Assessing the Karst Groundwater Quality and Hydrogeochemical Characteristics of a Prominent Dolomite Aquifer in Guizhou, China
Abstract
1. Introduction
2. Hydrogeochemical Characteristics of Karst Groundwater
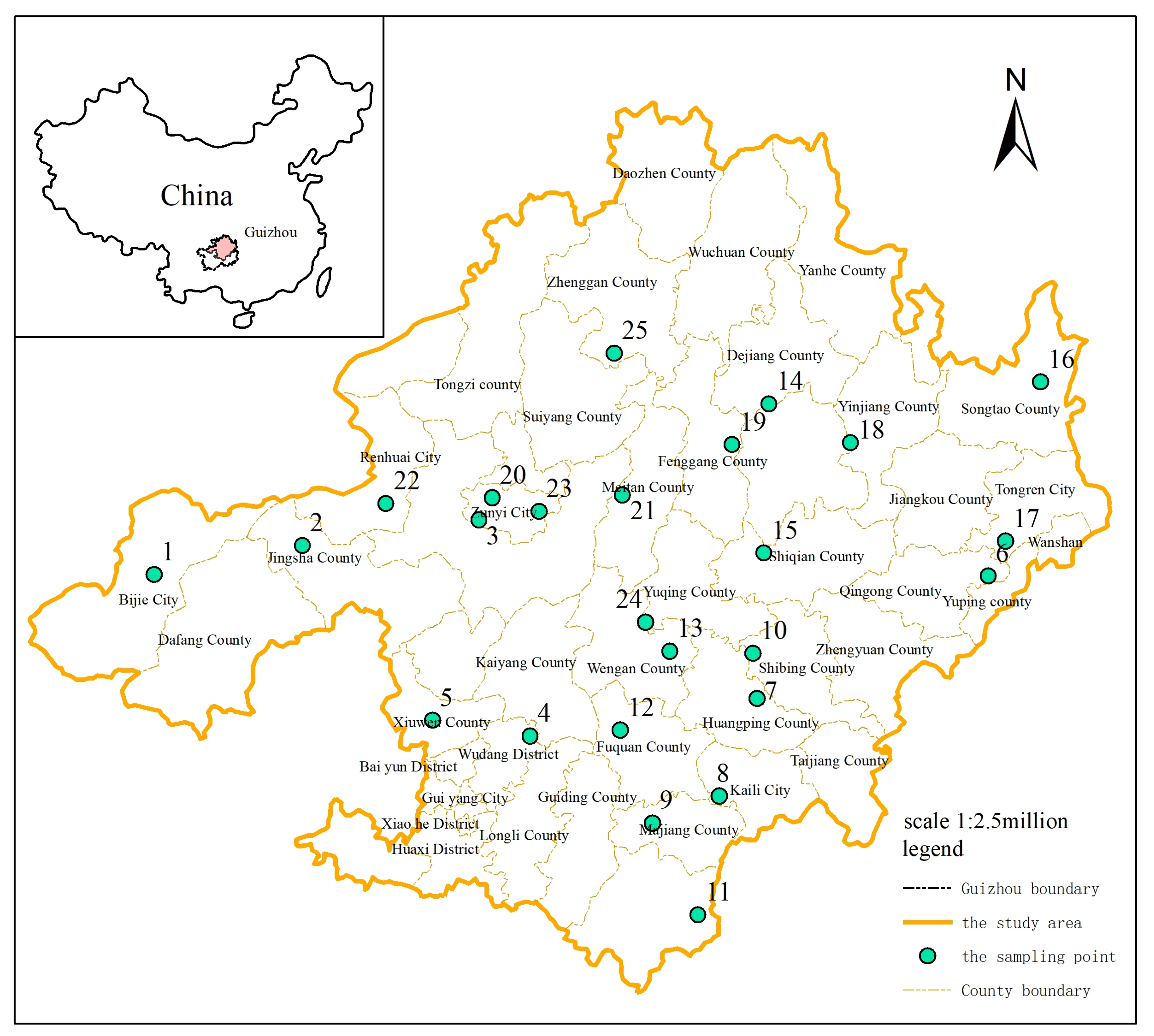
2.1. Groundwater Sampling and Testing in the Study Area
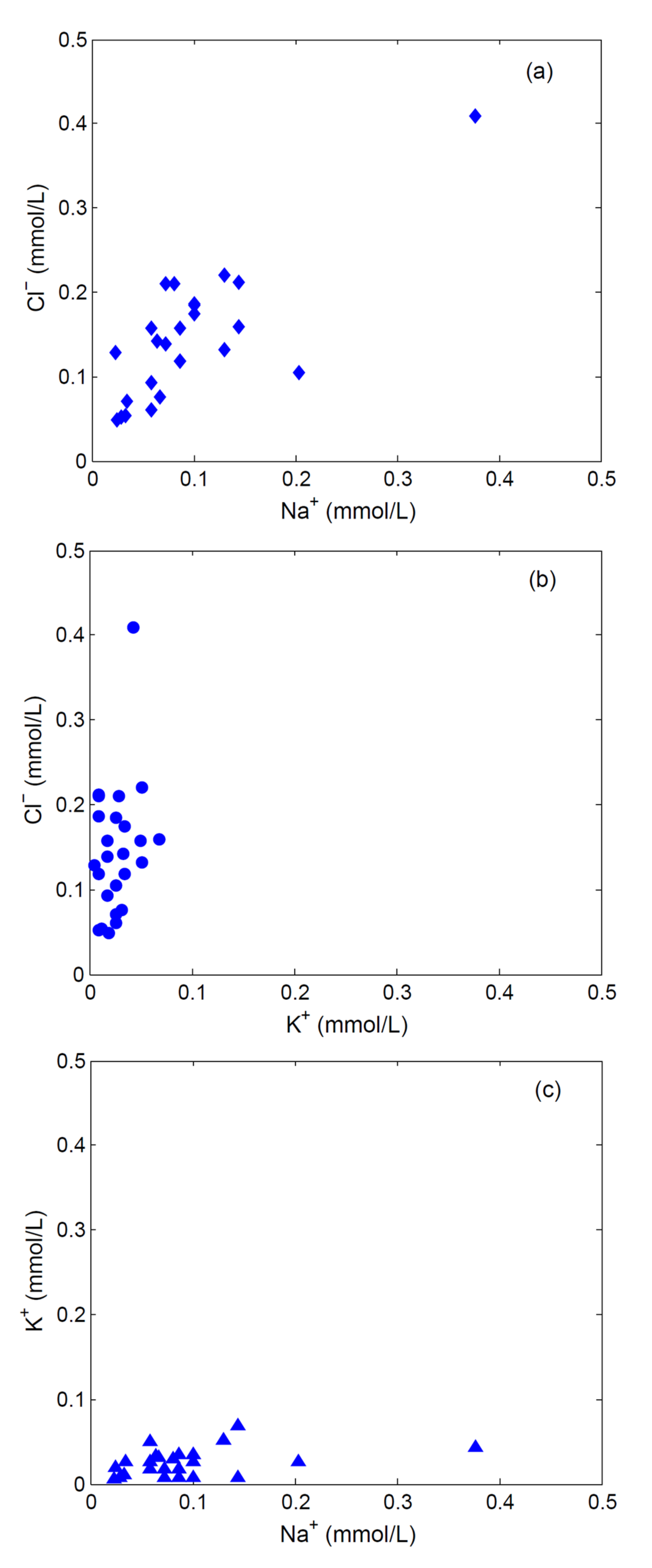
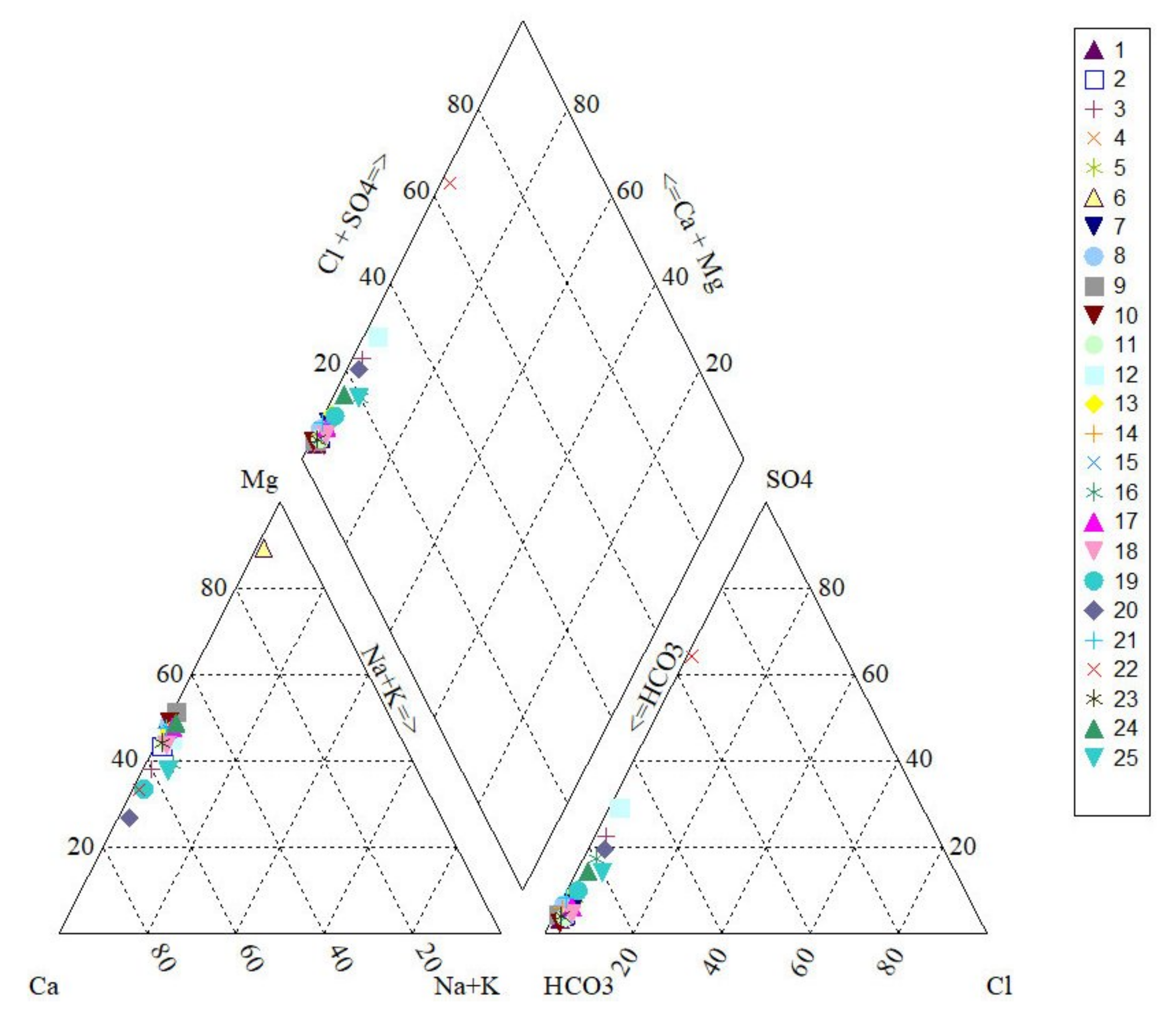
2.2. Results
3. Assessment of Groundwater Quality with Fuzzy Comprehensive Evaluation Method
3.1. Evaluation Method
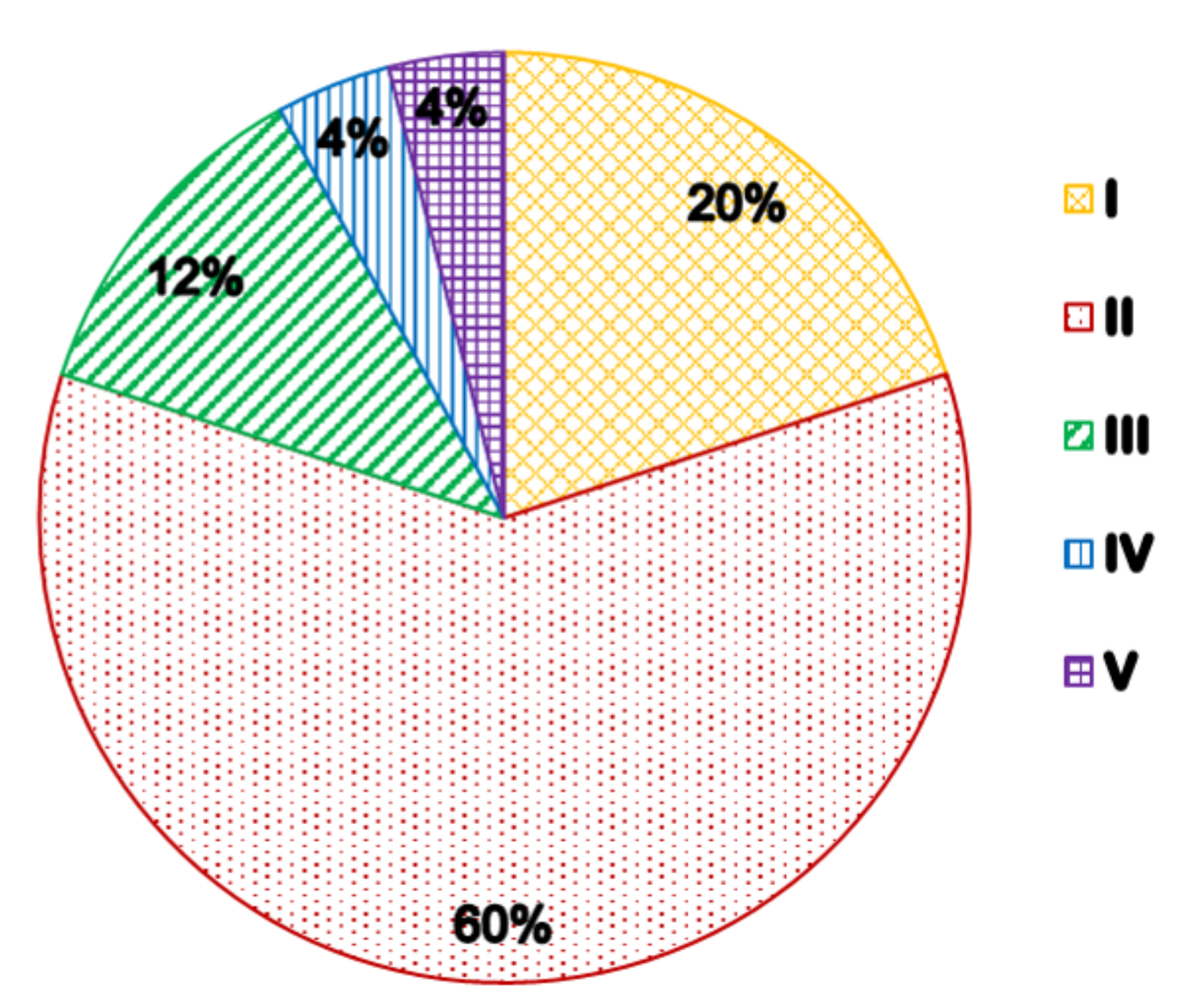
3.2. Evaluation Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kraiem, Z.; Zouari, K.; Chkir, N.; Agoune, A. Geochemical characteristics of arid shallow aquifers in Chott Djerid, south-western Tunisia. J. Hydro-Environ. Res. 2014, 8, 460–473. [Google Scholar] [CrossRef]
- de Fraiture, C.; Wichelns, D. Satisfying future water demands for agriculture. Agric. Water. Manag. 2008, 97, 502–511. [Google Scholar] [CrossRef]
- Zhu, W.X.; Li, P.; He, W.; Zhang, Y.; Li, H.Z. The key scientific and technological issues to engineering water shortage solution in Karst mountainous areas in Guizhou. J. Guizhou Sci. 2006, 24, 1–7. (In Chinese) [Google Scholar]
- Ford, D.; Williams, P. Karst Hydrogeology and Geomorphology; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- White, W.B. Geomorphology and Hydrology of Karst Terrains; Oxford University Press: Oxford, UK, 1988. [Google Scholar]
- White, W.B. Karst hydrology: Recent development and open questions. Eng. Geol. 2002, 65, 85–105. [Google Scholar] [CrossRef]
- Roy, S.; Gillardet, J.; Allegre, C.J. Geochemistry of dissolved and suspended loads of the Seine river, France: Anthropogenic impact, carbonate and silicate weathering. Geochim. Cosmochim. Acta 1999, 63, 77–1292. [Google Scholar] [CrossRef]
- Han, G.; Liu, C.Q. Water geochemistry controlled by carbonate dissolution: A study of the river waters Draining karst-dominated terrain, Guizhou, China. Chem. Geol. 2004, 204, l–21. [Google Scholar] [CrossRef]
- Yuan, D. Sensitivity of karst process to environmental change along the PEP II transect. Quat. Int. 1997, 35, 105–113. [Google Scholar] [CrossRef]
- Kacarog1u, F. Review of groundwater pollution and protection in karst areas. Water Air Soil Pollut. 1999, 113, 337–346. [Google Scholar] [CrossRef]
- Green, R.T.; Painter, S.L.; Sun, A.; Worthington, S.R.H. Groundwater contamination in karst terranes. Water Air Soil Pollut. Focus 2006, 6, 157–170. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, Y.; Groves, C.; Yuan, D.; Kambesis, P. Natural and anthropogenic factors affecting the groundwater quality in the Nandong karst underground river system in Yunan, China. J. Contam. Hydrol. 2009, 109, 49–61. [Google Scholar] [CrossRef]
- Zhao, M.; Zeng, C.; Liu, Z.; Wang, S. Effect of different land use/land cover on karst hydrogeochemistry: A paired catchment study of Chenqiand Dengzhanhe, Puding, Guizhou, SW China. J. Hydrol. 2010, 388, 121–130. [Google Scholar] [CrossRef]
- Mahler, B.J.; Personne, J.C.; Lods, G.F.; Drogue, C. Transport of free and particulate-associated bacteria in karst. J. Hydrol. 2008, 238, 179–193. [Google Scholar] [CrossRef]
- Berger, P. Viruses in Ground Water. In Dangerous Pollutants (Xenobiotics) in Urban Water Cycle. NATO Science for Peace and Security Series C: Environmental Security; Hlavinek, P., Bonacci, O., Marsalek, J., Mahrikova, I., Eds.; Springer: Heidelberg, Germany, 2008; pp. 131–149. [Google Scholar]
- Johnson, T.B.; McKay, L.D.; Layton, A.C.; Jones, S.W.; Johnson, G.C.; Cashdollar, J.L.; Dahling, D.R.; Villegas, L.F.; Fout, G.S.; Williams, D.E.; et al. Viruses and bacteria in karst and fractured rock aquifers in East Tennessee, USA. Ground Water 2011, 49, 98–110. [Google Scholar] [CrossRef]
- Rosiles-Gonzále, G.; Avila-Torres, G.; Moreno-Valenzuela, O.A.; Acosta-Gonzalez, G.; Leal-Bautista, R.M.; Grimaldo-Hernandez, C.D.; Brown, J.K.; Chaidez-Quiroz, C.; Betancourt, W.Q.; Gerba, C.P.; et al. Occurrence of Pepper Mild Mottle Virus (PMMoV) in Groundwater from a Karst Aquifer System in the Yucatan Peninsula, Mexico. Food Environ. Virol. 2017, 9, 487–497. [Google Scholar] [CrossRef]
- Heinz, B.; Birk, S.; Liedl, R.; Geyer, T.; Straub, K.L.; Andresen, J.; Bester, K.; Kappler, A. Water quality deterioration at a karst spring (Gallusquelle, Germany) due to combined sewer overflow: Evidence of bacterial and micro-pollutant contamination. Environ. Geol. 2008, 57, 797–808. [Google Scholar] [CrossRef]
- Boyer, D.G.; Kuczynska, E. Storm and seasonal distributions of fecal coliforms and cryptosporidium in a spring. J. Am. Water Resour. Assoc. 2003, 39, 1449–1456. [Google Scholar] [CrossRef]
- Butscher, C.; Auckenthaler, A.; Scheidler, S.; Huggenberger, P. Validation of a numerical indicator of microbial contamination for karst springs. Ground Water 2011, 49, 66–76. [Google Scholar] [CrossRef]
- Boyer, D.G.; Pasquarell, G.C. Agricultural land use impacts on bacterial water quality in a karst ground water aquifer. J. Am. Water Resour. Assoc. 1999, 35, 291–300. [Google Scholar] [CrossRef]
- Huebsch, M.; Horan, B.; Blum, P.; Richards, K.G.; Grant, J.; Fenton, O. Impact of agronomic practices of an intensive dairy farm on nitrogen concentrations in a karst aquifer in Ireland. Agric. Ecosyst. Environ. 2013, 179, 187–199. [Google Scholar] [CrossRef]
- Calijuri, M.L.; do Couto, E.D.A.; Santiago, A.D.F.; Camargo, R.D.A.; Silva, M.D.F.M. Evaluation of the Influence of Natural and Antrhopogenic Processes on Water Quality in Karstic Region. Water Air Soil Pollut. 2011, 223, 2157–2168. [Google Scholar] [CrossRef]
- Dar, F.A.; Ganai, J.A.; Ahmed, S.; Satyanarayanan, M. Groundwater trace element chemistry of the karstified limestone of Andhra Pradesh, India. Environ. Earth Sci. 2017, 76, 673. [Google Scholar] [CrossRef]
- Delgado, C.; Pacheco, J.; Cabrera, A.; Batllori, E.; Orellana, R.; Bautista, F. Quality of groundwater for irrigation in tropical karst environment: The case of Yucatan, Mexico. Agric. Water Manag. 2010, 97, 1423–1433. [Google Scholar] [CrossRef]
- Yang, L. Structural features and formation and evolution of the underground river in some parts of South China. J. Chengdu Univ. Technol. 1982, 2, 54–61. (In Chinese) [Google Scholar]
- Yang, L. Distribution of the underground river in South China. Carsologica Sin. 1985, 1, 92–100. (In Chinese) [Google Scholar]
- Yuan, J.; Xu, F.; Deng, G.; Tang, Y.; Li, P. Hydrogeochemistry of shallow groundwater in a karst aquifer system of Bijie City, Guizhou Province. Water 2017, 9, 625. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, D. The relationship between water quality and land use in the typical karst groundwater basin in Guizhou. Chin. J. Water Resour. Res. 2004, 18, 134–137. (In Chinese) [Google Scholar]
- Li, S.L.; Liu, C.Q.; Xiao, H.Y.; Tao, F.X.; Lang, Y.C.; Han, G.L. Identification and application of nitrogen isotope in the source of nitrate pollution of groundwater in Guiyang. Chin. J. Geochem. 2005, 34, 257–262. (In Chinese) [Google Scholar]
- Liu, C.-Q.; Tian, R.; Liu, R. Using δ15N- and δ18O-values to identify nitrate sources in karst ground water, Guiyang, southwest China. Environ. Sci. Technol. 2006, 40, 6928–6933. [Google Scholar] [CrossRef]
- Lang, Y.C.; Liu, C.Q.; Zhao, Z.Q.; Li, S.L.; Han, G.L. Geochemistry of surface and ground water in Guiyang, China: Water/rock interaction and pollution in a karst hydrological system. Appl. Geochem. 2006, 221, 887–903. [Google Scholar] [CrossRef]
- Lang, Y.; Liu, C.; Satake, H.; Wu, J.; Li, S. Sulfur and chlorine isotopic compositions and pollutant tracing significance of surface water and groundwater in Guiyang. Chin. J. Adv. Earth Sci. 2008, 23, 151–159. (In Chinese) [Google Scholar]
- Xiao, T.; Boyle, D.; Guha, J.; Rouleau, A.; Hong, Y.; Zheng, B. Groundwater-related thallium transfer processes and their impacts on the ecosystem: Southwest Guizhou Province, China. Appl. Geochem. 2003, 18, 675–691. [Google Scholar] [CrossRef]
- Horvat, M.; Nolde, N.; Fajon, V.; Jereb, V.; Logar, M.; Lojen, S.; Jacimovic, R.; Falnoga, I.; Liya, Q.; Faganeli, J.; et al. Total mercury, methylmercury and selenium in mercury polluted areas in the province Guizhou, China. Sci. Total Environ. 2003, 304, 231–256. [Google Scholar] [CrossRef]
- Bi, X.; Feng, X.; Yang, Y.; Qiu, G.; Li, G.; Li, F.; Liu, T.; Fu, Z.; Jin, Z. Environmental contamination of heavy metals from zinc smelting areas in Hezhang County, western Guizhou, China. Environ. Int. 2006, 32, 883–890. [Google Scholar] [CrossRef]
- Li, P.; Li, S.-L.; Lang, Y.-C.; Xiao, H.-Y. Solute geochemistry and multivariate analysis of water quality in the Guohua Phosphorite Mine, Guizhou Province, China. Expo. Health 2019, 11, 81–94. [Google Scholar] [CrossRef]
- Xu, C.; Li, Z.; Li, D. Heavy metal content and health risk assessment of karst cave water in Guizhou. Fresenius Environ. Bull. 2020, 29, 2378–2389. [Google Scholar]
- Yuan, D. Some opinions on geological survey of groundwater resources and ecological environment in karst mountain area of South China. Carsologica Sin. 2000, 19, 103–108. (In Chinese) [Google Scholar]
- Wu, P.; Tang, C.; Zhu, L.; Liu, C.; Cha, X.; Tao, X. Hydrogeochemical characteristics of surface water and groundwater in the karst basin, southwest China. Hydrol. Process. 2009, 23, 2012–2022. [Google Scholar] [CrossRef]
- Wei, Y.L.; Luo, S.W.; Chen, W.H.; Ouyang, Z.H.; Luo, Q.K.; Li, C.Z. Characteristics and formation and evolution analysis of the dolomite karst landscape of Suiyang Geopark, Guizhou Province. Acta Geosci. Sin. 2018, 39, 365–383. (In Chinese) [Google Scholar]
- Chen, L.; Zhou, Q. Sulfate thermochemical reduction (TSR) and rediscussion on the formation mechanism of mercury (gold/antimony) deposits in Guizhou. Geol. Rev. 2019, 65, 431–436. (In Chinese) [Google Scholar]
- Guo, F.; Jiang, G.H.; Fei, J.G.; Zhang, C.; Zhang, C. Water quality assessment and evolving trend of main groundwater in Guangxi. Carsologica Sin. 2002, 21, 195–201. (In Chinese) [Google Scholar]
- Moral, F.; Cruz-Sanjulián, J.; Olias, M. Geochemical evolution of groundwater in the carbonate aquifers of Sierra de Segura (Betic Cordillera, southern Spain). J. Hydrol. 2008, 360, 281–296. [Google Scholar]
- Katz, B.; Griffin, D.; Davis, J. Groundwater quality impacts from the land application of treated municipal wastewater in a large karstic spring basin: Chemical and microbiological indicators. Sci. Total Environ. 2009, 407, 2872–2886. [Google Scholar]
- Elliot, T.; Andrews, J.N.; Edmunds, M. Hydrochemical trends, Palaeoreeharge and groundwater ages in the fissured Chalk aquifer of the London and Berkshire Basins, UK. Appl. Geochem. 1999, 14, 333–363. [Google Scholar]
- Onwuka, O.S.; Omonona, O.V.; Anika, O.C. Hydrochemical characteristics and quality assessment of regolith aquifers in Enugu metropolis, southeastern Nigeria. Environ. Earth Sci. 2013, 70, 1135–1141. [Google Scholar]
- Klir, G.J.; Yuan, B. Fuzzy Sets and Fuzzy Logic: Theory and Applications; Prentice Hall: Upper Saddle River, NJ, USA, 1995. [Google Scholar]
- China Ministry of Water Resources. Standard for Groundwater Quality (GB/T14848-2017); China National Standardization Administration: Beijing, China, 2017. [Google Scholar]

| Sample | pH | Total Hardness | TDS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7.30 | 184 | 293 | 40.0 | 20.4 | 1.02 | 0.80 | 5.85 | 222 | 0 | 0 | 2.51 |
| 2 | 7.32 | 334 | 333 | 74.9 | 35.7 | 1.93 | 1.33 | 14.5 | 392 | 2.67 | 0 | 5.56 |
| 3 | 6.80 | 331 | 355 | 81.2 | 31.2 | 1.33 | 2.33 | 71.3 | 302 | 4.00 | 0 | 6.21 |
| 4 | 8.14 | 202 | 316 | 40.7 | 24.4 | 1.26 | 1.55 | 5.77 | 232 | 7.48 | 0.07 | 2.71 |
| 5 | 7.27 | 287 | 427 | 60.2 | 33.4 | 1.29 | 1.47 | 20.8 | 289 | 15.5 | 0.25 | 5.04 |
| 6 | 7.50 | 421 | 410 | 8.41 | 50.3 | 0.33 | 1.67 | 16.0 | 485 | 0.60 | 0.20 | 7.44 |
| 7 | 7.80 | 328 | 423 | 70.1 | 37.3 | 1.13 | 1.86 | 20.6 | 343 | 34.7 | 0.05 | 7.44 |
| 8 | 7.30 | 293 | 447 | 60.3 | 34.8 | 0.46 | 0.76 | 17.4 | 311 | 20.7 | 0.09 | 1.90 |
| 9 | 7.74 | 259 | 408 | 49.8 | 32.5 | 0.76 | 0.58 | 10.7 | 304 | 7.68 | 0.06 | 1.73 |
| 10 | 8.37 | 283 | 447 | 57.7 | 34.0 | 0.20 | 0.53 | 6.42 | 333 | 8.33 | 0.10 | 4.57 |
| 11 | 7.30 | 277 | 277 | 60.6 | 30.4 | 0.67 | 2.00 | 10.0 | 319 | 3.00 | 0 | 5.61 |
| 12 | 7.20 | 306 | 341 | 65.5 | 34.5 | 2.00 | 3.00 | 80.0 | 241 | 26.0 | 0.20 | 4.68 |
| 13 | 7.30 | 286 | 295 | 60.3 | 32.9 | 0.33 | 2.00 | 28.0 | 311 | 6.00 | 0.06 | 4.21 |
| 14 | 7.10 | 285 | 283 | 61.1 | 32.2 | 1.00 | 1.33 | 16.0 | 322 | 2.00 | 0.20 | 2.15 |
| 15 | 7.30 | 335 | 333 | 70.1 | 38.7 | 0.33 | 2.33 | 12.0 | 385 | 5.00 | 0.40 | 6.61 |
| 16 | 7.50 | 347 | 358 | 40.8 | 17.8 | 1.00 | 4.67 | 30.0 | 176 | 2.00 | 0.24 | 3.74 |
| 17 | 7.40 | 236 | 236 | 48.5 | 27.9 | 0.67 | 1.67 | 14.0 | 259 | 4.00 | 0.10 | 4.92 |
| 18 | 7.40 | 286 | 283 | 63.4 | 31.1 | 0.33 | 3.33 | 12.0 | 311 | 10.0 | 0 | 7.49 |
| 19 | 7.80 | 229 | 242 | 60.1 | 19.0 | 1.33 | 2.00 | 22.0 | 249 | 4.00 | 0.04 | 4.21 |
| 20 | 7.30 | 244 | 271 | 70.8 | 16.3 | 1.00 | 2.33 | 45.0 | 225 | 10.0 | 0.10 | 6.55 |
| 21 | 7.30 | 308 | 315 | 64.2 | 35.8 | 0.33 | 0.67 | 24.0 | 342 | 10.0 | 0.06 | 1.87 |
| 22 | 7.10 | 743 | 937 | 197 | 60.9 | 2.67 | 3.33 | 420 | 289 | 96.0 | 0.30 | 5.66 |
| 23 | 7.30 | 285 | 301 | 63.2 | 31.0 | 0.67 | 1.33 | 10.0 | 304 | 7.67 | 0.12 | 3.30 |
| 24 | 6.90 | 425 | 439 | 84.6 | 50.9 | 2.00 | 3.00 | 60.0 | 441 | 4.00 | 0 | 7.80 |
| 25 | 7.20 | 338 | 377 | 81.2 | 32.8 | 1.67 | 8.67 | 47.0 | 339 | 16.0 | 0.10 | 14.5 |
| Sample | pH | Total Hardness | TDS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | 6.80 | 184 | 236 | 8.40 | 16.3 | 0.20 | 0.53 | 5.77 | 176 | 0 | 0 | 1.73 |
| Max | 8.37 | 743 | 937 | 197 | 60.9 | 2.67 | 8.67 | 420 | 485 | 96.0 | 0.40 | 14.5 |
| Mean | 7.39 | 314 | 366 | 66.7 | 33.4 | 1.01 | 2.24 | 43.2 | 313 | 12.4 | 0.11 | 5.25 |
| Karst Area | Average Equivalent | Average Equivalent | Data Source |
|---|---|---|---|
| Concentration of Cations | Concentration of Anions | ||
| (meq/L) | (meq/L) | ||
| Dolomite aquifer of Guizhou (China) | 6.39 | 6.27 | Present study |
| Karst aquifer of Guangxi (China) | 4.05 | 3.99 | Guo et al. [43] |
| Karst aquifer of Sierra de Segura (Spain) | 4.03 | 4.28 | Moral et al. [44] |
| Wakulla limestone aquifer of Florida (USA) | 3.44 | 3.29 | Katz et al. [45] |
| Chalk aquifer of southern England (UK) | 8.33 | 8.01 | Elliot et al. [46] |
| No. | Index | I | II | III | IV | V |
|---|---|---|---|---|---|---|
| 1 | Total hardness | 150 | 300 | 450 | 550 | 650 |
| 2 | TDS | 300 | 500 | 1000 | 2000 | 3000 |
| 3 | 50 | 150 | 250 | 350 | 450 | |
| 4 | 50 | 150 | 250 | 350 | 450 | |
| 5 | 2 | 5 | 20 | 30 | 50 | |
| 6 | 1 | 1 | 1 | 2 | 3 |
| Sample | I | II | III | IV | V | Evaluation Outcome |
|---|---|---|---|---|---|---|
| 1 | 0.855 | 0.145 | 0 | 0 | 0 | I |
| 2 | 0.286 | 0.563 | 0.151 | 0 | 0 | II |
| 3 | 0.286 | 0.600 | 0.114 | 0 | 0 | II |
| 4 | 0.532 | 0.438 | 0.050 | 0 | 0 | I |
| 5 | 0.223 | 0.516 | 0.261 | 0 | 0 | II |
| 6 | 0.249 | 0.233 | 0.518 | 0 | 0 | III |
| 7 | 0.093 | 0.289 | 0.051 | 0.435 | 0.132 | IV |
| 8 | 0.119 | 0.409 | 0.423 | 0.032 | 0 | III |
| 9 | 0.289 | 0.609 | 0.100 | 0 | 0 | II |
| 10 | 0.180 | 0.763 | 0.057 | 0 | 0 | II |
| 11 | 0.404 | 0.596 | 0 | 0 | 0 | II |
| 12 | 0.216 | 0.319 | 0.192 | 0.273 | 0 | II |
| 13 | 0.360 | 0.626 | 0.013 | 0 | 0 | II |
| 14 | 0.482 | 0.518 | 0 | 0 | 0 | II |
| 15 | 0.326 | 0.557 | 0.117 | 0 | 0 | II |
| 16 | 0.386 | 0.438 | 0.176 | 0 | 0 | II |
| 17 | 0.579 | 0.421 | 0 | 0 | 0 | I |
| 18 | 0.245 | 0.647 | 0.108 | 0 | 0 | II |
| 19 | 0.607 | 0.393 | 0 | 0 | 0 | I |
| 20 | 0.454 | 0.444 | 0.102 | 0 | 0 | I |
| 21 | 0.228 | 0.648 | 0.123 | 0 | 0 | II |
| 22 | 0.024 | 0.010 | 0.068 | 0.058 | 0.840 | V |
| 23 | 0.299 | 0.655 | 0.046 | 0 | 0 | II |
| 24 | 0.231 | 0.299 | 0.470 | 0 | 0 | III |
| 25 | 0.225 | 0.424 | 0.351 | 0 | 0 | II |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Torres, M.; Paudel, P.; Hu, L.; Yang, G.; Chu, X. Assessing the Karst Groundwater Quality and Hydrogeochemical Characteristics of a Prominent Dolomite Aquifer in Guizhou, China. Water 2020, 12, 2584. https://doi.org/10.3390/w12092584
Wang Z, Torres M, Paudel P, Hu L, Yang G, Chu X. Assessing the Karst Groundwater Quality and Hydrogeochemical Characteristics of a Prominent Dolomite Aquifer in Guizhou, China. Water. 2020; 12(9):2584. https://doi.org/10.3390/w12092584
Chicago/Turabian StyleWang, Zhongmei, Martin Torres, Prakash Paudel, Liangbo Hu, Genlan Yang, and Xuewei Chu. 2020. "Assessing the Karst Groundwater Quality and Hydrogeochemical Characteristics of a Prominent Dolomite Aquifer in Guizhou, China" Water 12, no. 9: 2584. https://doi.org/10.3390/w12092584
APA StyleWang, Z., Torres, M., Paudel, P., Hu, L., Yang, G., & Chu, X. (2020). Assessing the Karst Groundwater Quality and Hydrogeochemical Characteristics of a Prominent Dolomite Aquifer in Guizhou, China. Water, 12(9), 2584. https://doi.org/10.3390/w12092584




