Cambodian Freshwater Fish Assemblage Structure and Distribution Patterns: Using a Large-Scale Monitoring Network to Understand the Dynamics and Management Implications of Species Clusters in a Global Biodiversity Hotspot
Abstract
1. Introduction
2. Materials and Methods
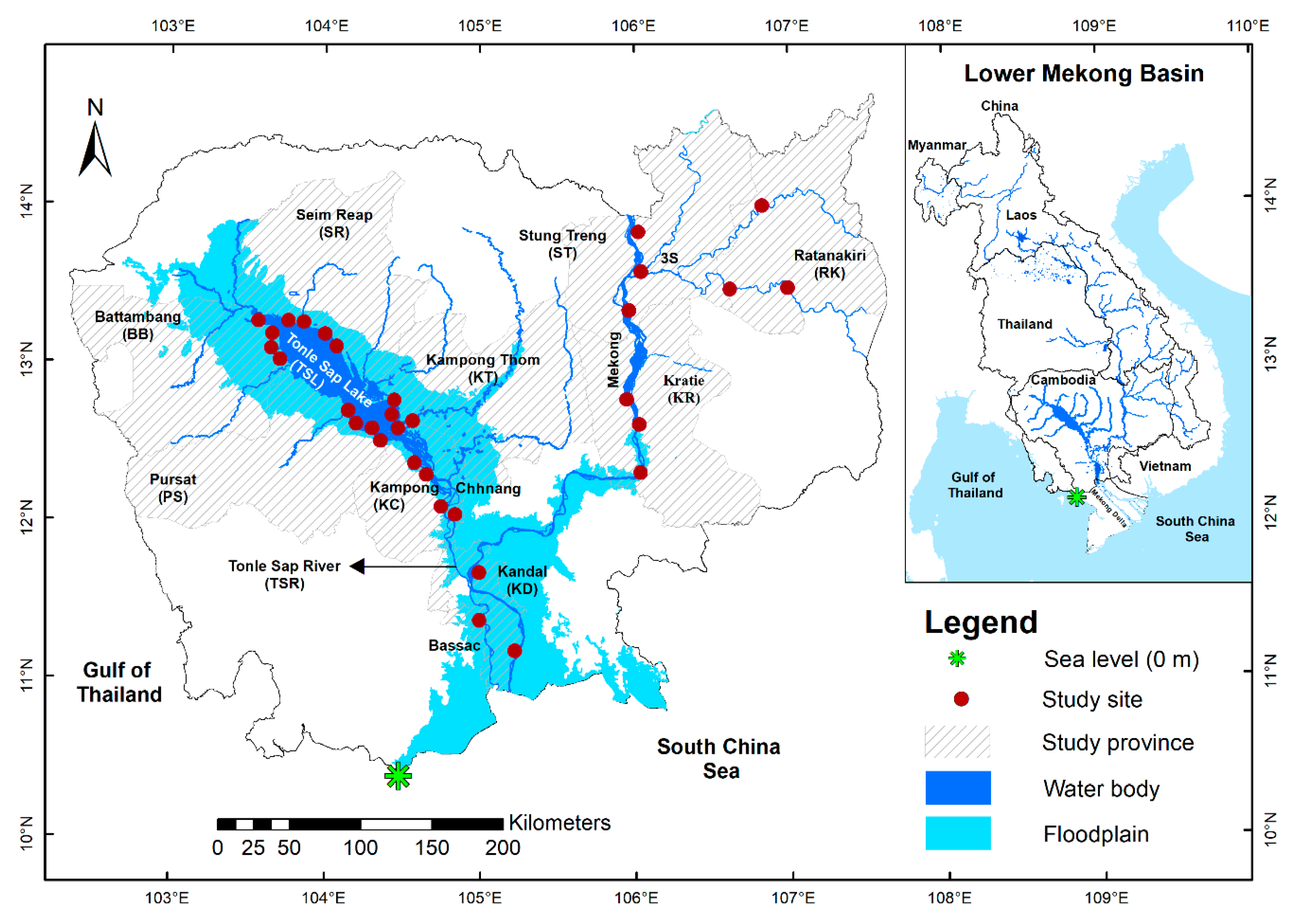
2.1. Study Site
2.2. Data Collection
2.3. Data Analyses
3. Results
3.1. Fish Community Composition
3.2. Spatial and Seasonal Variation of Cambodian Inland Fish Assemblages
3.3. Species Richness, Evenness and Diversity
3.4. Indicator Species by Cluster
3.5. Length–Weight Relationship of Indicator Species
4. Discussion
4.1. Spatial and Seasonal Variation of Fish Assemblages
4.2. Species Diversity
4.3. Fish Growth Conditions
4.4. Management Implications
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Winemiller, K.O.; McIntyre, P.B.; Castello, L.; Fluet-Chouinard, E.; Giarrizzo, T.; Nam, S.; Baird, I.G.; Darwall, W.; Lujan, N.K.; Harrison, I.; et al. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science 2016, 351, 128–129. [Google Scholar] [CrossRef] [PubMed]
- MRC. Overview of the Hydrology of the Mekong Basin; Mekong River Commission: Vientiane, Laos, 2005; ISSN 1728 3248. [Google Scholar]
- Adamson, P.T.; Rutherfurd, I.D.; Peel, M.C.; Conlan, I.A. The Hydrology of the Mekong River. In The Mekong Biophysical Environment of an International River Basin; Campbell, I.C., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2009; pp. 53–76. [Google Scholar]
- Mittermeier, R.A.; Turner, W.R.; Larsen, F.W.; Brooks, T.M.; Gascon, C. Global biodiversity conservation: The critical role of hotspots. In Biodiversity Hotspots, Proceedings of the Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas, Luxembourg, 26–28 March 2008; Zachos, F., Habel, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–22. [Google Scholar]
- Brooks, S.E.; Reynolds, J.D.; Allison, E.A.; Touch, B. The exploitation of Homalopsid water snakes at Tonlé Sap Lake, Cambodia. In Homalopsine Snakes: Evolutionary Experiments in Terrestrial-Aquatic Transitions; Murphy, J.C., Ed.; Krieger Publishing: Melbourne, FL, USA, 2007; pp. 31–37. [Google Scholar]
- Ngor, P.B.; Sor, R.; Prak, L.H.; So, N.; Hogan, Z.S.; Lek, S. Mollusc fisheries and length-weight relationship in Tonle Sap flood pulse system, Cambodia. Ann. Limnol. Int. J. Lim. 2018, 54, 34. [Google Scholar] [CrossRef]
- Rainboth, W.J. Fishes of the Cambodian Mekong; Food and Agriculture Organisation: Rome, Italy, 1996. [Google Scholar]
- Campbell, I.C.; Poole, C.; Giesen, W.; Valbo-Jorgensen, J. Species diversity and ecology of Tonle Sap Great Lake, Cambodia. Aquat. Sci. 2006, 68, 355–373. [Google Scholar] [CrossRef]
- Dudgeon, D. The contribution of scientific information to the conservation and management of freshwater biodiversity in tropical Asia. Hydrobiologia 2003, 500, 295–314. [Google Scholar] [CrossRef]
- Baran, E.; So, N.; Degen, P.; Chen, X.Y.; Starr, P. Updated information on fish and fisheries in the Mekong Basin. Catch Cult. 2013, 19, 24–25. [Google Scholar]
- Ngor, P.B. Fish Assemblages Dynamic in the Tropical Flood-Pulse System of the Lower Mekong River Basin. Ph.D. Thesis, University of Toulouse 3 Paul Sabatier, Toulouse, France, 2018. [Google Scholar]
- So, N.; Utsugi, K.; Shibukawa, K.; Thach, P.; Chhuoy, S.; Kim, S.; Chin, D.; Nen, P.; Chheng, P. Fishes of the Cambodian Freshwater Bodies; Inland Fisheries Research and Development Institute, Fisheries Administration: Phnom Penh, Cambodia, 2018. [Google Scholar]
- Chea, R.; Lek, S.; Ngor, P.; Grenouillet, G. Large-scale patterns of fish diversity and assemblage structure in the longest tropical river in Asia. Ecol. Freshw. Fish 2016, 26, 575–585. [Google Scholar] [CrossRef]
- Van Zalinge, N.; Nao, T.; Touch, S.T. Where there is water, there is fish? Fisheries issues in the Lower Mekong Basin from a Cambodian perspective. In Proceedings of the Seventh Common Property Conference of the International Association for the Study of Common Property, Vancouver, BC, Canada, 10–14 June 1998. [Google Scholar]
- Valbo-Jørgensen, J.; Poulsen, A.F. Using Local Knowledge as a Research Tool in the Study of River Fish Biology: Experiences from the Mekong. Environ. Dev. Sustain. 2000, 2, 253–376. [Google Scholar] [CrossRef]
- Sabo, J.L.; Ruhi, A.; Holtgrieve, G.W.; Elliott, V.; Arias, M.E.; Ngor, P.B.; Räsänen, T.A.; Nam, S. Designing river flows to improve food security futures in the Lower Mekong Basin. Science 2017, 358, eaao1053. [Google Scholar] [CrossRef]
- Ngor, P.B.; Oberdorff, T.; Phen, C.; Baehr, C.; Grenouillet, G.; Lek, S. Fish assemblage responses to flow seasonality and predictability in a tropical flood pulse system. Ecosphere 2018, 9, e02366. [Google Scholar] [CrossRef]
- Ngor, P.B.; Grenouillet, G.; Phem, S.; So, N.; Lek, S. Spatial and temporal variation in fish community structure and diversity in the largest tropical flood-pulse system of South-East Asia. Ecol. Freshw. Fish 2018, 2018, 1–14. [Google Scholar] [CrossRef]
- Van Zalinge, N.; Degen, P.; Pongsri, C.; Nuov, S.; Jensen, J.G.; Nguyen, V.H.; Choulamany, X. The Mekong River System. In Proceedings of the Second International Symposium on the Management of Large Rivers for Fisheries, Phnom Penh, Cambodia, 11–14 February 2003. [Google Scholar]
- MRC. State of the Basin Report 2010; Mekong River Commission: Vientiane, Laos, 2010. [Google Scholar]
- Valbo-Jørgensen, J.; Coates, D.; Hortle, K. Fish Diversity in the Mekong River Basin. In The Mekong; Campbell, I.C., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 161–196. ISBN 9780123740267. [Google Scholar]
- Welcomme, R.L. Inland Fisheries: Ecology and Management; Food and Agriculture Organization: Rome, Italy; Blackwell Science: Oxford, UK, 2001. [Google Scholar]
- ICEM. Strategic Environmental Assessment (SEA) of Hydropower on the Mekong Mainstream; Mekong River Commission: Hanoi, Vietnam, 2010; ISBN 9780642754301. [Google Scholar]
- Poulsen, A.F.; Ouch, P.; Viravong, S.; Suntornratana, U.; Nguyen, T.T. Fish Migrations of the Lower Mekong River Basin: Implications for Development, Planning and Environmental Management; Mekong River Commission: Phnom Penh, Cambodia, 2002. [Google Scholar]
- IFReDI. Food and Nutrition Security Vulnerability to Mainstream Hydropower Dam Development in Cambodia; Synthesis Report of the FiA/Danida/WWF/Oxfam Project “Food and Nutrition Security Vulnerability to Mainstream Hydropower Dam Development in Cambodia”; Inland Fisheries Research and Development Institude (IFRdDI): Phnom Penh, Cambodia, 2013. [Google Scholar]
- Hortle, K.G. Consumption and the Yield of Fish and Other Aquatic Animals from the Lower Mekong Basin; Mekong River Commission: Vientiane, Laos, 2007. [Google Scholar]
- Hortle, K.G.; Bamrungrach, P. Fisheries Habitat and Yield in the Lower Mekong Basin; Mekong River Commission: Phnom Penh, Cambodia, 2015. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2016; Contributing to Food Security and Nutrition for All; Food and Agriculture Organization: Rome, Italy, 2016; ISBN 9789251091852. [Google Scholar]
- So, N.; Phommakone, S.; Ly, V.; Samphawamana, T.; Nguyen, H.S.; Khumsri, M.; Ngor, P.B.; Kong, S.; Degen, P.; Starr, P. Lower Mekong fisheries estimated to be worth around $17 billion a year. Catch Cult. 2015, 21, 4–7. [Google Scholar]
- Starr, P. Fisheries production in Cambodia. Catch Cult. 2003, 9, 6. [Google Scholar]
- Lim, P.; Lek, S.; Touch, S.T.; Mao, S.O.; Chhouk, B. Diversity and spatial distribution of freshwater fish in Great Lake and Tonle Sap river (Cambodia, Southeast Asia). Aquat. Living Resour. 1999, 12, 379–386. [Google Scholar] [CrossRef]
- Chan, B.; So, N.; Lek, S.; Ngor, P.B. Spatial and temporal changes in fish yields and fish communities in the largest tropical floodplain lake in Asia. Ann. Limnol. Int. J. Limnol. 2017, 53, 485–493. [Google Scholar] [CrossRef]
- Pool, T.; Elliott, V.; Holtgrieve, G.; Arias, M.; Altman, I.; Kaufman, L.; McCann, K.; Fraser, E.D.G.; Tudesque, L.; Chevalier, M.; et al. Fish assemblage composition within the floodplain habitat mosaic of a tropical lake (Tonle Sap, Cambodia). Freshw. Biol. 2019, 64, 2026–2036. [Google Scholar] [CrossRef]
- Kong, H.; Chevalier, M.; Laffaille, P.; Lek, S. Spatio-temporal variation of fish taxonomic composition in a South-East Asian flood-pulse system. PLoS ONE 2017, 12, e0174582. [Google Scholar] [CrossRef]
- Ngor, P.B.; Legendre, P.; Oberdorff, T.; Lek, S. Flow alterations by dams shaped fish assemblage dynamics in the complex Mekong-3S river system. Ecol. Indic. 2018, 88, 103–114. [Google Scholar] [CrossRef]
- Montaña, C.G.; Ou, C.; Keppeler, F.W.; Winemiller, K.O. Functional and trophic diversity of fishes in the Mekong-3S river system: Comparison of morphological and isotopic patterns. Environ. Biol. Fishes 2020, 103, 185–200. [Google Scholar] [CrossRef]
- Ngor, P.B.; Hortle, G.K.; So, N. Standard Sampling Procedures for Fish Abundance and Diversity Monitoring in the Lower Mekong Basin; Mekong River Commission: Phnom Penh, Cambodia, 2016. [Google Scholar]
- Ngor, P.B.; McCann, K.S.; Grenouillet, G.; So, N.; McMeans, B.C.; Fraser, E.; Lek, S. Evidence of indiscriminate fishing effects in one of the world’s largest inland fisheries. Sci. Rep. 2018, 8, 8947. [Google Scholar]
- Lamberts, D. Tonle Sap Fisheries: A Case Study on Floodplain Gillnet Fisheries; Asia-Pacific Fisheries Commission, Food and Agriculture Organisation, Regional Office for Asia and the Pacific: Bangkok, Thailand, 2001. [Google Scholar]
- Hamid, M.A.; Mansor, M.; Nor, S.A.M. Length-weight Relationship and Condition Factor of Fish Populations in Temengor Reservoir: Indication of Environmental Health. Sains Malays. 2015, 44, 61–66. [Google Scholar] [CrossRef]
- Chan, B.; Brosse, S.; Hogan, Z.S.; Ngor, P.B.; Lek, S. Influence of Local Habitat and Climatic Factors on the Distribution of Fish Species in the Tonle Sap Lake. Water 2020, 12, 786. [Google Scholar] [CrossRef]
- Castello, L.; Hess, L.L.; Thapa, R.; McGrath, D.G.; Arantes, C.C.; Renó, V.; Isaac, V.J. Fishery yields vary with land cover on the Amazon River floodplain. Fish Fish. 2017, 19, 431–440. [Google Scholar] [CrossRef]
- Hilborn, R.; Waiters, C. Managing Fisheries; Chapman and Hall: London, UK, 1992; ISBN 0412022710. [Google Scholar]
- Das, S.K.; De, M.; Ghaffar, M.A. Length-weight relationship and trophic level of hard-tail scad Megalaspis cordyla. Sci. Asia 2014, 40, 317–322. [Google Scholar]
- Olson, K.; Morton, L.W. Tonle Sap Lake and River and confluence with the Mekong River in Cambodia. J. Soil Water Conserv. 2018, 73, 60A–66A. [Google Scholar] [CrossRef]
- MacQuarrie, P.R.; Welling, R.; Rammont, L.; Pangare, G. The 3S River Basin; IUCN: Gland, Switzerland, 2013. [Google Scholar]
- Sarkkula, J.; Kiirikki, M.; Koponen, J.; Kummu, M. Ecosystem Processes of the Tonle Sap Lake. In Proceedings of the 1st Workshop of Ecotone Phase II, Phnom Penh, Cambodia, 26 October–1 November 2003. [Google Scholar]
- Lu, X.; Kummu, M.; Oeurng, C. Reappraisal of sediment dynamics in the Lower Mekong River, Cambodia. Earth Surf. Process. Landforms 2014, 39, 1855–1865. [Google Scholar] [CrossRef]
- Boon, L.; Elliott, V.; Phauk, S.; Pheng, S.; Souter, N.; Payooha, K.; Jutagate, T.; Duong, V. Developing a Methodology for Standardized Fish Monitoring in the Mekong Basin; Inland Fisheries Research and Development Institute: Phnom Penh, Cambodia, 2016; ISBN 9789924904670. [Google Scholar]
- McCann, K.S.; Gellner, G.; McMeans, B.C.; Deenik, T.; Holtgrieve, G.; Rooney, N.; Hannah, L.; Cooperman, M.; Nam, S. Food webs and the sustainability of indiscriminate fisheries. Can. J. Fish. Aquat. Sci. 2016, 73, 656–665. [Google Scholar] [CrossRef]
- Bagnall, A.; Janacek, G. A Run Length Transformation for Discriminating Between Auto Regressive Time Series. J. Classif. 2014, 31, 154–178. [Google Scholar] [CrossRef][Green Version]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Science Ltd.: Malden, MA, USA, 2004. [Google Scholar]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R; Springer: Science + Business Media: New York, NY, USA, 2011; ISBN 978-1-4419-7975-9. [Google Scholar]
- De Cáceres, M.; Legendre, P.; Moretti, M. Improving indicator species analysis by combining groups of sites. Oikos 2010, 119, 1674–1684. [Google Scholar] [CrossRef]
- Dufrence, M.; Legendre, P. Species assemblage and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar]
- De Cáceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- De Cáceres, M.; Jansen, F. Indicspecies: Functions to Assess the Strength and Significance of Relationship of Species Site Group Associations. R Package Version 1.6.0. Available online: http://cran.r-project.org/web/packages/indicspecies (accessed on 23 January 2020).
- Ricker, W.E. Linear Regressions in Fishery Research. J. Fish. Res. Board Can. 1973, 30, 409–434. [Google Scholar] [CrossRef]
- Froese, R. Cube law, condition factor and weight-length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Jisr, N.; Younes, G.; Sukhn, C.; El-Dakdouki, M.H. Length-weight relationships and relative condition factor of fish inhabiting the marine area of the Eastern Mediterranean city, Tripoli-Lebanon. Egypt. J. Aquat. Res. 2018, 44, 299–305. [Google Scholar] [CrossRef]
- Un, B.; Pech, S.; Baran, E. Aquatic Agricultural Systems in Cambodia: National Situation Analysis; Program Report: AAS-2015-13; CGIAR Research Program on Aquatic Agricultural Systems: Penang, Malaysia, 2015. [Google Scholar]
- Joffre, O.; Kura, Y.; Pant, J.; Nam, S.; For, A. Aquaculture for the Poor in Cambodia—Lessons Learned; WorldFish Center: Phnom Penh, Cambodia, 2010. [Google Scholar]
- Froese, R.; Pauly, D. FishBase. World Wide Web Electronic Publication. Available online: https://www.fishbase.se/ (accessed on 1 December 2019).
- MRCS. Fisheries in the Lower Mekong Basin, Main Report and Annex; Review of the Fishery Sector in the Lower Mekong Basin; Interim Committee for the Coordination of Investigations of the Lower Mekong Basin, Mekong River Commission Secretariat: Bangkok, Thailand, 1992. [Google Scholar]
- Enomoto, K.; Ishikawa, S.; Hori, M.; Sitha, H.; Song, S.L.; Thuok, N.; Kurokura, H. Data mining and stock assessment of fisheries resources in Tonle Sap Lake, Cambodia. Fish. Sci. 2011, 77, 713–722. [Google Scholar] [CrossRef]
- Aloo, P. Biological diversity of the Yala Swamp lakes, with special emphasis on fish species composition, in relation to changes in the Lake Victoria Basin (Kenya): Threats and conservation measures. Biodivers. Conserv. 2003, 12, 905–920. [Google Scholar] [CrossRef]
- Păpuc, T.; Ladoși, D.; Boaru, A. The black bullhead (Ameiurus melas, Rafinesque 1820)—A new invasive fish species in Someș river, Romania. ELBA Bioflux 2018, 10, 18–24. [Google Scholar]
- Poulsen, A.F.; Hortle, K.G.; Valbo-Jorgensen, J.; Chan, S.; Chhuon, C.K.; Viravong, S.; Bouakhamvongsa, K.; Suntornratana, U.; Yoorong, N.; Nguyen, T.T.; et al. Distribution and Ecology of Some Important Riverine Fish Species of the Mekong River Basin; Mekong River Commission: Phnom Penh, Cambodia, 2004. [Google Scholar]
- Srun, P.; Ngor, P.B. The dry season migration pattern of five Mekong fish species: Riel (Henicorhynchus spp.), Chhkok (Cyclocheilichthys enoplos), Pruol (Cirrhinus microlepis), Pra (Pangasianodon hypophthalmus) and Trasork (Probarbus jullieni). In Proceedings of the Annual Meeting of the Department of Fisheries, Ministry of Agriculture, Forestry and Fisheries, Phnom Penh, Cambodia, 27–28 January 2000. [Google Scholar]
- Heng, K.; Ngor, P.B.; Deap, L. The Dry Season Migration Pattern of Five Mekong Fish Species: Trey Chhpin (Barbodes gonionotus), Trey Kaek (Morulius chrysophekadion), Trey Sloeuk Russey (Paralaubuca typus), Trey Klang Hay (Belodontichthys dinema) and Trey Po (Pangasius larnaudiei); Cambodia Fisheries Technical Paper Series; Inland Fisheries Research and Development Institute of Cambodia: Phnom Penh, Cambodia, 2001; Volume III, pp. 63–87. [Google Scholar]
- Halls, A.S.; Paxton, B.; Hall, N.; Hortle, K.; So, N.; Chea, T.; Chheng, P.; Putrea, S.; Lieng, S.; Peng Bun, N.; et al. Integrated Analysis of Data from MRC Fisheries Monitoring Programmes in the Lower Mekong Basin; Mekong River Commission: Phnom Penh, Cambodia, 2013. [Google Scholar]
- Ngor, P.B. Dai fisheries in the Tonle Sap River of Phnom Penh and Kandal province (including a Review of the Census Data of 1996–97). In Management aspects of Cambodia’s Freshwater Capture Fisheries and Management Implications, Proceedings of the Eleven Presentation Given at the Annual Meeting of the Department of Fisheries of the Ministry of Agriculture, Forestry and Fisheries, Phnom, Penh, Cambodia, 27–28 January 2000; van Zalinge, N.P., Nao, T., Lieng, S., Eds.; Mekong River Commission and Department of Fisheries: Phnom Penh, Cambodia, 2000; pp. 30–47. [Google Scholar]
- Halls, A.S.; Paxton, B.R.; Hall, N.; Ngor, P.B.; Lieng, S.; Ngor, P.; So, N. The Stationary Trawl (Dai) Fishery of the Tonle Sap-Great Lake System, Cambodia; Mekong River Commission: Phnom Penh, Cambodia, 2013; ISSN 1683-1489. [Google Scholar]
- Máiz-tomé, L. Freshwater Key Biodiversity Areas in the Lower Mekong River Basin; International Union for Conservation of Nature: Gland, Switzerland, 2019. [Google Scholar]
- IUCN International Union for Conservation of Nature and Natural Resources. Available online: https://www.iucnredlist.org/ (accessed on 6 June 2020).
- Baran, E.; Samadee, S.; Jiau, T.S.; Tran, T.C. Fish and Fisheries in the Sesan, Sekong and Srepok River Basins (Mekong Watershed); ICEM—International Centre for Environmental Management: Hanoi, Vietnam, 2014. [Google Scholar]
- The Ramsar Convention Secretariat Ramsar Sites Information Services 2014. Available online: http://www.ramsar.org/wetland/cambodia (accessed on 15 August 2019).
- Chan, S.; Putrea, S.; Sean, K.; Hortle, K.G. Using local knowledge to inventory deep pools, important fish habitats in Cambodia. In Proceedings of the 6th Technical Symposium on Mekong Fisheries, Pakse, Laos, 26–28 November 2003. [Google Scholar]
- MRC. Planning Atlas of the Lower Mekong River Basin; Mekong River Commission: Phnom Penh, Cambodia, 2011. [Google Scholar]
- Halls, A.S.; Conlan, I.; Wisesjindawat, W.; Phouthavongs, K.; Viravong, S.; Chan, S.; Vu, V.A. Atlas of Deep Pools in the Lower Mekong River and Some of its Tributaries; Mekong River Commission: Phnom Penh, Cambodia, 2013; ISSN 1683-1489. [Google Scholar]
- Chea, V. Fisheries activities in Stung Treng Province, Cambodia. In Proceedings of the Present Status of Cambodia’s Fisheries and Management Implications, Phnom Penh, Cambodia; van Zalinge, N., Nao, T., Eds.; Mekong River Commission and Department of Fisheries: Phnom Penh, Cambodia, 1999; pp. 54–66. [Google Scholar]
- Vu, V.A.; Nguyen, N.D.; Hidas, E.; Nguyen, M.N. Vam Nao deep pools: A critical habitat for Pangasius krempfi and other valuable species in the Mekong Delta, Vietnam. Asian Fish. Sci. 2009, 22, 631–639. [Google Scholar]
- Phan, C.; Hang, S.; Tan, S.B.; Lor, K. Population Monitoring of the Critically Endangered Mekong Dolphin Based on Rark-Resight Models; Cambodia Technical Report; World Wildlife Fund: Phnom Penh, Cambodia, 2015. [Google Scholar]
- Than, C. General Population Census of the Kingdom of Cambodia 2019. Natl. Inst. Stat. Minist. Plan. 2019, 53, 1–50. [Google Scholar]
- MoE. Cambodia Forest Cover 2016; The Ministry of Environment: Phnom Penh, Cambodia, 2018; pp. 1–22. [Google Scholar]
- Daly, K.; Ahmad, S.K.; Bonnema, M.; Beveridge, C.; Hossain, F.; Nijssen, B.; Holtgrieve, G. Recent warming of Tonle Sap Lake, Cambodia: Implications for one of the world’s most productive inland fisheries. Lakes Reserv. Res. Manag. 2020, 25, 133–142. [Google Scholar] [CrossRef]
- Vu, V.A.; Doan, V.T.; Ngor, P.B.; Nguyen, H.S.; Nam, S. Exotic species in southern VietNam. Catch Cult. 2013, 19, 18–23. [Google Scholar]
- Ng, T.H.; Jeratthitikul, E.; Sutcharit, C.; Chhuoy, S.; Pin, K.; Pholyotha, A.; Siriwut, W.; Srisonchai, R.; Hogan, Z.S.; Ngor, P.B. Annotated checklist of freshwater molluscs from the largest freshwater lake in Southeast Asia. ZooKeys 2020, 958, 107–141. [Google Scholar] [CrossRef] [PubMed]
- Ngor, P.B.; Chhuon, K.; Prak, L.H. Cambodia launches pilot study to assess Tonle Sap mollusc fishery. Catch Cult. 2014, 20, 8–13. [Google Scholar]
- Poulsen, A.F.; Valbo-Jørgensen, J. Fish Migrations and Spawning Habits in the Mekong Mainstream—A Survey Using Local Knowledge; AMFC Technical Report; Mekong River Commission: Phnom Penh, Cambodia, 2000; pp. 1–149. [Google Scholar]
- Baran, E. Fish Migration Triggers in the Lower Mekong Basin and Other Tropical Freshwater Systems; Burnhill, T., Ed.; Mekong River Commission: Vientiane, Laos, 2006; ISSN 1683-1489. [Google Scholar]
- Cowx, I.; Kamonrat, W.; Sukumasavin, N.; Sirimongkolthawon, R.; Suksri, S.; Phila, N. Larval and Juvenile Fish Communities of the Lower Mekong Basin; Mekong River Commission: Phnom Penh, Cambodia, 2015. [Google Scholar]
- Matthews, W.J. Patterns in Freshwater Fish Ecology; Chapman & Hall: London, UK, 1998. [Google Scholar]
- Ngor, P.B.; Aun, S.; Deap, L.; Hortle, K.G. Dai Trey Linh Fishery in Prey Veng Province. In Proceedings of the 6th Technical Symposium on Mekong Fisheries, Pakse, Laos, 26–28 November 2003; MRC Conference Series No.5; Burnhill, T.J., Hewitt, M.M., Eds.; Mekong River Commission: Vientiane, Laos, 2005; pp. 35–56. [Google Scholar]
- Shumway, C.; Musibono, D.; Ifuta, S.; Sullivan, J.; Schelly, R.; Punga, J.; Palata, J.C.; Puema, V. Biodiversity Survey: Systematics, Ecology and Conservation along the Congo River; Research Report for Congo River Environment and Development Project (CREDP); New England Aquarium: Boston, MA, USA, 2003; p. 127. [Google Scholar]
- MRC. Fish Migrations of the Lower Mekong River Basin: Implications for Development, Planning and Environmental Management; Mekong River Commission: Vientiane, Laos, 2002; pp. 36–45. [Google Scholar]
- Szuwalski, C.S.; Burgess, M.G.; Costello, C.; Gaines, S.D. High fishery catches through trophic cascades in China. Proc. Natl. Acad. Sci. USA 2017, 114, 717–721. [Google Scholar] [CrossRef]
- Deap, L.; Degen, P.; van Zalinge, N. Fishing Gears of the Cambodian Mekong; Inland Fisheries Research and Development Institute of Cambodia (IFReDI): Phnom Penh, Cambodia, 2003; ISSN 1726-3972. [Google Scholar]
- Ngor, P.B.; Lek, S.; McCann, K.S.; Hogan, Z.S. Dams threaten world’s largest inland fishery. Nature 2018, 563, 184. [Google Scholar] [CrossRef] [PubMed]
- Kc, K.B.; Bond, N.; Fraser, E.D.G.; Elliott, V.; Farrell, T.; McCann, K.; Rooney, N.; Bieg, C. Exploring tropical fisheries through fishers’ perceptions: Fishing down the food web in the Tonlé Sap, Cambodia. Fish. Manag. Ecol. 2017, 24, 452–459. [Google Scholar] [CrossRef]
- Allan, J.D.; Abell, R.; Hogan, Z.; Revenga, C.; Taylor, B.W.; Welcomme, R.L.; Winemiller, K. Overfishing of Inland Waters. Bioscience 2005, 55, 1041–1051. [Google Scholar]
- Cochrane, T.A.; Arias, M.E.; Piman, T. Historical impact of water infrastructure on water levels of the Mekong River and the Tonle Sap system. Hydrol. Earth Syst. Sci. 2014, 18, 4529–4541. [Google Scholar] [CrossRef]
- Lin, Z.; Qi, J. Hydro-dam—A nature-based solution or an ecological problem: The fate of the Tonlé Sap Lake. Environ. Res. 2017, 158, 24–32. [Google Scholar] [CrossRef]
- Arias, M.E.; Cochrane, T.A.; Kummu, M.; Lauri, H.; Holtgrieve, G.W.; Koponen, J.; Piman, T. Impacts of hydropower and climate change on drivers of ecological productivity of Southeast Asia’s most important wetland. Ecol. Model. 2014, 272, 252–263. [Google Scholar] [CrossRef]
- Sassoon, M.; Meta, K. A Wetland Laid to Waste: One Year after Cambodia’s Devastating Forest Fires; The Phnom Penh Post: Phnom Penh, Cambodia, 23 June 2017; pp. 1–8. [Google Scholar]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-Resolution Global Maps of 21st-Century Forest Cover Change. Science 2013, 342, 850–854. [Google Scholar] [CrossRef]
- Arias, M.E.; Holtgrieve, G.W.; Ngor, P.B.; Dang, T.D.; Piman, T. Maintaining perspective of ongoing environmental change in the Mekong floodplains. Curr. Opin. Environ. Sustain. 2019, 37, 1–7. [Google Scholar] [CrossRef]
- Cottingham, A.; Huang, P.; Hipsey, M.R.; Hall, N.G.; Ashworth, E.; Williams, J.; Potter, I.C. Growth, condition, and maturity schedules of an estuarine fish species change in estuaries following increased hypoxia due to climate change. Ecol. Evol. 2018, 8, 7111–7130. [Google Scholar] [CrossRef] [PubMed]
- De Robertis, A.; Williams, K. Weight-Length Relationships in Fisheries Studies: The Standard Allometric Model Should Be Applied with Caution. Trans. Am. Fish. Soc. 2008, 137, 707–719. [Google Scholar] [CrossRef]
- Le Cren, E.D. The Length-Weight Relationship and Seasonal Cycle in Gonad Weight and Condition in the Perch (Perca fluviatilis). J. Anim. Ecol. 1951, 20, 201. [Google Scholar] [CrossRef]
- MAFF. The Strategic Planning Framework for Fisheries: Update for 2015–2024 “Fishing for the Future”; Ministry of Agriculture, Forestry and Fisheries: Phnom Penh, Cambodia, 2015; p. 52. [Google Scholar]




| Cluster | Species | N | Total Length (cm) | Weight (g) | a | b | Adjusted R2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SE | Min | Max | Mean | SE | ||||||
| Cluster 1 | Albulichthys albuloides | 58 | 8.40 | 20.00 | 13.84 | 0.33 | 3.40 | 80.00 | 26.38 | 2.27 | 0.002070 | 3.54 | 0.93 |
| Anabas testudineus | 826 | 3.00 | 25.20 | 11.66 | 0.08 | 0.90 | 200.00 | 33.36 | 0.74 | 0.418952 | 1.71 | 0.32 | |
| Labiobarbus leptocheilus | 1058 | 3.30 | 19.50 | 12.11 | 0.07 | 3.50 | 77.50 | 19.77 | 0.39 | 0.083743 | 2.14 | 0.66 | |
| Mystus multiradiatus | 486 | 2.70 | 17.20 | 10.59 | 0.13 | 2.60 | 33.50 | 12.55 | 0.27 | 2.117000 | 0.72 | 0.27 | |
| Parachela siamensis | 992 | 3.00 | 15.00 | 9.96 | 0.05 | 2.20 | 35.60 | 9.93 | 0.13 | 0.786628 | 1.07 | 0.26 | |
| Parambassis wolffii | 1362 | 2.60 | 25.00 | 8.79 | 0.06 | 1.80 | 165.20 | 12.61 | 0.29 | 0.786628 | 1.20 | 0.30 | |
| Puntius brevis | 296 | 5.70 | 11.20 | 7.47 | 0.08 | 2.40 | 68.00 | 9.31 | 0.74 | 0.010889 | 3.20 | 0.61 | |
| Rasbora aurotaenia | 171 | 6.50 | 13.90 | 10.25 | 0.10 | 2.80 | 23.60 | 10.07 | 0.28 | 0.491644 | 1.27 | 0.21 | |
| Trichopodus microlepis | 683 | 2.30 | 14.00 | 10.25 | 0.08 | 2.00 | 68.00 | 15.10 | 0.38 | 0.548812 | 1.37 | 0.45 | |
| Trichopodus pectoralis | 239 | 2.90 | 24.20 | 12.54 | 0.31 | 2.10 | 208.10 | 42.97 | 2.87 | 0.055023 | 2.48 | 0.85 | |
| Trichopodus trichopterus | 2720 | 2.00 | 14.20 | 8.71 | 0.05 | 0.10 | 81.00 | 15.93 | 0.30 | 0.367879 | 1.62 | 0.53 | |
| Cluster 2 | Amblyrhynchichthys micracanthus | 244 | 8.00 | 23.10 | 11.65 | 0.14 | 4.70 | 134.50 | 18.36 | 1.21 | 0.004844 | 3.28 | 0.87 |
| Boesemania microlepis | 320 | 2.60 | 85.00 | 22.53 | 1.05 | 4.50 | 4000.00 | 452.96 | 52.12 | 0.110803 | 2.22 | 0.69 | |
| Cosmochilus harmandi | 52 | 10.00 | 70.00 | 24.61 | 2.11 | 8.80 | 4500.00 | 436.16 | 128.30 | 0.007227 | 3.13 | 0.99 | |
| Cyclocheilos enoplos | 916 | 4.20 | 65.00 | 15.86 | 0.22 | 3.30 | 2500.00 | 67.04 | 5.96 | 0.013982 | 2.83 | 0.88 | |
| Gymnostomus lobatus | 3673 | 1.00 | 18.90 | 11.31 | 0.03 | 1.40 | 86.70 | 17.43 | 0.18 | 0.088922 | 2.13 | 0.51 | |
| Labeo chrysophekadion | 898 | 1.00 | 68.10 | 14.09 | 0.24 | 2.60 | 4570.00 | 80.47 | 11.61 | 0.010998 | 2.97 | 0.90 | |
| Mystus albolineatus | 111 | 7.00 | 18.50 | 10.52 | 0.15 | 3.60 | 41.00 | 9.48 | 0.51 | 0.009658 | 2.89 | 0.81 | |
| Mystus bocourti | 494 | 7.20 | 26.50 | 12.25 | 0.11 | 4.70 | 113.50 | 18.92 | 0.77 | 0.168638 | 1.80 | 0.27 | |
| Osteochilus vittatus | 511 | 6.50 | 19.80 | 12.97 | 0.13 | 4.10 | 126.10 | 29.00 | 0.95 | 0.008148 | 3.11 | 0.89 | |
| Pangasius elongatus | 32 | 13.10 | 40.10 | 16.95 | 0.95 | 21.30 | 573.00 | 56.22 | 17.91 | 0.010462 | 2.91 | 0.97 | |
| Puntioplites proctozysron | 2997 | 3.20 | 33.40 | 12.79 | 0.07 | 2.20 | 600.00 | 38.00 | 0.85 | 0.047359 | 2.49 | 0.67 | |
| Thynnichthys thynnoides | 369 | 7.30 | 15.60 | 10.51 | 0.06 | 4.30 | 43.80 | 12.73 | 0.25 | 0.025991 | 2.61 | 0.68 | |
| Toxotes chatareus | 89 | 7.50 | 13.50 | 10.05 | 0.13 | 7.60 | 33.00 | 18.12 | 0.62 | 0.085435 | 2.30 | 0.69 | |
| Cluster 3 | Belodontichthys truncatus | 43 | 14.70 | 35.50 | 23.93 | 0.96 | 18.80 | 224.50 | 94.43 | 10.04 | 0.005462 | 3.00 | 0.90 |
| Labiobarbus siamensis | 700 | 7.40 | 24.80 | 11.12 | 0.08 | 2.80 | 153.80 | 15.42 | 0.53 | 0.005407 | 3.24 | 0.93 | |
| Oxygaster pointoni | 176 | 8.40 | 16.00 | 11.24 | 0.07 | 6.10 | 35.30 | 12.50 | 0.28 | 0.018873 | 2.67 | 0.80 | |
| Phalacronotus micronema | 179 | 9.00 | 51.50 | 23.77 | 0.77 | 2.80 | 560.00 | 109.98 | 8.95 | 0.004087 | 3.06 | 0.90 | |
| Cluster 4 | Barbonymus altus | 100 | 7.90 | 22.80 | 14.69 | 0.31 | 6.60 | 231.40 | 60.73 | 4.53 | 0.016408 | 2.98 | 0.72 |
| Chitala ornata | 37 | 10.00 | 72.00 | 34.34 | 2.88 | 21.40 | 2700.00 | 519.44 | 112.24 | 0.184520 | 2.11 | 0.84 | |
| Channa striata | 296 | 9.00 | 46.00 | 20.02 | 0.48 | 6.40 | 1100.00 | 106.97 | 7.74 | 0.039557 | 2.50 | 0.77 | |
| Clarias sp. | 35 | 12.50 | 31.60 | 20.87 | 0.69 | 16.90 | 263.50 | 79.21 | 9.25 | 0.003247 | 3.27 | 0.93 | |
| Hampala macrolepidota | 86 | 8.00 | 42.10 | 17.85 | 1.01 | 4.60 | 930.00 | 131.68 | 20.17 | 0.010673 | 3.03 | 0.94 | |
| Helicophagus leptorhynchus | 46 | 20.00 | 52.40 | 32.57 | 0.97 | 60.00 | 877.00 | 240.52 | 24.27 | 0.115325 | 2.15 | 0.59 | |
| Hemibagrus spilopterus | 445 | 7.80 | 43.00 | 19.92 | 0.31 | 5.10 | 900.00 | 102.48 | 5.76 | 0.016573 | 2.76 | 0.77 | |
| Hemibagrus wyckii | 107 | 16.00 | 75.50 | 33.77 | 1.13 | 25.00 | 3820.00 | 573.45 | 72.62 | 0.127454 | 2.26 | 0.52 | |
| Notopterus notopterus | 188 | 8.60 | 27.50 | 18.01 | 0.31 | 9.40 | 350.00 | 68.10 | 5.04 | 0.004844 | 3.19 | 0.79 | |
| Ompok siluroides | 177 | 3.10 | 35.00 | 13.92 | 0.37 | 3.10 | 260.00 | 26.50 | 2.72 | 0.165299 | 1.79 | 0.64 | |
| Osteochilus schlegelii | 150 | 7.50 | 21.00 | 11.94 | 0.15 | 4.10 | 186.20 | 20.98 | 1.39 | 0.004942 | 3.32 | 0.91 | |
| Oxyeleotris marmorata | 112 | 10.50 | 44.00 | 20.27 | 0.58 | 14.30 | 1800.00 | 162.36 | 23.77 | 0.005976 | 3.26 | 0.94 | |
| Pangasius conchophilus | 44 | 22.00 | 68.00 | 36.95 | 1.47 | 40.00 | 2135.00 | 490.30 | 74.37 | 0.001747 | 3.39 | 0.88 | |
| Pangasius larnaudii | 56 | 12.40 | 56.20 | 34.97 | 1.36 | 19.20 | 2608.00 | 735.28 | 102.20 | 0.001782 | 3.51 | 0.84 | |
| Pristolepis fasciata | 676 | 4.00 | 19.00 | 9.92 | 0.10 | 1.90 | 172.50 | 25.47 | 1.00 | 0.016083 | 3.10 | 0.88 | |
| Scaphognathops bandanensis | 97 | 9.30 | 29.00 | 17.34 | 0.48 | 7.30 | 320.00 | 82.92 | 7.11 | 0.804615 | 3.28 | 0.89 | |
| Systomus rubripinnis | 61 | 10.00 | 16.20 | 13.49 | 0.20 | 14.80 | 75.10 | 35.89 | 2.10 | 0.007083 | 3.25 | 0.79 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pin, K.; Nut, S.; Hogan, Z.S.; Chandra, S.; Saray, S.; Touch, B.; Chheng, P.; Ngor, P.B. Cambodian Freshwater Fish Assemblage Structure and Distribution Patterns: Using a Large-Scale Monitoring Network to Understand the Dynamics and Management Implications of Species Clusters in a Global Biodiversity Hotspot. Water 2020, 12, 2506. https://doi.org/10.3390/w12092506
Pin K, Nut S, Hogan ZS, Chandra S, Saray S, Touch B, Chheng P, Ngor PB. Cambodian Freshwater Fish Assemblage Structure and Distribution Patterns: Using a Large-Scale Monitoring Network to Understand the Dynamics and Management Implications of Species Clusters in a Global Biodiversity Hotspot. Water. 2020; 12(9):2506. https://doi.org/10.3390/w12092506
Chicago/Turabian StylePin, Kakada, Savat Nut, Zeb S. Hogan, Sudeep Chandra, Samadee Saray, Bunthang Touch, Phen Chheng, and Peng Bun Ngor. 2020. "Cambodian Freshwater Fish Assemblage Structure and Distribution Patterns: Using a Large-Scale Monitoring Network to Understand the Dynamics and Management Implications of Species Clusters in a Global Biodiversity Hotspot" Water 12, no. 9: 2506. https://doi.org/10.3390/w12092506
APA StylePin, K., Nut, S., Hogan, Z. S., Chandra, S., Saray, S., Touch, B., Chheng, P., & Ngor, P. B. (2020). Cambodian Freshwater Fish Assemblage Structure and Distribution Patterns: Using a Large-Scale Monitoring Network to Understand the Dynamics and Management Implications of Species Clusters in a Global Biodiversity Hotspot. Water, 12(9), 2506. https://doi.org/10.3390/w12092506







