Patterns of Mekong Mollusc Biodiversity: Identification of Emerging Threats and Importance to Management and Livelihoods in a Region of Globally Significant Biodiversity and Endemism
Abstract
1. Introduction
2. Materials and Methods
2.1. Mollusc Collection and Physical-Chemical Measurements
2.2. Land Cover Data
2.3. Climatic Data
2.4. Data Treatment and Analysis
3. Results
3.1. Overall Biodiversity and Conservation Status
3.2. Community Variation
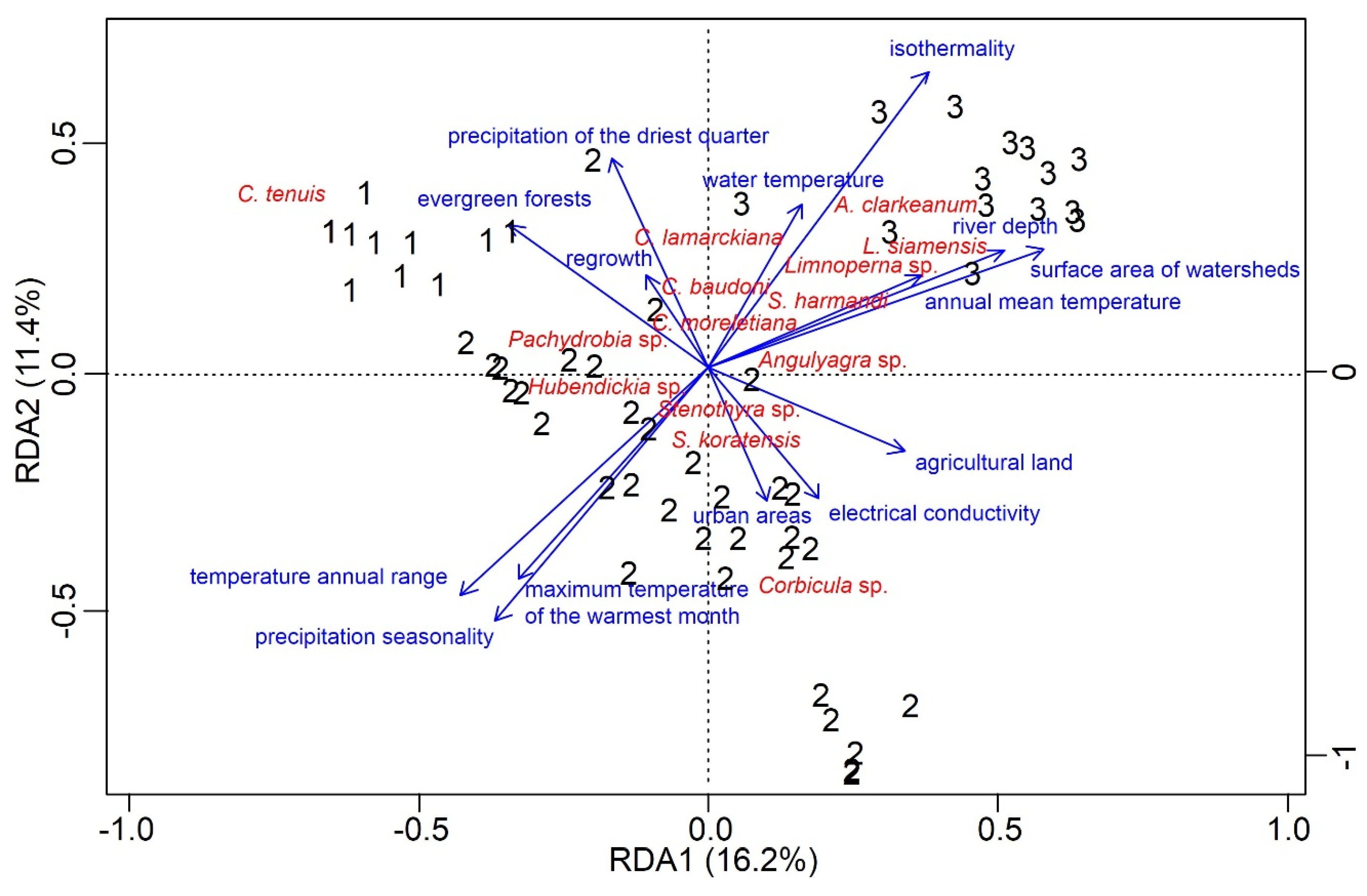
3.3. Drivers of Biodiversity Patterns
4. Discussion
4.1. Mollusc Biodiversity, Conservation Status, and Threats
4.2. Environmental Gradients as Drivers of Mollusc Diversity
4.3. Management Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bogan, A.E. Global diversity of freshwater mussels (Mollusca, Bivalvia) in freshwater. Hydrobiologia 2008, 595, 139–147. [Google Scholar] [CrossRef]
- Palmer, M.; Covich, A.; Finlay, B.; Gibert, J.; Hyde, K.; Johnson, R.; Kairesalo, T.; Lake, S.; Lovell, C.; Naiman, R.; et al. Biodiversity and ecosystem processes in freshwater sediments. Ambio 1997, 26, 571–577. [Google Scholar]
- Covich, A.P.; Palmer, M.A.; Crowl, T.A. The role in of species invertebrate freshwater ecosystems—Zoobenthic species influence energy flows and nutrient cycling. Bioscience 1999, 49, 119–127. [Google Scholar] [CrossRef]
- Vaughn, C.C.; Nichols, S.J.; Spooner, D.E. Community and foodweb ecology of freshwater mussels. J. N. Am. Benthol. Soc. 2008, 27, 409–423. [Google Scholar] [CrossRef]
- Green, R.H. A multivariate statistical approach to hutchinsonian niche: Bivalve molluscs of Central Canada. Ecology 1971, 52, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Köhler, F.; Seddon, M.; Bogan, A.E.; Van Tu, D.; Sri-Aroon, P.; Allen, D. The status and distribution of freshwater molluscs of the Indo-Burma region. In The Status and Distribution of Freshwater Biodiversity in Indo-Burma; Allen, D., Smith, K., Darwall, W., Eds.; Gland: Cambridge, UK, 2012; pp. 66–89. [Google Scholar]
- Coates, D.; Poeu, O.; Suntornratana, U.; Tung, N.T.; Viravong, S. Biodiversity and Fisheries in the Mekong River Basin; IWA Publishing: London, UK, 2003. [Google Scholar]
- Cannicci, S.; Bartolini, F.; Dahdouh-Guebas, F.; Fratini, S.; Litulo, C.; Macia, A.; Mrabu, E.J.; Penha-Lopes, G.; Paula, J. Effects of urban wastewater on crab and mollusc assemblages in equatorial and subtropical mangroves of East Africa. Estuar. Coast. Shelf Sci. 2009, 84, 305–317. [Google Scholar] [CrossRef]
- Skilleter, G.A.; Warren, S. Effects of habitat modification in mangroves on the structure of mollusc and crab assemblages. J. Exp. Mar. Bio. Ecol. 2000, 244, 107–129. [Google Scholar] [CrossRef]
- Barash, A.; Danin, Z. Contribution to the knowledge of Mollusca in the bardawil lagoon. Boll. Malacol. 1982, 18, 107–128. [Google Scholar]
- Jones, G.; Ferrell, D.; Sale, P. Spatial pattern in the abundance and structure of mollusc populations in the soft sediments of a coral reef lagoon. Mar. Ecol. Prog. Ser. 1990, 62, 109–120. [Google Scholar] [CrossRef]
- Albuquerque, F.; Peso-Aguiar, M.; Assunção-Albuquerque, M.; Gálvez, L. Do climate variables and human density affect Achatina fulica (Bowditch) (Gastropoda: Pulmonata) shell length, total weight and condition factor? Braz. J. Biol. 2009, 69, 879–885. [Google Scholar] [CrossRef][Green Version]
- Sor, R.; Boets, P.; Chea, R.; Goethals, P.; Lek, S. Spatial organization of macroinvertebrate assemblages in the Lower Mekong Basin. Limnologica 2017, 64, 20–30. [Google Scholar] [CrossRef]
- Strong, E.E.; Gargominy, O.; Ponder, W.F.; Bouchet, P. Global diversity of gastropods (Gastropoda; Mollusca) in freshwater. Hydrobiologia 2008, 595, 149–166. [Google Scholar] [CrossRef]
- Ng, T.H.; Jeratthitikul, E.; Sutcharit, C.; Chhuoy, S.; Pin, K.; Pholyotha, A.; Siriwut, W.; Srisonchai, R.; Hogan, Z.S.; Ngor, P.B. Annotated checklist of freshwater molluscs from the largest freshwater lake in Southeast Asia. Zookeys 2020, 958, 107. [Google Scholar] [CrossRef] [PubMed]
- Altobelli, A.; Baird, I.G.; Borsani, J.F.; Brambati, A.; Carulli, G.B.; Cunningham, P.; Daconto, G.; Elliott, S.; Friend, R.; Maxwell, J.F. Siphandone Wetlands; Daconto, G., Ed.; CESVI Cooperazione e Sviluppo: Bergamo, Italy, 2001. [Google Scholar]
- Sor, R. Modelling Spatio-Temporal Changes of Benthic Macroinvertebrate Communities in Asian and European Rivers. Ph.D. Thesis, Université Paul Sabatier—Toulouse III and Ghent University, Toulouse, France, 2017. [Google Scholar]
- Davis, G.; Kitikoon, V.; Temcharoen, P. Monograph on “Lithoglyphopsis” aperta, the snail host of Mekong River schistosomiasis. Malacologia 1976, 15, 241–287. [Google Scholar]
- Köhler, F.; Dames, C. Phylogeny and systematics of the Pachychilidae of mainland South-East Asia—Novel insights from morphology and mitochondrial DNA (Mollusca, Caenogastropoda, Cerithioidea). Zool. J. Linn. Soc. 2009, 157, 679–699. [Google Scholar] [CrossRef]
- Davis, G.M. The Stenothyridae of China. No. 2: Stenothyra hunanensis. Proc. Acad. Nat. Sci. Phila. 1988, 140, 247–266. [Google Scholar]
- Davis, G.M. The origin and evolution of the Gastropod family Pomatiopsidae, with emphasis in the Mekong River Triculinae. Syst. Zool. 1981, 30, 115–118. [Google Scholar]
- Glaubrecht, M.; Fehér, Z.; Von Rintelen, T. Brooding in Corbicula madagascariensis (Bivalvia, Corbiculidae) and the repeated evolution of viviparity in corbiculids. Zool. Scr. 2006, 35, 641–654. [Google Scholar] [CrossRef]
- Bros, V.; Torre, I.; Santos, X. Uncovering the environmental factors that influence diversity patterns of Mediterranean terrestrial Gastropod communities: A useful tool for conservation. Ecol. Res. 2016, 31, 39–47. [Google Scholar] [CrossRef]
- Cortes, R.; Varandas, S.; Teixeira, A.; Hughes, S.; Magalhaes, M.; Barquín, J.; Álvarez-Cabria, M.; Fernández, D. Effects of landscape metrics and land use variables on macroinvertebrate communities and habitat characteristics. Limnetica 2011, 30, 347–362. [Google Scholar]
- MRC. Biomonitoring of the lower Mekong River and selected tributaries, 2004–2007. MRC Tech. Pap. 2008, 20, 77. [Google Scholar]
- MRC. Identification of Freshwater Invertebrates of the Mekong River and Its Tributaries; Mekong River Commission (MRC): Vientiane, Laos, 2006. [Google Scholar]
- McCluskey, A.; Lalkhen, A.G. Statistics II: Central tendency and spread of data. Contin. Educ. Anaesth. Crit. Care Pain 2007, 7, 127–130. [Google Scholar] [CrossRef]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R; Springer: Berlin, Germany, 2011; ISBN 9788578110796. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 978-0-444-53868-0. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2016. [Google Scholar]
- Dębkowska, K.; Jarocka, M. The impact of the methods of the data normalization on the result of linear ordering. Acta Univ. Lodz. Folia Oecon. 2013, 286, 181–188. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. Version 2020-1.. Available online: https://www.iucnredlist.org (accessed on 19 February 2020).
- Sor, R.; Legendre, P.; Lek, S. Uniqueness of sampling site contributions to the total variance of macroinvertebrate communities in the Lower Mekong Basin. Ecol. Indic. 2018, 84, 425–432. [Google Scholar] [CrossRef]
- Ngor, P.B.; Sor, R.; Prak, L.H.; So, N.; Hogan, Z.S.; Lek, S. Mollusc fisheries and length-weight relationship in Tonle Sap flood pulse system, Cambodia. Ann. Limnol. Int. J. Limnol. 2018, 54. [Google Scholar] [CrossRef]
- Morris, T.J.; Corkum, L.D. Unionid growth patterns in rivers of differing riparian vegetation. Freshw. Biol. 1999, 42, 59–68. [Google Scholar] [CrossRef]
- Pérez-Quintero, J.C. Distribution patterns of freshwater molluscs along environmental gradients in the southern Guadiana River basin (SW Iberian Peninsula). Hydrobiologia 2011, 678, 65–76. [Google Scholar] [CrossRef]
- Haag, W.R.; Warren, M.L., Jr. Role of ecological factors and reproductive strategies in structuring freshwater mussel communities. Can. J. Fish. Aquat. Sci. 1998, 55, 297–306. [Google Scholar] [CrossRef]
- Vaughn, C.; Hakenkamp, C. The functional role of burrowing bivalves in freshwater ecosystems. Freshw. Biol. 2001, 46, 1431–1446. [Google Scholar] [CrossRef]
- Bradley, B.A.; Laginhas, B.B.; Whitlock, R.; Allen, J.M.; Bates, A.E.; Bernatchez, G.; Diez, J.M.; Early, R.; Lenoir, J.; Sorte, C.J.B. Disentangling the abundance—Impact relationship for invasive species. Proc. Natl. Acad. Sci. USA 2019, 116, 9919–9924. [Google Scholar] [CrossRef]
- GBIF. GBIF Occurrence Download; GBIF: Copenhagen, Denmark, 2015. [Google Scholar]
- Cai, Y.; Zhang, M.; Xu, J.; Heino, J. Geographical gradients in the biodiversity of Chinese freshwater molluscs: Implications for conservation. Divers. Distrib. 2018, 24, 485–496. [Google Scholar] [CrossRef]
- Brandt, R.; Temcharoen, P. The molluscan fauna of the Mekong at the foci of Schistosomiasis in South Laos and Cambodia. Arch. Fur Mollusken Kufide 1971, 101, 111–140. [Google Scholar]
- Brandt, R. The non-marine aquatic Mollusca of Thailand. Arch. Fur Mollusken Kufide 1974, 105, 1–423. [Google Scholar]
- Lopes-Lima, M.; Sousa, R.; Geist, J.; Aldridge, D.C.; Araujo, R.; Bergengren, J.; Bespalaya, Y.; Bódis, E.; Burlakova, L.; Van Damme, D.; et al. Conservation status of freshwater mussels in Europe: State of the art and future challenges. Biol. Rev. 2016, 92, 572–607. [Google Scholar] [CrossRef]
- Quang, N.; Sinh, N.; Tu, N.; Lam, P.; Lan, N. Biodiversity of littoral macroinvertebrates in the Mekong River. Tạp Chí Khoa Học 2013, 51, 16–28. [Google Scholar]
- Musig, Y.; Musig, W.; Satienjit, S. Filtration rates of tropical freshwater bivalve mollusks: Pilsbryoconcha excilis compressa, Ensidens ingallsianus ingallsianus, Corbicula boudoni and Corbicula moreletiana. Kasetsart Univ. Fish. Res. Bull. 2012, 36, 23–29. [Google Scholar]
- Downing, J.; Meter, P.; Woolnough, D. Suspects and evidence: A review of the causes of extirpation and decline in freshwater mussels. Anim. Biodivers. Conserv. 2010, 33, 151–185. [Google Scholar]
- Mehlhop, P.; Vaughn, C.C. Threats to and Sustainability of Ecosystems for Freshwater Mollusks. In Sustainable Ecological Systems: Implementing an Ecological Approach to Land Management; Covington, L., Dehand, W., Eds.; US Department of Agriculture: Fort Collins, CO, USA, 1994; pp. 68–77. [Google Scholar]
- Ng, T.H.; Tan, S.K.; Wong, W.H.; Meier, R.; Chan, S.Y.; Tan, H.H.; Yeo, D.C.J. Molluscs for sale: Assessment of freshwater gastropods and bivalves in the ornamental pet trade. PLoS ONE 2016, 11, e0161130. [Google Scholar] [CrossRef]
- Ngor, P.; Legendre, P.; Oberdorff, T.; Lek, S. Flow alterations by dams shaped fish assemblage dynamics in the complex Mekong-3S river system. Ecol. Indic. 2018, 88, 103–114. [Google Scholar] [CrossRef]
- Collier, K.J.; Lill, A. Spatial patterns in the composition of shallow-water macroinvertebrate communities of a large New Zealand river. N. Z. J. Mar. Freshw. Res. 2008, 42, 129–141. [Google Scholar] [CrossRef]
- Lorenz, C.M.; Van Dijk, G.M.; Van Hattum, A.G.M.; Cofino, W.P. Concepts in river ecology: Implications for indicator development. Regul. Rivers Res. Manag. 1997, 13, 501–516. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The River Continuum Concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Thorp, J.H.; Delong, M.D. The Riverine Productivity Model: An Heuristic View of Carbon Sources and Organic Processing in Large River Ecosystems. Oikos 1994, 70, 305. [Google Scholar] [CrossRef]
- Hecky, R.E.; Kilham, P. Nutrient limitation of phytoplankton in freshwater and marine environments: A review of recent evidence on the effects of enrichment. Limnol. Oceanogr. 1988, 33, 796–822. [Google Scholar] [CrossRef]
- Maltchik, L.; Stenert, C.; Kotzian, C.; Pereira, D. Responses of freshwater molluscs to environmental factors in Southern Brazil wetlands. Braz. J. Biol. 2010, 70, 473–482. [Google Scholar] [CrossRef]
- Chea, R.; Lek, S.; Ngor, P.; Grenouillet, G. Large-scale patterns of fish diversity and assemblage structure in the longest tropical river in Asia. Ecol. Freshw. Fish 2016, 25, 1–11. [Google Scholar] [CrossRef]
- Rezende, R.S.; Santos, A.M.; Henke-oliveira, C.; Gonçalves, J.F., Jr. Effects of spatial and environmental factors on benthic a macroinvertebrate community. Zoologia 2014, 31, 426–434. [Google Scholar] [CrossRef]
- Bae, M.; Park, Y. Key determinants of freshwater gastropod diversity and distribution: The implications for conservation and management. Water 2020, 12, 1908. [Google Scholar] [CrossRef]
- Oyem, H.; Oyem, I.; Ezeweali, D. Temperature, pH, electrical conductivity, total dissolved solids and chemical oxygen demand of groundwater in Boji-BojiAgbor/Owa area and immediate suburbs. Res. J. Environ. Sci. 2014, 8, 444. [Google Scholar] [CrossRef]
- Sheil, D.; Murdiyarso, D. How Forests Attract Rain: An Examination of a New Hypothesis. Bioscience 2009, 59, 341–347. [Google Scholar] [CrossRef]
- Junk, W.J.; Bayley, P.B.; Sparks, R.E. The Flood Pulse Concept in river-floodplain systems. Can. Spec. Publ. Fish. Aquat. Sci. 1989, 106, 110–127. [Google Scholar]
- Gutiérrez, J.L.; Jones, C.G.; Strayer, D.L.; Iribarne, O.O. Mollusks as ecosystem engineers: The role of shell production in aquatic habitats. Oikos 2003, 101, 79–90. [Google Scholar] [CrossRef]
- Attwood, S.W. Mekong Schistosomiasis: Where did it Come from and Where is it Going? 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Davis, G.M.; Chen, C.; Liu, Y. Snail hosts of Paragonimus in Asia and the Americas. Biomed. Environ. Sci. 1994, 7, 369–382. [Google Scholar] [PubMed]
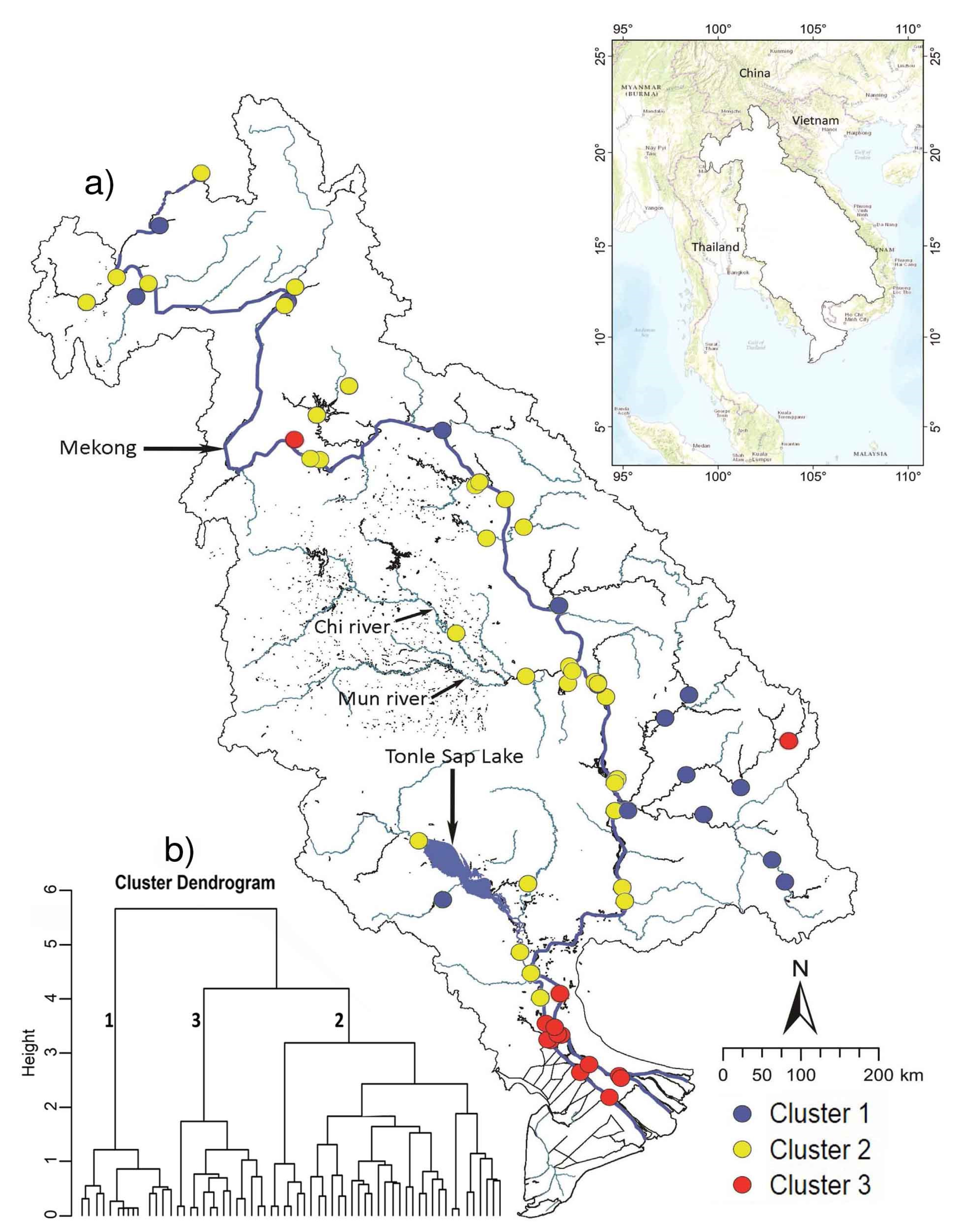

| Cluster 1 | Cluster 2 | Cluster 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Metrics | Tributaries | Upstream Main Channel | Delta | p-Value | ||||||
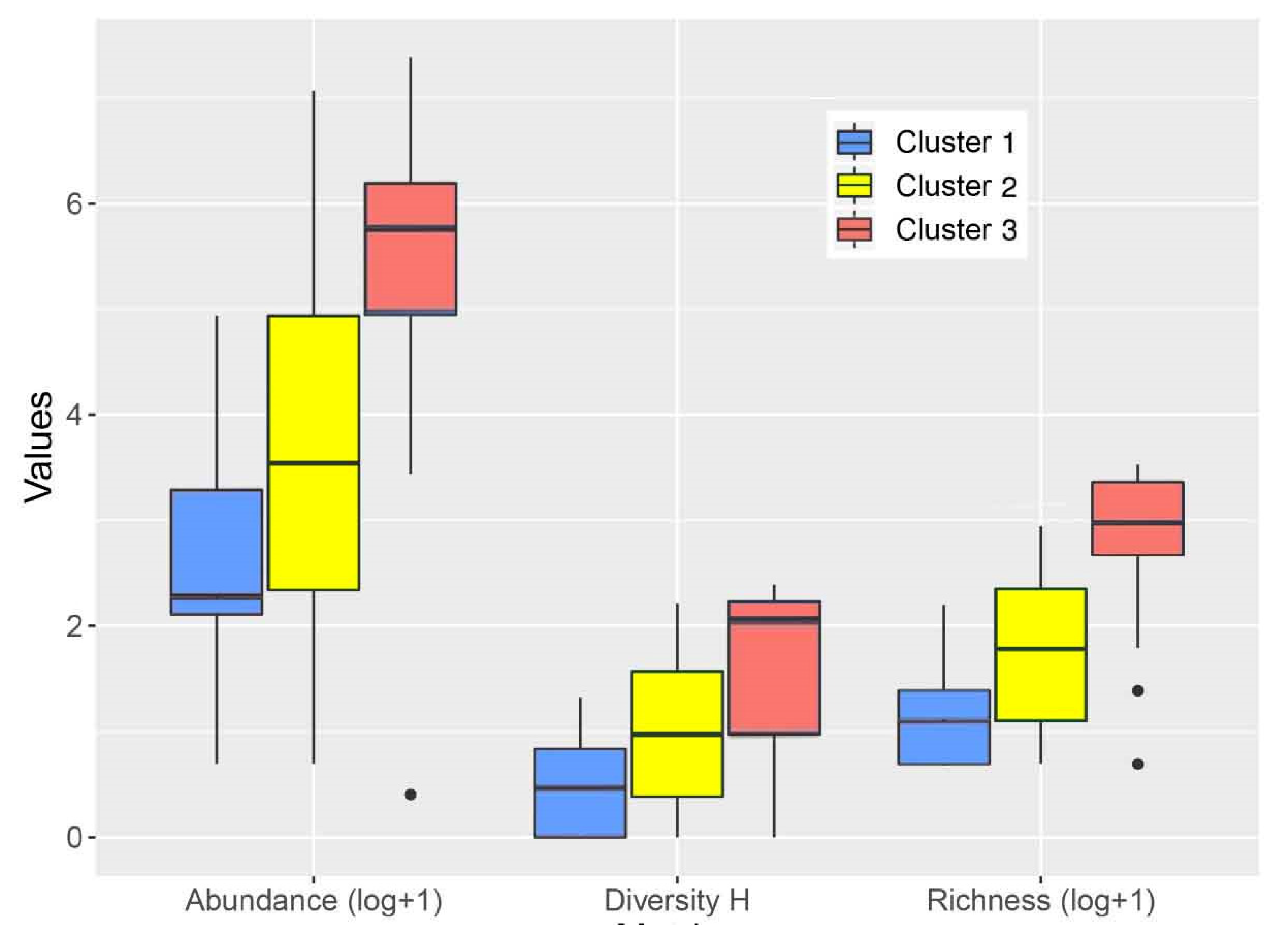
| Taxonomic Richness | 2.0 | 2.9 | 2.4 | 5 | 6.3 | 4.7 | 18.5 | 18.7 | 10.6 | 0.0000 |
| Taxonomic Abundance | 8.8 | 26.2 | 39.6 | 33.5 | 130 | 235.6 | 316 | 406.1 | 417.4 | 0.0004 |
| Shannon Diversity H | 0.5 | 0.5 | 0.4 | 1 | 1 | 0.7 | 2.1 | 1.6 | 0.8 | 0.0011 |
| Sub-group richness | ||||||||||
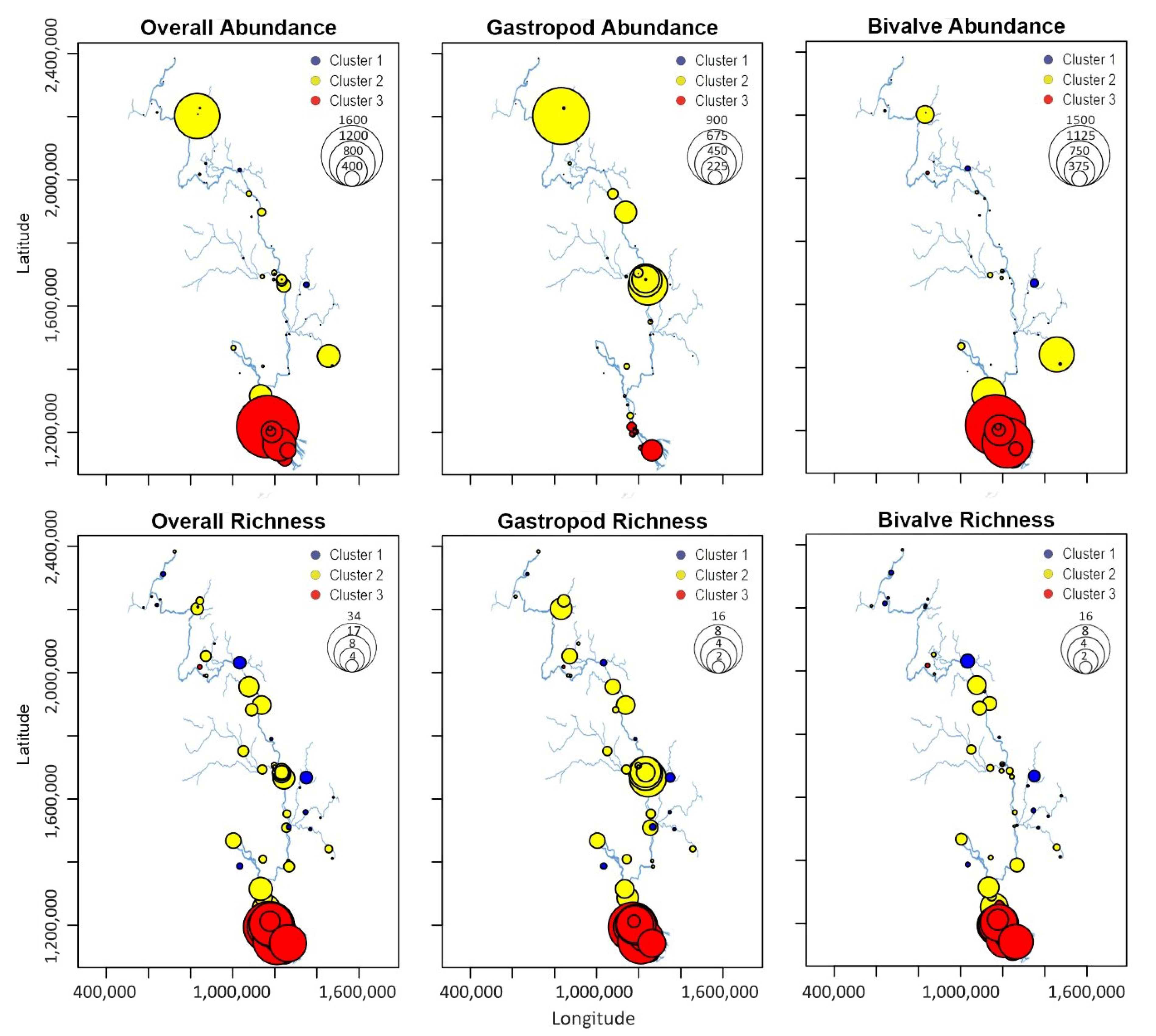
| Gastropods | 1 | 0.9 | 1 | 3 | 3.6 | 3.2 | 8 | 7.8 | 5.3 | 0.0001 |
| Bivalves | 1 | 1.9 | 1.6 | 2 | 2.7 | 2.8 | 11.5 | 10.9 | 5.4 | 0.0000 |
| Sub-group abundance | ||||||||||
| Gastropods | 0.8 | 1.4 | 1.6 | 10 | 69.4 | 164 | 36.8 | 43.9 | 49.3 | 0.0002 |
| Bivalves | 7.5 | 24.8 | 38.7 | 11.5 | 60.6 | 139.3 | 238.5 | 362.2 | 403.7 | 0.0002 |
| FFG richness | ||||||||||
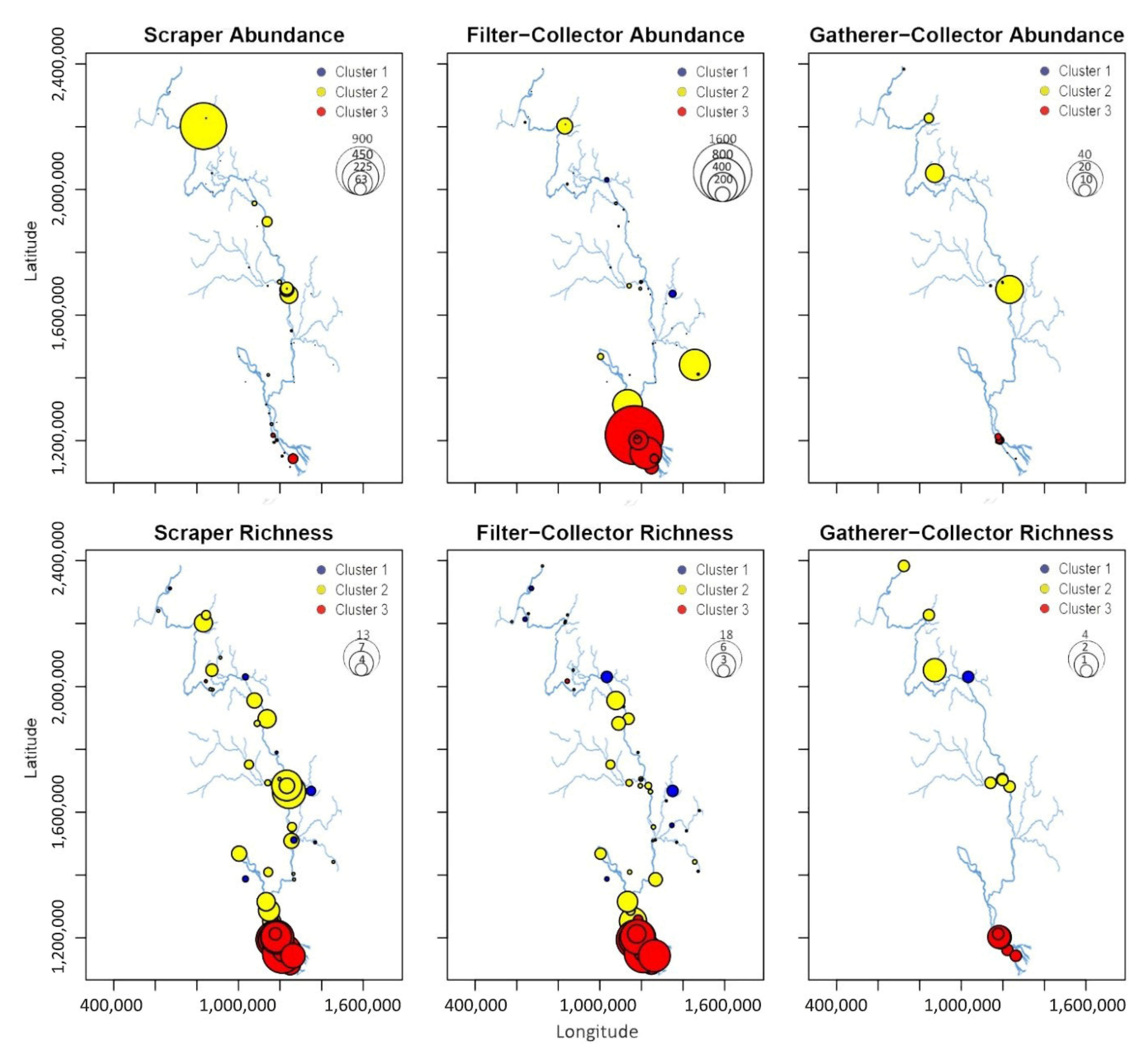
| SC | 0.5 | 0.9 | 1 | 2 | 3.2 | 3 | 7.5 | 6.7 | 4.3 | 0.0002 |
| FC | 1 | 1.9 | 1.4 | 2 | 2.6 | 2.8 | 11.5 | 10.6 | 5.3 | 0.0000 |
| GC | 0 | 0.1 | 0.3 | 0 | 0.2 | 0.5 | 0.5 | 0.6 | 0.7 | 0.0179 |
| FFG abundance | ||||||||||
| SC | 0.3 | 1.2 | 1.5 | 9 | 67.5 | 162 | 26.5 | 40.6 | 49.4 | 0.0004 |
| FC | 7.5 | 24.8 | 38.7 | 10.5 | 60.2 | 139.1 | 238.5 | 361.3 | 404.2 | 0.0002 |
| GC | 0 | 0.1 | 0.1 | 0 | 1.7 | 5.4 | 0.3 | 1.7 | 2.7 | 0.0274 |
| FFG diversity H | ||||||||||
| SC | 0 | 0.2 | 0.4 | 0.5 | 0.5 | 0.6 | 1.3 | 1.2 | 0.8 | 0.0009 |
| FC | 0 | 0.2 | 0.3 | 0.03 | 0.4 | 0.6 | 1.7 | 1.3 | 0.7 | 0.0002 |
| GC | 0 | 0 | 0 | 0 | 0 | 0.1 | 0 | 0.1 | 0.1 | 0.1685 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sor, R.; Ngor, P.B.; Boets, P.; Goethals, P.L.M.; Lek, S.; Hogan, Z.S.; Park, Y.-S. Patterns of Mekong Mollusc Biodiversity: Identification of Emerging Threats and Importance to Management and Livelihoods in a Region of Globally Significant Biodiversity and Endemism. Water 2020, 12, 2619. https://doi.org/10.3390/w12092619
Sor R, Ngor PB, Boets P, Goethals PLM, Lek S, Hogan ZS, Park Y-S. Patterns of Mekong Mollusc Biodiversity: Identification of Emerging Threats and Importance to Management and Livelihoods in a Region of Globally Significant Biodiversity and Endemism. Water. 2020; 12(9):2619. https://doi.org/10.3390/w12092619
Chicago/Turabian StyleSor, Ratha, Peng Bun Ngor, Pieter Boets, Peter L. M. Goethals, Sovan Lek, Zeb S. Hogan, and Young-Seuk Park. 2020. "Patterns of Mekong Mollusc Biodiversity: Identification of Emerging Threats and Importance to Management and Livelihoods in a Region of Globally Significant Biodiversity and Endemism" Water 12, no. 9: 2619. https://doi.org/10.3390/w12092619
APA StyleSor, R., Ngor, P. B., Boets, P., Goethals, P. L. M., Lek, S., Hogan, Z. S., & Park, Y.-S. (2020). Patterns of Mekong Mollusc Biodiversity: Identification of Emerging Threats and Importance to Management and Livelihoods in a Region of Globally Significant Biodiversity and Endemism. Water, 12(9), 2619. https://doi.org/10.3390/w12092619








