Performance of Anaerobic Digestion of Acidified Palm Oil Mill Effluent under Various Organic Loading Rates and Temperatures
Abstract
1. Introduction
2. Material and Methods
2.1. Acidified Palm Oil Mill Effluent (POME) and Inoculum of methanogens
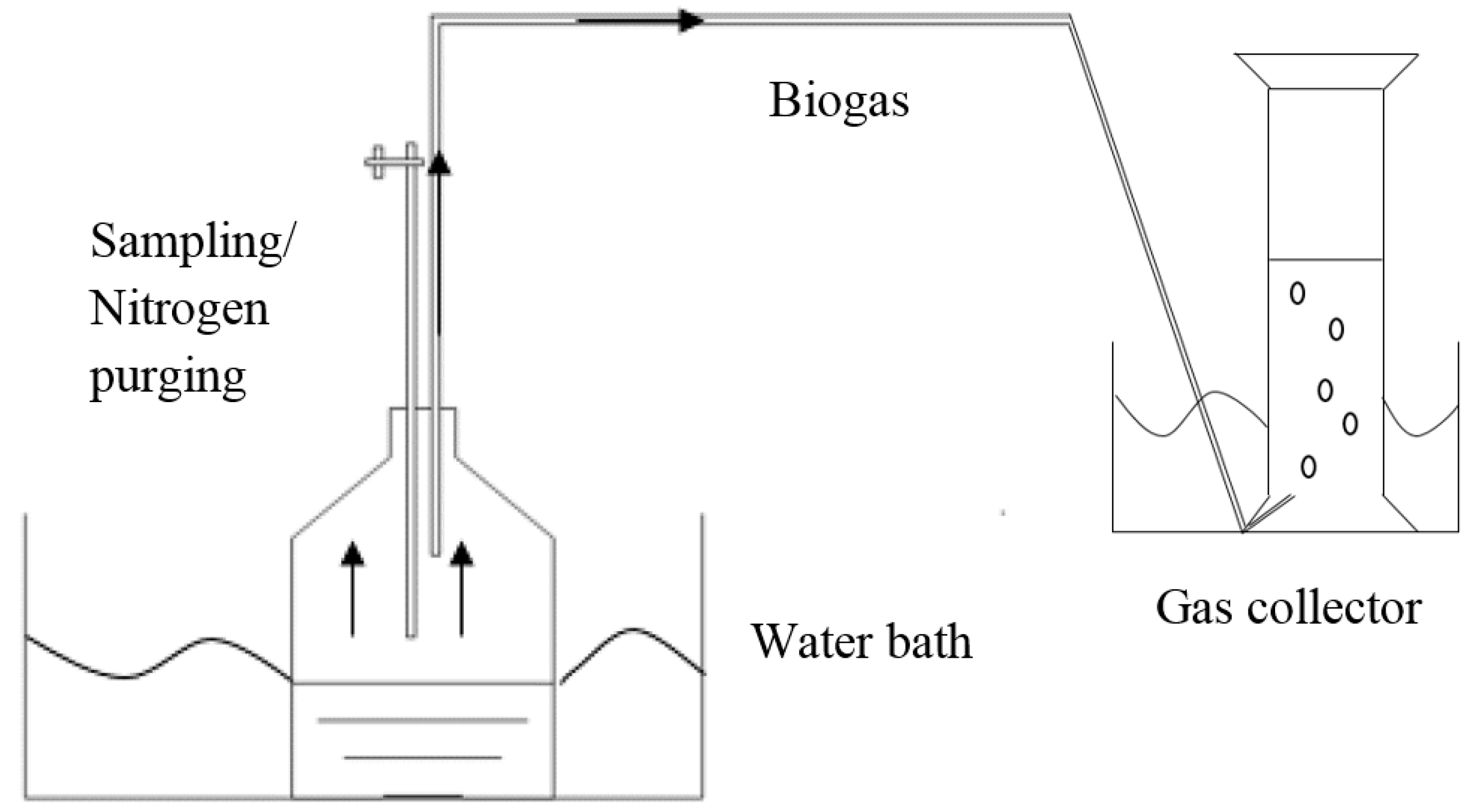
2.2. Anaerobic Digestion Operation
2.3. Start-Up Operation
2.4. Analytical Method
2.5. Microbial Community Analysis
2.6. Heat Requirement and Heat Loss during Anaerobic Digestion
3. Results and Discussion
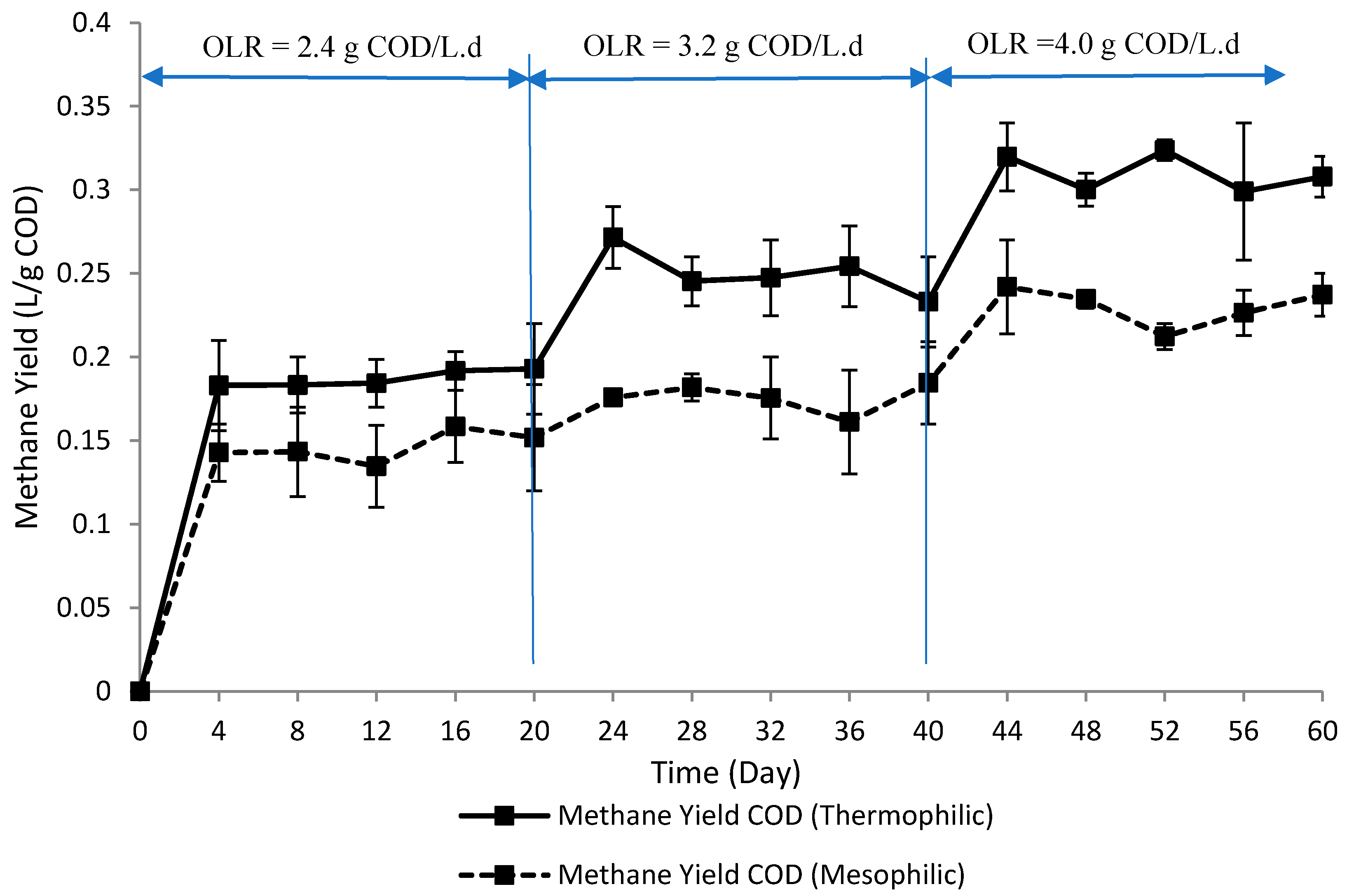
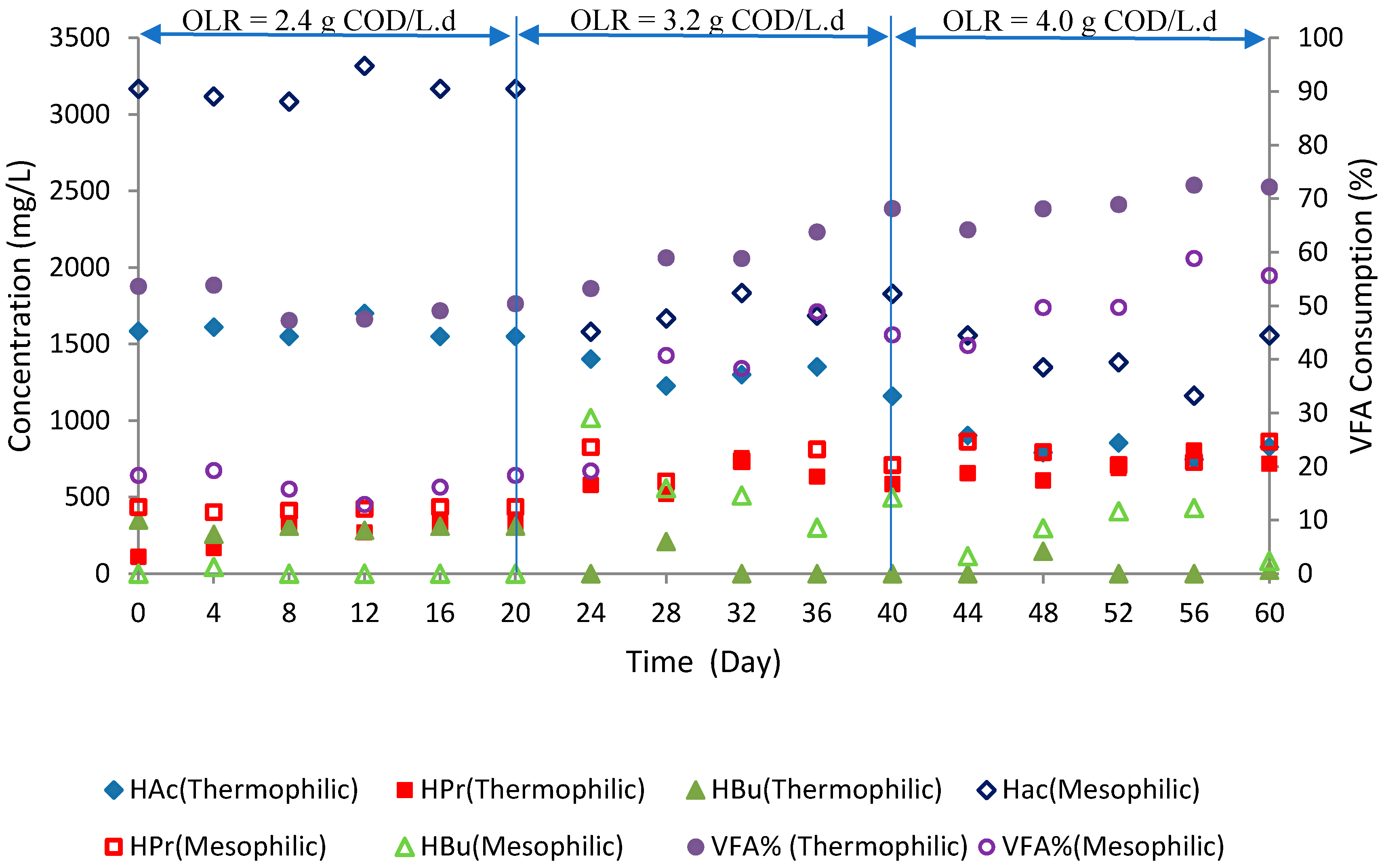
3.1. Methane Yield
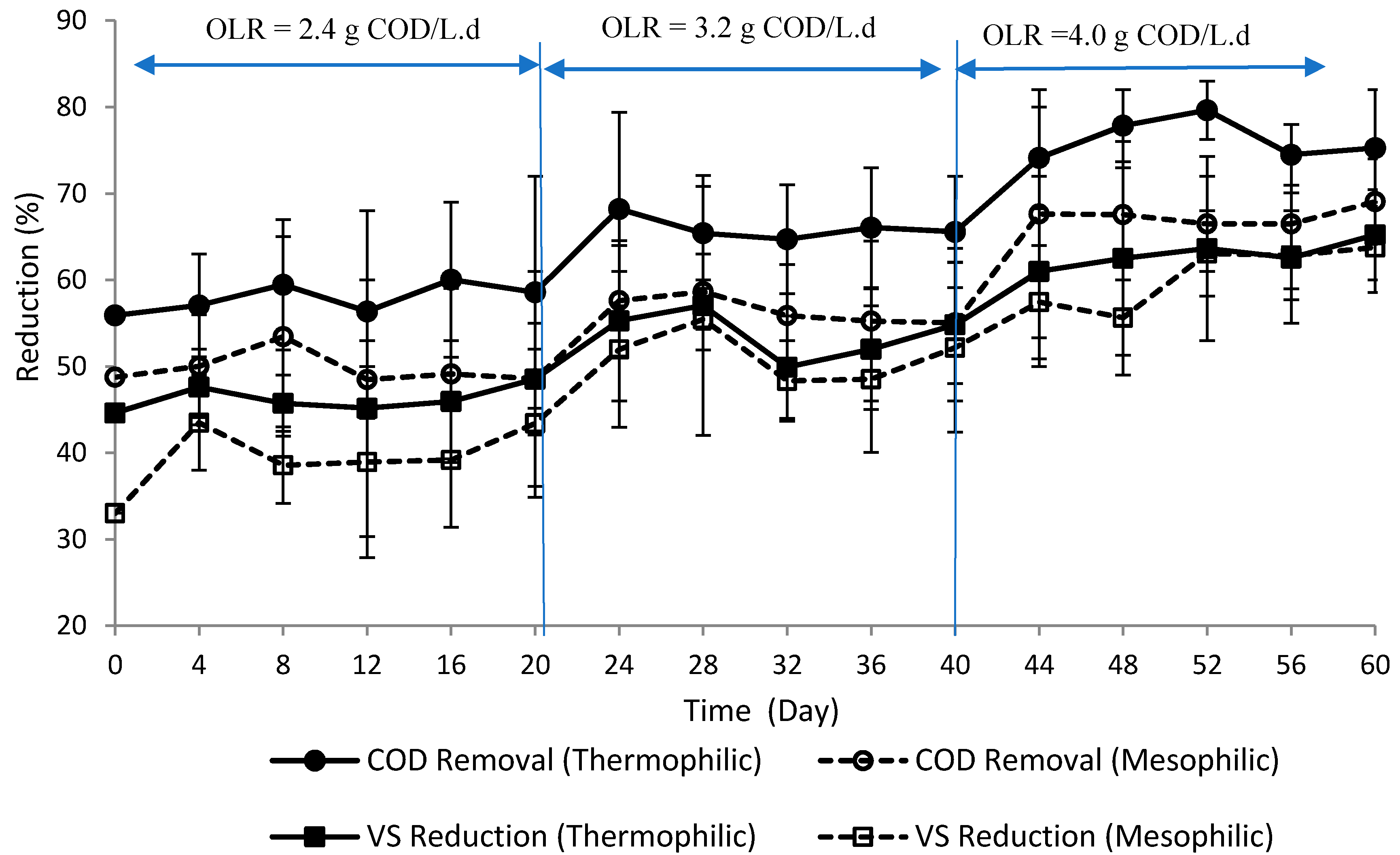
3.2. Substrate Removal
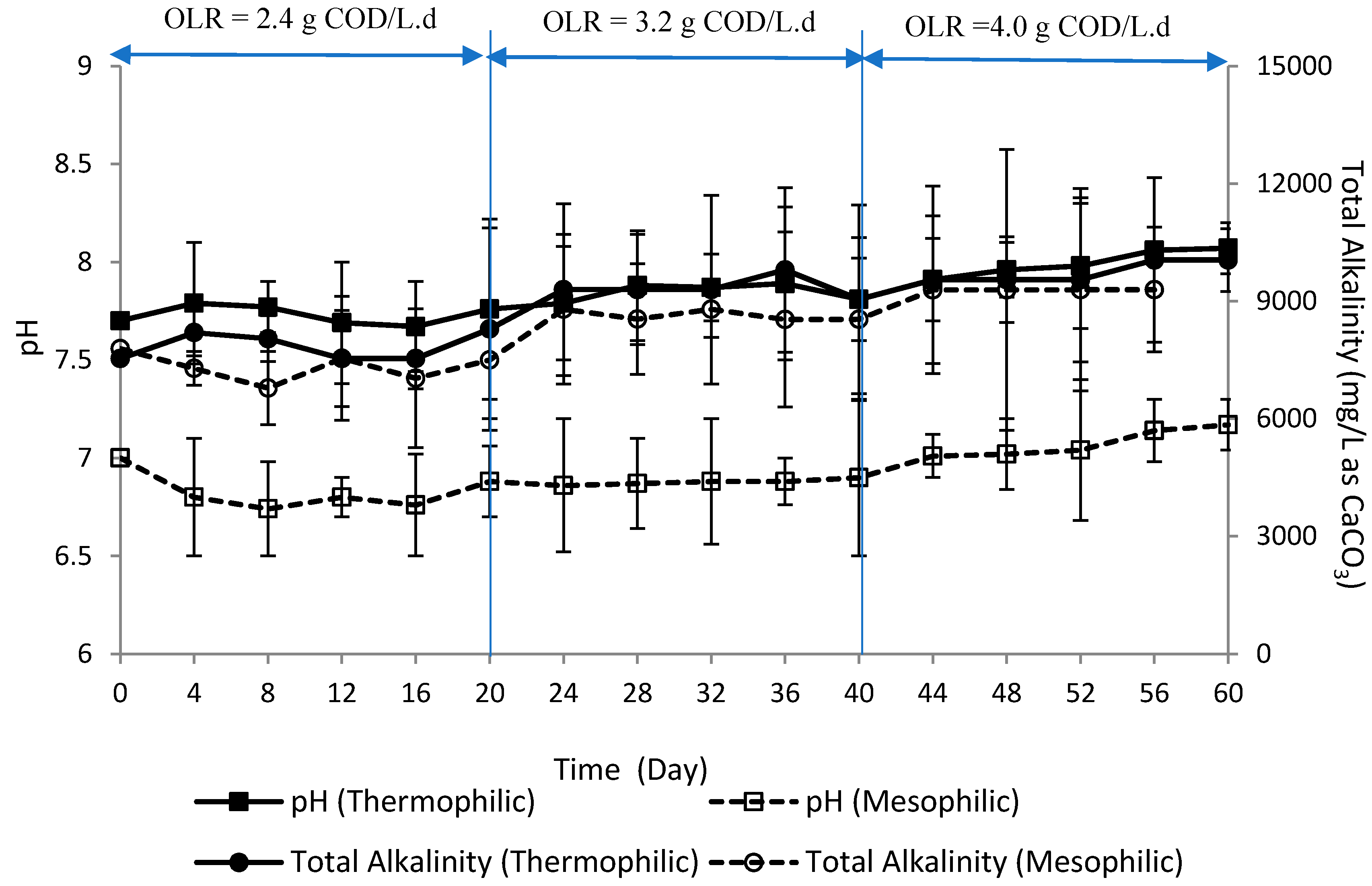
3.3. Total Alkalinity and pH
3.4. Metabolite Production
3.5. Microbial Community at Best Performance OLR
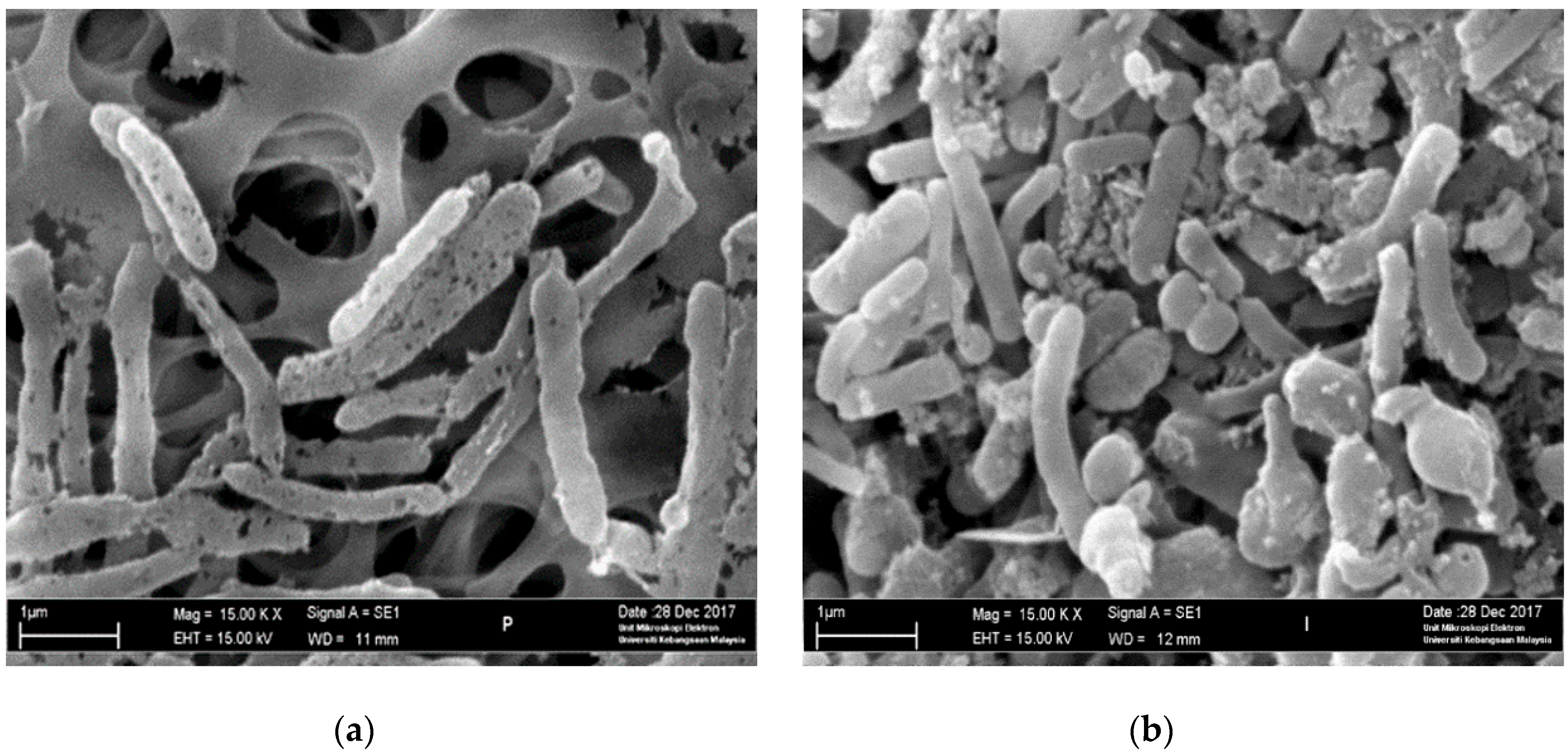
3.6. Scanning Electronic Microscopy
3.7. Overall Performance and Energy Requirement
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mahmod, S.S.; Jahim, J.M.; Abdul, P.M. Pretreatment Conditions of Palm Oil Mill Effluent (POME) for Thermophilic Biohydrogen Production by Mixed Culture. Int. J. Hydrogen Energy 2017, 42, 27512–27522. [Google Scholar] [CrossRef]
- Choorit, W.; Wisarnwan, P. Effect of Temperature on the Anaerobic Digestion of Palm Oil Mill Effluent. Electron. J. Biotechnol. 2007, 10, 376–385. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Feasibility Study of Biogas Production and Utilization as a Source of Renewable Energy in Malaysia. Renew. Sustain. Energy Rev. 2013, 19, 454–462. [Google Scholar] [CrossRef]
- Chan, Y.J.; Chong, M.F. Palm Oil Mill Effluent (POME) Treatment—Current Technologies, Biogas Capture and Challenges; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Yanti, F.M.; Hastuti, Z.D.; Murti, S.D.S.; Valentino, N.; Sholihah, A.; Juwita, A.R. Utilization Palm Oil Mill Effluent for Biogas Using Continous-Stirred-Tank-Reactor: Production and Biogas Cleaning. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2019; Volume 2097. [Google Scholar] [CrossRef]
- Hamzah, M.A.F.; Jahim, J.; Abdul, P.M.; Asis, A.J. Investigation of Temperature Effect on Start-Up Operation from Anaerobic Digestion of Acidified. Energies 2019, 12, 2473. [Google Scholar] [CrossRef]
- Mamimin, C.; Singkhala, A.; Kongjan, P. Two-Stage Thermophilic Fermentation and Mesophilic Methanogen Process for Biohythane Production from Palm Oil Mill Effluent. Int. J. Hydrogen Energy 2015, 40, 6319–6328. [Google Scholar] [CrossRef]
- Wu, T.Y.; Mohammad, A.W.; Jahim, J.M.; Anuar, N. Pollution Control Technologies for the Treatment of Palm Oil Mill Effluent (POME) through End-of-Pipe Processes. J. Environ. Manag. 2010, 91, 1467–1490. [Google Scholar] [CrossRef]
- Kamarudin, S.K.; Muniandy, A.; Shamsul, N.S.; Kofli, H.T. Enhanced Biogas Production from Agro Wastes by Co-Digestion with Crude Glycerol. J. Kejuruter. 2018, 1, 47–57. [Google Scholar] [CrossRef]
- Rabii, A.; Aldin, S.; Dahman, Y.; Elbeshbishy, E. A Review on Anaerobic Co-Digestion with a Focus on the Microbial Populations and the Effect of Multi-Stage Digester Configuration. Energies 2019, 12, 1106. [Google Scholar] [CrossRef]
- Vindis, P.; Mursec, B.; Janzekovic, M.; Cus, F. The Impact of Mesophilic and Thermophilic Anaerobic Digestion on Biogas Production. J. Achiev. Mater. Manuf. Eng. 2009, 36, 192–198. [Google Scholar]
- Meegoda, J.N.; Li, B.; Patel, K.; Wang, L.B. A Review of the Processes, Parameters, and Optimization of Anaerobic Digestion. Int. J. Environ. Res. Public Health 2018, 15, 2224. [Google Scholar] [CrossRef]
- Maaroff, R.M.; Md Jahim, J.; Azahar, A.M.; Abdul, P.M.; Masdar, M.S.; Nordin, D.; Nasir, M.A.A. Biohydrogen Production from Palm Oil Mill Effluent (POME) by Two Stage Anaerobic Sequencing Batch Reactor (ASBR) System for Better Utilization of Carbon Sources in POME. Int. J. Hydrogen Energy 2019, 44, 3395–3406. [Google Scholar] [CrossRef]
- Badiei, M.; Jahim, J.; Anuar, N.; Rozaimah, S.; Abdullah, S. Effect of Hydraulic Retention Time on Biohydrogen Production from Palm Oil Mill Effluent in Anaerobic Sequencing Batch Reactor. Int. J. Hydrogen Energy 2011, 36, 5912–5919. [Google Scholar] [CrossRef]
- Jeong, J.-Y.; Son, S.-M.; Pyon, J.-H.; Park, J.-Y. Performance Comparison between Mesophilic and Thermophilic Anaerobic Reactors for Treatment of Palm Oil Mill Effluent. Bioresour. Technol. 2014, 165, 122–128. [Google Scholar] [CrossRef]
- Krishnan, S.; Singh, L.; Sakinah, M.; Thakur, S.; Wahid, Z.A.; Sohaili, J. Effect of Organic Loading Rate on Hydrogen (H2) and Methane (CH4) Production in Two-Stage Fermentation under Thermophilic Conditions Using Palm Oil Mill Effluent (POME). Energy Sustain. Dev. 2016, 34, 130–138. [Google Scholar] [CrossRef]
- Abdullah, M.F.; Md Jahim, J.; Abdul, P.M.; Mahmod, S.S. Effect of Carbon/Nitrogen Ratio and Ferric Ion on the Production of Biohydrogen from Palm Oil Mill Effluent (POME). Biocatal. Agric. Biotechnol. 2020, 23, 101445. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Roussel, E.G.; Sauvadet, A.L.; Chaduteau, C.; Fouquet, Y.; Charlou, J.L.; Prieur, D.; Cambon Bonavita, M.A. Archaeal Communities Associated with Shallow to Deep Subseafloor Sediments of the New Caledonia Basin. Environ. Microbiol. 2009, 11, 2446–2462. [Google Scholar] [CrossRef]
- Nübel, U.; Engelen, B.; Felsre, A.; Snaidr, J.; Wieshuber, A.; Amann, R.I.; Ludwig, W.; Backhaus, H. Sequence Heterogeneities of Genes Encoding 16S RRNAs in Paenibacillus Polymyxa Detected by Temperature Gradient Gel Electrophoresis. J. Bacteriol. 1996, 178, 5636–5643. [Google Scholar] [CrossRef]
- Mohd Yasin, N.H.; Rahman, N.A.; Man, H.C.; Mohd Yusoff, M.Z.; Hassan, M.A. Microbial Characterization of Hydrogen-Producing Bacteria in Fermented Food Waste at Different PH Values. Int. J. Hydrogen Energy 2011, 36, 9571–9580. [Google Scholar] [CrossRef]
- Maidak, B.L.; Olsen, G.J.; Larsen, N.; Overbeek, R.; McCaughey, M.J.; Woese, C.R. The Ribosomal Database Project (RDP). Nucleic Acids Res. 1997, 25, 109–110. [Google Scholar] [CrossRef]
- Nordlander, E.; Olsson, J.; Thorin, E.; Nehrenheim, E. Simulation of Energy Balance and Carbon Dioxide Emission for Microalgae Introduction in Wastewater Treatment Plants. Algal Res. 2017, 24, 251–260. [Google Scholar] [CrossRef]
- Zupančič, G.D.; Roš, M. Heat and Energy Requirements in Thermophilic Anaerobic Sludge Digestion. Renew. Energy 2003, 28, 2255–2267. [Google Scholar] [CrossRef]
- Davis, M.L. Water and Wastewater Engineering: Design Principles and Practice; McGraw-Hill Education: New York, NY, USA, 2010. [Google Scholar]
- Musa, M.A.; Idrus, S.; Hasfalina, C.M.; Daud, N.N.N. Effect of Organic Loading Rate on Anaerobic Digestion Performance of Mesophilic (UASB) Reactor Using Cattle Slaughterhouse Wastewater as Substrate. Int. J. Environ. Res. Public Health 2018, 15, 2220. [Google Scholar] [CrossRef] [PubMed]
- Varel, V.H.; Chen, T.H.; Hashimoto, A.G. Thermophilic and Mesophilic Methane Production from Anaerobic Degradation of the Cyanobacterium Spirulina Maxima. Resour. Conserv. Recycl. 1988, 1, 19–26. [Google Scholar] [CrossRef]
- Fang, H.H.P.; Chui, H.K. Comparison of Startup Performance of Four Anaerobic Reactors for the Treatment of High-Strength Wastewater. Resour. Conserv. Recycl. 1994, 11, 123–138. [Google Scholar] [CrossRef]
- Kanimozhi, R.; Vasudevan, N. An Overview of Wastewater Treatment in Distillery Industry. Int. J. Environ. Eng. 2010, 2, 159–184. [Google Scholar] [CrossRef]
- Westerholm, M.; Schnürer, A. Microbial Responses to Different Operating Practices for Biogas Production Systems. In Anaerobic Digestion; In Tech: Vienna, Austria, 2019. [Google Scholar] [CrossRef]
- Cavinato, C.; Bolzonella, D.; Pavan, P.; Fatone, F.; Cecchi, F. Mesophilic and Thermophilic Anaerobic Co-Digestion of Waste Activated Sludge and Source Sorted Biowaste in Pilot- and Full-Scale Reactors. Renew. Energy 2013, 55, 260–265. [Google Scholar] [CrossRef]
- Nasir, M.A.A.; Jahim, J.; Mohamed, P.; Silvamany, H.; Muzhafar, R.; Yunus, M.F.M. The Use of Acidified Palm Oil Mill Effluent for Thermophilic Biomethane Production by Changing the Hydraulic Retention Time in Anaerobic Sequencing Batch Reactor. Int. J. Hydrogen Energy 2019, 44, 3373–3381. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, J.; Meng, L. Effects of Volatile Fatty Acid Concentrations on Methane Yield and Methanogenic Bacteria. Biomass Bioenergy 2009, 33, 848–853. [Google Scholar] [CrossRef]
- Babaee, A.; Shayegan, J. Effect of Organic Loading Rates (OLR) on Production of Methane from Anaerobic Digestion of Vegetables Waste. In Proceedings of the World Renewable Energy Congress-Sweden, Linköping, Sweden, 8–13 May 2011; Volume 57, pp. 411–417. [Google Scholar] [CrossRef]
- Khanal, S.K. Anaerobic Biotechnology for Bioenergy Production: Principles and Applications, 1st ed.; Wiley-Blackwell: Iowa, IA, USA, 2009. [Google Scholar]
- Najafpour, G.D.; Zinatizadeh, A.A.L.; Mohamed, A.R.; Hasnain Isa, M.; Nasrollahzadeh, H. High-Rate Anaerobic Digestion of Palm Oil Mill Effluent in an Upflow Anaerobic Sludge-Fixed Film Bioreactor. Process Biochem. 2006, 41, 370–379. [Google Scholar] [CrossRef]
- Yu, G.H.; He, P.J.; Shao, L.M.; He, P.P. Toward Understanding the Mechanism of Improving the Production of Volatile Fatty Acids from Activated Sludge at PH 10.0. Water Res. 2008, 42, 4637–4644. [Google Scholar] [CrossRef]
- Demirel, B.; Yenigün, O. Two-Phase Anaerobic Digestion Processes: A Review. J. Chem. Technol. Biotechnol. 2002, 77, 743–755. [Google Scholar] [CrossRef]
- Junicke, H.; van Loosdrecht, M.C.M.; Kleerebezem, R. Kinetic and Thermodynamic Control of Butyrate Conversion in Non-Defined Methanogenic Communities. Appl. Microbiol. Biotechnol. 2016, 100, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Inanc, B.; Matsui, S.; Ide, S. Propionic Acid Accumulation in Anaerobic Digestion of Carbohydrates: An Investigation on the Role of Hydrogen Gas. Water Sci. Technol. 1999, 40, 93–100. [Google Scholar] [CrossRef]
- Ziels, R.M.; Beck, D.A.C.; Martí, M.; Gough, H.L.; Stensel, H.D.; Svensson, B.H. Monitoring the Dynamics of Syntrophic β-Oxidizing Bacteria during Anaerobic Degradation of Oleic Acidby Quantitative PCR. FEMS Microbiol. Ecol. 2015, 91, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mahmod, S.S.; Azahar, A.M.; Amru, A.; Luthfi, I.; Jahim, J.M.D. Potential Utilisation of Dark- Fermented Palm Oil Mill Effluent in Continuous Production of Biomethane by Self-Granulated Mixed Culture. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Franke-Whittle, I.H.; Walter, A.; Ebner, C.; Insam, H. Investigation into the Effect of High Concentrations of Volatile Fatty Acids in Anaerobic Digestion on Methanogenic Communities. Waste Manag. 2014, 34, 2080–2089. [Google Scholar] [CrossRef]
- Kim, Y.M.; Jang, H.M.; Lee, K.; Chantrasakdakul, P.; Kim, D.; Park, K.Y. Changes in Bacterial and Archaeal Communities in Anaerobic Digesters Treating Different Organic Wastes. Chemosphere 2015, 141, 134–137. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Zakaria, M.R.; Rahim, R.A.; Wright, A.D.G.; Shirai, Y.; Abdullah, N.; Sakai, K.; Ikeno, S.; Mori, M.; Kazunori, N.; et al. PCR-Based DGGE and FISH Analysis of Methanogens in an Anaerobic Closed Digester Tank for Treating Palm Oil Mill Effluent. Electron. J. Biotechnol. 2009, 12. [Google Scholar] [CrossRef]
- Conklin, A.; Stensel, H.D.; Ferguson, J. Growth Kinetics and Competition Between Methanosarcina and Methanosaeta in Mesophilic Anaerobic Digestion. Water Environ. Res. 2006, 78, 486–496. [Google Scholar] [CrossRef]
- Patil, S.S.; Kumar, M.S.; Ball, A.S. Microbial Community Dynamics in Anaerobic Bioreactors and Algal Tanks Treating Piggery Wastewater. Appl. Microbiol. Biotechnol. 2010, 87, 353–363. [Google Scholar] [CrossRef]
- David, A.; Govil, T.; Tripathi, A.K.; McGeary, J.; Farrar, K.; Sani, R.K. Thermophilic Anaerobic Digestion: Enhanced and Sustainable Methane Production from Co-Digestion of Food and Lignocellulosic Wastes. Energies 2018, 11, 2058. [Google Scholar] [CrossRef]
- Kor-bicakci, G.; Ubay-cokgor, E.; Eskicioglu, C. Comparative Analysis of Bacterial and Archaeal Community Structure in Microwave Pretreated Thermophilic and Mesophilic Anaerobic Digesters Utilizing Mixed Sludge under Organic Overloading. Water 2020, 12, 887. [Google Scholar] [CrossRef]
- Kouzuma, A.; Tsutsumi, M.; Ishii, S.; Ueno, Y.; Abe, T.; Watanabe, K. Non-Autotrophic Methanogens Dominate in Anaerobic Digesters. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Krakat, N.; Westphal, A.; Schmidt, S.; Scherer, P. Anaerobic Digestion of Renewable Biomass: Thermophilic Temperature Governs Methanogen Population Dynamics. Appl. Environ. Microbiol. 2010, 76, 1842–1850. [Google Scholar] [CrossRef] [PubMed]
- Enitan, A.M.; Kumari, S.; Swalaha, F.M.; Odiyo, J.O.; Bux, F. Microbiota of a Full-Scale UASB Reactor Treating Brewery Wastewater Using Illumina MiSeq Sequencing. Open Microbiol. J. 2019, 13, 1–9. [Google Scholar] [CrossRef]
- Zellner, G.; Macario, A.J.L.; Conway De Macario, E. Microbial Subpopulations in the Biofilm Attached to the Substratum and in the Free Flocs of a Fixed-Bed Anaerobic Bioreactor. Appl. Microbiol. Biotechnol. 1996, 46, 443–449. [Google Scholar] [CrossRef]
- Boone, D.R.; Whitman, W.B.; Koga, Y. The Archaea and the Deeply Branching and Phototrophic Bacteria. In Bergey’s Manual of Systematic Bacteriology; Springer-Verlag: New York, NY, USA, 2001; Volume 1, pp. 268–294. [Google Scholar]
- Kern, T.; Fischer, M.A.; Deppenmeier, U.; Schmitz, R.A.; Rother, M. Methanosarcina flavescens sp. nov., a Methanogenic Archaeon Isolated from a Full-Scale Anaerobic Digester. Int. J. Syst. Evol. Microbiol. 2016, 66, 1533–1538. [Google Scholar] [CrossRef]
- Borja, R.; Banks, C.J.; Khalfaoui, B.; Martín, A. Performance Evaluation of an Anaerobic Hybrid Digester Treating Palm Oil Mill Effluent. J. Environ. Sci. Health Part A 1996, 31, 1379–1393. [Google Scholar] [CrossRef]
- Amani, T.; Nosrati, M.; Sreekrishnan, T.R. A Precise Experimental Study on Key Dissimilarities between Mesophilic and Thermophilic Anaerobic Digestion of Waste Activated Sludge. Int. J. Environ. Res. 2011, 5, 333–342. [Google Scholar] [CrossRef]

| ID | Parameter | Concentration Range |
|---|---|---|
| Acidified POME | ||
| 1 | pH | 5.08 ± 0.1 |
| 2 | Chemical oxygen demand (g/L) | 35.5 ± 3.3 |
| 3 | Total solid (g/L) | 31.0 ± 3.6 |
| 4 | Total suspended solid (g/L) | 24.1 ± 4.2 |
| 5 | Volatile suspended solid (g/L) | 19.0 ± 5.0 |
| 6 | Volatile fatty acid (mg/L as CH3COOH) | 6703.6 ± 1515.1 |
| 7 | Total nitrogen (mg/L) | 263 ± 28 |
| Mesophilic | Thermophilic | ||||
|---|---|---|---|---|---|
| Band | Nearest Relative | Accession | Band | Nearest Relative | Accession |
| 1 | Methanosarcina thermophila | CP009502 | 1 | Methanothermobacter crinale sp. | HQ293273 |
| 2 | Uncultured Methanothermobacter sp. | MF471083 | 2 | Methanothermobacter sp. | JN983061 |
| 3 | Methanothermobacter sp. | JN983061 | 3 | Methanothermobacter crinale sp. | HQ293273 |
| 4 | Uncultured Methanosarcina sp. | KU661854 | 4 | Methanothermobacter crinale sp. | HQ293273 |
| 5 | Methanosarcina thermophila | MH708238 | 5 | Methanothermobacter crinale sp. | HQ293273 |
| 6 | Methanosarcina thermophila | MG008505 | 6 | Methanothermobacter tenebrarum | AB5237865 |
| 7 | Methanothermobacter sp. | JN983061 | |||
| 8 | Methanothermobacter tenebrarum | AB5237865 | |||
| Substrate | Temperature | Best OLR Performance (g COD/L.d) | Methane Yield | COD Removal (%) | Reference |
|---|---|---|---|---|---|
| Acidified POME Acidified POME | Mesophilic | 4.0 | 0.23 | 67.5 | Present study |
| Thermophilic | 4.0 | 0.31 | 76.3 | ||
| POME | Mesophilic | 12.9 | 0.35 | 94.1 | [56] |
| POME | Thermophilic | 12.0 | 0.16 | 85.0 | [16] |
| POME | Thermophilic | 12.9 | 0.20 | 87.8 | [32] |
| Independent Samples Test | |||||||
|---|---|---|---|---|---|---|---|
| Levene’s Test for Equality of Variances | t-test for Equality of Means | ||||||
| F | Sig. | t | df | Sig. (2-tailed) | Mean Difference | ||
| Productivity | Equal variances assumed | 2.503 | 0.125 | 3.884 | 28 | 0.001 | 0.065 |
| Equal variances not assumed | 3.884 | 25 | 0.001 | 0.065 | |||
| Group Statistics | |||||||
| Condition | N | Mean | Std Deviation | Std Error Mean | |||
| Methane yield | Thermophilic | 15 | 0.31 | 0.05 | 0.01 | ||
| Mesophilic | 15 | 0.23 | 0.04 | 0.01 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fikri Hamzah, M.A.; Abdul, P.M.; Mahmod, S.S.; Azahar, A.M.; Jahim, J.M. Performance of Anaerobic Digestion of Acidified Palm Oil Mill Effluent under Various Organic Loading Rates and Temperatures. Water 2020, 12, 2432. https://doi.org/10.3390/w12092432
Fikri Hamzah MA, Abdul PM, Mahmod SS, Azahar AM, Jahim JM. Performance of Anaerobic Digestion of Acidified Palm Oil Mill Effluent under Various Organic Loading Rates and Temperatures. Water. 2020; 12(9):2432. https://doi.org/10.3390/w12092432
Chicago/Turabian StyleFikri Hamzah, Muhammad Arif, Peer Mohamed Abdul, Safa Senan Mahmod, Azratul Madihah Azahar, and Jamaliah Md. Jahim. 2020. "Performance of Anaerobic Digestion of Acidified Palm Oil Mill Effluent under Various Organic Loading Rates and Temperatures" Water 12, no. 9: 2432. https://doi.org/10.3390/w12092432
APA StyleFikri Hamzah, M. A., Abdul, P. M., Mahmod, S. S., Azahar, A. M., & Jahim, J. M. (2020). Performance of Anaerobic Digestion of Acidified Palm Oil Mill Effluent under Various Organic Loading Rates and Temperatures. Water, 12(9), 2432. https://doi.org/10.3390/w12092432







