Isotope Composition of Precipitation, Groundwater, and Surface and Lake Waters from the Plitvice Lakes, Croatia
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Sampling
2.3. Meteorological and Hydrological Data
2.4. Measurement
2.5. Data Analysis
3. Results and Discussion
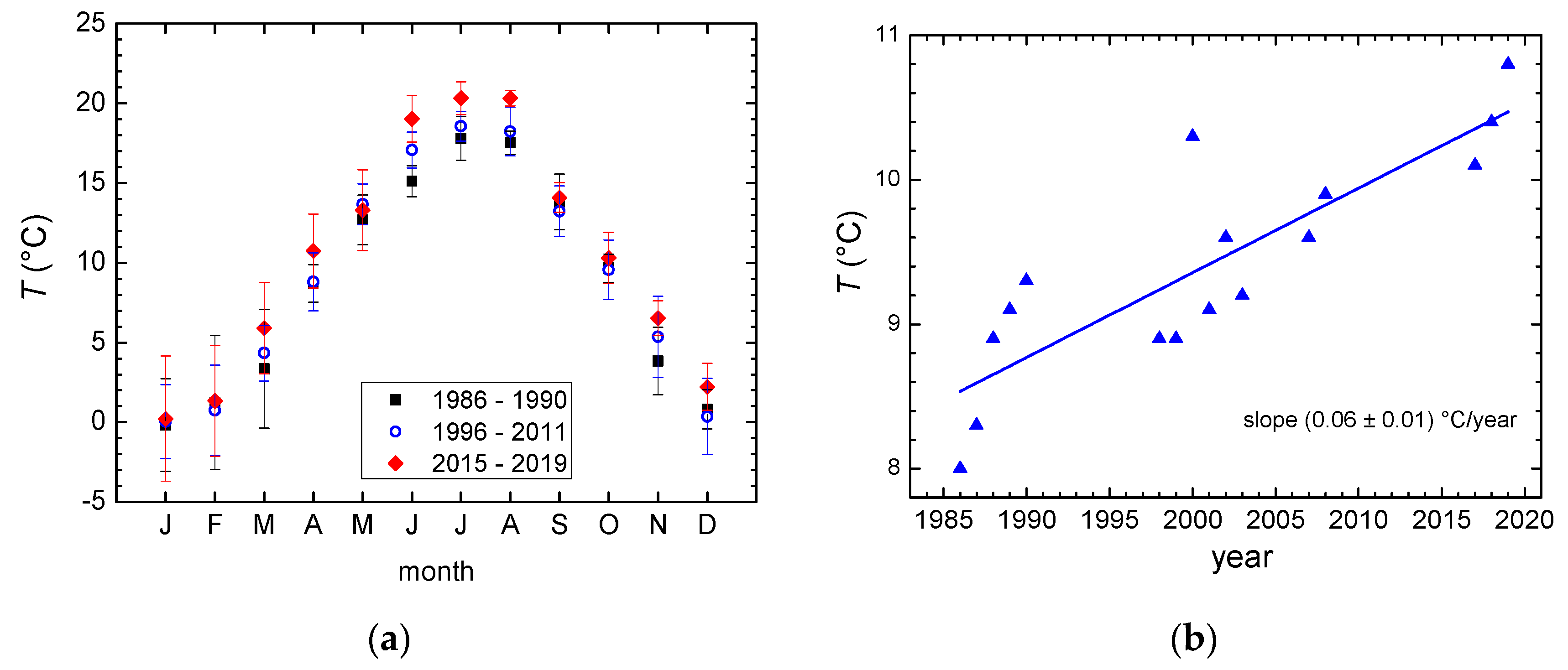
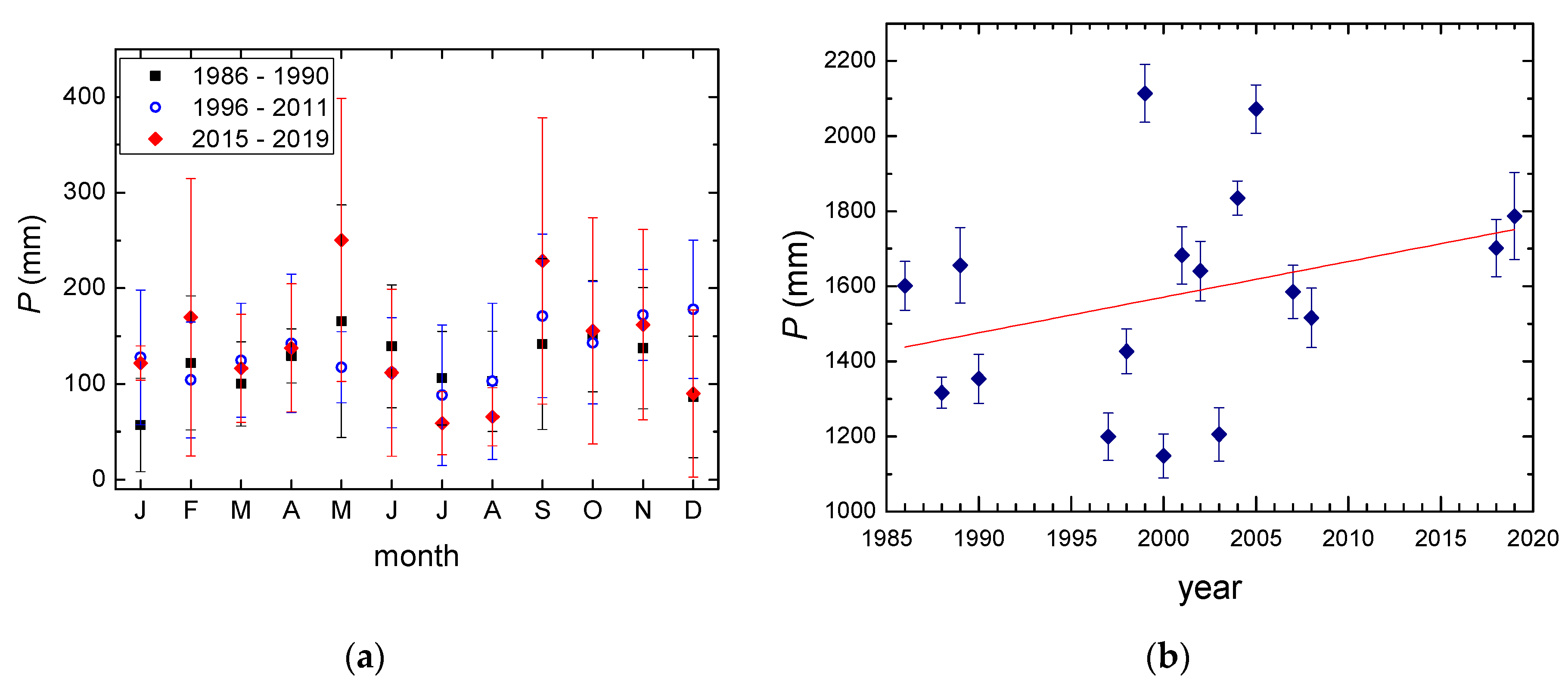
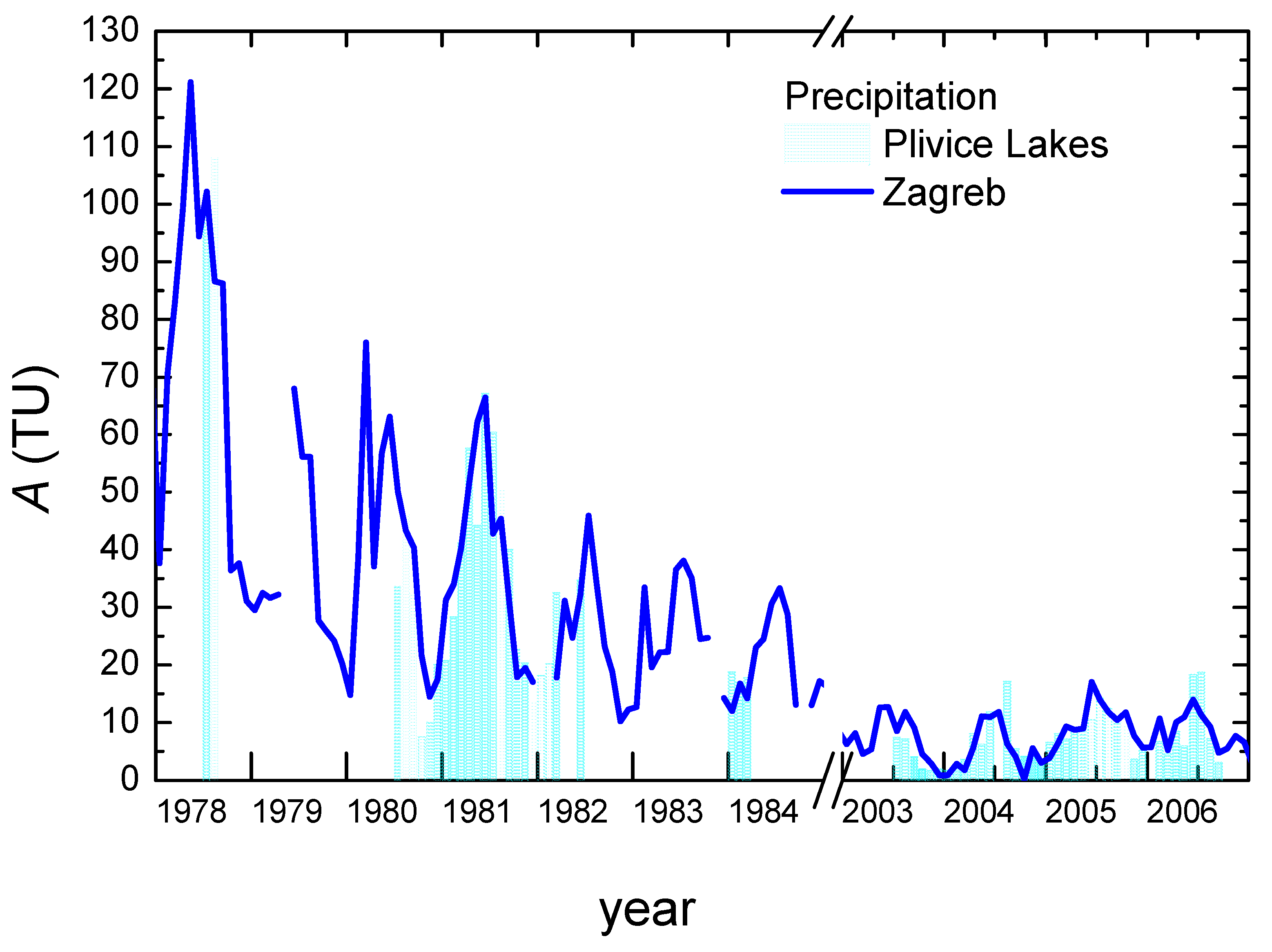
3.1. Temperature and Precipitation Amount
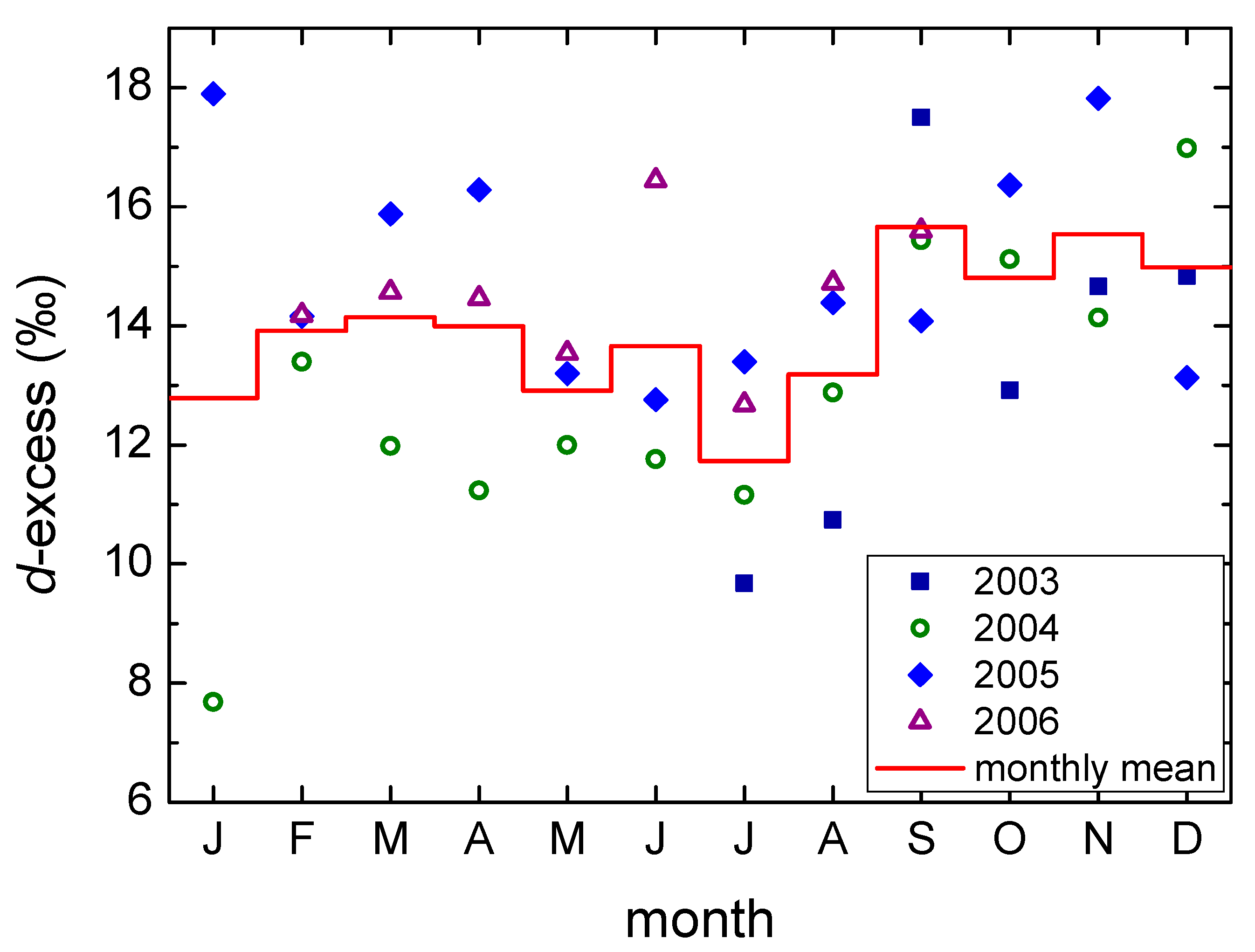
3.2. Isotope Composition of Precipitation
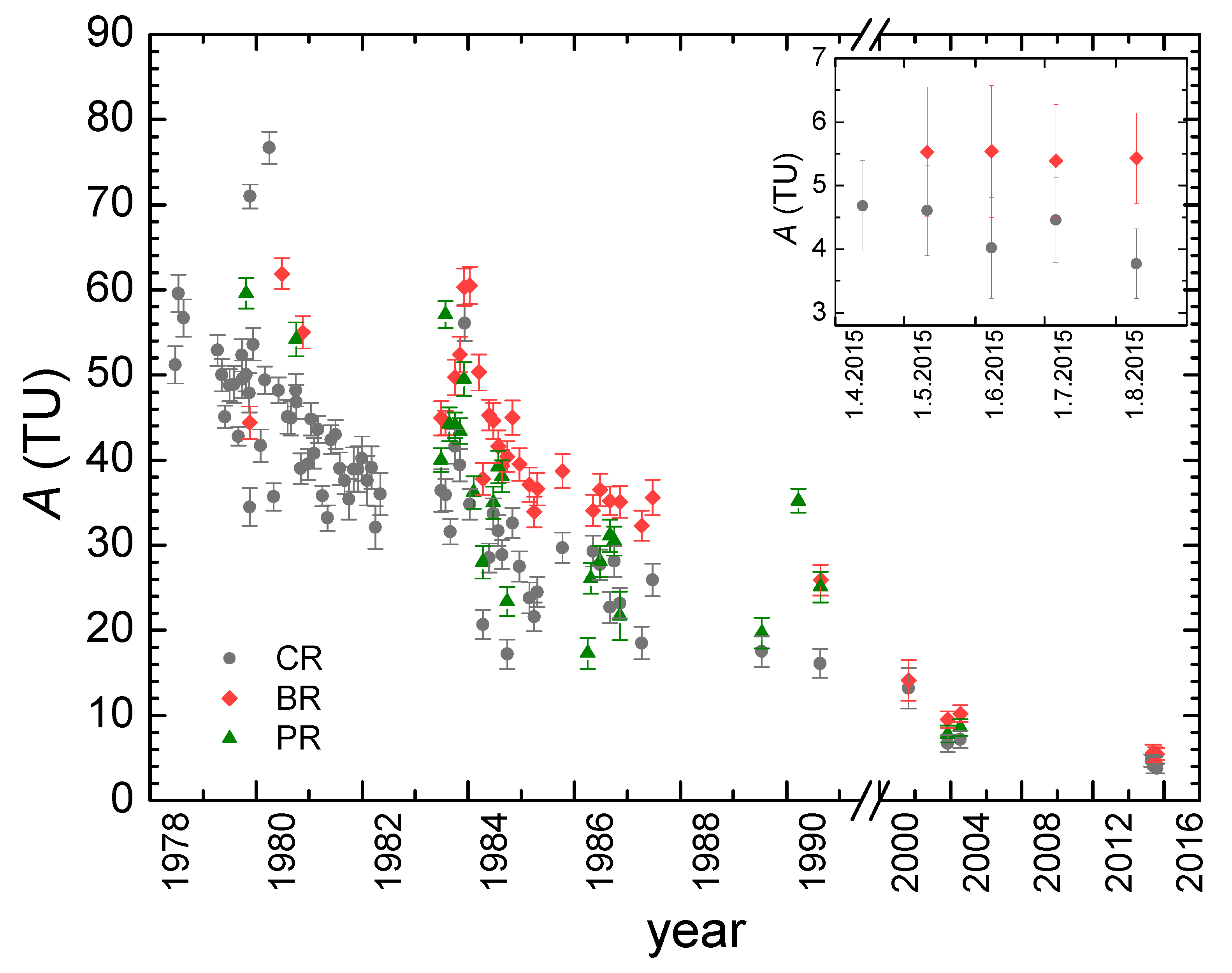
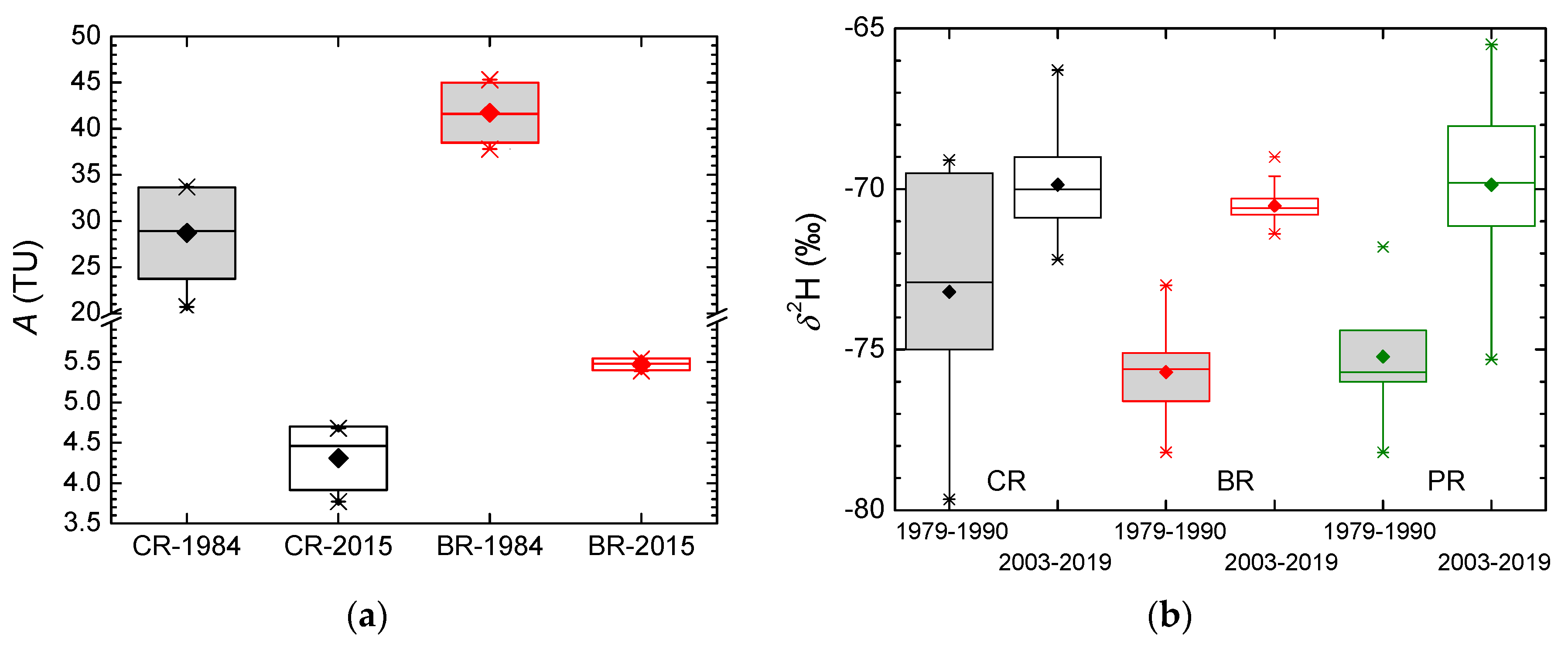
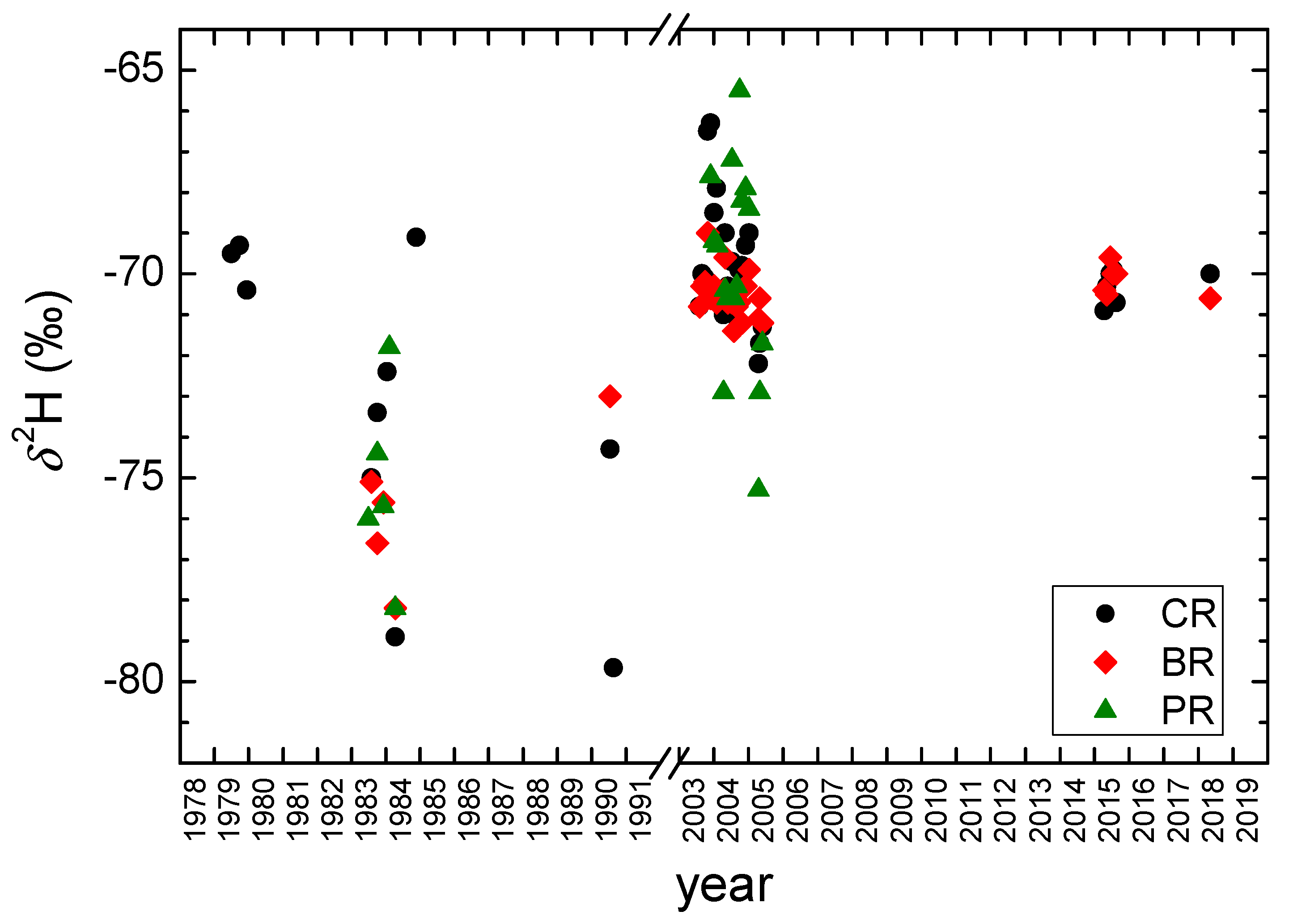
3.3. Groundwater
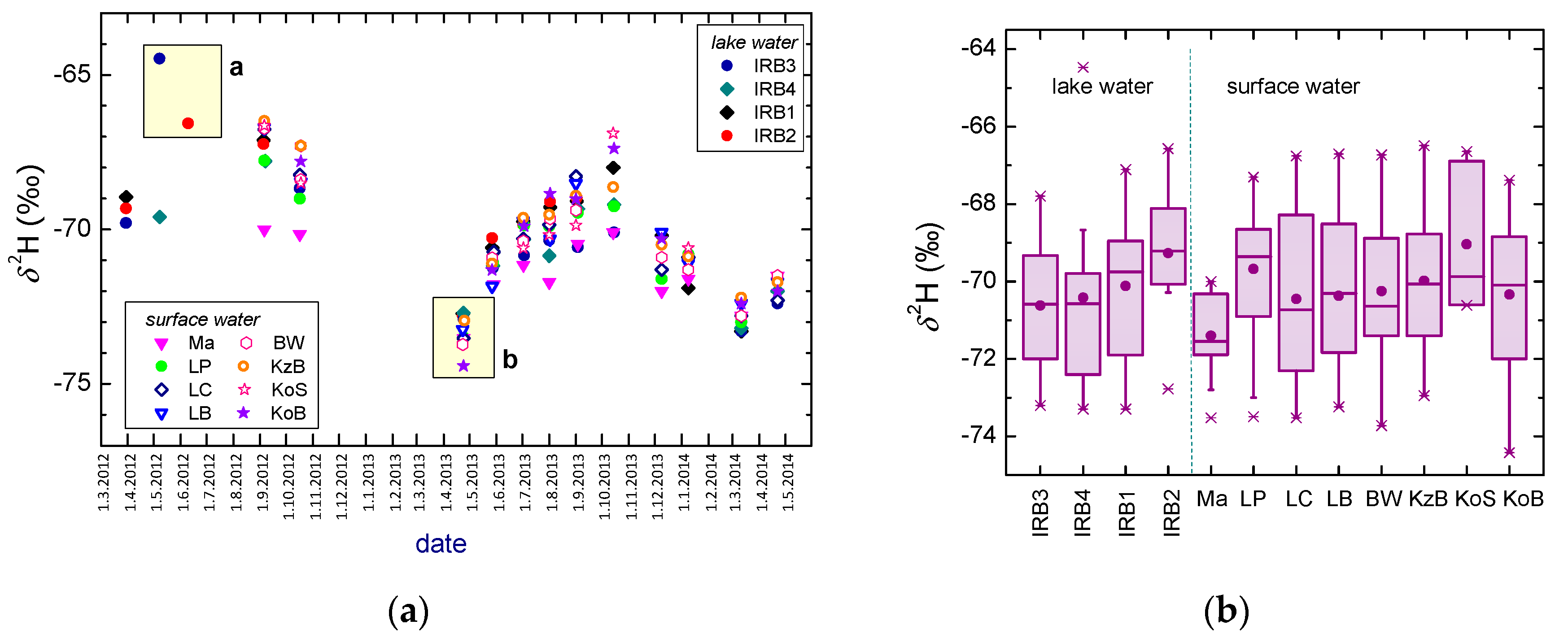
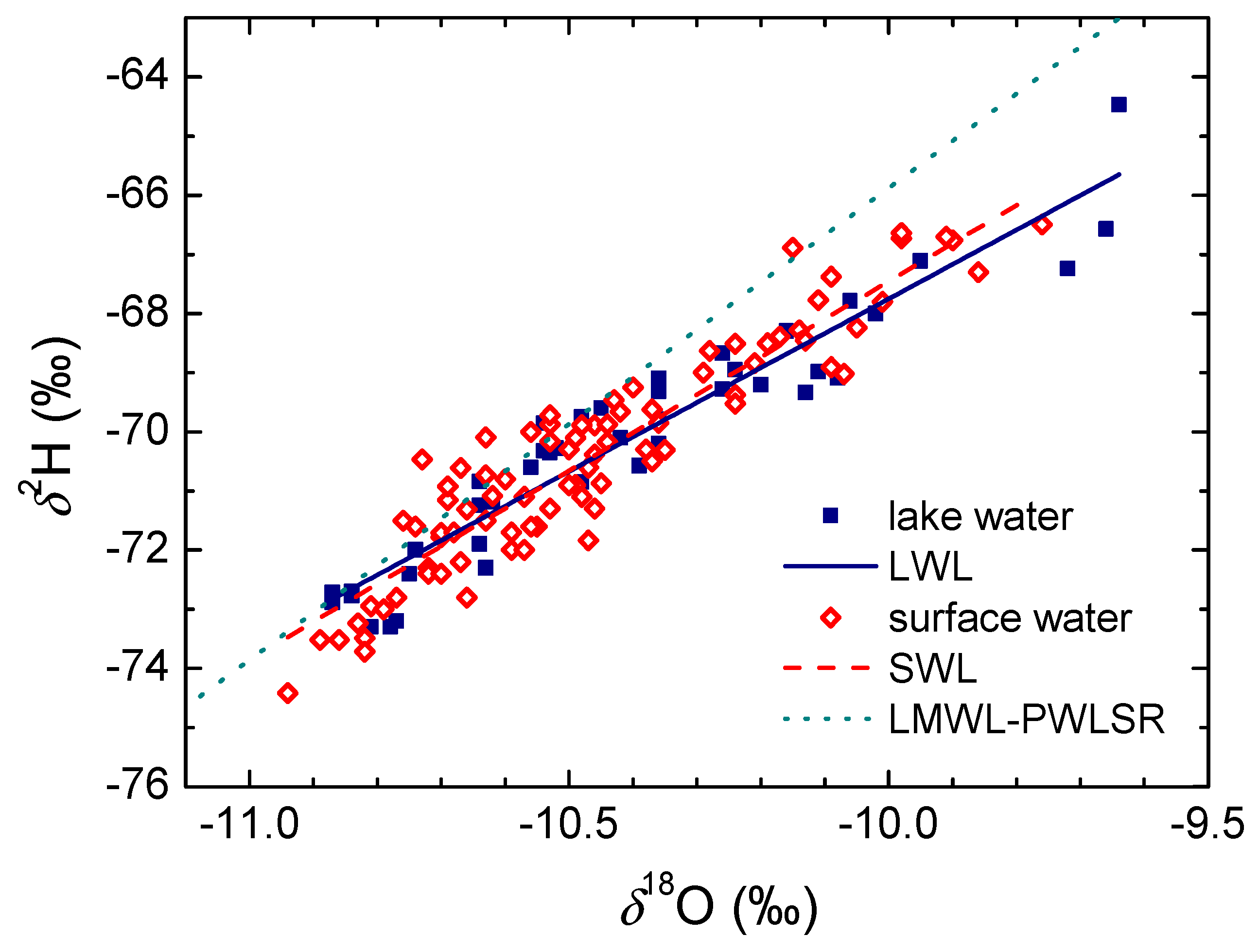
3.4. Surface and Lake Waters
4. Conclusions
- An increase in the mean annual air temperatures of 0.06 °C per year is observed for the period 1986–2019. An increase in the annual precipitation amount is also observed, at about 10 mm per year, and the range of the monthly precipitation amounts is higher in recent years.
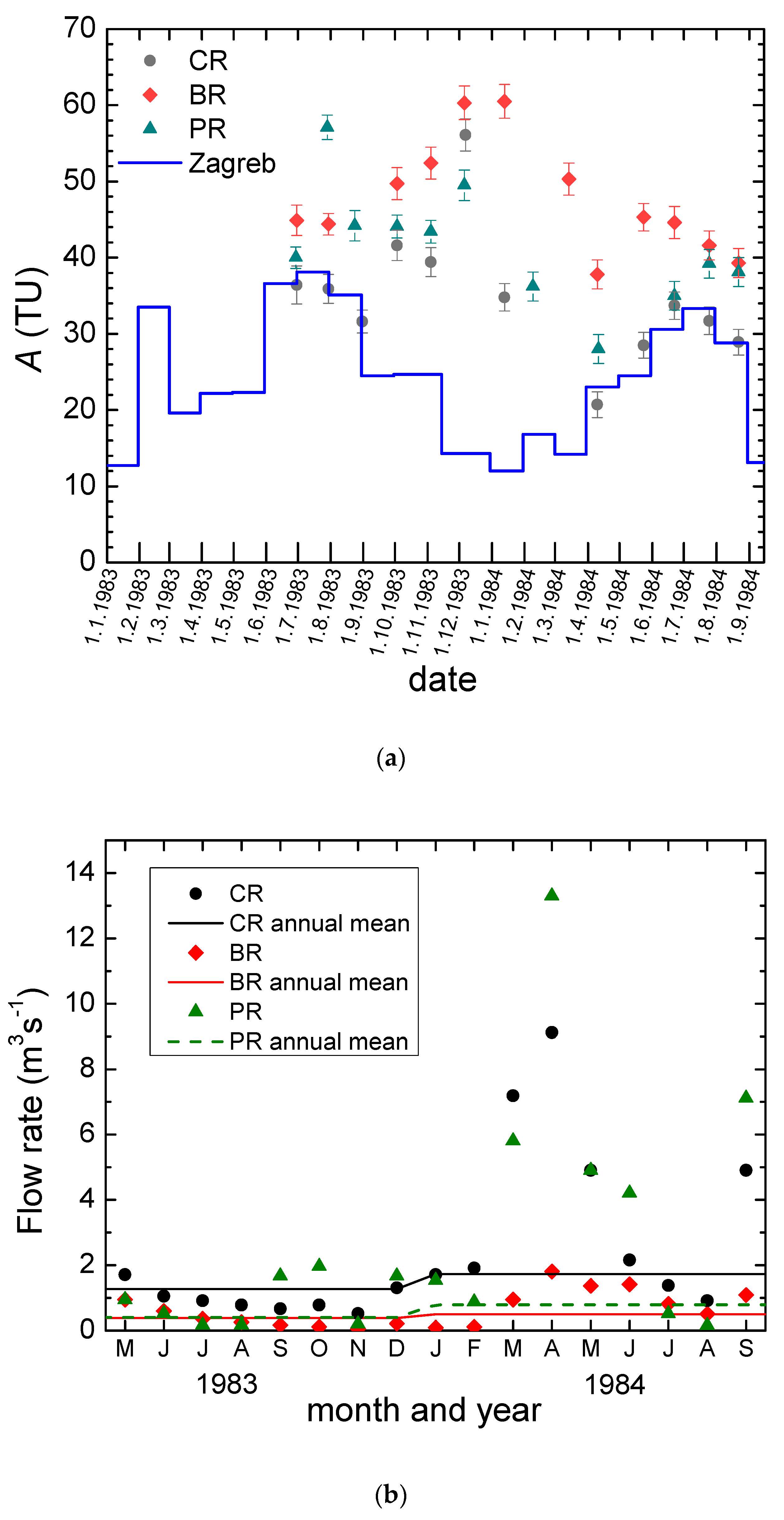
- Tritium activity concentration in the Plitvice Lakes precipitation shows characteristics typical for the northern hemisphere. A good correlation of the tritium activity concentration in the precipitation at the Plitvice Lakes with that in the Zagreb precipitation is observed, implying that the tritium data for Zagreb can be safely used for the study of the PL area if/when data do not exist for the PL precipitation.
- The range of the δ18O in precipitation was about 14‰, and in δ2H it was about 110‰. Various regression approaches for the determination of LMWL were applied, and the best results were obtained by the RMA and PWLSR approaches, which gave also the best results for the stations Zagreb and Zadar. The LMWLPWLSR for PL is δ2H = (7.97 ± 0.12) δ18O + (13.8 ± 1.3), n = 36, for the period 2003–2006. This LMWL lies in between the LMWL for Zagreb and Zadar.
- The deuterium excess for precipitation at the Plitvice Lakes is higher than that for the Zagreb precipitation. This is caused by the combination of higher altitude and the more intense influence of the Mediterranean air masses. The seasonal pattern in d-excess is similar to that in Zagreb, with higher values in the autumn precipitation due to the higher influence of precipitation of Mediterranean origin.
- The ranges in the δ values in groundwaters (at springs) were much narrower than those in precipitation, at less than 1‰ in δ18O and less than 8‰ in δ2H, indicating the good mixing of waters in karst aquifers.
- The higher mean δ values in all three karst springs were observed in recent decades (2003–2019) than in the older period 1979–1990. It can be attributed to the increase in the mean air temperature.
- Different values of mean residence time (MRT) were obtained for three main springs (between 2 years for Crna Rijeka spring and 4 years for Bijela Rijeka spring). Extreme climatological and hydrological conditions in 1983–1984 enabled the estimation of the proportions of precipitation in the spring water: the shorter the MRT, the higher proportions of precipitation.
- The amplitude in the δ2H of surface and lake water is less than 10‰, and < 1.4‰ in δ18O, similar to that in the groundwaters. A slight increase in both the mean δ2H and δ18O values and their seasonal variations is observed for locations along the water course due to the evaporation of surface waters. No difference between lake and surface waters is observed if the mean values are concerned. There is no significant difference observed between the Surface Water Line (SWL) and Lake Water Line (LWL) (2011–2014), both having lower slopes than the LMWLPWLSR obtained for the PL area. The stable isotope composition of the surface and lake waters reacts to the extreme hydrological conditions.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CMHS | Croatian Meteorological and Hydrological Service |
| GMWL | Global Meteoric Water Line |
| GNIP | Global Network of Isotopes in Precipitation |
| GPC | Gas Proportional Counting |
| GWL | Groundwater Water Line |
| IAEA | International Atomic Energy Agency |
| IRMS | Isotope Ratio Mass Spectrometry |
| LMWL | Local Meteoric Water Line |
| LSC-EE | Liquid Scintillation Counting with Electrolytic Enrichment |
| LWL | Lake Water Line |
| MA | Major Axis least squared regression |
| MRT | mean residence time |
| OLSR | Ordinary Least Square Regression |
| PL | Plitvice Lakes |
| PWLSR | Precipitation weighted OLSR |
| PWMA | Precipitation weighted MA |
| PWRMA | Precipitation weighted RMA |
| RBI | Ruđer Bošković Institute |
| RMA | reduced major axis regression |
| rmSSEav | average of the root mean square sum of squared errors |
| SMOW | Standard Mean Ocean Water |
| SWL | Surface Water Line |
| TU | Tritium Unit |
| UNESCO | The United Nations Educational, Scientific and Cultural Organisation |
| WMO | World Meteorological Organization |
References
- Bradley, C.; Baker, A.; Jex, C.N.; Leng, M.J. Hydrological uncertainties in the modelling of cave drip-water δ18O and the implications for stalagmite palaeoclimate reconstructions. Quat. Sci. Rev. 2010, 29, 2201–2214. [Google Scholar] [CrossRef]
- Biondić, B.; Biondić, R.; Dukanić, F. Protection of karst aquifers in the Dinarides in Croatia. Environ. Geol. 1998, 34, 309–319. [Google Scholar] [CrossRef]
- Fajković, H.; Hasan, O.; Miko, S.; Juračić, M.; Mesić, S.; Prohić, E. Vulnerability of the karst area related to potentially toxic elements. Geol. Croat. 2011, 64, 41–48. [Google Scholar] [CrossRef]
- Terzić, J.; Marković, T.; Lukač Reberski, J. Hydrogeological properties of a complex Dinaric karst catchment: Miljacka Spring case study. Environ. Earth Sci. 2014, 72, 1129–1142. [Google Scholar] [CrossRef]
- Kazakis, N.; Chalikakis, K.; Mazzilli, N.; Ollivier, C.; Manakos, A.; Voudouris, K. Management and research strategies of karst aquifers in Greece: Literature overview and exemplification based on hydrodynamic modelling and vulnerability assessment of a strategic karst aquifer. Sci. Total Environ. 2018, 643, 592–609. [Google Scholar] [CrossRef]
- Małoszewski, P.; Rauert, W.; Stichler, W.; Herrmann, A. Application of flow models in an alpine catchment area using tritium and deuterium data. J. Hydrol. 1983, 66, 319–330. [Google Scholar] [CrossRef]
- Srdoč, D.; Horvatinčić, N.; Obelić, B.; Krajcar Bronić, I.; Sliepčević, A. Procesi taloženja kalcita u krškim vodama s posebnim osvrtom na Plitvička jezera (Calcite deposition processes in karstwaters with special emphasis on the Plitvice Lakes, Yugoslavia). Carsus Iugoslaviae 1985, 11, 101–204. [Google Scholar]
- Srdoč, D.; Krajcar Bronić, I. The application of stable and radioactive isotopes in karst water research. Naš Krš 1986, 12, 37–47. [Google Scholar]
- Małoszewski, P.; Zuber, A. Lumped parameter models for the interpretation of environmental tracer data. In Manual on Mathematical Models in Isotope Hydrology; IAEA-TECDOC-910; IAEA: Vienna, Austria, 1996; pp. 9–58. [Google Scholar]
- Babinka, S. Multi-tracer study of karst waters and lake sediments in Croatia and Bosnia-Herzegovina: Plitvice Lakes National Park and Bihać area. Ph.D. Thesis, Rheinischen Friedrich-Wilhelms-Universität, Bonn, Germany, 2007; p. 167. [Google Scholar]
- Babinka, S.; Obelić, B.; Krajcar Bronić, I.; Horvatinčić, N.; Barešić, J.; Kapelj, S.; Suckow, A. Mean residence time of water from springs of the Plitvice Lakes and Una River area. In Advances in Isotope Hydrology and Its Role in Sustainable Water Resources Management (HIS-2007), Proceedings of a Symposium, Vienna, Austria, 21–25 May 2007; IAEA-CN-15/45; IAEA: Vienna, Austria, 2007; pp. 327–336. Available online: https://www-pub.iaea.org/MTCD/publications/PDF/Pub1310_web.pdf (accessed on 5 August 2020).
- Horvatinčić, N.; Kapelj, S.; Sironić, A.; Krajcar Bronić, I.; Kapelj, J.; Marković, T. Investigation of water resources and water protection in the karst area of Croatia using isotopic and geochemical analyses. In Advances in Isotope Hydrology and Its Role in Sustainable Water Resources Management (HIS-2007), Proceedings of a Symposium, Vienna, Austria, 21–25 May 2007; IAEA: Vienna, Austria, 2007; pp. 295–304. ISBN 978-92-0-110207-2. Available online: https://www-pub.iaea.org/MTCD/publications/PDF/Pub1310Vol2_web.pdf (accessed on 5 August 2020).
- Häusler, H.; Frančišković-Bilinski, S.; Rank, D.; Stadler, P.; Bilinski, H. Preliminary results from stable isotope investigations in the Kupa Drainage Basin (Western Croatia). In BALWOIS 2012 Conference Web-Site, Ohrid, Macedonia, 28 May–3 June 2012; Morell, M., Popovska, C., Morell, O., Stojov, V., Eds.; BALWOIS: Ohrid, Macedonia, 2012. [Google Scholar]
- Mandić, M.; Bojić, D.; Roller-Lutz, Z.; Lutz, H.O.; Krajcar Bronić, I. Note on the spring region of Gacka River (Croatia). Isot. Environ. Health Stud. 2008, 44, 201–208. [Google Scholar] [CrossRef]
- Cartwright, I.; Morgenstern, U. Transit times from rainfall to baseflow in headwater catchments estimated using tritium: The Ovens River, Australia. Hydrol. Earth Syst. Sci. 2015, 19, 1–15. [Google Scholar] [CrossRef]
- Nikolov, J.; Krajcar Bronić, I.; Todorović, N.; Barešić, J.; Petrović Pantić, T.; Marković, T.; Bikit-Schroeder, K.; Stojković, I.; Tomić, M. A survey of isotopic composition (2H, 3H, 18O) of groundwater from Vojvodina. J. Radioanal. Nucl. Chem. 2019, 320, 385–394. [Google Scholar] [CrossRef]
- Parlov, J.; Kovač, Z.; Nakić, Z.; Barešić, J. Using Water Stable Isotopes for Identifying Groundwater Recharge Sources of the Unconfined Alluvial Zagreb Aquifer (Croatia). Water 2019, 11, 2177. [Google Scholar] [CrossRef]
- Madonia, P.; Cangemi, M.; Oliveri, Y.; Germani, C. Hydrogeochemical Characters of Karst Aquifers in Central Italy and Relationship with Neotectonics. Water 2020, 12, 1926. [Google Scholar] [CrossRef]
- Vreča, P.; Krajcar Bronić, I.; Horvatinčić, N.; Barešić, J. Isotopic characteristics of precipitation in Slovenia and Croatia: Comparisom of continental and maritime stations. J. Hydrol. 2006, 330, 457–469. [Google Scholar] [CrossRef]
- IAEA/WMO. Global Network of Isotopes in Precipitation. The GNIP Database. Available online: https://nucleus.iaea.org/wiser (accessed on 22 November 2019).
- Rozanski, K.; Araguás-Araguás, L.; Gonfiantini, R. Isotopic patterns in modern global precipitation. Geophys. Monogr. Ser. 1993, 78, 1–36. [Google Scholar] [CrossRef]
- Rozanski, K.; Gonfiantini, R.; Araguas-Araguas, L. Tritium in the global atmosphere: Distribution patterns and recent trends. J. Phys. G Nucl. Part. Phys. 1991, 17, 523–536. [Google Scholar] [CrossRef]
- Krajcar Bronić, I.; Barešić, J.; Borković, D.; Sironić, A.; Lovrenčić Mikelić, I.; Vreča, P. Long-Term Isotope Records of Precipitation in Zagreb, Croatia. Water 2020, 12, 226. [Google Scholar] [CrossRef]
- Bognar, A.; Faivre, S.; Buzjak, N.; Pahernik, M.; Bočić, N. Recent Landform Evolution in the Dinaric and Pannonian Regions of Croatia. In Recent Landform Evolution; Lóczy, D., Stankoviansky, M., Kotarba, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 313–344. [Google Scholar] [CrossRef]
- Surić, M.; Czuppon, G.; Lončarić, R.; Bočić, N.; Lončar, N.; Bajo, P.; Drysdale, R.N. Stable Isotope Hydrology of Cave Groundwater and its Relevance for Speleothem-Based Palaeoenvironmental Reconstruction in Croatia. Water 2020, 12, 2386. [Google Scholar] [CrossRef]
- Bonacci, O. Karst hydrogeology/hydrology of Dinaric chain and isles. Environ. Earth Sci. 2015, 74, 37–55. [Google Scholar] [CrossRef]
- Bonacci, O.; Terzić, J.; Roje-Bonacci, T.; Frangen, T. An Intermittent Karst River: The Case of the Čikola River (Dinaric Karst, Croatia). Water 2019, 11, 2415. [Google Scholar] [CrossRef]
- Garašić, M.; Garašić, D. The new insights about deep karst springs in the Dinaric karst of Croatia. In Proceedings of the 17th International Congress of Speleology—Volume 2, The 17th International Congress of Speleology, Sydney, NSW, Australia, 23–29 July 2017; Moore, K., White, S., Eds.; Australian Speleological Federation Inc and Speleo: Sydney, Australia, 2017; pp. 26–29. [Google Scholar]
- Bencetić Klaić, Z.; Rubinić, J.; Kapelj, S. Review of research on Plitvice Lakes, Croatia in the fields of meteorology, climatology, hydrology, hydrogeochemistry and physical limnology. Geofizika 2018, 35, 189–278. [Google Scholar] [CrossRef]
- Brkić, Ž.; Kuhta, M.; Hunjak, T.; Larva, O. Regional Isotopic Signatures of Groundwater in Croatia. Water 2020, 12, 1983. [Google Scholar] [CrossRef]
- Horvatinčić, N.; Krajcar Bronić, I.; Obelić, B. Diferences in the 14C age, δ13C and δ18O of Holocene tufa and speleothem in the Dinaric Karst. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2003, 193, 139–157. [Google Scholar] [CrossRef]
- Horvatinčić, N.; Sironić, A.; Barešić, J.; Sondi, I.; Krajcar Bronić, I.; Borković, D. Mineralogical, Organic and Isotopic Composition as Palaeoenvironmental Records in the Lake Sediments of Two Lakes, the Plitvice Lakes, Croatia. Quat. Int. 2018, 494, 300–313. [Google Scholar] [CrossRef]
- Sironić, A.; Barešić, J.; Horvatinčić, N.; Brozinčević, A.; Vurnek, M.; Kapelj, S. Changes in the geochemical parameters of karst lakes over the past three decades—The case of Plitvice Lakes, Croatia. Appl. Geochem. 2017, 78, 12–22. [Google Scholar] [CrossRef]
- Horvatinčić, N. Radiocarbon and tritium measurements in water samples and application of isotopic analyses in hydrology. Fizika 1980, 12, 201–218. [Google Scholar]
- Horvatinčić, N.; Krajcar Bronić, I.; Pezdič, J.; Srdoč, D.; Obelić, B. The distribution or radioactive (3H, 14C) and stable (2H, 18O) isotopes in precipitation, surface and groundwaters during the last decade in Yugoslavia. Nucl. Instrum. Methods Phys. Res. B 1986, 1, 550–553. [Google Scholar] [CrossRef]
- Krajcar Bronić, I.; Horvatinčić, N.; Obelić, B. Two decades of environmental isotope records in Croatia: Reconstruction of the past and prediction of future levels. Radiocarbon 1998, 40, 399–416. [Google Scholar] [CrossRef]
- Krajcar Bronić, I.; Vreča, P.; Horvatinčić, N.; Barešić, J.; Obelić, B. Distribution of hydrogen, oxygen and carbon isotopes in the atmosphere of Croatia and Slovenia. Arch. Ind. Hyg. Toxicol. 2006, 57, 23–29. [Google Scholar]
- Biondić, B.; Biondić, R.; Meaški, H. The conceptual hydrogeological model of the Plitvice Lakes. Geol. Croat. 2010, 63, 195–206. [Google Scholar] [CrossRef]
- Biondić, R.; Meaški, H.; Biondić, B. Hydrogeology of the sinking zone of the Korana River downstream of the Plitvice Lakes, Croatia. Acta Carsol. 2016, 45, 43–56. [Google Scholar] [CrossRef]
- Rubinić, J.; Zwicker, G.; Dragičević, N. Contribution to knowing Plitvice Lakes hydrology—lake water level variation dynamics and significant changes. In Proceedings of the Meeting Hydrological Measurements and Data Processing (“Hidrološka mjerenja i obrada podataka”), NP Plitvice Lakes, Croatia, 26–28 November 2008; pp. 207–230, (In Croatian, English abstract). [Google Scholar]
- Polšak, A.; Juriša, M.; Šparica, M.; Šimunić, A. Basic Geological Map of SFRJ, Sheet Bihać, 1:100 000; Federal Geological Office: Belgrade, Yugoslavia, 1976.
- Halamić, J. Explanatory Notes of the Geological Map of the Republic of Croatia 1:300.000; Velić, I., Vlahović, I., Eds.; Croatian Geological Institute: Zagreb, Croatia, 2009; p. 141. [Google Scholar]
- Köppen, W. Das geographische System der Klimate. In Handbuch der Klimatologie; Köppen, W., Geiger, G., Eds.; Gebrüder Borntraeger: Berlin, Germany, 1936; pp. 1–44. [Google Scholar]
- Peel, M.C.; Finlyanson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- CMHS, 2020 Croatian Meteorological and Hydrological Service. Available online: https://meteo.hr/index_en.php (accessed on 25 August 2020).
- Sironić, A.; Krajcar Bronić, I.; Horvatinčić, N.; Barešić, J.; Borković, D.; Vurnek, M.; Lovrenčić Mikelić, I. Carbon isotopes in dissolved inorganic carbon as tracers of carbon sources in karst waters of the Plitvice Lakes, Croatia. Geol. Soc. Lond. 2020. [Google Scholar] [CrossRef]
- Vreča, P.; Krajcar Bronić, I.; Leis, A.; Demšar, M. Isotopic composition of precipitation at the station Ljubljana (Reaktor), Slovenia—period 2007–2010. Geologija 2014, 57, 217–230. [Google Scholar] [CrossRef]
- Morrison, J.; Brockwell, T.; Merren, T.; Fourel, F.; Phillips, A.M. On-line high-precision stable hydrogen isotopic analyses on nanoliter water samples. Anal. Chem. 2001, 73, 3570–3575. [Google Scholar] [CrossRef]
- Coplen, T.B.; Wassenaar, L.I. LIMS for Lasers for achieving long-term accuracy and precision of δ2H, δ17O, and δ18O of waters using laser absorption spectrometry. Rapid Commun. Mass Spectrom. 2015, 29, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- Krajcar Bronić, I.; Obelić, B.; Srdoč, D. The simultaneous measurement of tritium activity and background count rate in a proportional counter by the Povinec method: Three years experience at the Rudjer Bošković Institute. Nucl. Instrum. Methods Phys. Res. 1986, 17, 498–500. [Google Scholar] [CrossRef]
- Barešić, J.; Krajcar Bronić, I.; Horvatinčić, N.; Obelić, B.; Sironić, A.; Kožar-Logar, J. Tritium activity measurement of water samples using liquid scintillation counter and electrolytical enrichment. In Proceedings of the 8th Symposium of the Croatian Radiation Protection Association, Krk, Croatia, 13–15 April 2011; Krajcar Bronić, I., Kopjar, N., Milić, M., Branica, G., Eds.; HDZZ: Zagreb, Croatia, 2011; pp. 461–467. [Google Scholar]
- Coplen, T.B. Guidelines and recommended terms for expression of stable isotope-ratio and gas-ratio measurement results. Rapid Commun. Mass Spectrom. 2011, 25, 2538–2560. [Google Scholar] [CrossRef] [PubMed]
- IAEA. Stable Isotope Hydrology: Deuterium and Oxygen-18 in Water Cycle; Technical Reports Series 210; Gat, J.R., Gonfiantini, R., Eds.; IAEA: Vienna, Austria, 1981; p. 339. [Google Scholar]
- Brand, W.A.; Coplen, T.B.; Vogl, J.; Rosner, M.; Prohaska, T. Assessment of International Reference Materials for Isotope-Ratio Analysis (IUPAC Technical Report). Pure Appl. Chem. 2014, 86, 425–467. [Google Scholar] [CrossRef]
- Craig, H. Isotope variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef]
- Dansgaard, W. Stable isotopes in precipitation. Tellus 1964, 16, 436–468. [Google Scholar] [CrossRef]
- Dansgaard, W. The isotope composition of natural waters. Medd. Grønland 1961, 165, 120. [Google Scholar]
- Mook, W.G. Environmental Isotopes in the Hydrological Cycle, Principles and Applications, Volumes I, IV and V; Technical Documents in Hydrology 39; IAEA-UNESCO: Paris, France, 2001. [Google Scholar]
- Gat, J.R.; Carmi, I. Evolution of the Isotopic Composition of Atmospheric Waters in the Mediterranean Sea Area. J. Geophys. Res. 1970, 75, 3039–3048. [Google Scholar] [CrossRef]
- Gat, J.R.; Shemesh, A.; Tziperman, E.; Hecht, A.; Georgopoulos, D.; Basturk, O. The stable isotope composition of waters of the eastern Mediterranean Sea. J. Geophys. Res. 1996, 101, 6441–6451. [Google Scholar] [CrossRef]
- Crawford, J.; Hughes, C.E.; Lykoudis, S. Alternative least squares methods for determining the meteoric water line, demonstrated using GNIP data. J. Hydrol. 2014, 519, 2331–2340. [Google Scholar] [CrossRef]
- Hughes, C.E.; Crawford, J. A new precipitation weighted method for determining the meteoric water line for hydrological applications demonstrated using Australian and global GNIP data. J. Hydrol. 2012, 464, 344–351. [Google Scholar] [CrossRef]
- IAEA. Statistical Treatment of Environmental Isotopes in Precipitation; Technical Report Series 331; IAEA: Vienna, Austria, 1992; p. 781. [Google Scholar]
- ANSTO. Australian Nuclear Science and Technology Organisation. Local Meteoric Water Line Freeware. 2012. Available online: https://openscience.ansto.gov.au/collection/879 (accessed on 28 November 2019).
- Dolinar, M.; Vertačnik, G. Spremenljivost temperaturnih in padavinskih razmer v Sloveniji. In Okolje se Spreminja: Podnebna Spremenljivost Slovenije in Njen Vpliv na Vodno Okolje; Cegnar, T., Ed.; Ministry of Environment: Ljubljana, Slovenia, 2010; pp. 37–42. ISBN 978-961-6024-55-6. [Google Scholar]
- NASA. Available online: https://www.nasa.gov/press-release/nasa-noaa-data-show-2016-warmest-year-on-record-globally/ (accessed on 19 August 2020).
- Global Temperature Vital Signs—Climate Change: Vital Signs of the Planet. Available online: https://climate.nasa.gov/vital-signs/global-temperature/ (accessed on 19 August 2020).
- Bastin, J.F.; Clark, E.; Elliott, T.; Hart, S.; van den Hoogen, J.; Hordijk, I.; Ma, H.; Majumder, M.; Manoli, G.; Maschler, J.; et al. Understanding climate change from a global analysis of city analogues. PLoS ONE 2019, 14, e0217592. [Google Scholar] [CrossRef]
- Barešić, J.; Horvatinčić, N.; Krajcar Bronić, I.; Obelić, B.; Vreča, P. Stable isotope composition of daily and monthly precipitation in Zagreb. Isot. Environ. Health Stud. 2006, 42, 239–249. [Google Scholar] [CrossRef]
- Geyh, M.A. Basic studies in hydrology and 14C and 3H measurements. In General Proceedings of the 24th International Geological Congress, The 24th International Geological Congress, Montreal, QC Canada, 21–20 August 1972; Armstrong, J.E., Ed.; International Geological Congress: Montreal, QC, Canada, 1973; Volume 11, pp. 227–234. [Google Scholar]
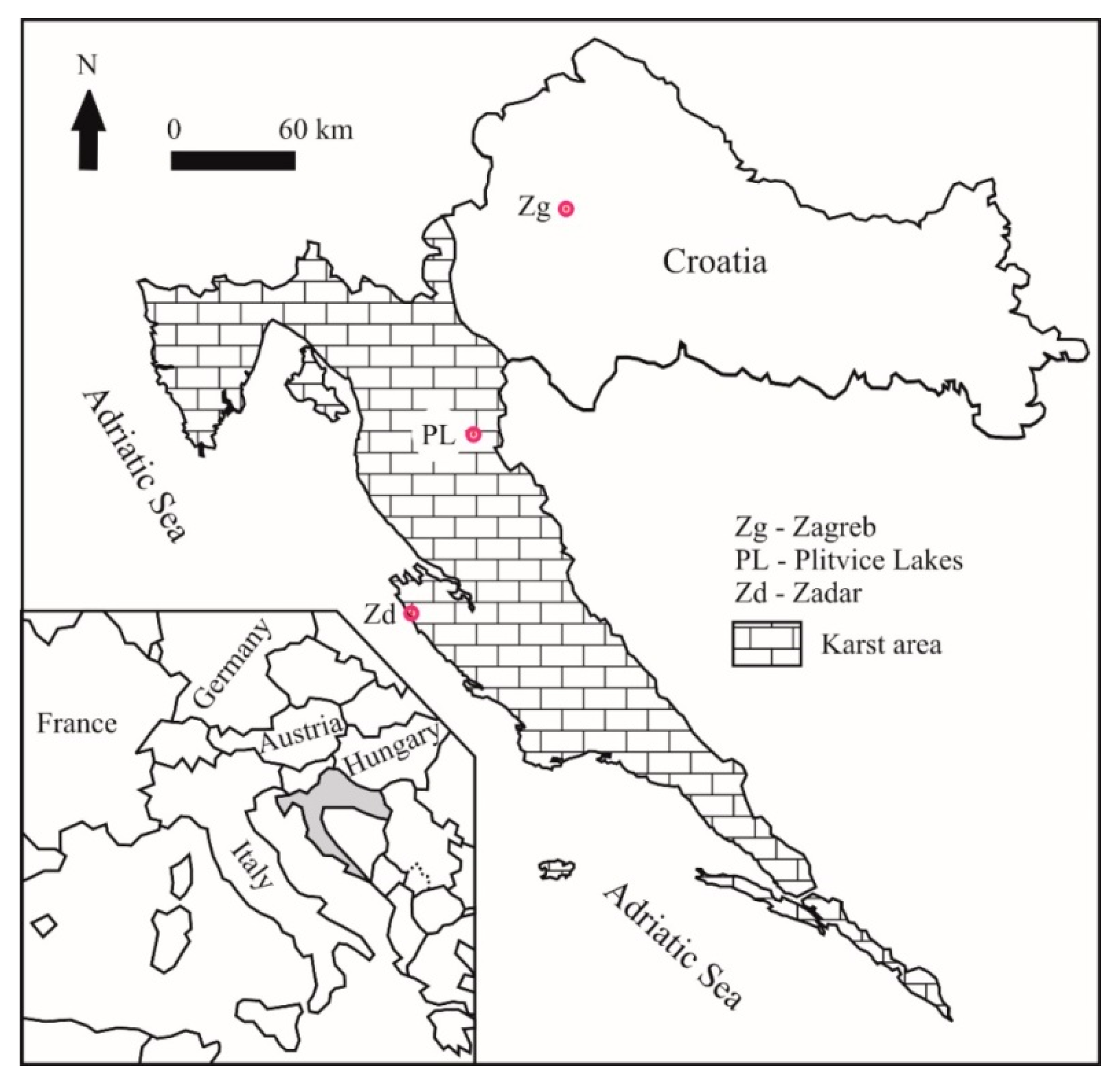
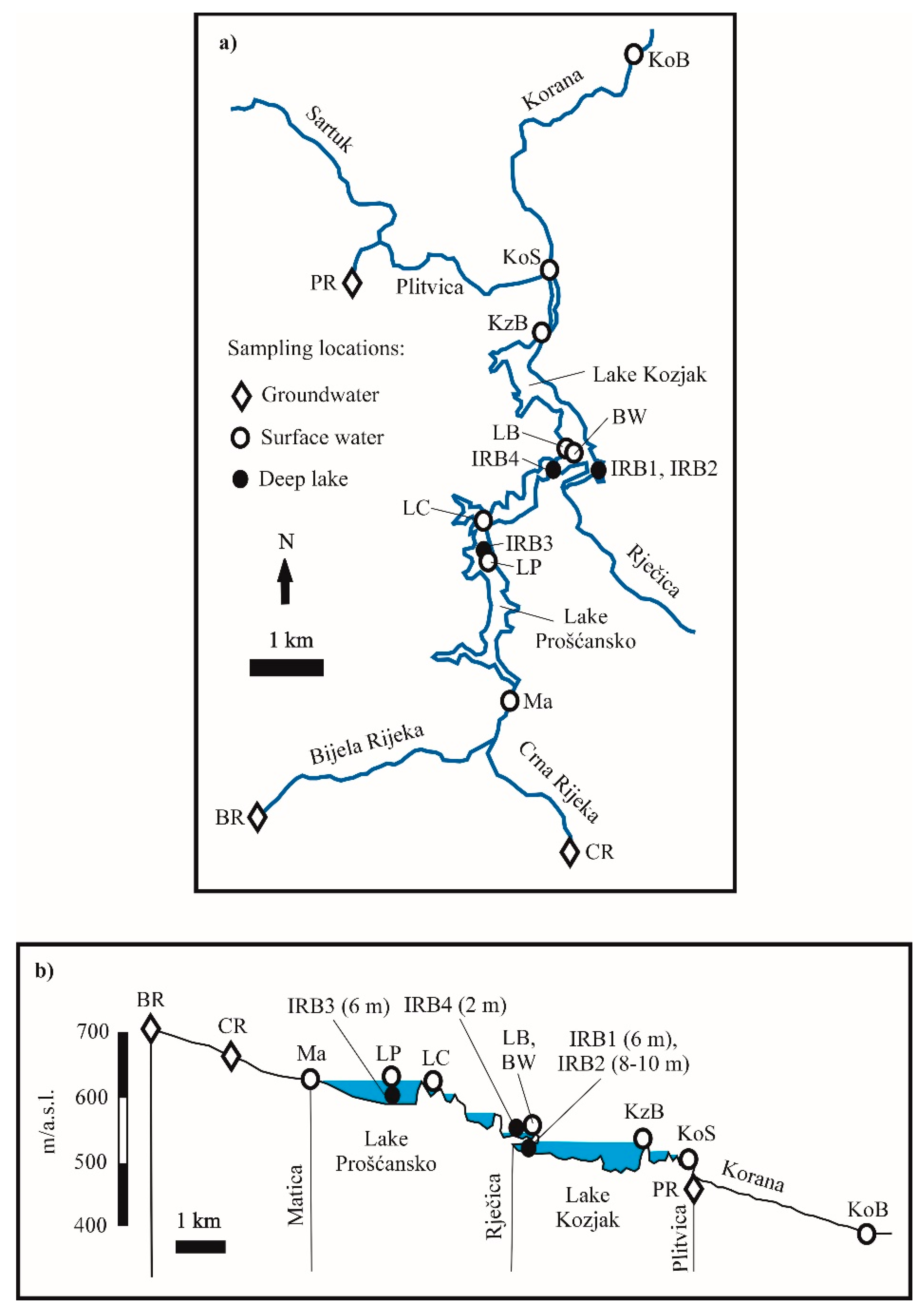




| Location | Code | Sample Type | Comment |
|---|---|---|---|
| Plitvice Lakes | PL | precipitation | study area |
| Zagreb | Zg | precipitation | comparison location |
| Zadar | Zd | precipitation | comparison location |
| Crna Rijeka River | CR | groundwater | spring |
| Bijela Rijeka River | BR | groundwater | spring |
| Plitvica River | PR | groundwater | spring |
| Matica | Ma | surface water | |
| Lake Prošćansko | LP | surface water | |
| Lake Ciginovac | LC | surface water | |
| Lake Burget | LB | surface water | |
| Burget—Waterfall | BW | surface water | |
| Lake Kozjak—Bridges | KzB | surface water | |
| Korana River—Sastavci | KoS | surface water | |
| Korana River—Bridge | KoB | surface water | |
| Lake Kozjak | IRB1 | lake water | water depth 6 m |
| Lake Kozjak | IRB2 | lake water | water depth 8–10 m |
| Lake Prošćansko | IRB3 | lake water | water depth 6 m |
| Lake Gradinsko | IRB4 | lake water | water depth 2 m |
| Period | T (°C) | P (mm) |
|---|---|---|
| 1986–1990 | 8.7 ± 0.5 | 1480 ± 170 |
| 1996–2011 | 9.4 ± 0.5 | 1580 ± 330 |
| 2016–2019 | 10.4 ± 0.4 | 1745 ± 60 |
| Location and Period | Method | n | a ± σa | b ± σb | rmSSEav |
|---|---|---|---|---|---|
| Plitvice Lakes | OLSR | 36 | 7.85 ± 0.12 | 12.46 ± 1.23 | 1.0016 |
| 2003–2006 | RMA | 36 | 7.88 ± 0.12 | 12.77 ± 1.20 | 1.0006 |
| MA | 36 | 7.91 ± 0.12 | 13.06 ± 1.24 | 1.0016 | |
| PWLSR | 36 | 7.97 ± 0.12 | 13.82 ± 1.32 | 1.0000 | |
| PWRMA | 36 | 8.00 ± 0.12 | 14.15 ± 1.32 | 1.0073 | |
| PWMA | 36 | 8.04 ± 0.12 | 14.47 ± 1.32 | 1.0162 | |
| 2003–2005 [10] | OLSR | 24 | 7.85 ± 0.12 | 12.46 ± 1.23 | 1.0016 |
| Zadar | OLSR | 36 | 6.57 ± 0.41 | 1.55 ± 2.37 | 1.0262 |
| 2003–2004 | RMA | 36 | 6.70 ± 0.40 | 3.76 ± 2.31 | 1.0100 |
| Data from [19] | MA | 36 | 7.43 ± 0.440 | 6.01 ± 2.52 | 1.0259 |
| PWLSR | 36 | 7.27 ± 0.50 | 5.95 ± 3.03 | 1.0000 | |
| PWRMA | 36 | 7.84 ± 0.51 | 9.23 ± 3.09 | 1.0525 | |
| PWMA | 36 | 8.44 ± 0.54 | 12.67 ± 3.26 | 1.1433 | |
| Zagreb | OLSR | 121 | 7.43 ± 0.11 | 2.59 ± 0.97 | 1.0048 |
| 1996–2006 | RMA | 121 | 7.52 ± 0.10 | 3.35 ± 0.96 | 1.0019 |
| [23] | MA | 121 | 7.60 ± 0.11 | 4.08 ± 0.98 | 1.0047 |
| PWLSR | 121 | 7.38 ± 0.11 | 2.68 ± 0.95 | 1.0095 | |
| PWRMA | 121 | 7.47 ± 0.11 | 3.44 ± 0.95 | 1.0024 | |
| PWMA | 121 | 7.56 ± 0.11 | 4.19 ± 0.96 | 1.0018 | |
| 1980–2018 [23] | RMA | 389 | 7.74 ± 0.06 | 5.57 ± 0.55 | 1.0019 |
| RMWL_cont 2008–2013 [30] | OLSR | 524 | 7.4 ± 0.005 | 4.1 ± 0.5 | R = 0.99 |
| RMWL_coast 2008–2013 [30] | OLSR | 655 | 7.0 ± 0.08 | 4.4 ± 0.5 | R = 0.96 |
| Type of Line | Slope | Intercept | n | r | Ref. |
|---|---|---|---|---|---|
| GWL, 1983–1984 | 7.8 | 8.5 | 13 | [7] | |
| RGWL_karst | 7.0 | 4.2 | 340 | R2 = 0.98 | [30] |
| GWL, 2000–2018 | 5.7 ± 0.7 | −10.1 ± 7.3 | 31 | 0.84 | |
| SWL, 2003–2005 | 5.0 ± 0.6 | −17.3 ± 5.9 | 35 | 0.86 | [10] |
| SWL, 2011–2014 | 6.3 ± 0.3 | −3.4 ± 2.7 | 87 | 0.94 | |
| LWL, 2011–2014 | 5.8 ± 0.3 | −9.4 ± 3.0 | 42 | 0.95 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krajcar Bronić, I.; Barešić, J.; Sironić, A.; Lovrenčić Mikelić, I.; Borković, D.; Horvatinčić, N.; Kovač, Z. Isotope Composition of Precipitation, Groundwater, and Surface and Lake Waters from the Plitvice Lakes, Croatia. Water 2020, 12, 2414. https://doi.org/10.3390/w12092414
Krajcar Bronić I, Barešić J, Sironić A, Lovrenčić Mikelić I, Borković D, Horvatinčić N, Kovač Z. Isotope Composition of Precipitation, Groundwater, and Surface and Lake Waters from the Plitvice Lakes, Croatia. Water. 2020; 12(9):2414. https://doi.org/10.3390/w12092414
Chicago/Turabian StyleKrajcar Bronić, Ines, Jadranka Barešić, Andreja Sironić, Ivanka Lovrenčić Mikelić, Damir Borković, Nada Horvatinčić, and Zoran Kovač. 2020. "Isotope Composition of Precipitation, Groundwater, and Surface and Lake Waters from the Plitvice Lakes, Croatia" Water 12, no. 9: 2414. https://doi.org/10.3390/w12092414
APA StyleKrajcar Bronić, I., Barešić, J., Sironić, A., Lovrenčić Mikelić, I., Borković, D., Horvatinčić, N., & Kovač, Z. (2020). Isotope Composition of Precipitation, Groundwater, and Surface and Lake Waters from the Plitvice Lakes, Croatia. Water, 12(9), 2414. https://doi.org/10.3390/w12092414









