Use of a Novel Biopellet to Treat Total Petroleum Hydrocarbon Contaminated Groundwater
Abstract
1. Introduction
2. Material and Methods
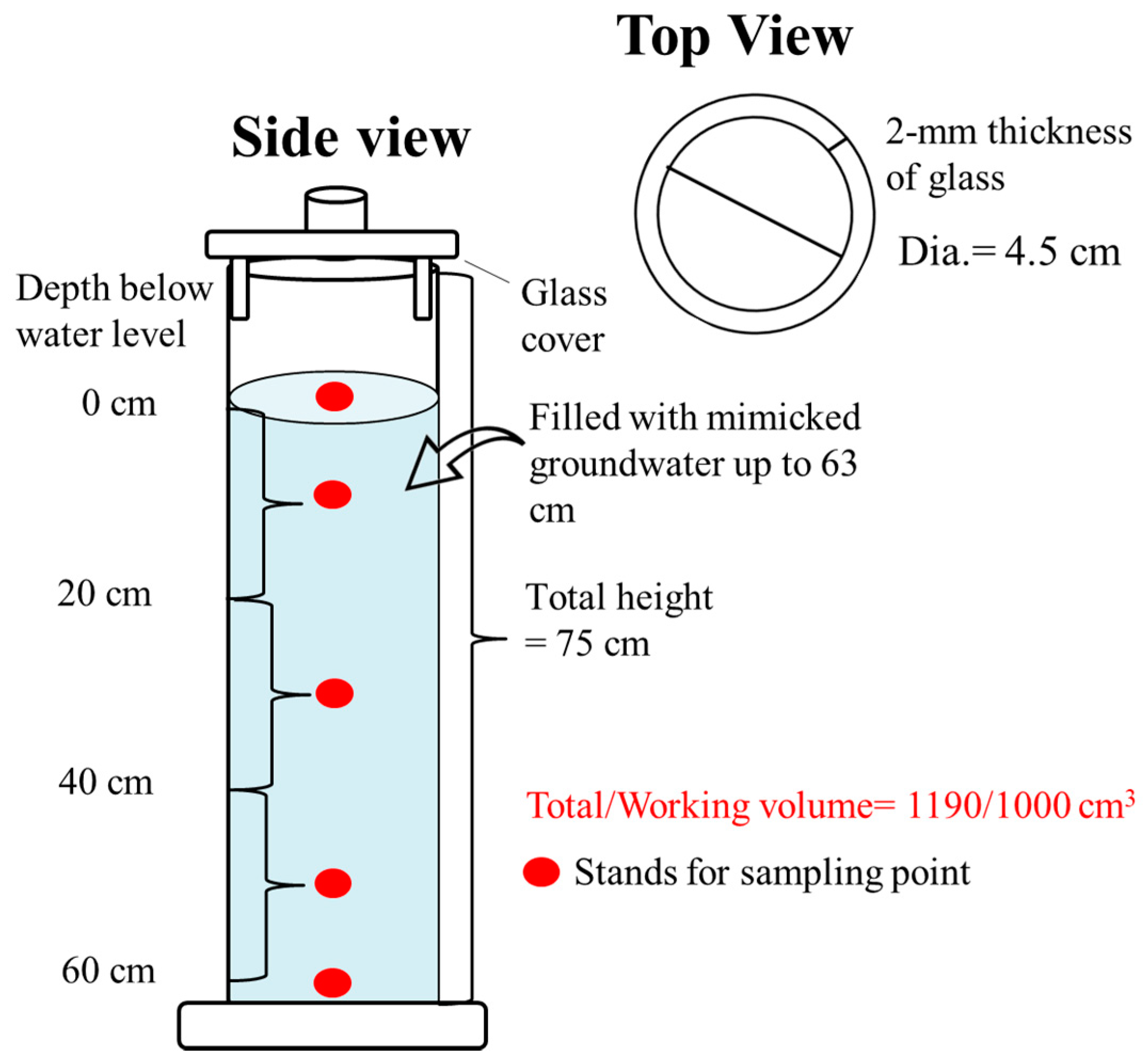
2.1. The Reactor
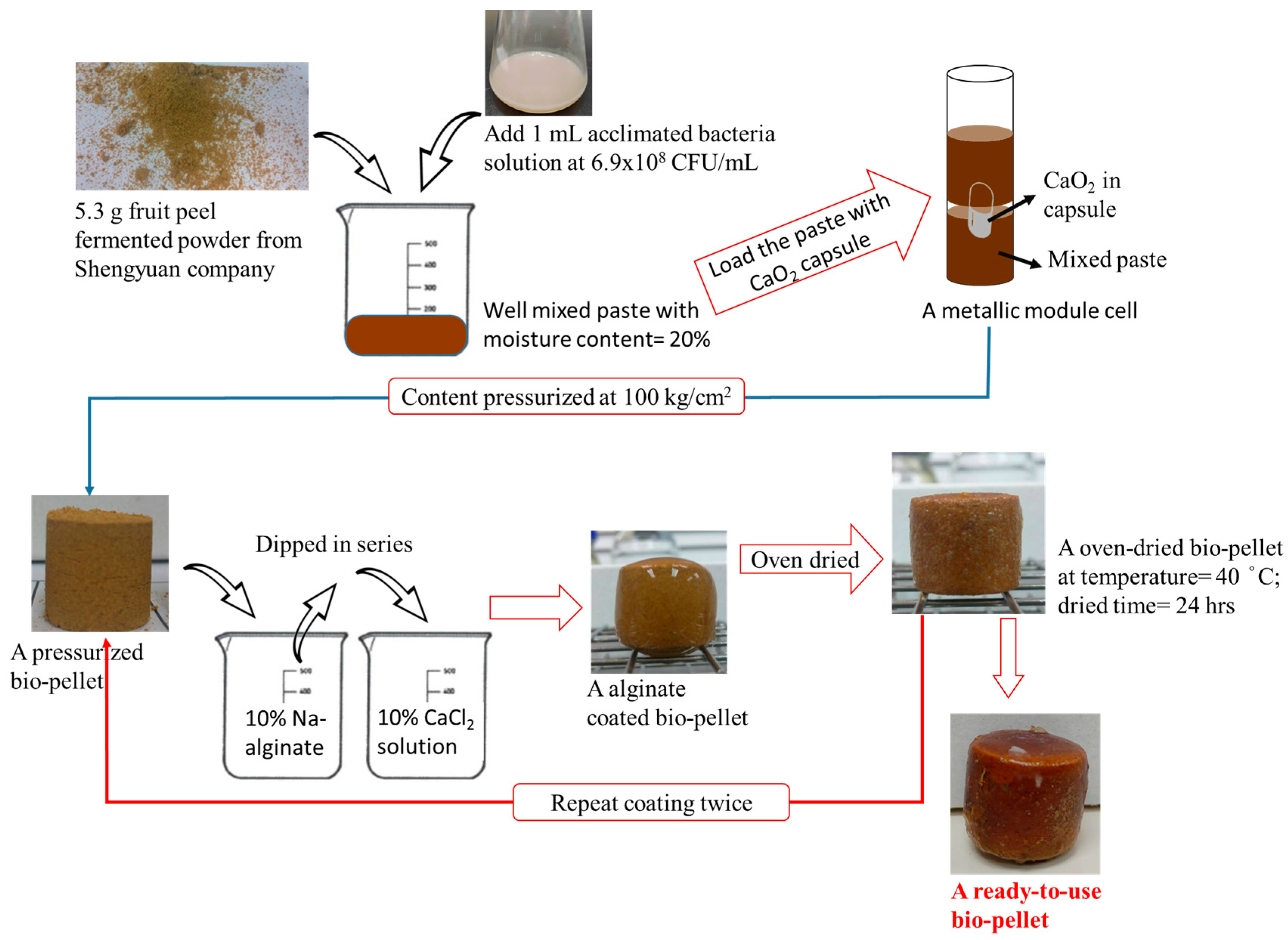
2.2. Novel Biopellets
2.3. TPH Treatment and Its Analysis
2.4. Data Quality
3. Results and Discussion
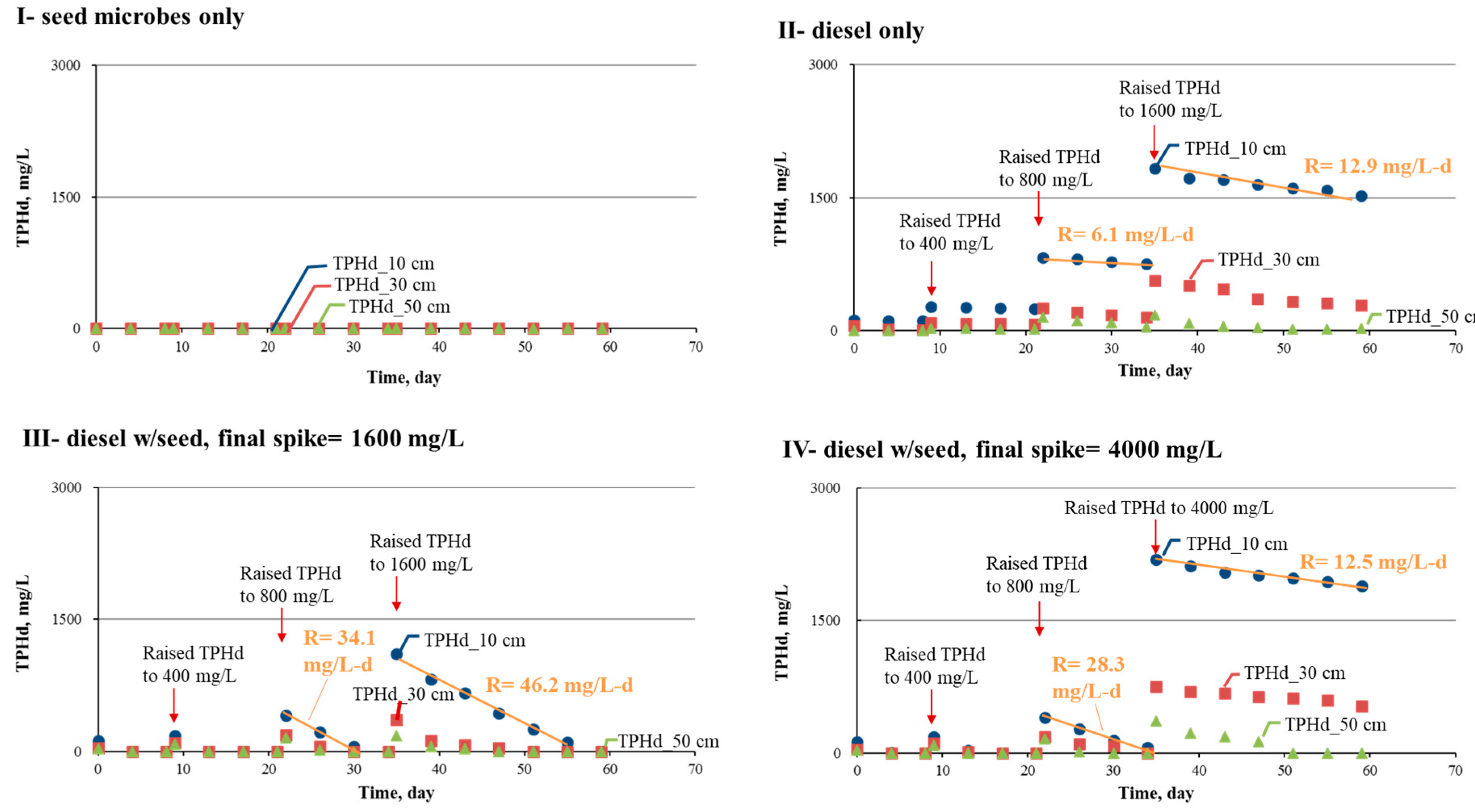
3.1. Microbial Acclimation
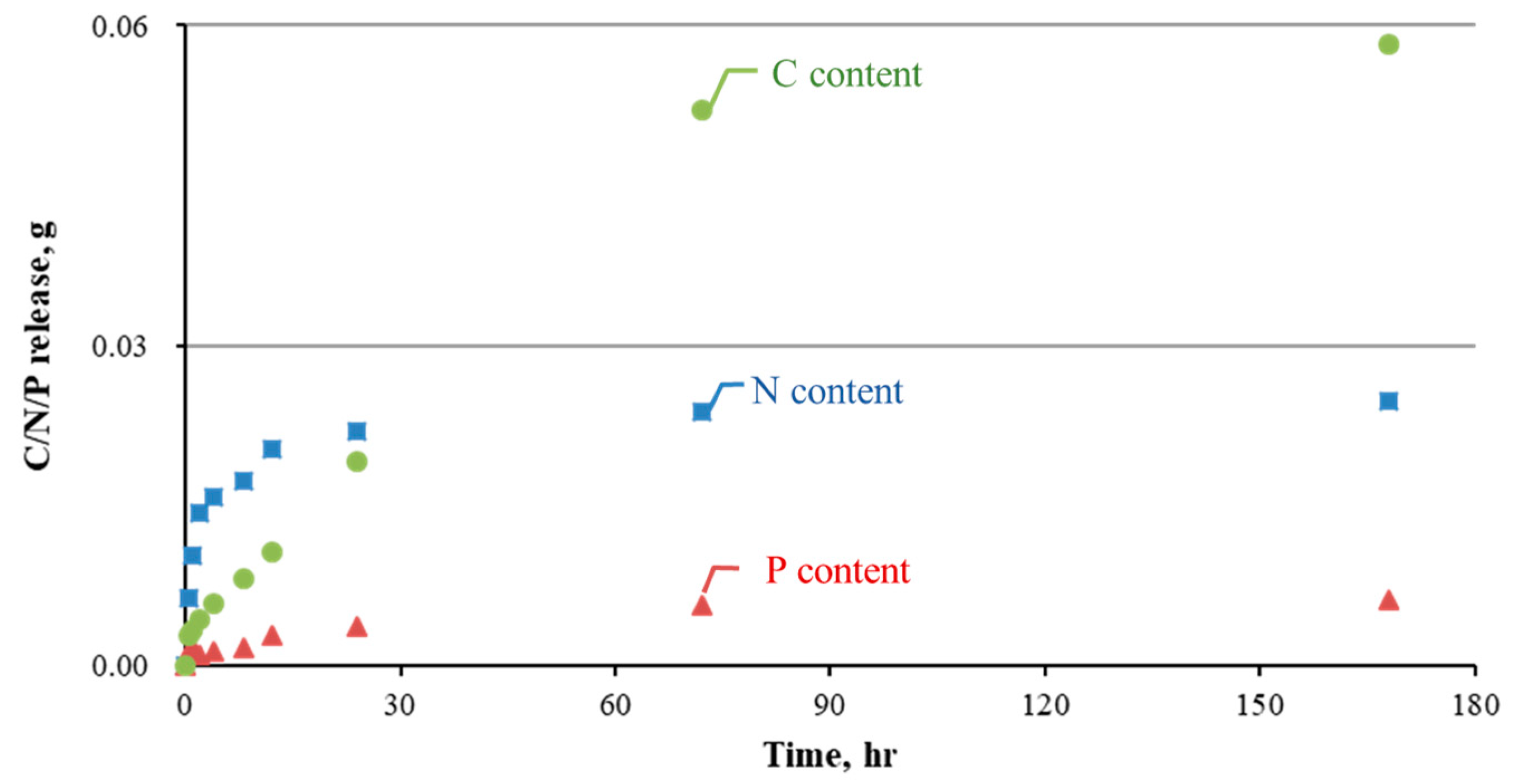
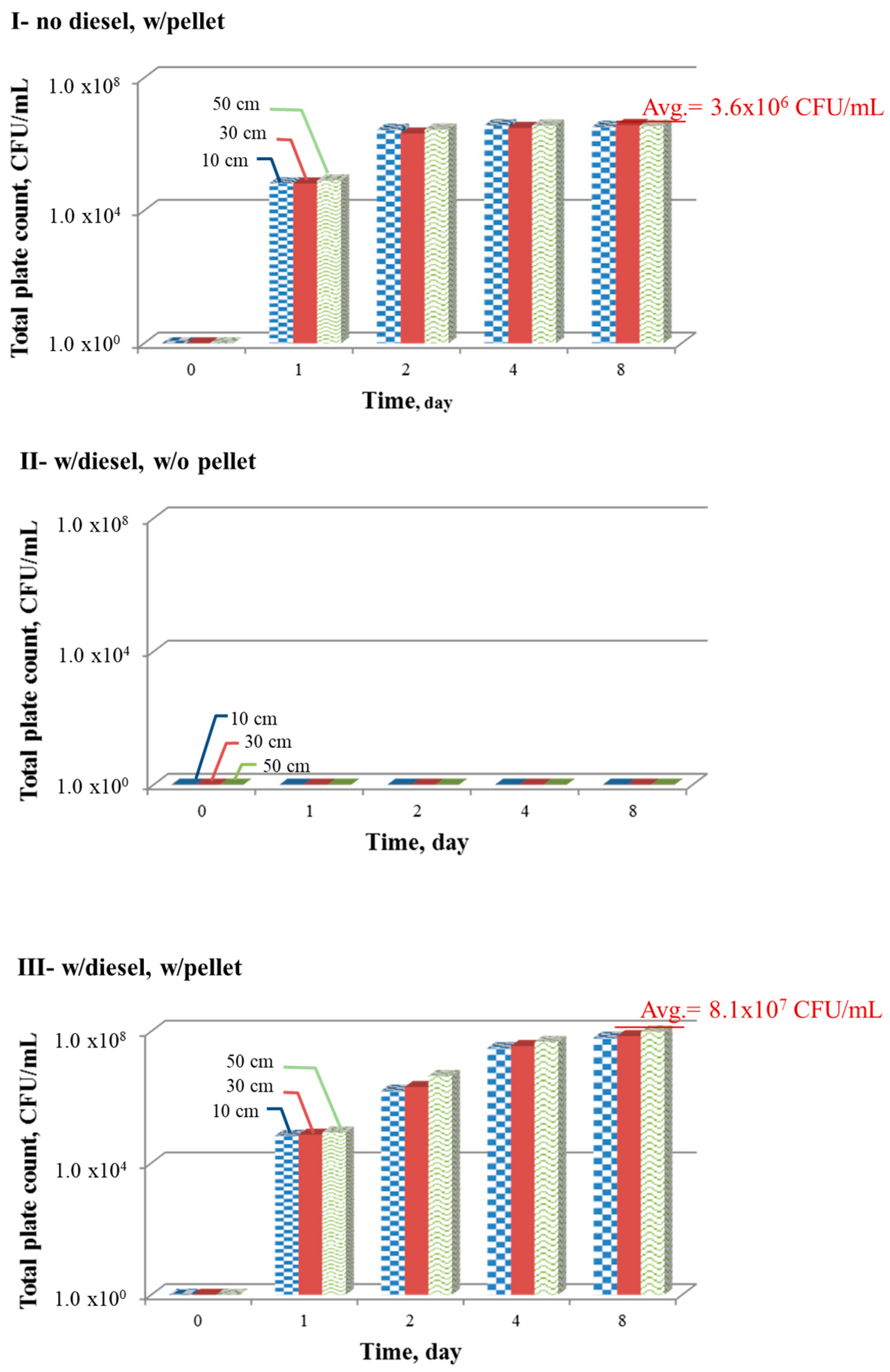
3.2. Biopellet Manufacturing and Performances
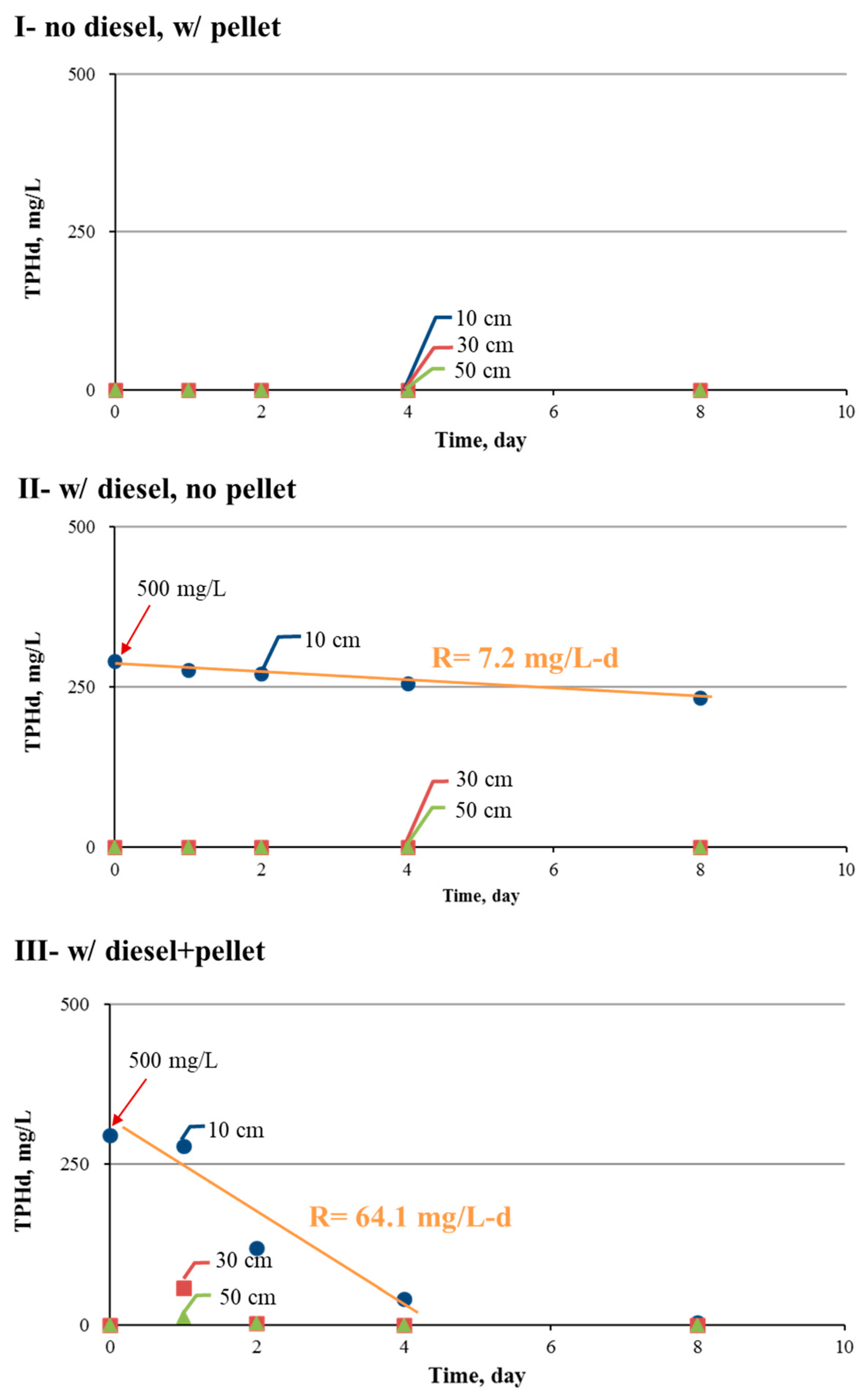
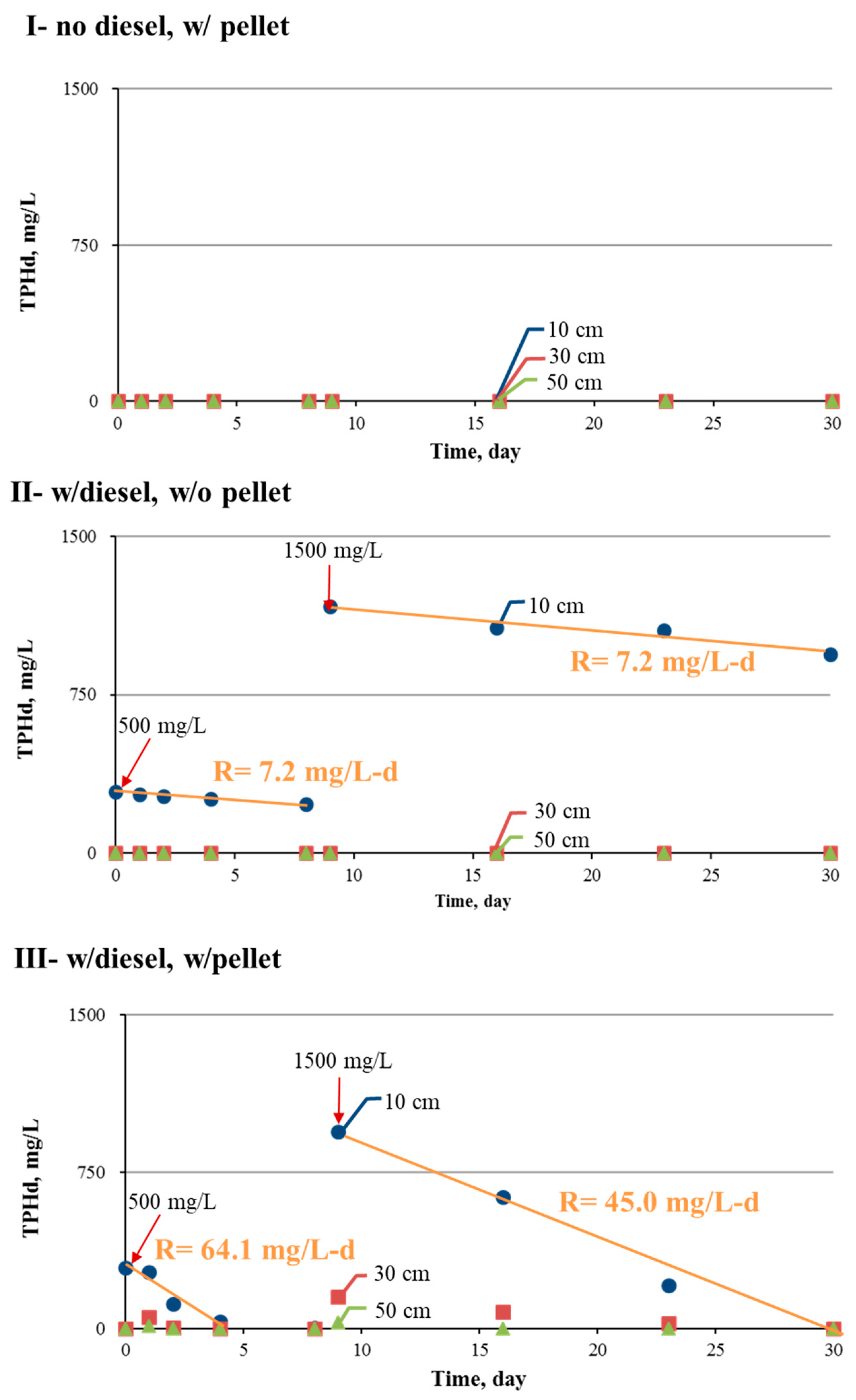
3.3. TPHd Removal
4. Conclusions
- Novel biopellets made from acclimated microbes, fermented fruit peels, and CaO2 recycled from eggshells appeared to effectively treat TPH-contaminated groundwater. The saturated C:N:P ratio reached 10:4:1 (by weight), with oxygen being released at approximately 1.0 mg/L after the addition of biopellets. Furthermore, triple alginate coating yielded a pellet floating time of up to 25 days.
- Microbes acclimated through gradually increasing TPHd additions up to concentrations of 1600 mg/L over a 60-day period were considered adequate, and this operation can be adopted to acclimate TPHd degraders. Through this acclimation process, the TPHd removal rate reached 46.2 mg/L per day.
- The biopellet treatment of TPHd in water at an initial concentration of 500 mg/L removed nearly 99% of the spiked TPHd at a removal rate of 64.1 mg/L per day. The remaining TPHd mainly consisted of carbon (C13–C18), which was more than 98% degraded after 8 days. When the TPHd spike was raised to 1500 mg/L, the daily removal rate increased to approximately 45.0 mg/L per day, and all spiked TPHs were completely removed by 22 days.
- During the first 8 days of treatment, the microbial count increased from near zero to 8.1 × 107 CFU/mL, the microbial consumption of N, P, and oxygen was considerable, the oxygen level remained high at 2.1 mg/L, and the pH was low at approximately 4.7.
Author Contributions
Funding
Conflicts of Interest
Data Availability
References
- TWEPA. TW NPL Site Query. 2020. Available online: https://sgw.epa.gov.tw/ContaminatedSitesMap/Default.aspx (accessed on 17 March 2020).
- USEPA. Groundwater Remedies Selected at Superfund Sites; EPA-542-R-01-022; USEPA: Washington, DC, USA, 2002.
- Karthick, A.; Banasri, R.; Pradipta, C. A review on the application of chemical surfactant and surfactant foam for remediation of petroleum oil contaminated soil. J. Environ. Manag. 2019, 243, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-C.; Chadol, L.; Jeung-Sun, L.; Kitae, B. Simultaneous application of chemical oxidation and extraction processes is effective at remediating soil Co-contaminated with petroleum and heavy metals. J. Environ. Manag. 2017, 186, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.Y. Application of natural attenuation for the control of petroleum hydrocarbon plume: Mechanisms and effectiveness evaluation. J. Hydrol. 2013, 505, 126–137. [Google Scholar] [CrossRef]
- Sullivana, G.L.; Prigmore, R.M.; Knight, P.; Godfrey, A.R. Activated carbon biochar from municipal waste as a sorptive agent for the removal of polyaromatic hydrocarbons (PAHs), phenols and petroleum based compounds in contaminated liquids. J. Environ. Manag. 2019, 251, 109551. [Google Scholar] [CrossRef] [PubMed]
- Falciglia, P.P.; Maddalena, R.; Mancuso, G.; Messina, V.; Vagliasindi, F.G. Lab-scale investigation on remediation of diesel-contaminated aquifer using microwave energy. J. Environ. Manag. 2016, 167, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.Y.; Kao, C.-T.; Chen, S.-C.; Chen, Y.L. Bioremediation of a petroleum-hydrocarbon contaminated aquifer by in situ biosparging system. J. Biotechnol. 2008, 136, S653. [Google Scholar] [CrossRef]
- Daghio, M.; Tatangelo, V.; Franzetti, A.; Gandolfi, I.; Papacchini, M.; Careghini, A.; Bestetti, G. Hydrocarbon degrading microbial communities in bench scale aerobic biobarriers for gasoline contaminated groundwater treatment. Chemosphere 2015, 130, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Poi, G.; Shahsavari, E.; Aburto-Medina, A.; Mok, P.C.; Ball, A.S. Large scale treatment of total petroleum-hydrocarbon contaminated groundwater using bioaugmentation. J. Environ. Manag. 2018, 214, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, J.A.; Hartog, N.; Gerritse, J.; Gallacher, C.; Helmus, R.; Brock, O.; Parsons, J.R.; Hassanizadeh, S.M. The dissolution and microbial degradation of mobile aromatic hydrocarbons from a Pintsch gas tar DNAPL source zone. Sci. Total Environ. 2020. accepted for publication. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.F.; Liu, S.H.; Su, Y.M.; Chen, Y.R.; Lin, C.W.; Lin, K.L. Bioremediation capability evaluation of benzene and sulfolane contaminated groundwater: Determination of bioremediation parameters. Sci. Total Environ. 2019, 648, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Le Thanh, T.; Kim, E.J.; Gong, J.; Chang, Y.Y.; Chang, Y.S. Fabrication of novel oxygen-releasing alginate beads as an efficient oxygen carrier for the enhancement of aerobic bioremediation of 1,4-dioxane contaminated groundwater. Bioresour. Technol. 2014, 171, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Wu, C.H.; Tang, C.T.; Chang, S.H. Novel oxygen-releasing immobilized cell beads for bioremediation of BTEX-contaminated water. Bioresour. Technol. 2012, 124, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Wu, C.H.; Guo, P.Y.; Chang, S.H. Innovative encapsulated oxygen-releasing beads for bioremediation of BTEX at high concentration in groundwater. J. Environ. Manag. 2017, 204, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater. American Public Health Association; American Water Works Association; Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]
- Shen, M.W.; Yan, C.Y.; Chen, H.C.; Chen, S.T. Removal of heavy oil using rhinoceros beetle, Oryctes rhinoceros. J. Environ. Manag. 2019, 249, 109418. [Google Scholar] [CrossRef] [PubMed]
- Kucharzyk, K.H.; Benotti, M.; Darlington, R.; Lalgudi, R. Enhanced biodegradation of sediment-bound heavily weathered crude oil with ligninolytic enzymes encapsulated in calcium-alginate beads. J. Hazard. Mater. 2018, 357, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Speight, J.G. 8—Remediation technologies. In Natural Water Remediation: Chemistry and Technology; Butterworth-Heinemann: Oxford, UK, 2020; pp. 263–303. [Google Scholar]
- Speight, J.G.; El-Gendy, N.S. Chapter 10—Bioremediation of Contaminated Soil. In Introduction to Petroleum Biotechnology; Gulf Professional Publishing: Boston, MA, USA, 2017; pp. 361–417. [Google Scholar]
- Brusseau, M.L. Chapter 19—Soil and Groundwater Remediation. In Environmental and Pollution Science, 3rd ed.; Brusseau, M.L., Gerba, C.P., Peper, I.L., Eds.; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]

| No. of Peaks | Compounds | Carbon Number | Density (g/cm³) | Eluted Time (min) |
|---|---|---|---|---|
| 1 | Naphthalene | C10 | 1.14 | 13.350 |
| 2 | Dodecane | C12 | 0.75 | 13.522 |
| 3 | Tridecane | C13 | 0.756 | 15.137 |
| 4 | Naphthalene, 2-methyl- | C11 | NA | 15.204 |
| 5 | Naphthalene, 1-methyl- | C11 | NA | 15.452 |
| 6 | Tetradecane | C14 | 0.764 | 16.613 |
| 7 | 2,6,10-Trimethyltridecane | C16 | NA | 17.446 |
| 8 | Heptadecane | C17 | 0.777 | 17.985 |
| 9 | Butane, 2,2-dimethyl- | C6 | NA | 19.455 |
| 10 | Hexadecane | C16 | 0.770 | 19.457 |
| 11 | Eicosane | C20 | NA | 28.921 |
| 12 | Phenanthrene, 2,3-dimethyl- | C16 | NA | 29.784 |
| 13 | 1,4-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester | C24 | 0.946 | 41.426 |
| 14 | Hexatriacontane | C36 | NA | 42.331 |
| Runs | 0 Day | 1 Day | 2 Day | 4 Day | 8 Day |
|---|---|---|---|---|---|
| I—no diesel, w/pellet |  |  |  |  |  |
| II—w/diesel, w/o pellet | 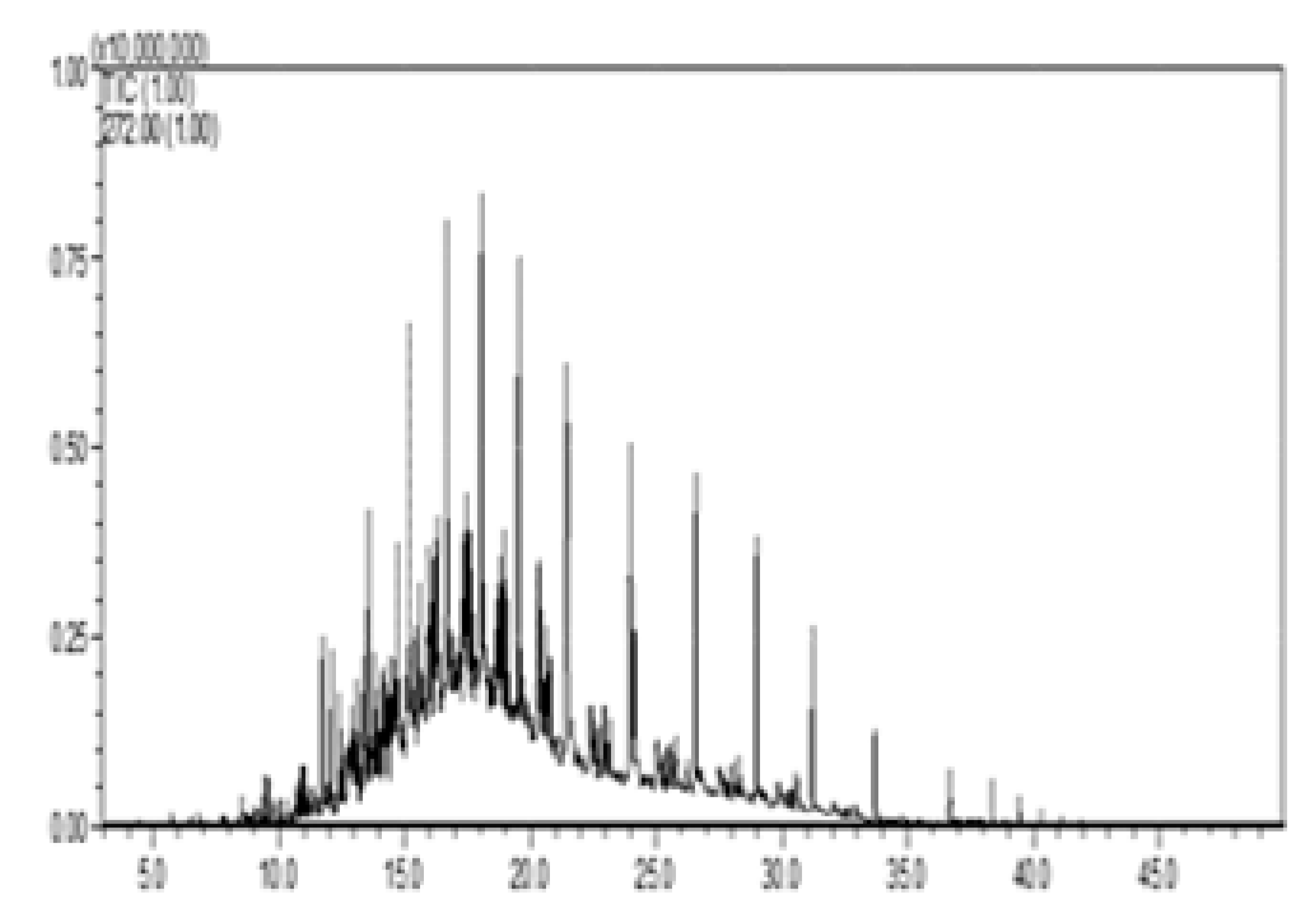 |  | 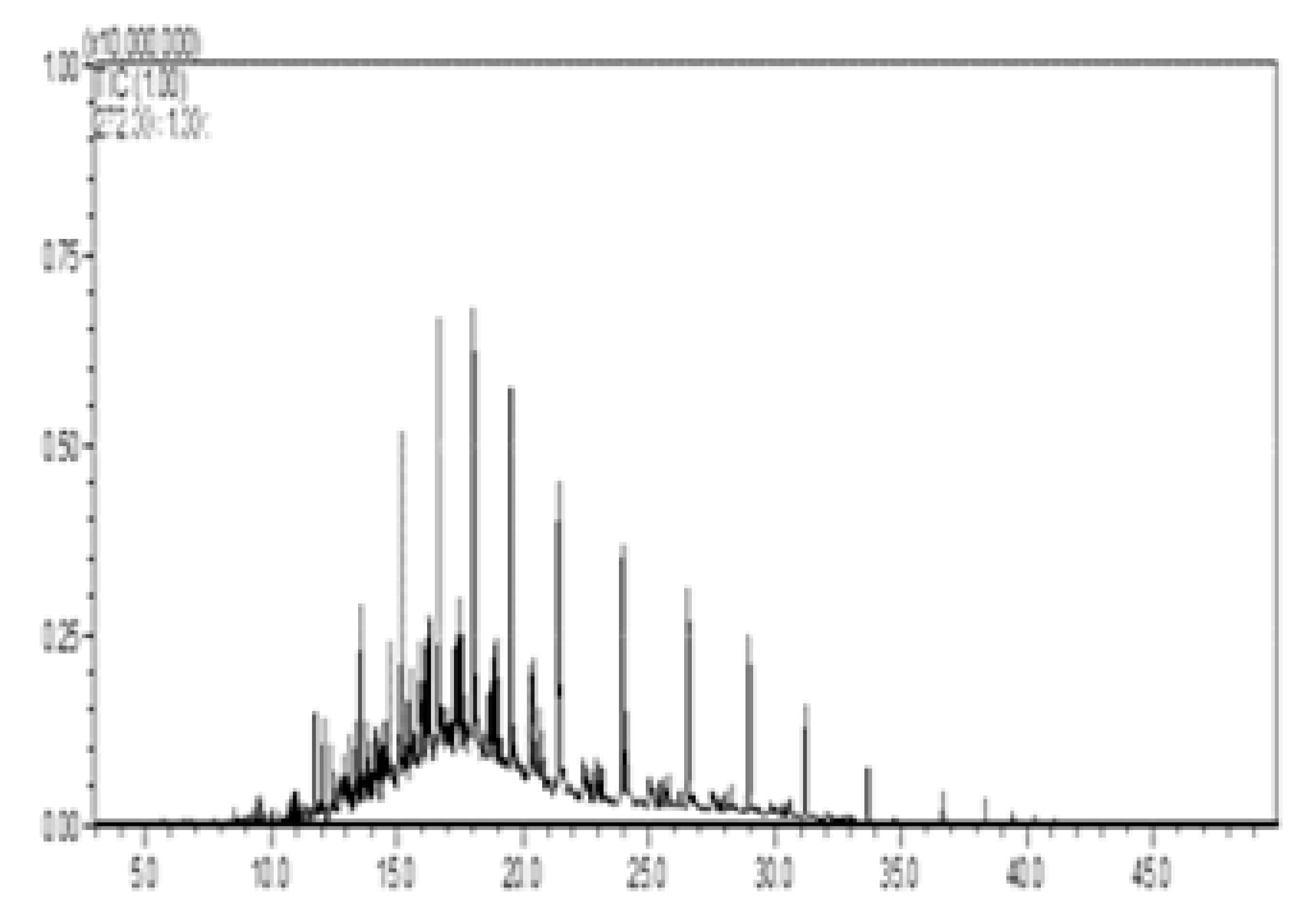 | 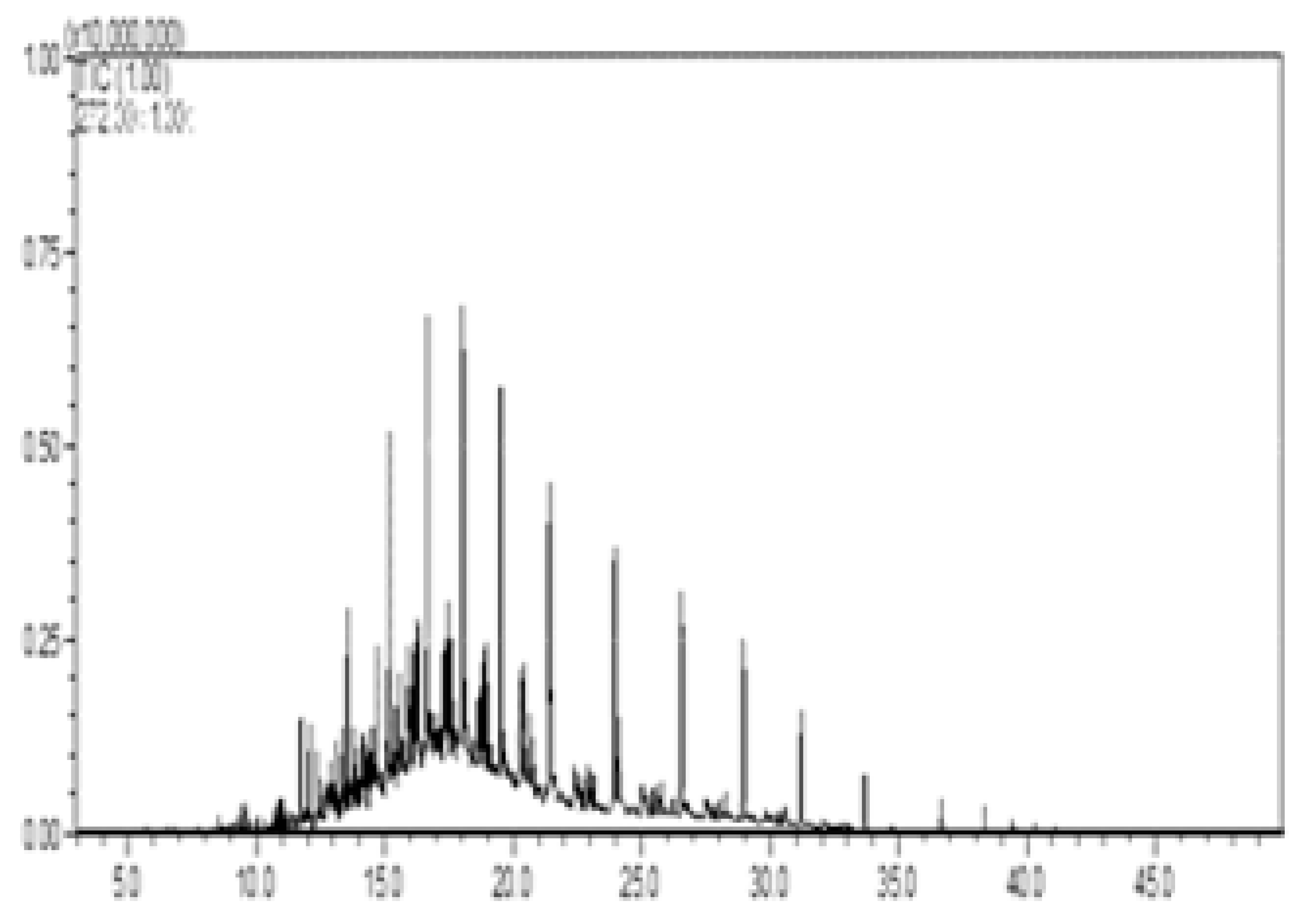 | 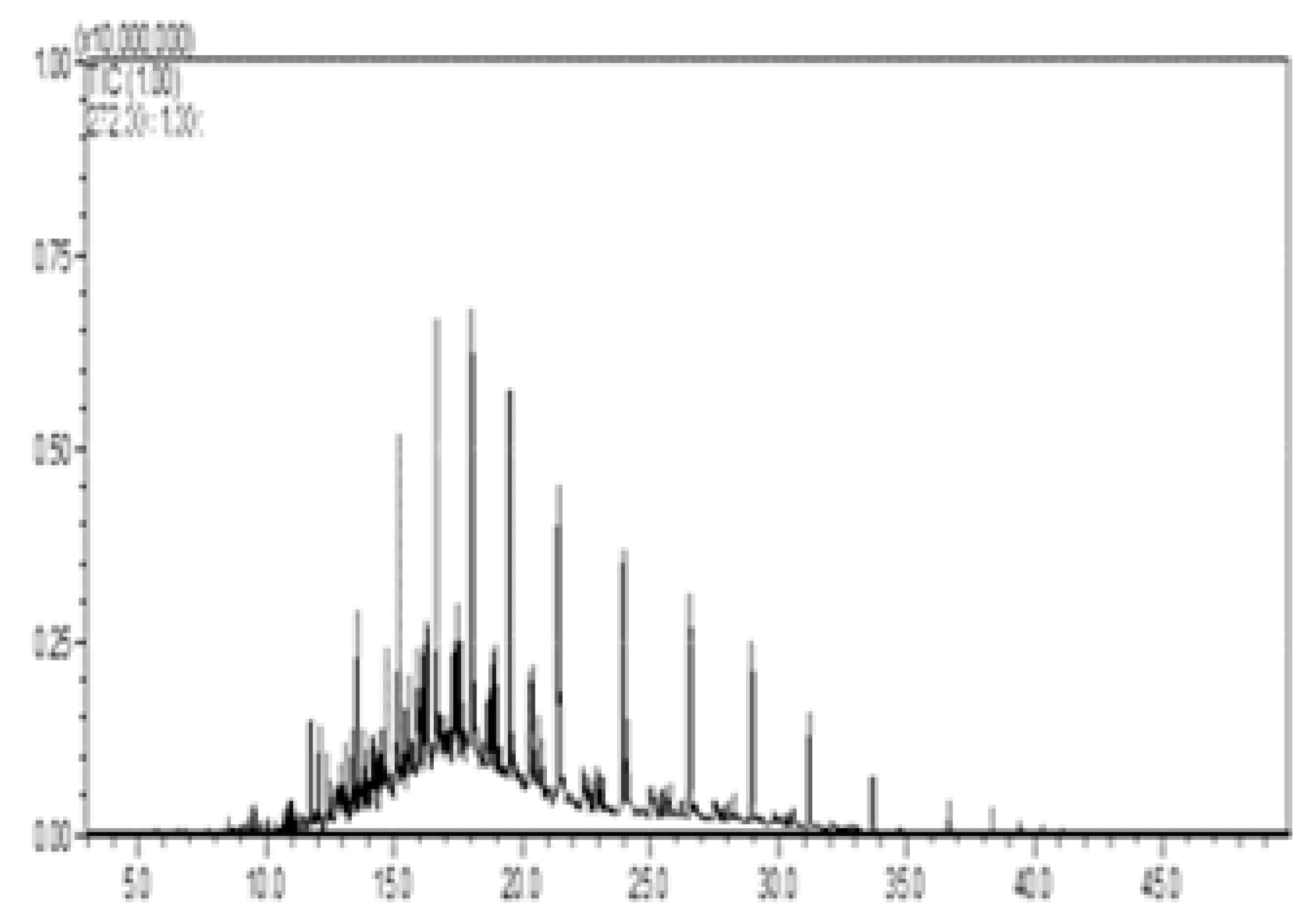 |
| III—w/diesel, w/pellet | 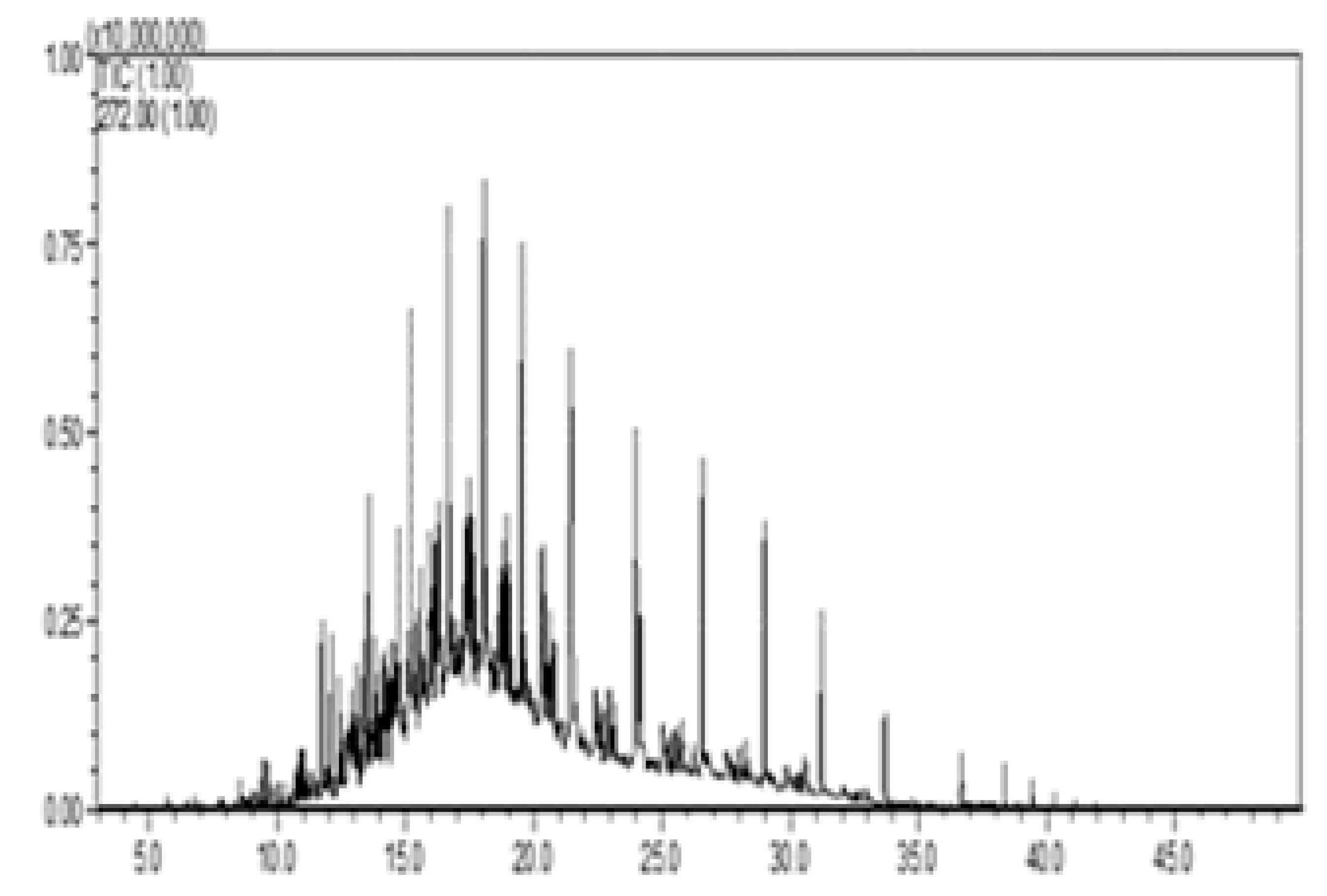 |  | 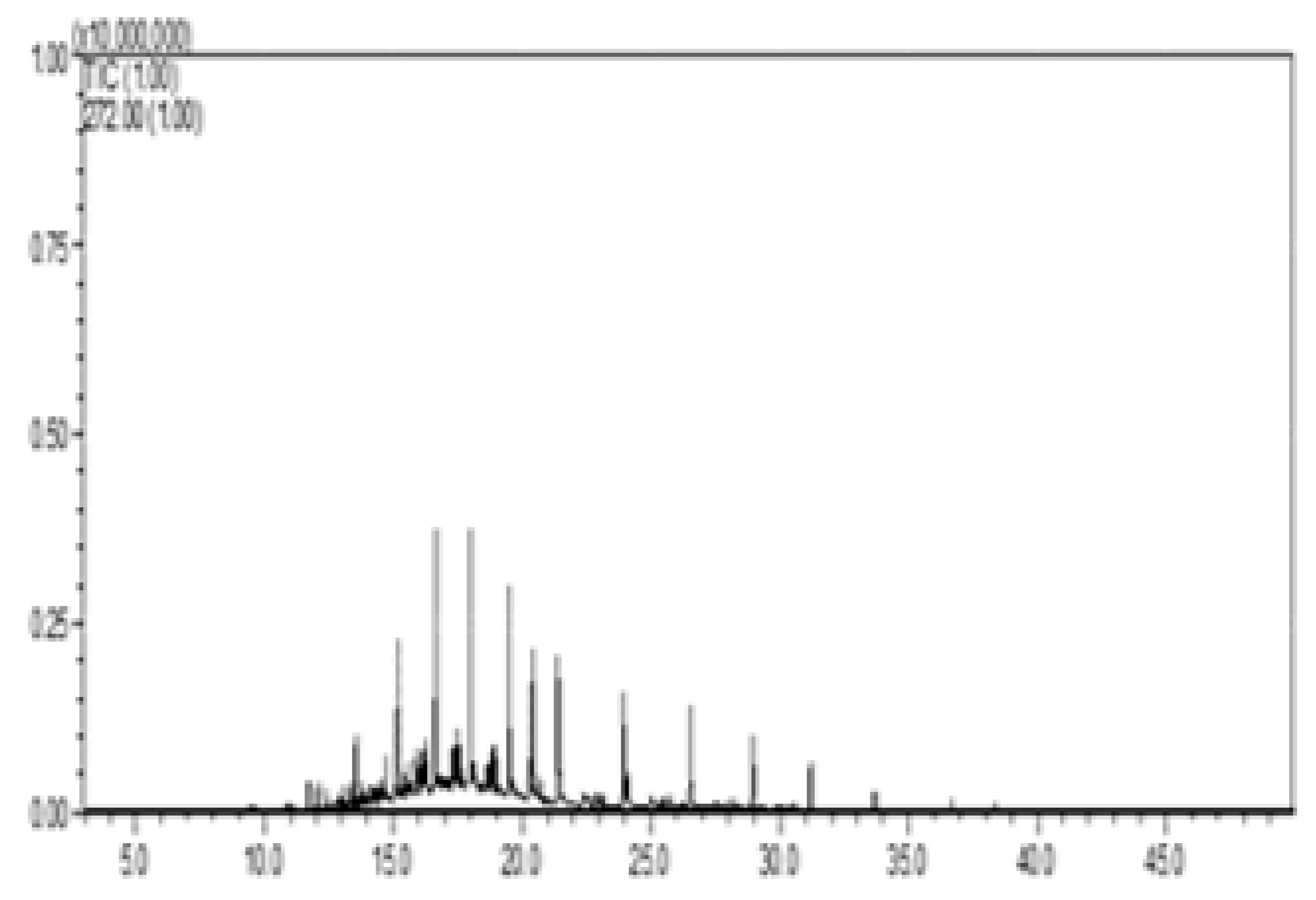 | 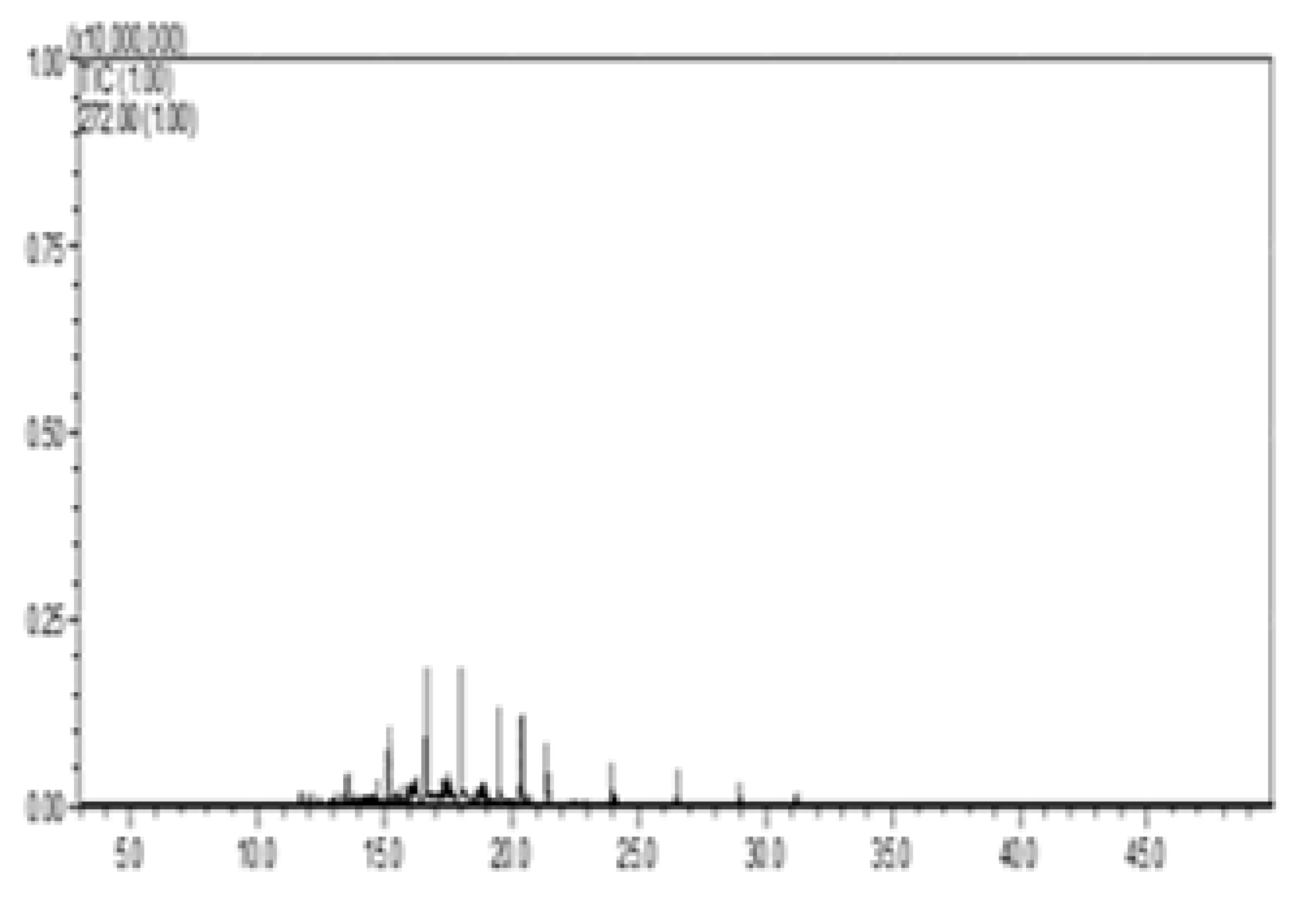 |  |
| C # | Compounds Left | Peak Area/% Area at Day 0 | Peak Area/% Area at Day 8 | % Removed on Day 8 |
|---|---|---|---|---|
| C13 | Tridecane | 24,135,133/1.6 | 275,068/14.6 | 98.9 |
| C14 | Tetradecane | 44,592,741/2.9 | 592,722/31.5 | 98.7 |
| C15 | Tetradecane, 2-methyl- | 13,138,933/0.9 | 100,706/5.4 | 99.2 |
| C15 | Pentadecane | 38,815,206/2.5 | 628,999/33.4 | 98.4 |
| C18 | Octadecane | 29,464,709/1.9 | 283,232/15.1 | 99.0 |
| Percent of total peak areas | 9.8% | 100% | ||
| Time Elapsed (Day) | Runs | TOC (mg/L) | TN (mg/L) | TP (mg/L) | DO (mg/L) | pH |
|---|---|---|---|---|---|---|
| 0 | All runs | 0.0 | 0.0 | 0.0 | 0.39 | 7.08 |
| 4 | I—no diesel, w/pellet | 119.1 | 396.7 | 178.8 | 2.35 | 6.58 |
| II—w/diesel, w/o pellet | 2.5 | 0.0 | 0.0 | 0.39 | 6.91 | |
| III—w/diesel, w/pellet | 68.1 | 119.7 | 6.3 | 2.14 | 5.46 | |
| 8 | I—no diesel, w/pellet | 91.5 | 289.0 | 214.3 | 2.10 | 6.51 |
| II—w/diesel, w/o pellet | 2.3 | 0.0 | 0.0 | 0.39 | 6.88 | |
| III—w/diesel, w/pellet | 10.1 | 37.3 | 2.1 | 2.06 | 4.65 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.-S.; Chen, S.-T. Use of a Novel Biopellet to Treat Total Petroleum Hydrocarbon Contaminated Groundwater. Water 2020, 12, 2512. https://doi.org/10.3390/w12092512
Yang D-S, Chen S-T. Use of a Novel Biopellet to Treat Total Petroleum Hydrocarbon Contaminated Groundwater. Water. 2020; 12(9):2512. https://doi.org/10.3390/w12092512
Chicago/Turabian StyleYang, Dun-Sheng, and Shyi-Tien Chen. 2020. "Use of a Novel Biopellet to Treat Total Petroleum Hydrocarbon Contaminated Groundwater" Water 12, no. 9: 2512. https://doi.org/10.3390/w12092512
APA StyleYang, D.-S., & Chen, S.-T. (2020). Use of a Novel Biopellet to Treat Total Petroleum Hydrocarbon Contaminated Groundwater. Water, 12(9), 2512. https://doi.org/10.3390/w12092512






