Removal of Pb2+ from Aqueous Solutions Using K-Type Zeolite Synthesized from Coal Fly Ash
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Physicochemical Properties of the Adsorbents
2.3. Adsorption Capacity of Pb2+
2.4. Effect of Initial Concentration, Temperature, pH, Contact Time, and Coexisting Ions on Pb2+ Adsorption
3. Results and Discussion
3.1. Physicochemical Properties
3.2. Adsorption Capacity of Pb2+
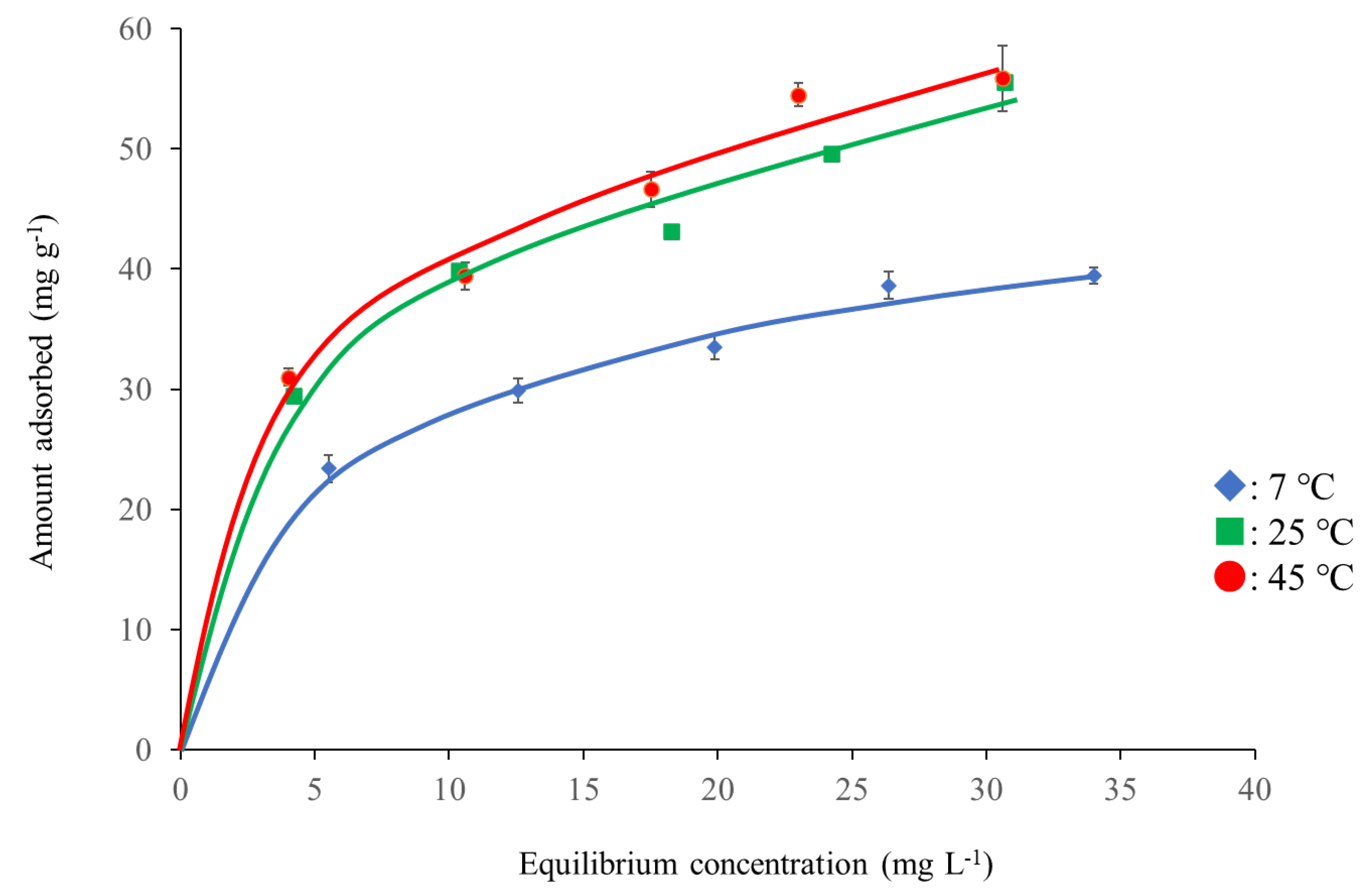
3.3. Pb2+ Adsorption Isotherms
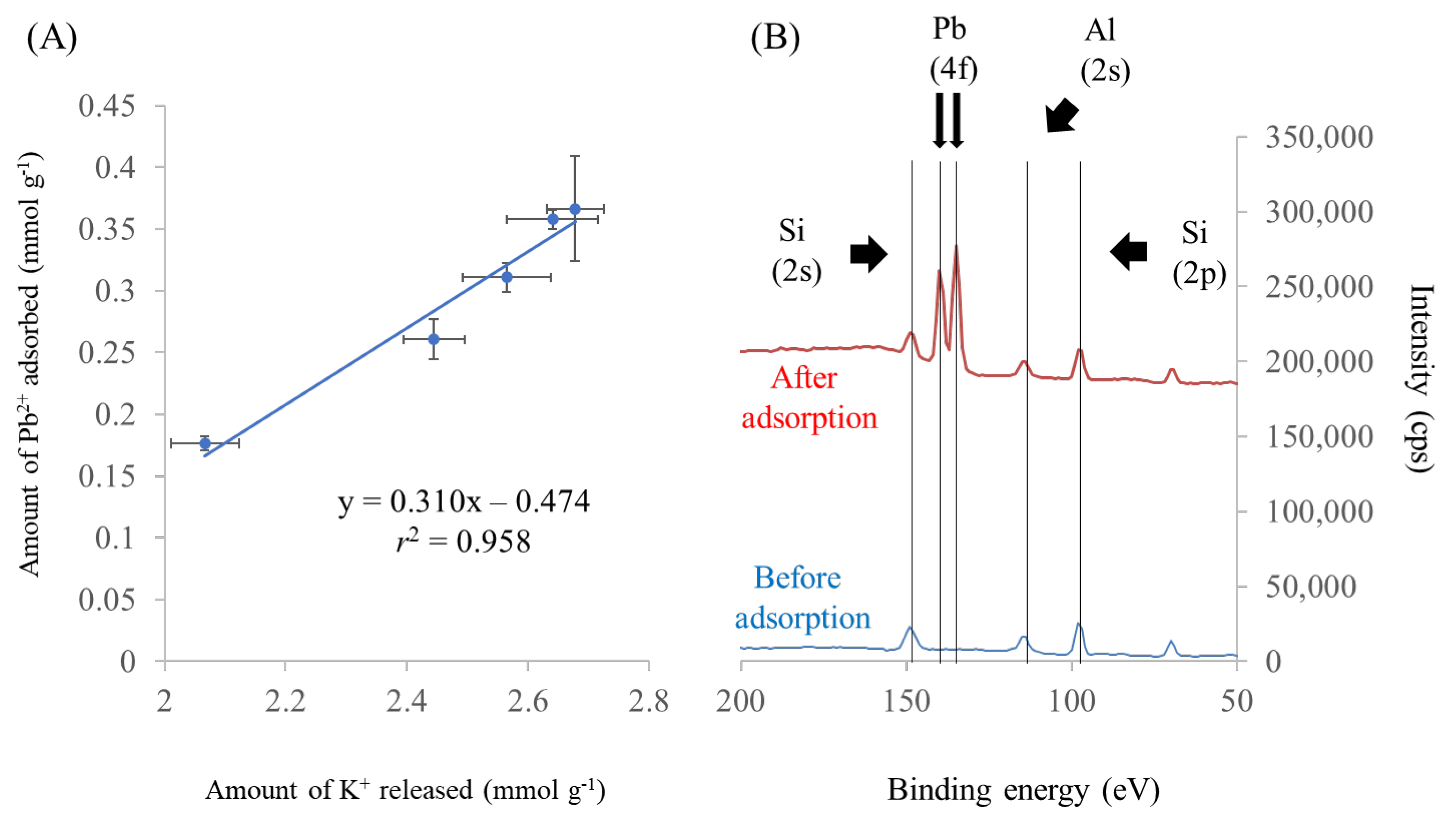
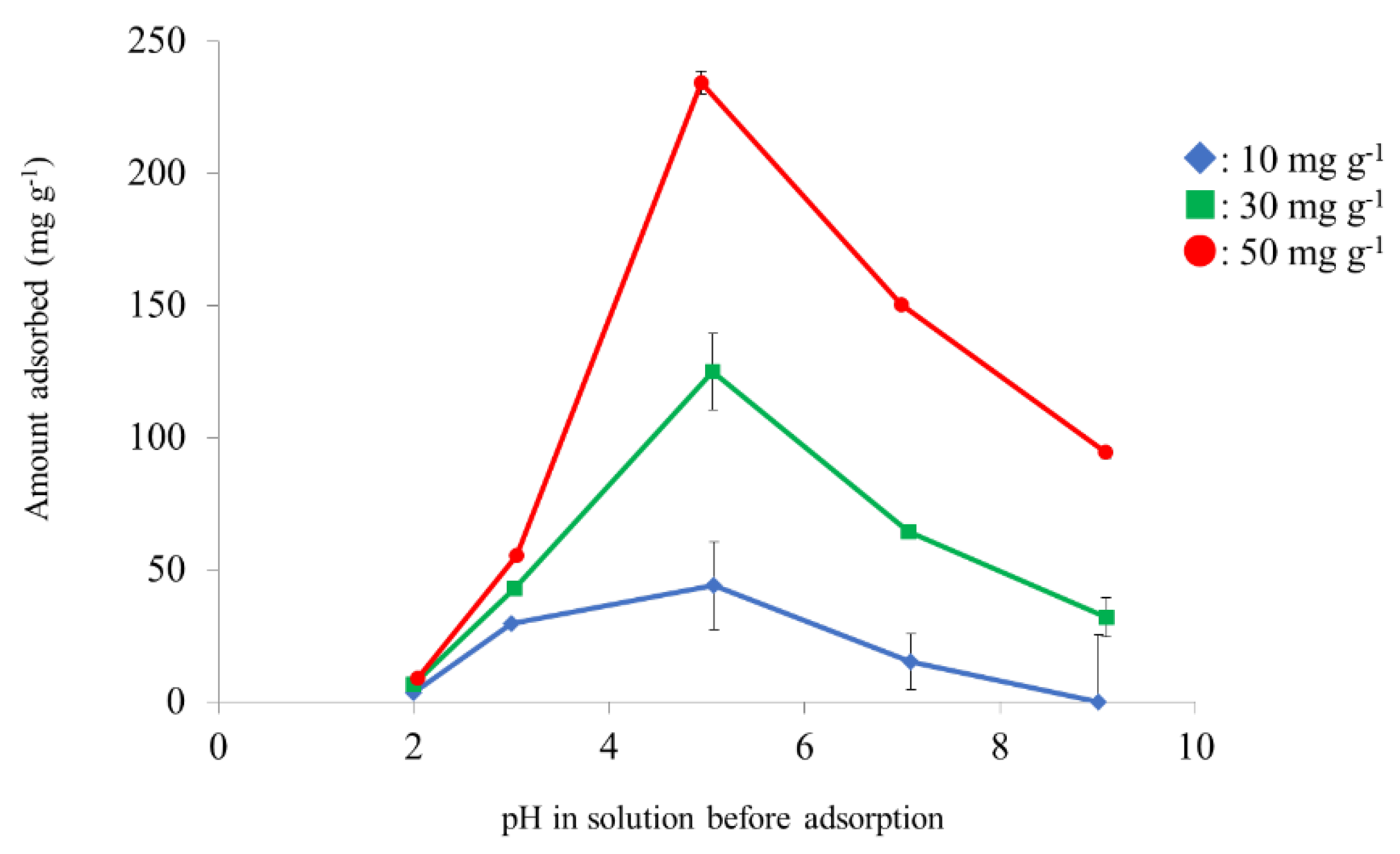
3.4. Effect of pH on Pb2+ Adsorption
–SiOH + H2O (H+ + OH−) → –SiOH2+ + OH−
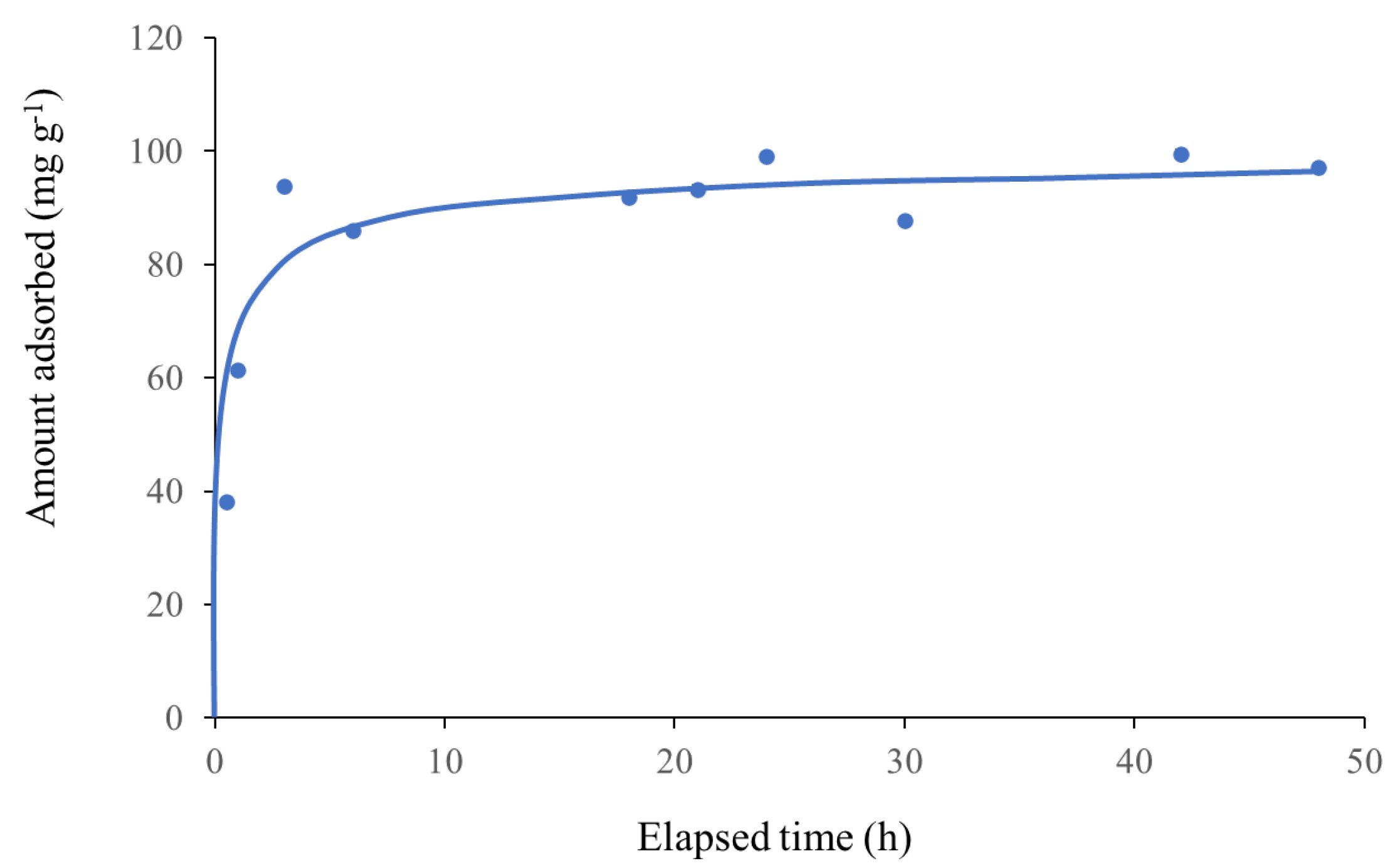
3.5. Effect of Contact Time on Pb2+ Adsorption
3.6. Effect of Coexisting Ions on Pb2+ Adsorption
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- About the Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/sustainable-development-goals/ (accessed on 19 May 2020).
- Venkata Ramana, D.K.; Kumar Reddy, D.H.; Kumar, B.N.; Seshaiah, K.; Chandra Rao, G.P.; Lu, C. Adsorption of Pb(II) from aqueous solutions by chemically modified zeolite supported carbon nanotubes: Equilibrium, kinetic, and thermodynamic studies. Sep. Sci. Technol. 2013, 48, 403–412. [Google Scholar] [CrossRef]
- Kragovic, M.; Dakovic, A.; Markovic, M.; Krstic, J.; Gatta, G.D.; Rotiroti, N. Characterization of lead sorption by the natural and Fe(III)-modified zeolite. Appl. Surf. Sci. 2013, 283, 764–774. [Google Scholar] [CrossRef]
- Hamidpour, M.; Kalbasib, M.; Afyunib, M.; Shariatmadarib, H.; Holmic, P.E.; Hansenc, H.C.B. Sorption hysteresis of Cd(II) and Pb(II) on natural zeolite and bentonite. J. Hazard. Mater. 2010, 181, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Lead Poisoning and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health (accessed on 19 May 2020).
- Sarma, P.R.; Chattopadhyay, A.; Zhan, C.; Sharma, S.K.; Geng, L.; Hsiao, B.S. Lead removal from water using carboxycellulose nanofibers prepared by nitro-oxidation method. Nature 2018, 25, 1961–1973. [Google Scholar]
- Torrice, M. How lead ended up in flint’s tap water. Chem. Eng. News 2016, 94, 26–29. [Google Scholar]
- Edwards, M.; Triantafyllidou, S.; Best, D. Elevated blood lead in young children due to lead-contaminated drinking water: Washington, DC, 2001–2004. Environ. Sci. Technol. 2009, 43, 1618–1623. [Google Scholar] [CrossRef] [PubMed]
- Mankikar, D.; Campbell, C.; Greenberg, R. Evaluation of a home-based environmental and educational intervention to improve health in vulnerable households: Southeastern Pennsylvania lead and healthy homes program. Int. J. Environ. Res. Public Health 2016, 13, 900. [Google Scholar] [CrossRef] [PubMed]
- Pieper, K.J.; Krometis, L.A.; Gallagher, D.; Benham, B.; Edwards, M. Profiling private water system to identify patterns of waterborne lead exposure. Environ. Sci. Technol. 2015, 49, 12697–12704. [Google Scholar] [CrossRef] [PubMed]
- Japan Coal Energy Center. Available online: http://www.jcoal.or.jp/ashdb/ashstatistics/upload/H30_ashstatistics.pdf (accessed on 19 May 2020).
- Franus, W.; Wdowin, M.; Franus, M. Synthesis and characterization of zeolites prepared from industrial fly ash. Environ. Monit. Assess. 2014, 186, 5721–5729. [Google Scholar] [CrossRef]
- Blissett, R.S.; Rowson, N.A. A review of the multi-component utilization of coal fly ash. Fuel 2012, 97, 1–23. [Google Scholar] [CrossRef]
- Flores, C.G.; Schneider, H.; Marcillio, N.R.; Ferret, L.; Oliveira, J.C.P. Potassic zeolites from Brazilian coal ash for use as a fertilizer in agriculture. Waste Manag. 2017, 70, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Visa, M.; Isac, L.; Dute, A. Fly ash adsorbents for multi-cation wastewater treatment. App. Surf. Sci. 2012, 258, 6345–6352. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- He, K.; Chen, Y.; Tang, Z.; Hu, Y. Removal of heavy metal ions from aqueous solution by zeolite synthesized from fly ash. Environ. Sci. Pollut. Res. 2016, 23, 2778–2788. [Google Scholar] [CrossRef]
- Wdowin, M.; Franus, M.; Panek, R.; Badura, L.; Rranus, W. The conversation technology of fly ash into zeolites. Clean Technol. Environ. Policy 2014, 16, 1217–1223. [Google Scholar] [CrossRef]
- Derkowski, A.; Franus, W.; Beran, E.; Czimerova, A. Properties and potential applications of zeolitic materials produced from fly ash using simple method of synthesis. Powder Technol. 2006, 166, 47–54. [Google Scholar] [CrossRef]
- Derkowski, A.; Franus, W.; Waniak-Nowicka, H.; Czimerova, A. Textural properties vs. CEC and EGME retention of Na-X zeolite prepared from fly ash at room temperature. Int. J. Miner. Process. 2007, 82, 57–68. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Derbalah, A.S.; Moghanm, F.S. Removal of heavy metals from aqueous solution by zeolite in competitive sorption system. Int. J. Environ. Sci. Dev. 2012, 3, 362–367. [Google Scholar] [CrossRef]
- Siddique, R. Performance characteristics of high-volume class F fly ash concrete. Cem. Concr. Res. 2004, 34, 487–493. [Google Scholar] [CrossRef]
- Izidoro, J.C.; Fungaro, D.A.; Abbott, J.E.; Wang, S. Synthesis of zeolite X and A from fly ashes for cadmium and zinc removal from aqueous solutions in single and binary ion systems. Fuel 2013, 103, 827–834. [Google Scholar] [CrossRef]
- Golbad, S.; Khoshnoud, P.; Abu-Zahra, N. Hydrothermal synthesis of hydroxyl sodalite from fly ash for the removal of lead ions from water. Int. J. Environ. Sci. Technol. 2017, 14, 135–142. [Google Scholar] [CrossRef]
- Ogata, F.; Tominaga, H.; Yabutani, H.; Taga, A.; Kawasaki, N. Recovery of molybdenum from fly ash by gibbsite. Toxicol. Environ. Chem. 2011, 93, 635–642. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ogata, F.; Nakamura, T.; Kawasaki, N. Synthesis of novel zeolites produced from fly ash hydrothermal treatment in alkaline solution and its evaluation as an adsorbent for heavy metal. J. Environ. Chem. Eng. 2020, 8, 103687. [Google Scholar] [CrossRef]
- Ogata, F.; Tominaga, H.; Kangawa, M.; Inoue, K.; Kawasaki, N. Adsorption capacity of Cu(II) and Pb(II) onto carbon fiber produced from wool. J. Oleo Sci. 2012, 3, 149–154. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, B.; Zhang, Y.; Shukla, A.; Shukla, S.S.; Dorris, K.L. The removal of heavy metals from aqueous solutions by sawdust adsorption removal of lead and comparison of its adsorption with copper. J. Hazard. Mater. 2001, B84, 83–94. [Google Scholar] [CrossRef]
- Pehlivan, E.; Yamk, B.H.; Ahmetli, G.; Pehlivan, M. Equilibrium isotherm studies for the uptake of cadmium and lead ions onto sugar beet pulp. Bioresour. Technol. 2008, 99, 3520–3527. [Google Scholar] [CrossRef] [PubMed]
- Battacharyya, K.G.; Sharma, A. Adsorption of Pb(II) from aqueous solution by Azadirachta indiea (Neem) leaf powder. J. Hazard. Mater. 2004, 113, 97–109. [Google Scholar] [CrossRef]
- Slater, J.C. Atomic radii in crystals. J. Chem. Phys. 1964, 41, 3199–3204. [Google Scholar] [CrossRef]
- Ogata, F.; Iwata, Y.; Kawasaki, N. Properties of novel adsorbent produced by hydrothermal treatment of waste fly ash in alkaline solution and its capability for adsorption of tungsten from aqueous solution. J. Environ. Chem. Eng. 2015, 3, 333–338. [Google Scholar] [CrossRef]
- Faria, P.C.C.; Orfao, J.J.M.; Pereira, M.F.R. Adsorption of anionic and cationic dyes on activated carbons with different surface chemistries. Water Res. 2004, 38, 2043–2052. [Google Scholar] [CrossRef]
- Boehm, H.P. Chemical identification of surface groups. J. Adv. Catal. Sci. Technol. 1966, 16, 179–274. [Google Scholar]
- Nassar, N.N. Rapid removal and recovery of Pb(II) from wastewater by magnetic nanoadsorbents. J. Hazard. Mater. 2010, 184, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.T.; Lee, C.W. Ecological risk assessment of sediment in wastewater discharging area by means of metal speciation. Microchem. J. 2001, 70, 255–264. [Google Scholar] [CrossRef]
- Wang, C.; Hu, X.; Chen, M.L.; Wu, Y.H. Total concentrations and fractions of Cd, Cr, Pb, Cu, Ni and Zn in sewage sludge municipal and industrial wastewater treatment plants. J. Hazard. Mater. 2005, B119, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Montoya, V.; Perez-Cruz, M.; Mendoza-Castillo, D.I.; Moreno-Virgen, M.R.; Bonilla-Petriciolet, A. Competitive adsorption of syes and heavy metals on zeolitic structures. J. Environ. Manag. 2013, 116, 213–221. [Google Scholar] [CrossRef]
- Murayama, N.; Ymamamoto, H.; Shibata, J. Mechansim of zeolite synthesis from coal fly ash by alkali hydrothermal reaction. Int. J. Miner. Process. 2002, 64, 1–17. [Google Scholar] [CrossRef]
- Freundlich, H.M.T. Over the adsorption in solution. J. Phy. Chem. 1906, 57, 385–471. [Google Scholar]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Abe, I.; Hayashi, K.; Kitagawa, M. Studies on the adsorption of surfactants on activated carbons. I. Adsorption of nonionic surfactants. Yukagaku 1976, 25, 145–150. [Google Scholar]
- Myroslav, S.; Boguslaw, B.; Artur, T.P.; Jacek, N. Study of the selection mechanism of heavy metal (Pb2+, Cu2+, Ni2+, and Cd2+) adsorption on clinoptilolite. J. Colloid Interface Sci. 2006, 304, 21–28. [Google Scholar]
- Sari, A.; Tuzen, M.; Citak, D.; Soylak, M. Equilibrium, kinetic and thermodynamic studies of adsorption of Pb(II) from aqueous solution onto Turkish kaolinite clay. J. Hazard. Mater. 2007, 149, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, S.; Yang, S.; Hu, J.; Tan, X.; Wang, X. Effect of pH, ionic strength and temperature on sorption of Pb(II) on NKF-6 zeolite studied by batch technique. Chem. Eng. J. 2011, 168, 86–93. [Google Scholar] [CrossRef]
- Alswat, A.A.; Ahmad, M.B.; Saleh, T.A. Zeolite modified with copper oxide and iron oxide for lead and arsenic adsorption from aqueous solutions. J. Water Supply Res. Technol. AQUA 2016, 65, 465–479. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffie, Kunglia Svenska Vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption process. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Z.; Wu, D.; Zhang, Z.; Kong, H. Synthesis of zeolite/aluminum oxide hydrate from coal ash: A new type of adsorbent for simultaneous removal of cationic and anionic pollutants. J. Ind. Eng. Chem. 2013, 52, 14890–14897. [Google Scholar] [CrossRef]
- Yadanaparthi, S.K.P.; Graybill, D.; Wandruszka, R.V. Adsorbents for the removal of arsenic, cadmium, and lead from contaminated waters. J. Hazard. Mater. 2009, 171, 1–15. [Google Scholar] [CrossRef]
- Merrikhpour, H.; Jalali, M. Comparative and competitive adsorption of cadmium, copper, nickel, and lead ions by Iranian natural zeolite. Clean Technol. Environ. Policy 2013, 15, 303–316. [Google Scholar] [CrossRef]
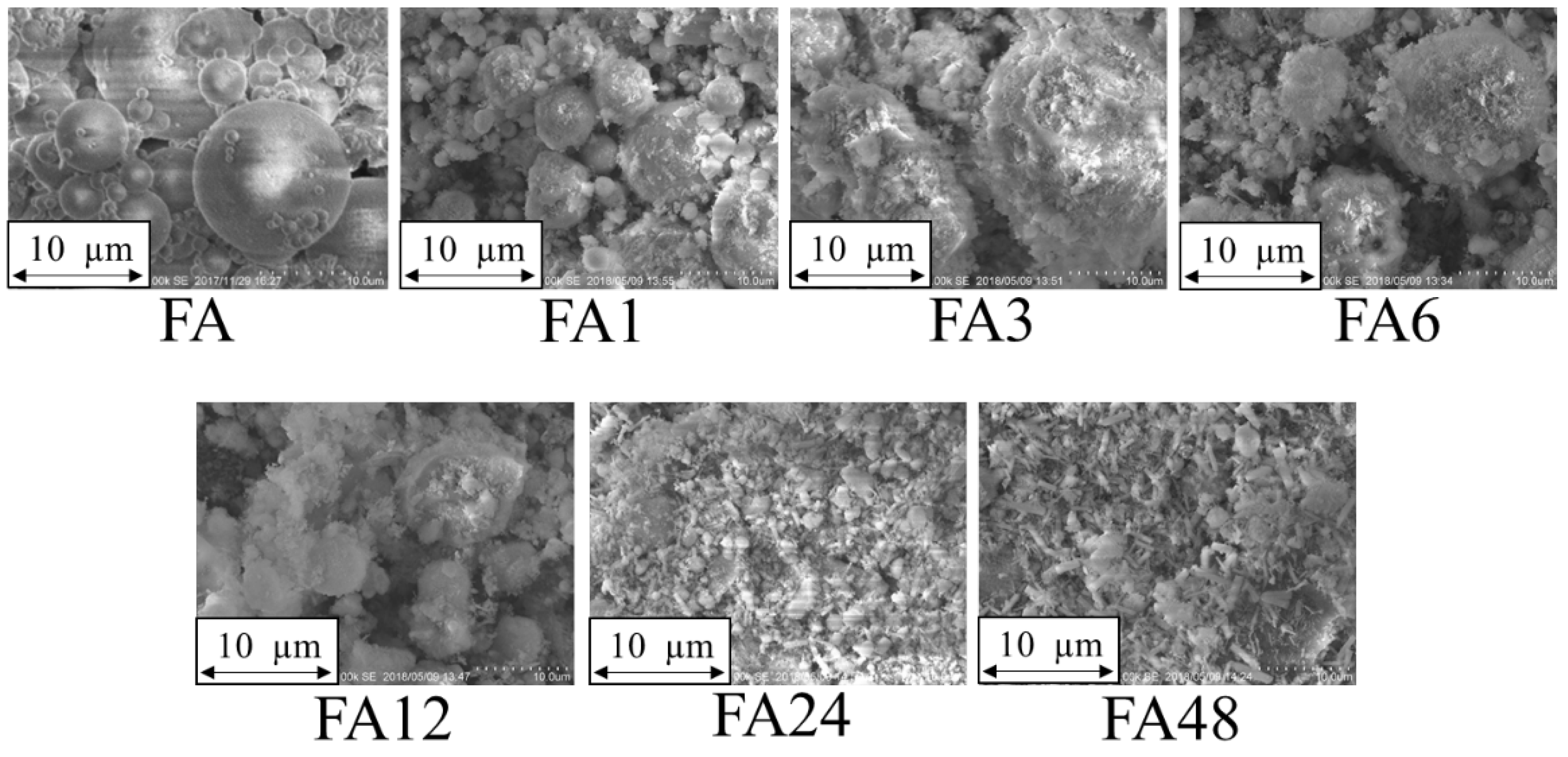
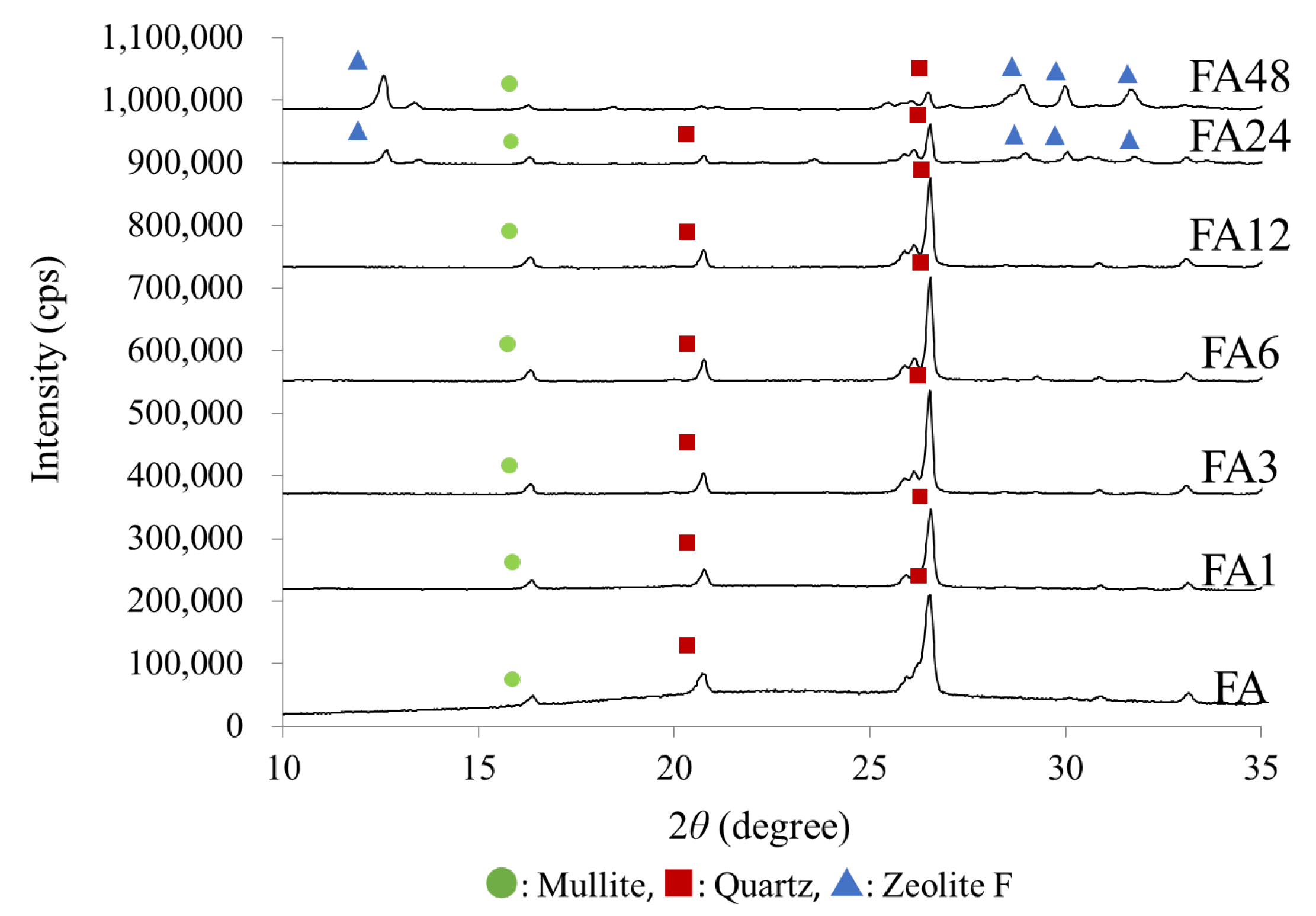
| Adsorbents | FA | FA1 | FA3 | FA6 | FA12 | FA24 | FA48 | |
|---|---|---|---|---|---|---|---|---|
| Concentration of basic functional groups (mmol g−1) | 0 | 0.41 | 0.77 | 1.38 | 1.36 | 1.48 | 1.43 | |
| Concentration of acidic functional groups (mmol g−1) | 0.10 | 0.24 | 0.30 | 0.31 | 0.31 | 0.24 | 0.24 | |
| CEC (mmol g−1) | pH 5 | 0.34 | 1.98 | 1.17 | 1.63 | 2.27 | 7.90 | 8.98 |
| pH 10 | 0.19 | 0.65 | 1.55 | 2.09 | 3.46 | 11.17 | 11.17 | |
| pHpzc | 9.8 | 9.3 | 9.3 | 9.5 | 9.7 | 10.4 | 10.4 | |
| Specific surface area (m2 g−1) | 1.4 | 15.1 | 31.5 | 53.3 | 54.5 | 50.3 | 47.3 | |
| Pore volume (µL g−1) | d ≤ 20 (Å) | 0.1 | 0.9 | 0 | 0.5 | 0.2 | 10.0 | 10.0 |
| 20 < d ≤ 500 (Å) | 2.0 | 41.9 | 97.4 | 161.5 | 185.0 | 105.0 | 99.0 | |
| Total | 2.2 | 63.0 | 139.0 | 221.0 | 220.0 | 151.0 | 131.0 | |
| Mean pore diameter (Å) | 57.0 | 167.2 | 176.7 | 165.9 | 161.6 | 120.1 | 110.7 | |
| Adsorbents | FAs | |
|---|---|---|
| Concentration of basic functional groups (mmol g−1) | 0.677 | |
| Concentration of acidic functional groups (mmol g−1) | 0.044 | |
| CEC (mmol g-1) | pH 5 | 0.986 |
| pH 10 | 0.999 | |
| pHpzc | 0.921 | |
| Specific surface area (m2 g−1) | 0.549 | |
| Pore volume (µL g−1) | d ≤ 20 (Å) | 0.980 |
| 20 < d ≤ 500 (Å) | 0.201 | |
| Total | 0.227 | |
| Mean pore diameter (Å) | 0.262 | |
| Adsorbent | Temperatures (°C) | Langmuir Constants | Freundlich Constants | ||||
|---|---|---|---|---|---|---|---|
| Ws (mg g−1) | a (L mg−1) | r | logk | 1/n | r | ||
| FA48 | 7 | 74.1 | 0.20 | 0.982 | 1.38 | 0.30 | 0.994 |
| 25 | 98.0 | 0.24 | 0.987 | 1.51 | 0.30 | 0.997 | |
| 45 | 102.0 | 0.25 | 0.960 | 1.52 | 0.25 | 0.960 | |
| Adsorbent | qe,exp | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|
| k1 (h−1) | qe,cal (mg g−1) | r | k2 (g mg−1 h−1) | qe,cal (mg g−1) | r | ||
| FA48 | 99.5 | 0.057 | 19.7 | 0.602 | 0.010 | 98.0 | 0.998 |
| Components in Binary Solution | Removal Percentage of Pb2+ (%) | Removal of Other Cations (%) |
|---|---|---|
| Pb2+ + Na+ | 60.7 | 0.2 |
| Pb2+ + Mg2+ | 69.4 | 0 |
| Pb2+ + K+ | 75.7 | 0 |
| Pb2+ + Ca2+ | 62.4 | 0 |
| Pb2+ + Ni+ | 73.2 | 0 |
| Pb2+ + Cu2+ | 62.7 | 3.7 |
| Pb2+ + Zn2+ | 63.7 | 0.9 |
| Pb2+ + Sr2+ | 70.0 | 0.7 |
| Pb2+ + Cd2+ | 64.4 | 4.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, Y.; Ogata, F.; Saenjum, C.; Nakamura, T.; Kawasaki, N. Removal of Pb2+ from Aqueous Solutions Using K-Type Zeolite Synthesized from Coal Fly Ash. Water 2020, 12, 2375. https://doi.org/10.3390/w12092375
Kobayashi Y, Ogata F, Saenjum C, Nakamura T, Kawasaki N. Removal of Pb2+ from Aqueous Solutions Using K-Type Zeolite Synthesized from Coal Fly Ash. Water. 2020; 12(9):2375. https://doi.org/10.3390/w12092375
Chicago/Turabian StyleKobayashi, Yuhei, Fumihiko Ogata, Chalermpong Saenjum, Takehiro Nakamura, and Naohito Kawasaki. 2020. "Removal of Pb2+ from Aqueous Solutions Using K-Type Zeolite Synthesized from Coal Fly Ash" Water 12, no. 9: 2375. https://doi.org/10.3390/w12092375
APA StyleKobayashi, Y., Ogata, F., Saenjum, C., Nakamura, T., & Kawasaki, N. (2020). Removal of Pb2+ from Aqueous Solutions Using K-Type Zeolite Synthesized from Coal Fly Ash. Water, 12(9), 2375. https://doi.org/10.3390/w12092375





