A Biosorption-Pyrolysis Process for Removal of Pb from Aqueous Solution and Subsequent Immobilization of Pb in the Char
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass and PA Pretreatment
2.2. Pb Removal and Sorption Experiments
2.3. Pyrolysis of Pb-Contaminated Biomass
2.4. Evaluation of the Stability of Pb in the Solid Samples
2.5. Sample Analysis
3. Results and Discussion
3.1. Influence of PA Pretreatment on the Removal of Pb by Biomass
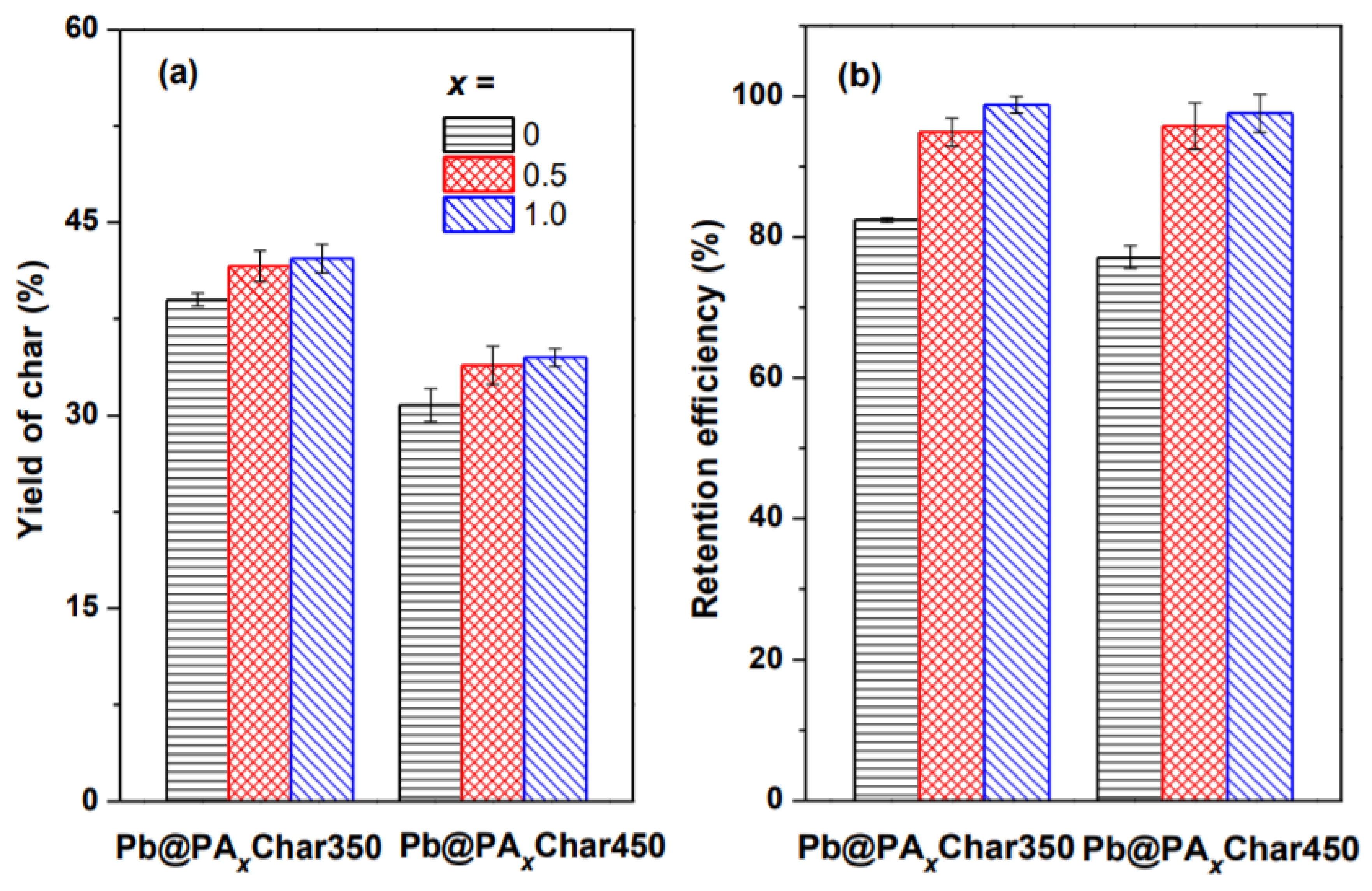
3.2. Influence of Pyrolysis on Pb Retention in the Char
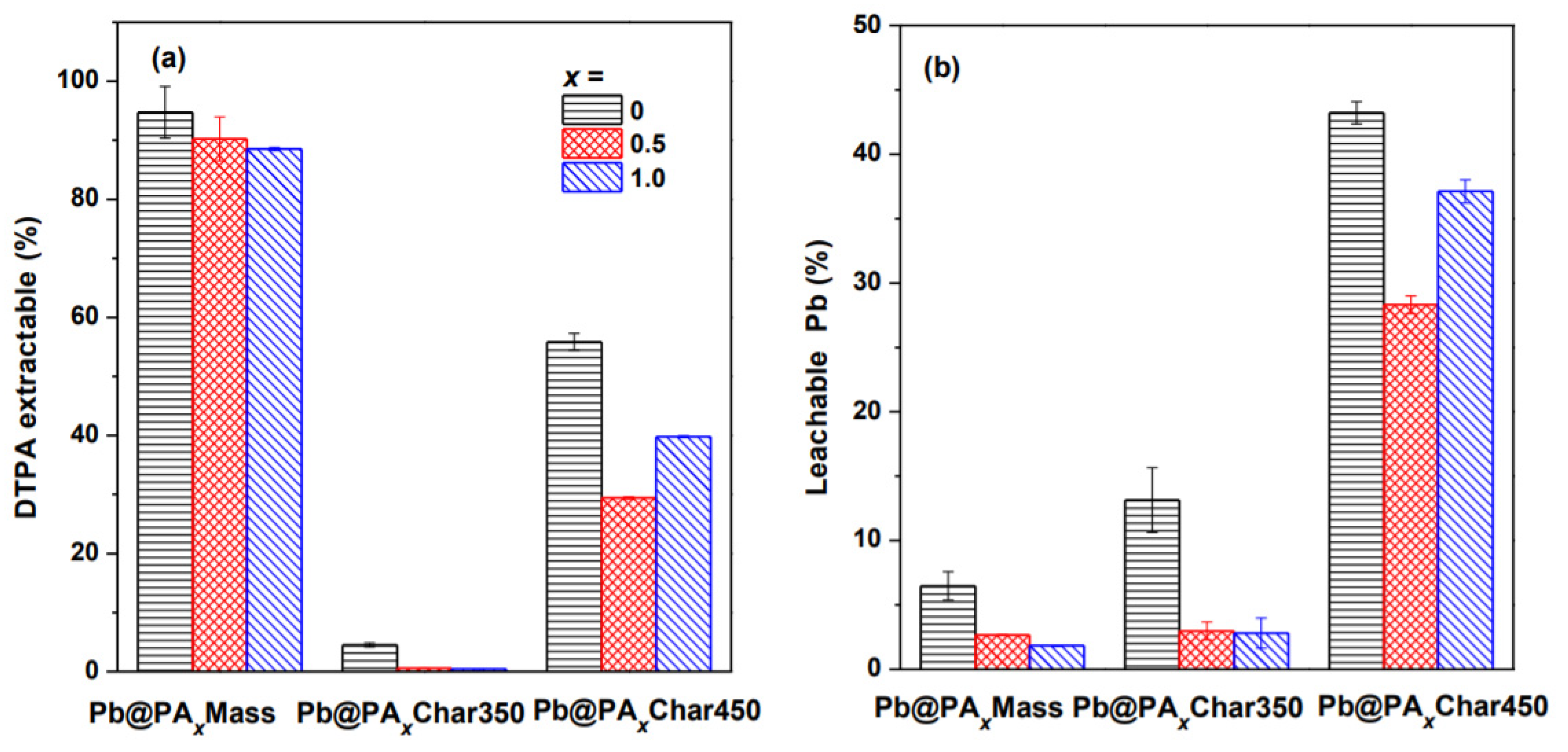
3.3. Influence of Pyrolysis on Stability of Pb in the Char
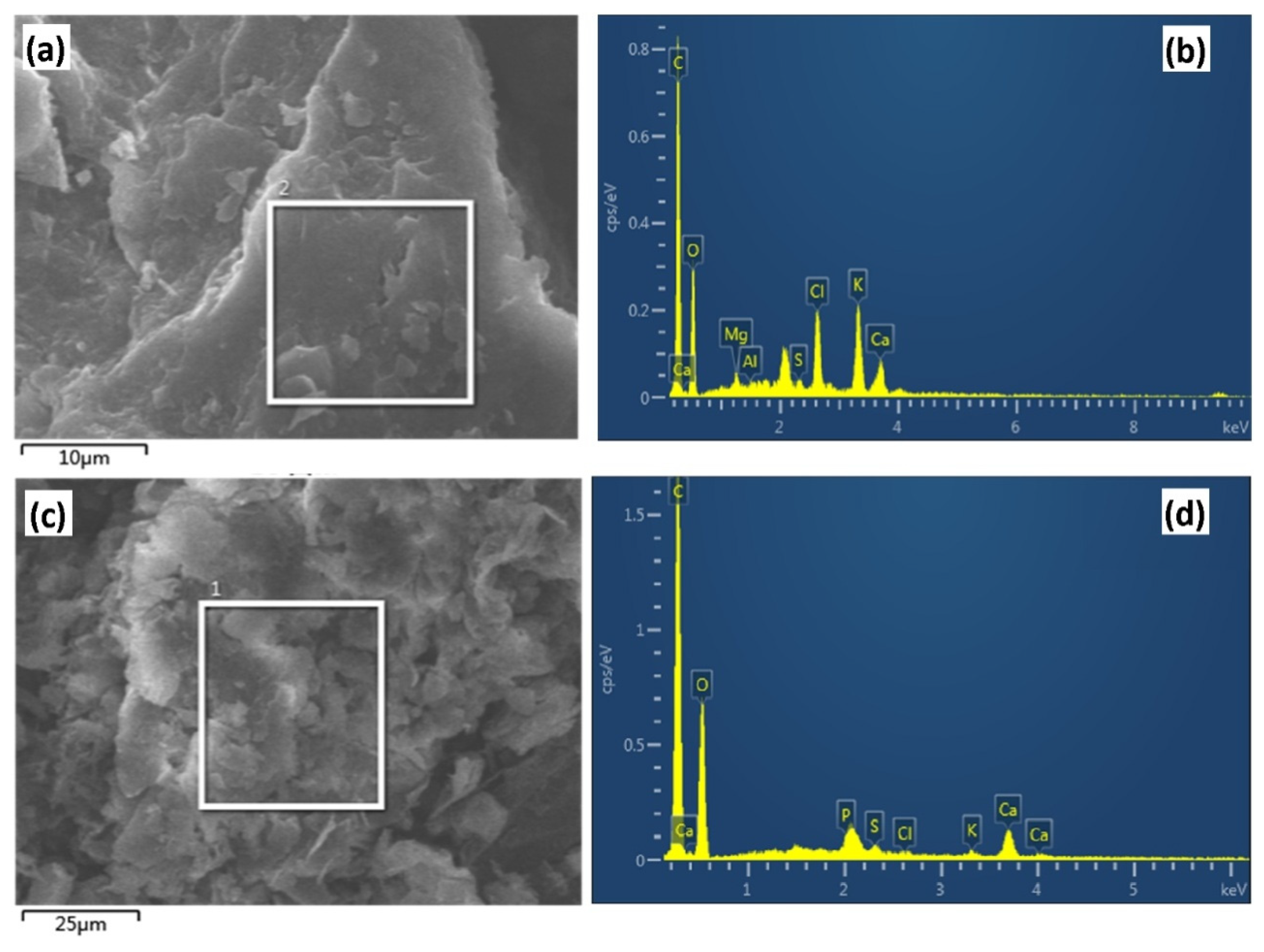
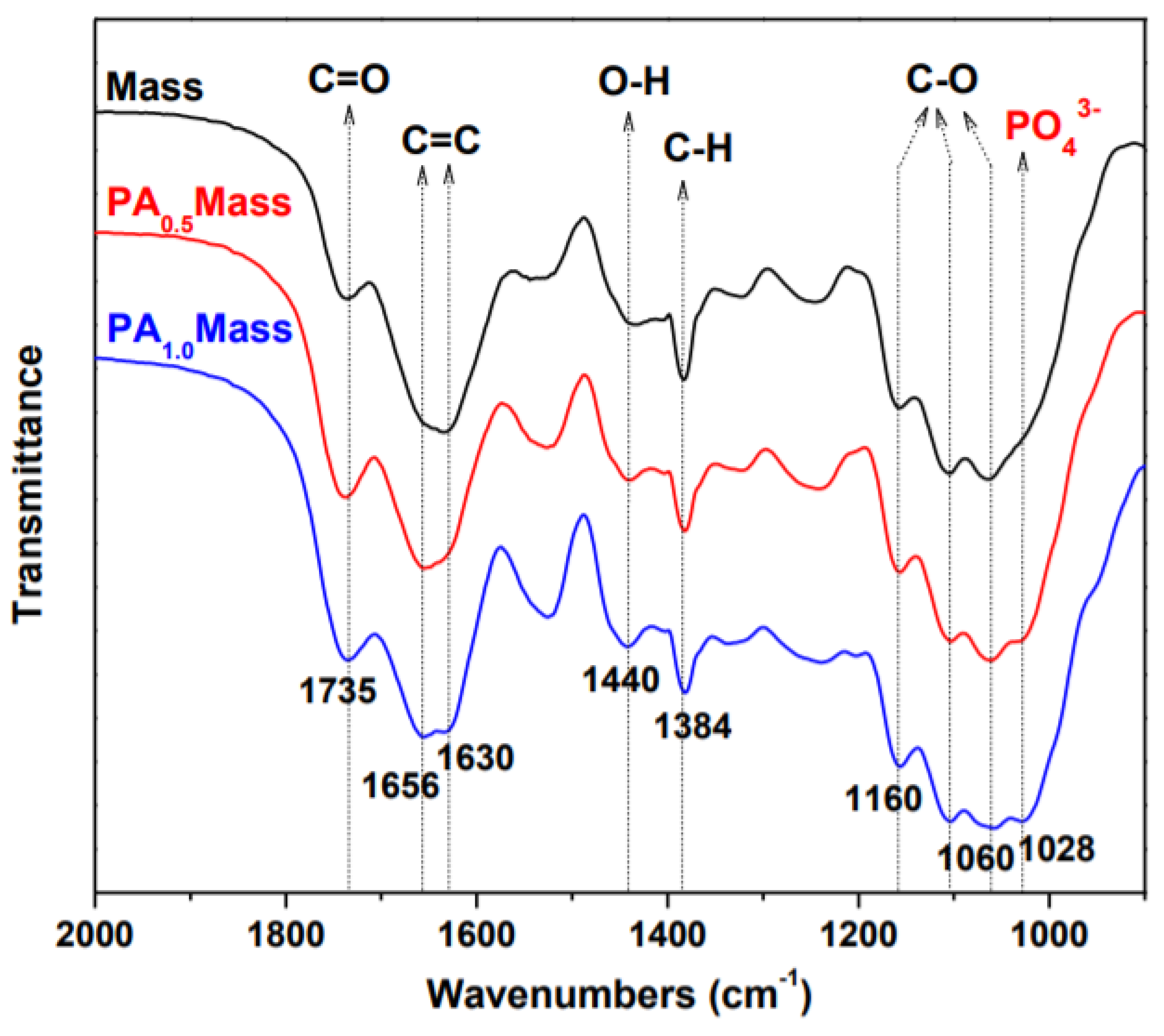
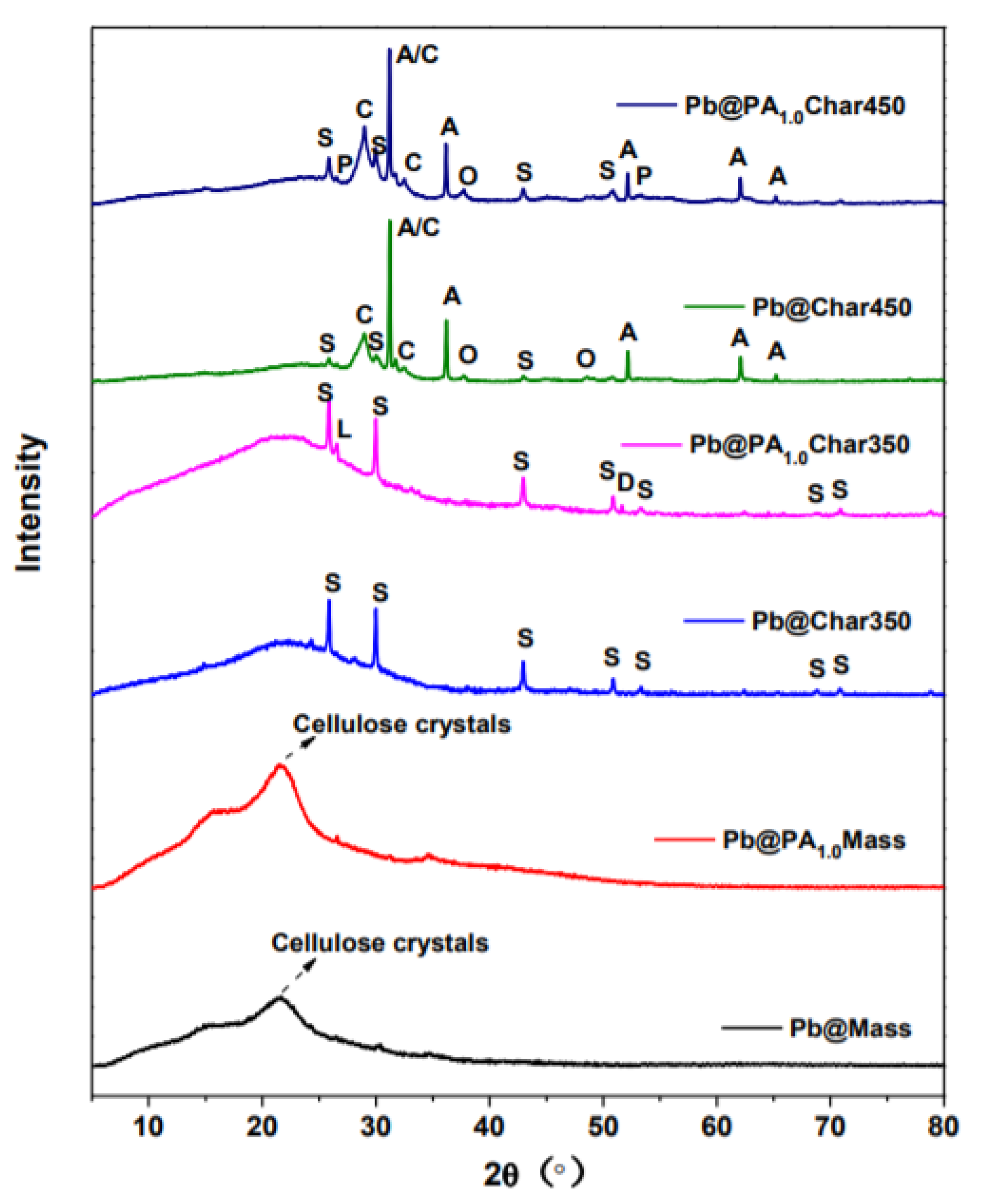
3.4. Investigations about the Immobilization Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ungureanu, G.; Santos, S.; Boaventura, R.; Botelho, C. Arsenic and antimony in water and wastewater: Overview of removal techniques with special reference to latest advances in adsorption. J. Environ. Manag. 2015, 151, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Ayangbenro, A.S.; Babalola, O.O. A new strategy for heavy metal polluted environments: A review of microbial biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Czikkely, M.; Neubauer, E.; Fekete, I.; Ymeri, P.; Fogarassy, C. Review of heavy metal adsorption processes by several organic matters from wastewaters. Water 2018, 10, 1377. [Google Scholar] [CrossRef]
- Fernández-González, R.; Martín-Lara, M.Á.; Blázquez, G.; Pérez, A.; Calero, M. Recovering metals from aqueous solutions by biosorption onto hydrolyzed olive cake. Water 2019, 11, 2519. [Google Scholar] [CrossRef]
- Filote, C.; Volf, I.; Santos, S.C.; Botelho, C.M. Bioadsorptive removal of Pb(II) from aqueous solution by the biorefinery waste of Fucusspiralis. Sci. Total Environ. 2019, 648, 1201–1209. [Google Scholar] [CrossRef]
- Farooq, U.; Kozinski, J.A.; Khan, M.A.; Athar, M. Biosorption of heavy metal ions using wheat based biosorbents—A review of the recent literature. Bioresour. Technol. 2010, 101, 5043–5053. [Google Scholar] [CrossRef]
- Abdolali, A.; Guo, W.S.; Ngo, H.H.; Chen, S.S.; Nguyen, N.C.; Tung, K.L. Typical lignocellulosic wastes and by-products for biosorption process in water and wastewater treatment: A critical review. Bioresour. Technol. 2014, 160, 57–66. [Google Scholar] [CrossRef]
- Shaikh, R.B.; Saifullah, B.; Rehman, F.U. Greener method for the removal of toxic metal ions from the wastewater by application of agricultural waste as an adsorbent. Water 2018, 10, 1316. [Google Scholar] [CrossRef]
- Salman, M.; Athar, M.; Farooq, U. Biosorption of heavy metals from aqueous solutions using indigenous and modified lignocellulosic materials. Rev. Environ. Sci. BioTechnol. 2015, 14, 211–228. [Google Scholar] [CrossRef]
- Feng, N.; Guo, X.; Liang, S.; Zhu, Y.; Liu, J. Biosorption of heavy metals from aqueous solutions by chemically modified orange peel. J. Hazard. Mater. 2011, 185, 49–54. [Google Scholar] [CrossRef]
- Abdolali, A.; Ngo, H.H.; Guo, W.; Zhou, J.L.; Du, B.; Wei, Q.; Wang, X.C.; Nguyen, P.D. Characterization of a multi-metal binding biosorbent: Chemical modification and desorption studies. Bioresour. Technol. 2015, 193, 477–487. [Google Scholar] [CrossRef]
- Pintor, A.M.; Vieira, B.R.; Boaventura, R.A.; Botelho, C.M. Removal of antimony from water by iron-coated cork granulates. Sep. Purif. Technol. 2020, 233, 116020. [Google Scholar] [CrossRef]
- Martín-Lara, M.A.; Pagnanelli, F.; Mainelli, S.; Calero, M.; Toro, L. Chemical treatment of olive pomace: Effect on acid-basic properties and metal biosorption capacity. J. Hazard. Mater. 2008, 156, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Buasri, A.; Chaiyut, N.; Tapang, K.; Jaroensin, S.; Panphrom, S. Equilibrium and kinetic studies of biosorption of Zn(II) ions from wastewater using modified corn cob. APCBEE Procedia 2012, 3, 60–64. [Google Scholar] [CrossRef]
- Dada, A.O.; Olalekan, A.P.; Olatunya, A.M.; Dada, O.J.I.J.C. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar]
- Xu, Y.L.; Song, S.Y.; Chen, J.D.; Chi, R.A.; Yu, J.X. Simultaneous recovery of Cu2+ and Pb2+ from metallurgical wastewater by two tandem columns fixed respectively with tetraethylenepentamine and phosphoric acid modified bagasse. J. Taiwan Inst. Chem. Eng. 2019, 99, 132–141. [Google Scholar] [CrossRef]
- Naeem, M.A.; Imran, M.; Amjad, M.; Abbas, G.; Tahir, M.; Murtaza, B.; Zakir, A.; Shahid, M.; Bulgariu, L.; Ahmad, I. Batch and column scale removal of cadmium from water using raw and acid activated wheat straw biochar. Water 2019, 11, 1438. [Google Scholar] [CrossRef]
- Nzihou, A.; Stanmore, B. The fate of heavy metals during combustion and gasification of contaminated biomass—A brief review. J. Hazard. Mater. 2013, 256, 56–66. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.; Shiralipour, A.; Harris, W. Biomass reduction and arsenic transformation during composting of arsenic-rich hyperaccumulator Pterisvittata L. Environ. Sci. Pollut. Res. 2010, 17, 586–594. [Google Scholar] [CrossRef]
- Bădescu, I.S.; Bulgariu, D.; Ahmad, I.; Bulgariu, L. Valorisation possibilities of exhausted biosorbents loaded with metal ions—A review. J. Environ. Manag. 2018, 224, 288–297. [Google Scholar] [CrossRef]
- Liu, W.J.; Zeng, F.X.; Jiang, H.; Zhang, X.S.; Yu, H.Q. Techno-economic evaluation of the integrated biosorption–pyrolysis technology for lead (Pb) recovery from aqueous solution. Bioresour. Technol. 2011, 102, 6260–6265. [Google Scholar] [CrossRef] [PubMed]
- Gonsalvesh, L.; Yperman, J.; Carleer, R.; Mench, M.; Herzig, R.; Vangronsveld, J. Valorisation of heavy metals enriched tobacco biomass through slow pyrolysis and steam activation. J. Chem. Technol. Biotechnol. 2016, 91, 1585–1595. [Google Scholar] [CrossRef]
- Martín-Lara, M.Á.; Iáñez-Rodríguez, I.; Blázquez, G.; Quesada, L.; Pérez, A.; Calero, M. Kinetics of thermal decomposition of some biomasses in an inert environment: An investigation of the effect of lead loaded by biosorption. Waste Manag. 2017, 70, 101–113. [Google Scholar] [CrossRef]
- Han, Z.; Guo, Z.; Zhang, Y.; Xiao, X.; Peng, C. Potential of pyrolysis for the recovery of heavy metals and bioenergy from contaminated Broussonetia papyrifera biomass. BioResources 2018, 13, 2932–2944. [Google Scholar] [CrossRef]
- Gong, X.; Huang, D.; Liu, Y.; Zeng, G.; Wang, R.; Wei, J.; Huang, C.; Xu, P.; Wan, J.; Zhang, C. Pyrolysis and reutilization of plant residues after phytoremediation of heavy metals contaminated sediments: For heavy metals stabilization and dye adsorption. Bioresour. Technol. 2018, 253, 64–71. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Strezov, V.; Zhou, X.; Kumar, R.; Kan, T. Pyrolysis of heavy metal contaminated biomass pre-treated with ferric salts: Product characterisation and heavy metal deportment. Bioresour. Technol. 2020, 313, 123641. [Google Scholar] [CrossRef] [PubMed]
- Stals, M.; Thijssen, E.; Vangronsveld, J.; Carleer, R.; Schreurs, S.; Yperman, J. Flash pyrolysis of heavy metal contaminated biomass from phytoremediation: Influence of temperature, entrained flow and wood/leaves blended pyrolysis on the behaviour of heavy metals. J. Anal. Appl. Pyrol. 2010, 87, 1–7. [Google Scholar] [CrossRef]
- Dastyar, W.; Raheem, A.; He, J.; Zhao, M. Biofuel production using thermochemical conversion of heavy metal-contaminated biomass (HMCB) harvested from phytoextraction process. Chem. Eng. J. 2018, 358, 759–785. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z.; Lv, S. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour. Technol. 2011, 102, 716–723. [Google Scholar] [CrossRef]
- Li, L.; Zou, D.; Xiao, Z.; Zeng, X.; Zhang, L.; Jiang, L.; Wang, A.; Ge, D.; Zhang, G.; Liu, F. Biochar as a sorbent for emerging contaminants enables improvements in waste management and sustainable resource use. J. Clean. Prod. 2019, 210, 1324–1342. [Google Scholar] [CrossRef]
- Kim, H.; Ko, R.-A.; Lee, S.; Chon, K. Removal efficiencies of manganese and iron using pristine and phosphoric acid pre-treated biochars made from banana peels. Water 2020, 12, 1173. [Google Scholar] [CrossRef]
- Stals, M.; Carleer, R.; Reggers, G.; Schreurs, S.; Yperman, J. Flash pyrolysis of heavy metal contaminated hardwoods from phytoremediation: Characterisation of biomass, pyrolysis oil and char/ash fraction. J. Anal. Appl. Pyrol. 2010, 89, 22–29. [Google Scholar] [CrossRef]
- He, J.; Strezov, V.; Kan, T.; Weldekidan, H.; Asumadu-Sarkodie, S.; Kumar, R. Effect of temperature on heavy metal (loid) deportment during pyrolysis of Avicennia marina biomass obtained from phytoremediation. Bioresour. Technol. 2019, 278, 214–222. [Google Scholar] [CrossRef]
- Li, S.; Zou, D.; Li, L.; Wu, L.; Liu, F.; Zeng, X.; Wang, H.; Zhu, Y.; Xiao, Z. Evolution of heavy metals during thermal treatment of manure: A critical review and outlooks. Chemosphere 2020, 247, 125962. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Hu, S.; Syed-Hassan, S.S.A.; Xiao, Y.; Wang, Y.; Xu, J.; Xiang, J. Effects of reaction conditions on the emission behaviors of arsenic, cadmium and lead during sewage sludge pyrolysis. Bioresour. Technol. 2017, 236, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Al Chami, Z.; Amer, N.; Smets, K.; Yperman, J.; Carleer, R.; Dumontet, S.; Vangronsveld, J. Evaluation of flash and slow pyrolysis applied on heavy metal contaminated Sorghum bicolor shoots resulting from phytoremediation. Biomass Bioenergy 2014, 63, 268–279. [Google Scholar] [CrossRef]
- He, J.; Strezov, V.; Kumar, R.; Weldekidan, H.; Jahan, S.; Dastjerdi, B.H.; Zhou, X.; Kan, T. Pyrolysis of heavy metal contaminated Avicennia marina biomass from phytoremediation: Characterisation of biomass and pyrolysis products. J. Clean. Prod. 2019, 234, 1235–1245. [Google Scholar] [CrossRef]
- Lievens, C.; Yperman, J.; Vangronsveld, J.; Carleer, R. Study of the potentialvalorization of heavy metal contaminated biomass via phytoremediation by fast pyrolysis: Part I. Influence of temperature, biomass species and solid heat carrier on the behaviour of heavy metals. Fuel 2008, 87, 1894–1905. [Google Scholar] [CrossRef]
- Li, S.; Zhang, T.; Li, J.; Shi, L.; Zhu, X.; Lü, J.; Li, Y. Stabilization of Pb(II) accumulated in biomass through phosphate-pretreated pyrolysis at low temperatures. J. Hazard. Mater. 2017, 324, 464–471. [Google Scholar] [CrossRef]
- Shi, L.; Wang, L.; Zhang, T.; Li, J.; Huang, X.; Cai, J.; Wang, Y. Reducing the bioavailability and leaching potential of lead in contaminated water hyacinth biomass by phosphate-assisted pyrolysis. Bioresour. Technol. 2017, 241, 908–914. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Y.; Liu, X.; Lü, J.; Li, J. Functions of phosphorus additives on immobilizing heavy metal cadmium in the char through pyrolysis of contaminated biomass. J. Anal. Appl. Pyrol. 2019, 144, 104721. [Google Scholar] [CrossRef]
- Zeng, X.; Xiao, Z.; Zhang, G.; Wang, A.; Li, Z.; Liu, Y.; Wang, H.; Zeng, Q.; Liang, Y.; Zou, D. Speciation and bioavailability of heavy metals in pyrolytic biochar of swine and goat manures. J. Anal. Appl. Pyrol. 2018, 132, 82–93. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, W.; Yang, Y.; Huang, X.; Wang, S.; Qiu, R. Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res. 2012, 46, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cao, X.; Zhao, L. Comparison of rice husk-and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: Role of mineral components in biochars. Chemosphere 2013, 92, 955–961. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, X.; Shi, L.; Li, J.; Li, S.; Lü, J.; Li, Y. Efficient removal of lead from solution by celery-derived biochars rich in alkaline minerals. Bioresour. Technol. 2017, 235, 185–192. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- Blázquez, G.; Ronda, A.; Martín-Lara, M.A.; Pérez, A.; Calero, M. Comparative study of isotherm parameters of lead biosorption by two wastes of olive-oil production. Water Sci. Technol. 2015, 72, 711–720. [Google Scholar] [CrossRef]
- Patra, C.; Shahnaz, T.; Subbiah, S.; Narayanasamy, S. Comparative assessment of raw and acid-activated preparations of novel Pongamiapinnata shells for adsorption of hexavalent chromium from simulated wastewater. Environ. Sci. Pollut. R. 2020, 27, 14836–14851. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Martınez-Alonso, A.; Suárez-Garcıa, F.; Tascón, J.M.D. Synthetic carbons activated with phosphoric acid: I. Surface chemistry and ion binding properties. Carbon 2002, 40, 1493–1505. [Google Scholar] [CrossRef]
- Shu, Y.; Tang, C.; Hu, X.; Jiang, L.; Hu, X.; Zhao, Y. H3PO4-activated cattail carbon production and application in chromium removal from aqueous solution: Process optimization and removal mechanism. Water 2018, 10, 754. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, X.; Zheng, W.; Kan, Y. Phosphorus-assisted biomass thermal conversion: Reducing carbon loss and improving biochar stability. PLoS ONE 2014, 9, e115373. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cao, X.; Zheng, W.; Scott, J.W.; Sharma, B.K.; Chen, X. Copyrolysis of biomass with phosphate fertilizers to improve biochar carbon retention, slow nutrient release, and stabilize heavy metals in soil. ACS Sustain. Chem. Eng. 2016, 4, 1630–1636. [Google Scholar] [CrossRef]
- Yuan, J.H.; Xu, R.K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
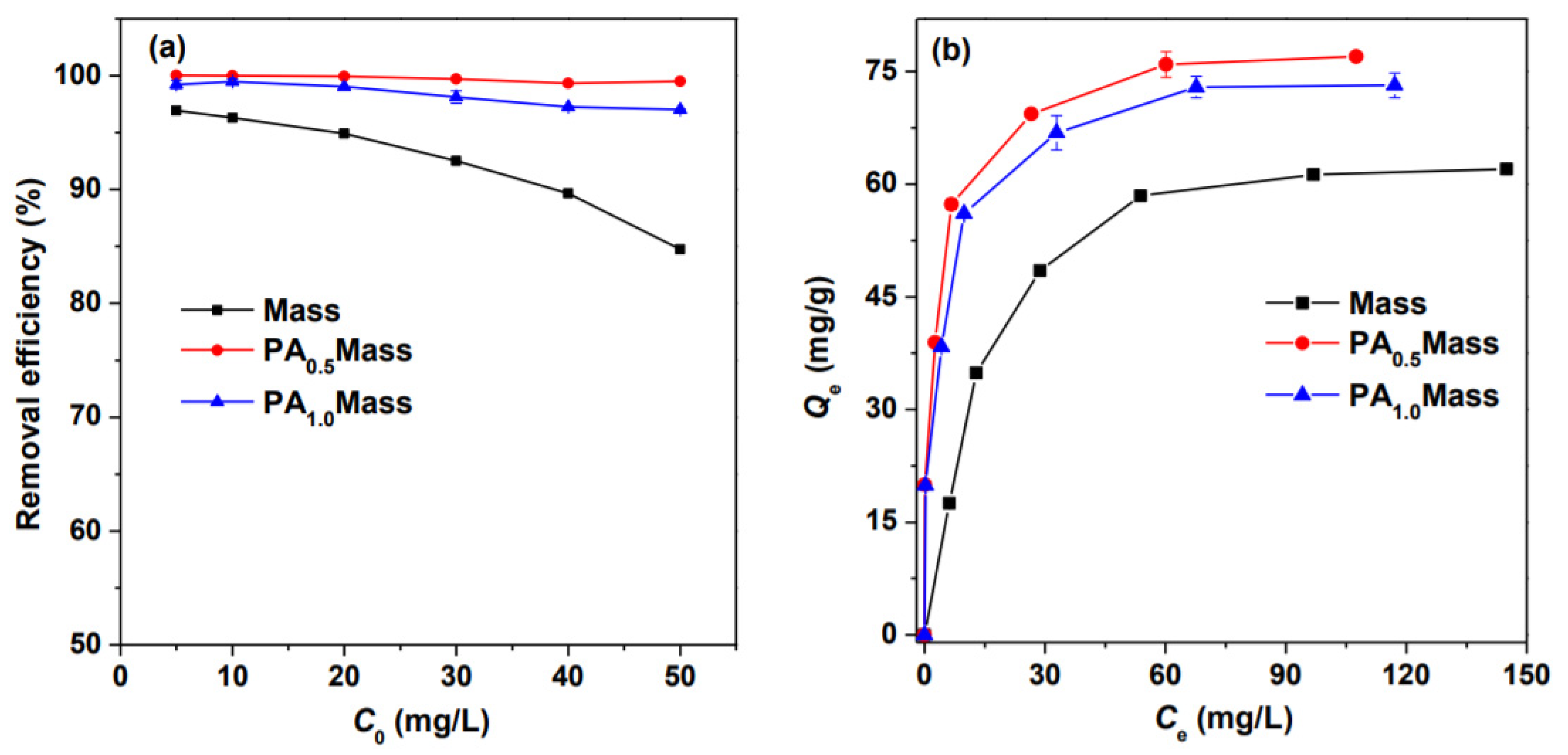

| Models | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qm (mg/g) | b (L/mg) | R2 | kF | n | R2 | |
| Mass | 69.6 | 0.0788 | 0.992 | 20.6 | 4.21 | 0.855 |
| PA0.5Mass | 78.7 | 0.373 | 0.996 | 36.7 | 5.72 | 0.853 |
| PA1.0Mass | 76.9 | 0.242 | 0.993 | 32.8 | 5.39 | 0.839 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Lü, J.; Feng, D.; Guo, S.; Li, J. A Biosorption-Pyrolysis Process for Removal of Pb from Aqueous Solution and Subsequent Immobilization of Pb in the Char. Water 2020, 12, 2381. https://doi.org/10.3390/w12092381
Wang Y, Lü J, Feng D, Guo S, Li J. A Biosorption-Pyrolysis Process for Removal of Pb from Aqueous Solution and Subsequent Immobilization of Pb in the Char. Water. 2020; 12(9):2381. https://doi.org/10.3390/w12092381
Chicago/Turabian StyleWang, Yue, Jinhong Lü, Dongqing Feng, Sen Guo, and Jianfa Li. 2020. "A Biosorption-Pyrolysis Process for Removal of Pb from Aqueous Solution and Subsequent Immobilization of Pb in the Char" Water 12, no. 9: 2381. https://doi.org/10.3390/w12092381
APA StyleWang, Y., Lü, J., Feng, D., Guo, S., & Li, J. (2020). A Biosorption-Pyrolysis Process for Removal of Pb from Aqueous Solution and Subsequent Immobilization of Pb in the Char. Water, 12(9), 2381. https://doi.org/10.3390/w12092381





