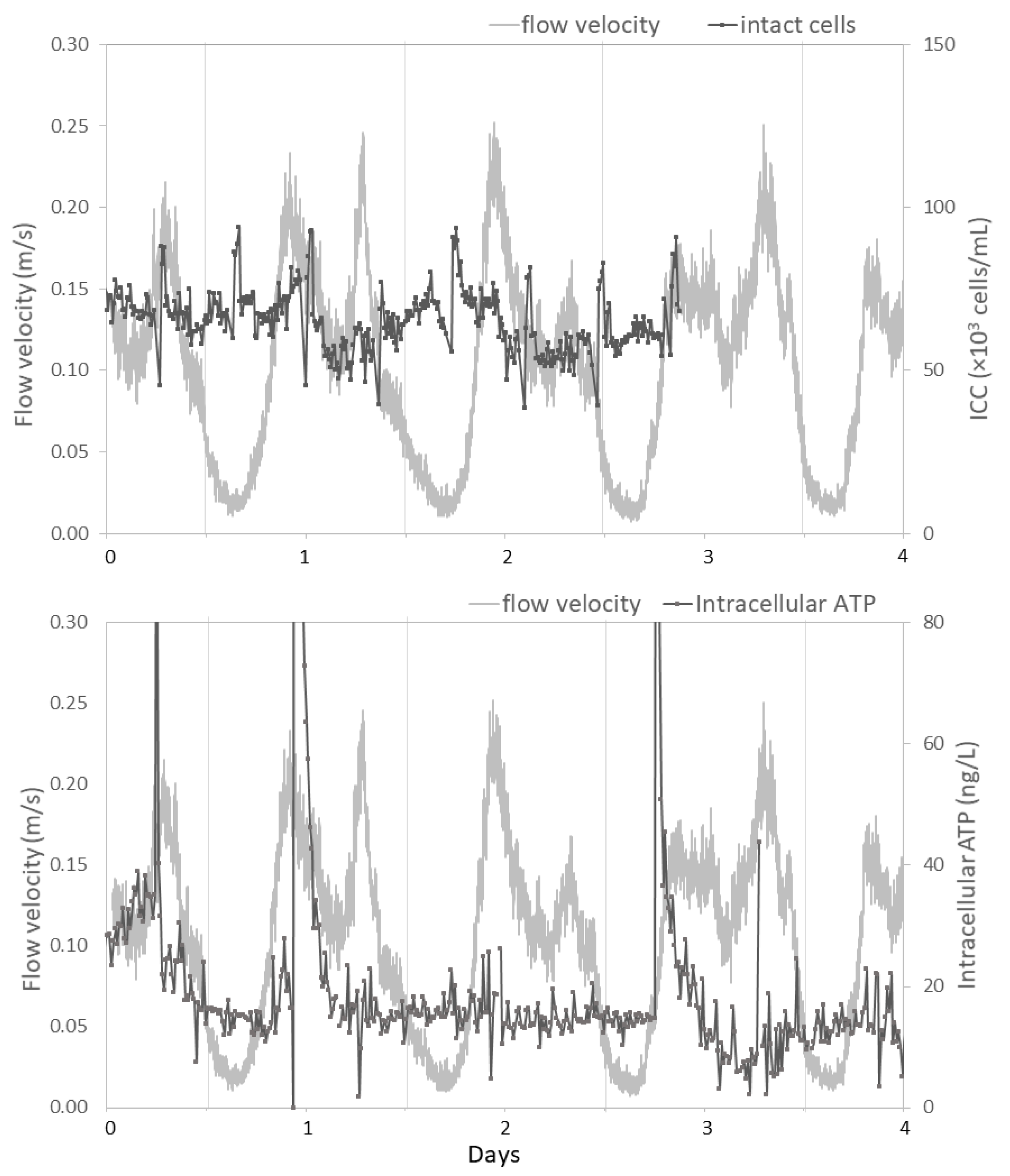
3.2. Temporal Dynamics Reveal Hydraulic Influences
3.2.1. Studied Locations are Subject to Large Daily Variations in Hydraulic Conditions
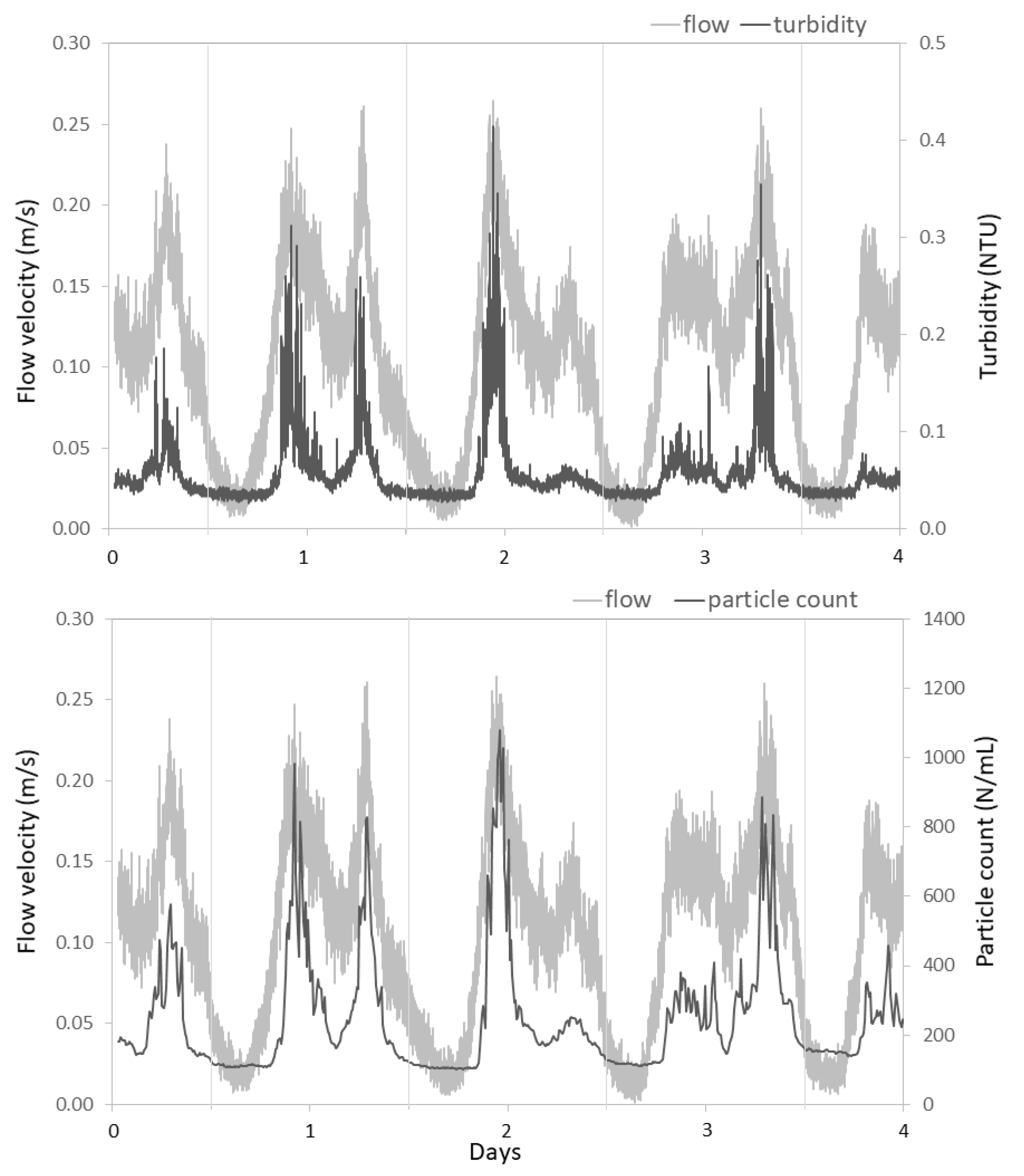
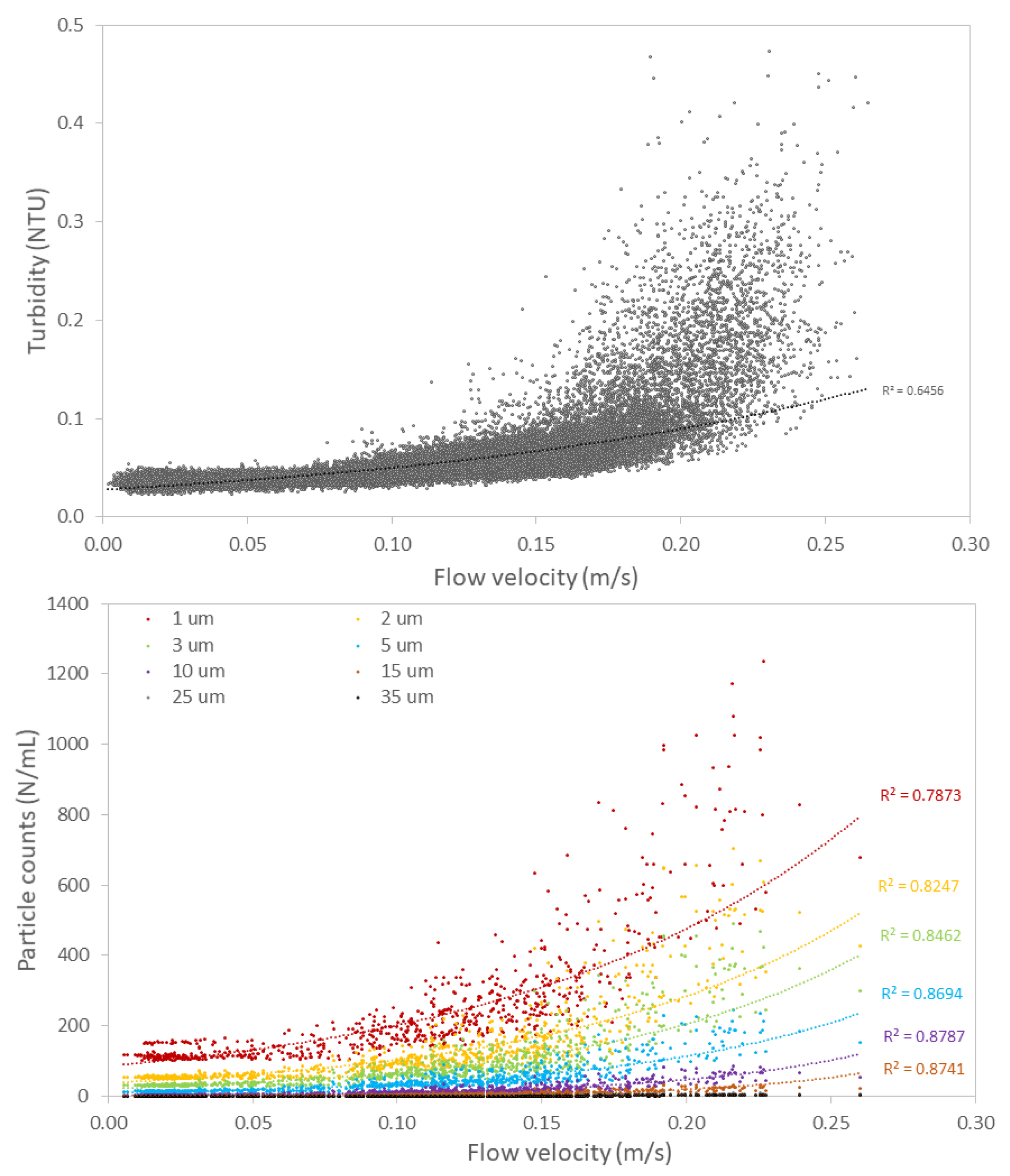
3.2.2. Hydraulic Conditions Influence Particle Counts and Turbidity
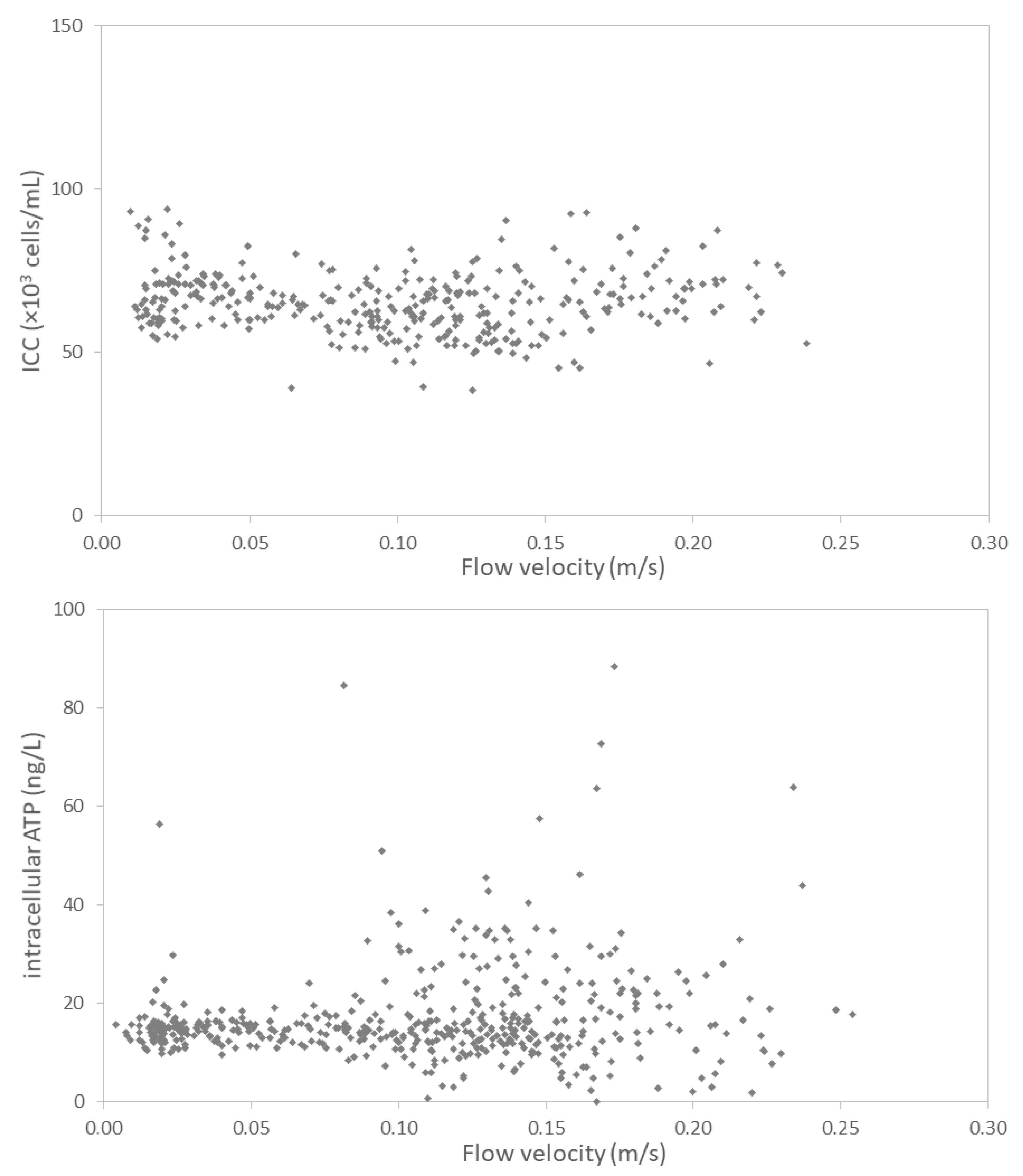
3.2.3. Hydraulic Conditions Barely Impact Online Microbial Parameters
4. Discussion
4.1. Impact of Hydraulic Conditions on Particle Deposition and Re-Suspension
4.2. Impact of Hydraulic Conditions on Microbiology
4.3. Different Microbial Methods Provide Complementary Information
4.4. Online Measurements and Large Datasets
5. Conclusions
- Hydraulic changes in drinking water distribution systems are highly dynamic and inevitable.
- Flow velocity variations have a strong impact on turbidity and particle counts as a result of particle deposition and re-suspension.
- Concentration of bacteria in suspension in water (free flowing) is mostly influenced by water age and concentration of the residual disinfectant (when relevant). Hydraulic conditions had limited impact on suspended microbiology, as assessed by flow cytometry.
- A weak correlation between flow velocity and ATP concentrations suggests an impact of the hydraulic condition on particle-bound bacteria. Resuspension of particle-bound bacteria by either biofilm detachment or release from sediment is a random process, which can be incidentally enhanced by increased flow velocities.
- Combinations of complimentary methods are required to describe complex microbial dynamics in drinking water systems.
- Online measurements provide a range of values expected at a specific location or distribution area, and therefore help with the interpretation of routine monitoring data, understanding of dynamics inherent to the system, and better design sampling programs for water utilities.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Moel, P.J.; Verberk, J.Q.J.C.; Van Dijk, J.C. Drinking Water: Principles and Practices; World Scientific: Singapore, 2006. [Google Scholar]
- National Research Council. Drinking Water Distribution Systems: Assessing and Reducing Risks; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar] [CrossRef]
- Blokker, E.J.M.; Vreeburg, J.H.G.; Buchberger, S.G.; van Dijk, J.C. Importance of demand modelling in network water quality models: A review. Drink. Water Eng. Sci. 2008, 1, 27–38. [Google Scholar] [CrossRef]
- Pothof, I.W.M.; Blokker, E.J.M. Dynamic hydraulic models to study sedimentation in drinking water networks in detail. Drink. Water Eng. Sci. 2012, 5, 87–92. [Google Scholar] [CrossRef][Green Version]
- Blokker, E.J.M.; Vreeburg, J.H.G.; Beverloo, H.; Klein Arfman, M.; van Dijk, J.C. A bottom-up approach of stochastic demand allocation in water quality modelling. Drink. Water Eng. Sci. 2010, 3, 43–51. [Google Scholar] [CrossRef]
- Husband, P.S.; Boxall, J.B.; Saul, A.J. Laboratory studies investigating the processes leading to discolouration in water distribution networks. Water Res. 2008, 42, 4309–4318. [Google Scholar] [CrossRef] [PubMed]
- Van Summeren, J.; Blokker, M. Modeling particle transport and discoloration risk in drinking water distribution networks. Drink. Water Eng. Sci. 2017, 10, 99–107. [Google Scholar] [CrossRef][Green Version]
- Zacheus, O.M.; Lehtola, M.J.; Korhonen, L.K.; Martikainen, P.J. Soft deposits, the key site for microbial growth in drinking water distribution networks. Water Res. 2001, 35, 1757–1765. [Google Scholar] [CrossRef]
- Liu, G.; Bakker, G.L.; Li, S.; Vreeburg, H.G.; Verberk, J.Q.J.C.; Medema, G.J.; Liu, W.T.; Van Dijk, J.C. Pyrosequencing Reveals Bacterial Communities in Unchlorinated Drinking Water Distribution System: An Integral Study of Bulk Water, Suspended Solids, Loose Deposits, and Pipe Wall Biofilm. Environ. Sci. Technol. 2014, 48, 5467–5476. [Google Scholar] [CrossRef]
- Van der Wielen, P.W.J.J.; Lut, M.C. Distribution of microbial activity and specific microorganisms across sediment size fractions and pipe wall biofilm in a drinking water distribution system. Water Supply 2016, 16, 896–904. [Google Scholar] [CrossRef]
- Flemming, H.-C. Biofouling in water systems—Cases, causes and countermeasures. Appl. Microbiol. Biotechnol. 2002, 59, 629–640. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Y.; van der Mark, E.; Magic-Knezev, A.; Pinto, A.; van den Bogert, B.; Liu, W.T.; van der Meer, W.; Medema, G. Assessing the origin of bacteria in tap water and distribution system in an unchlorinated drinking water system by SourceTracker using microbial community fingerprints. Water Res. 2018, 138, 86–96. [Google Scholar] [CrossRef]
- Telgmann, U.; Horn, H.; Morgenroth, E. Influence of growth history on sloughing and erosion from biofilms. Water Res. 2004, 38, 3671–3684. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-P. Impact of flow velocity on the dynamic behaviour of biofilm bacteria. Biofouling 2005, 21, 267–277. [Google Scholar] [CrossRef]
- Besmer, M.D.; Hammes, F. Short-term microbial dynamics in a drinking water plant treating groundwater with occasional high microbial loads. Water Res. 2016, 107, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Besmer, M.D.; Hammes, F.; Sigrist, J.A.; Ort, C. Evaluating monitoring strategies to detect precipitation-induced microbial contamination events in karstic springs used for drinking water. Front. Microbiol. 2017, 8, 2229. [Google Scholar] [CrossRef] [PubMed]
- Skovhus, L.T.; Højris, B. Microbiological Sensors for the Drinking Water Industry; IWA Publishing: London, UK, 2018. [Google Scholar] [CrossRef]
- Besmer, M.D.; Weissbrodt, D.G.; Kratochvil, B.E.; Sigrist, J.A.; Weyland, M.S.; Hammes, F. The feasibility of automated online flow cytometry for in-situ monitoring of microbial dynamics in aquatic ecosystems. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef]
- Prest, E.I.; Hammes, F.; Kotzsch, S.; van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Monitoring microbiological changes in drinking water systems using a fast and reproducible flow cytometric method. Water Res. 2013, 47, 7131–7142. [Google Scholar] [CrossRef]
- Vreeburg, J.H.G.; Boxall, J.B. Discolouration in potable water distribution systems: A review. Water Res. 2007, 41, 519–529. [Google Scholar] [CrossRef]
- Vreeburg, J.H.G.; Schaap, P.G.; van Dijk, J.C. Particles in the drinking water system: From source to discolouration. Water Supply 2004, 4, 431–438. [Google Scholar] [CrossRef]
- Matsui, Y.; Yamagishi, T.; Terada, Y.; Matsushita, T.; Inoue, T. Suspended particles and their characteristics in water mains: Developments of sampling methods. AQUA 2007, 56, 13–24. [Google Scholar] [CrossRef]
- Husband, S.; Fish, K.E.; Douterelo, I.; Boxall, J. Linking discolouration modelling and biofilm behaviour within drinking water distribution systems. Water Sci. Technol. Water Supply 2016, 16, 942–950. [Google Scholar] [CrossRef]
- Gauthier, V.; Gérard, B.; Portal, J.-M.; Block, J.-C.; Gatel, D. Organic matter as loose deposits in a drinking water distribution system. Water Res. 1999, 33, 1014–1026. [Google Scholar] [CrossRef]
- Ackers, J.; Brandt, M.; Powell, J. Hydraulic Characterisation of Deposits and Review of Sediment Modelling; Drinking water quality and health—Distribution Systems. Project 01/DW/03/18; UK Water Industry Research: London, UK, 2001. [Google Scholar]
- Slaats, P.G.G.; Rosenthal, L.P.M.; Siegers, W.G.; van den Boomen, M.; Beuken, R.H.S.; Vreeburg, J.H.G. Processes Involved in the Generation of Discolored Water; AWWARF Report 90966F; AWWARF: Denver, CO, USA, 2003. [Google Scholar]
- Ryan, G.; Mathes, P.; Haylock, G.; Jayaratne, A.; Wu, J.; Noui-Mehidi, N.; Grainger, C.; Nguyen, B.V. Particles in Water Distribution Systems; Research Report 33; Cooperative Research Centre for Water Quality and Treatment: Salisbury, Australia, 2008. [Google Scholar]
- Blokker, E.J.M.; Schaap, P.G.; Vreeburg, J.H.G. Self-cleaning networks put to the test. In Strategic Asset Management of Water Supply and Wastewater Infrastructures 2008, Proceedings of the Invited Papers from the IWA Leading Edge Conference on Strategic Asset Management (LESAM), Lisbon, Portugal, 17 October 2007; Alegre, H., Almeida, M.C., Eds.; IWA Publishing: London, UK, 2009; pp. 407–417. [Google Scholar]
- Blokker, E.J.M.; Vreeburg, J.H.G.; Schaap, P.G.; van Dijk, J.C. The Self-Cleaning Velocity in Practice; WDSA: Tuscon, AZ, USA, 2010. [Google Scholar]
- Vreeburg, J.H.G.; Blokker, E.J.M.; Horst, P.; Van Dijk, J.C. Velocity-based self-cleaning residential drinking water distribution systems. Water Sci. Technol. Water Supply 2009, 9, 635–641. [Google Scholar] [CrossRef]
- Husband, P.S.; Boxall, J.B. Asset deterioration and discolouration in water distribution systems. Water Res. 2011, 45, 113–124. [Google Scholar] [CrossRef]
- Sunny, I.; Husband, P.S.; Boxall, J.B. Impact of hydraulic interventions on chronic and acute material loading and discolouration risk in drinking water distribution systems. Water Res. 2020, 169, 115224. [Google Scholar] [CrossRef] [PubMed]
- Fish, K.; Osborn, A.M.; Boxall, J.B. Characterising and understanding the impact of microbial biofilms and the extracellular polymeric substance (EPS) matrix in drinking water distribution systems. Environ. Sci. Water Res. Technol. 2016, 2, 614. [Google Scholar] [CrossRef]
- Fish, K.; Osborn, A.M.; Boxall, J.B. Biofilm structures (EPS and bacterial communities) in drinking water distribution systems are conditioned by hydraulics and influence discolouration. Sci. Total Environ. 2017, 593–594, 571–580. [Google Scholar] [CrossRef]
- Cowle, M.; Webster, G.; Babatunde, A.; Bockelmann-Evans, B.; Weightman, A. Impact of flow hydrodynamics and pipe material properties on biofilm development within drinking water systems. Environ. Technol. 2019. [Google Scholar] [CrossRef]
- Douterelo, I.; Husband, S.; Loza, V.; Boxall, J. Dynamics of biofilm regrowth in drinking water distribution systems. Appl. Environ. Microbiol. 2016, 82, 4155–4168. [Google Scholar] [CrossRef]
- Douterelo, I.; Dutilh, B.E.; Arkhipova, K.; Calero, C.; Husband, S. Microbial diversity, ecological networks and functional traits associated to materials used in drinking water distribution systems. Water Res. 2020, 173, 115586. [Google Scholar] [CrossRef]
- Van Nevel, S.; Koetzsch, S.; Proctor, C.R.; Besmer, M.D.; Prest, E.I.; Vrouwenvelder, J.S.; Knezev, A.; Boon, N.; Hammes, F. Flow cytometric bacterial cell counts challenge conventional heterotrophic plate counts for routine microbiological drinking water monitoring. Water Res. 2017, 113, 191–206. [Google Scholar] [CrossRef]
- Hammes, F.; Berney, M.; Egli, T. Cultivation-independent assessment of bacterial viability. Adv. Biochem. Engin. Biotechnol. 2011, 124, 123–150. [Google Scholar]
- Nescerecka, A.; Juhna, T.; Hammes, F. Behavior and stability of adenosine triphosphate (ATP) during chlorine disinfection. Water Res. 2016, 101, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Hammes, F.; Goldschmidt, F.; Vital, M.; Wang, Y.; Egli, T. Measurement and interpretation of microbial adenosine tri-phosphate (ATP) in aquatic environments. Water Res. 2010, 44, 3915–3923. [Google Scholar] [CrossRef] [PubMed]
- Seviour, T.; Derlon, N.; Dueholm, M.; Flemming, H.; Girbal-Neuhauser, E.; Horn, H.; Kjelleberg, S.; van Loosdrecht, M.; Lotti, T.; Malpei, M.; et al. Extracellular polymeric substances of biofilms: Suffering from an identity crisis. Water Res. 2019, 151, 1–7. [Google Scholar] [CrossRef]
- Favere, J.; Buysschaert, B.; Boon, N.; De Gusseme, B. Online microbial fingerprinting for quality management of drinking water: Full-scale event detection. Water Res. 2020, 170, 115353. [Google Scholar] [CrossRef]
- Buysschaert, B.; Vermijs, L.; Naka, A.; Boon, N.; De Gusseme, B. Online flow cytometric monitoring of microbial water quality in a full-scale water treatment plant. NPJ Clean Water 2018, 1, 16. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prest, E.I.; Schaap, P.G.; Besmer, M.D.; Hammes, F. Dynamic Hydraulics in a Drinking Water Distribution System Influence Suspended Particles and Turbidity, But Not Microbiology. Water 2021, 13, 109. https://doi.org/10.3390/w13010109
Prest EI, Schaap PG, Besmer MD, Hammes F. Dynamic Hydraulics in a Drinking Water Distribution System Influence Suspended Particles and Turbidity, But Not Microbiology. Water. 2021; 13(1):109. https://doi.org/10.3390/w13010109
Chicago/Turabian StylePrest, Emmanuelle I., Peter G. Schaap, Michael D. Besmer, and Frederik Hammes. 2021. "Dynamic Hydraulics in a Drinking Water Distribution System Influence Suspended Particles and Turbidity, But Not Microbiology" Water 13, no. 1: 109. https://doi.org/10.3390/w13010109
APA StylePrest, E. I., Schaap, P. G., Besmer, M. D., & Hammes, F. (2021). Dynamic Hydraulics in a Drinking Water Distribution System Influence Suspended Particles and Turbidity, But Not Microbiology. Water, 13(1), 109. https://doi.org/10.3390/w13010109





