Spatiotemporal Variation in Phytoplankton Community Driven by Environmental Factors in the Northern East China Sea
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Site and Water Sampling
2.2. Phytoplankton Pigment Analysis
- Area = area of the peak in the sample [area]
- Rf = standard response factor [ngL−1 area−1]
- Ve = AIS/(peak area of IS added to sample) × (Volume of IS added to sample) [L]
- Vs = volume of filtered water sample [L]
- AIS = peak area of IS when 1 mL IS is mixed with 300 μL of H2O
- IS = Internal Standard
2.3. Dissolved Inorganic Nutrient Concentration
2.4. Statistical Analysis
3. Results
3.1. Physical Environments
3.2. Dissolved Inorganic Nutrient Concentrations
3.3. Phytoplankton Biomass and Community Structure
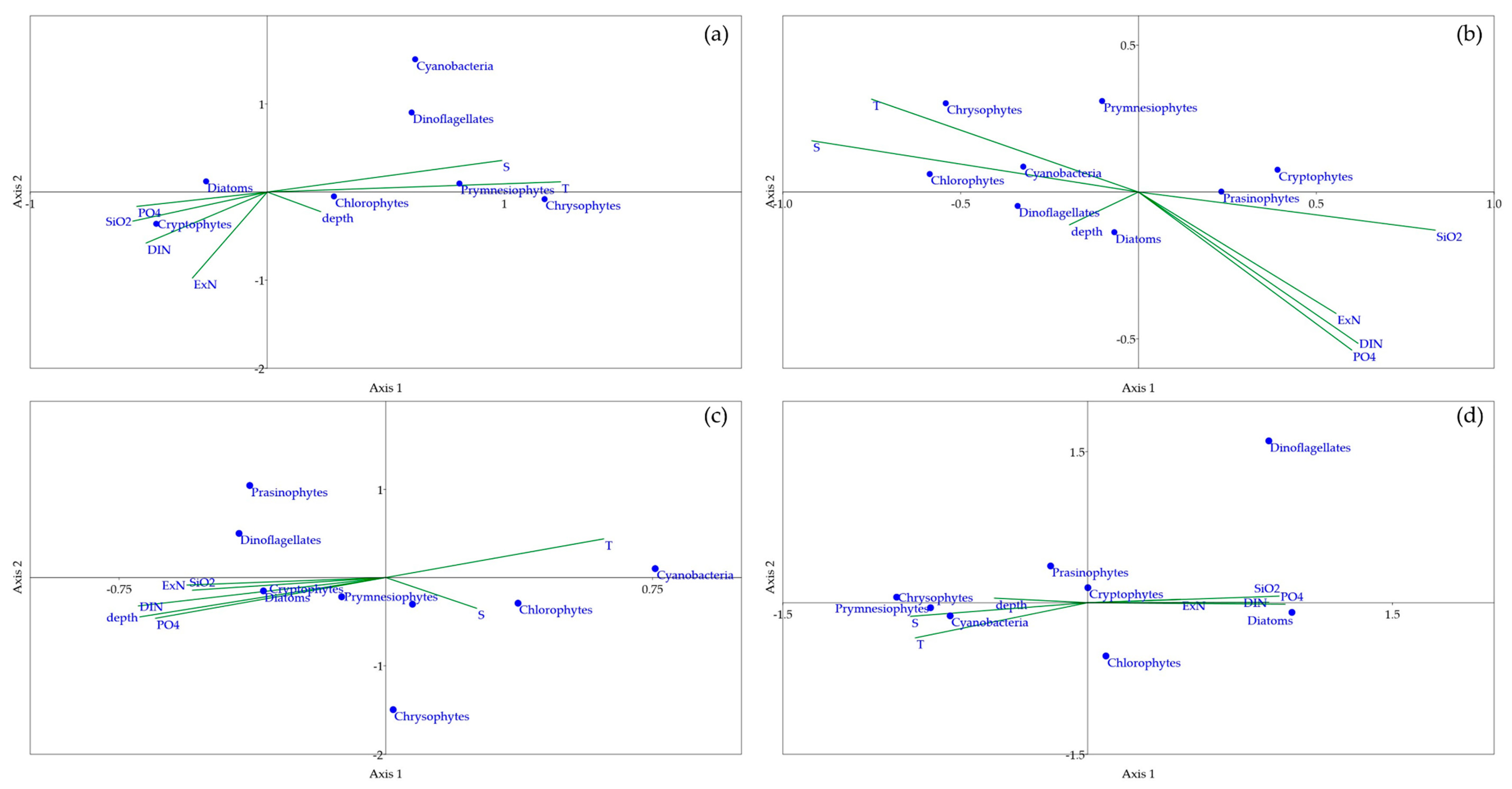
3.4. Canonical Correspondence Analysis (CCA)
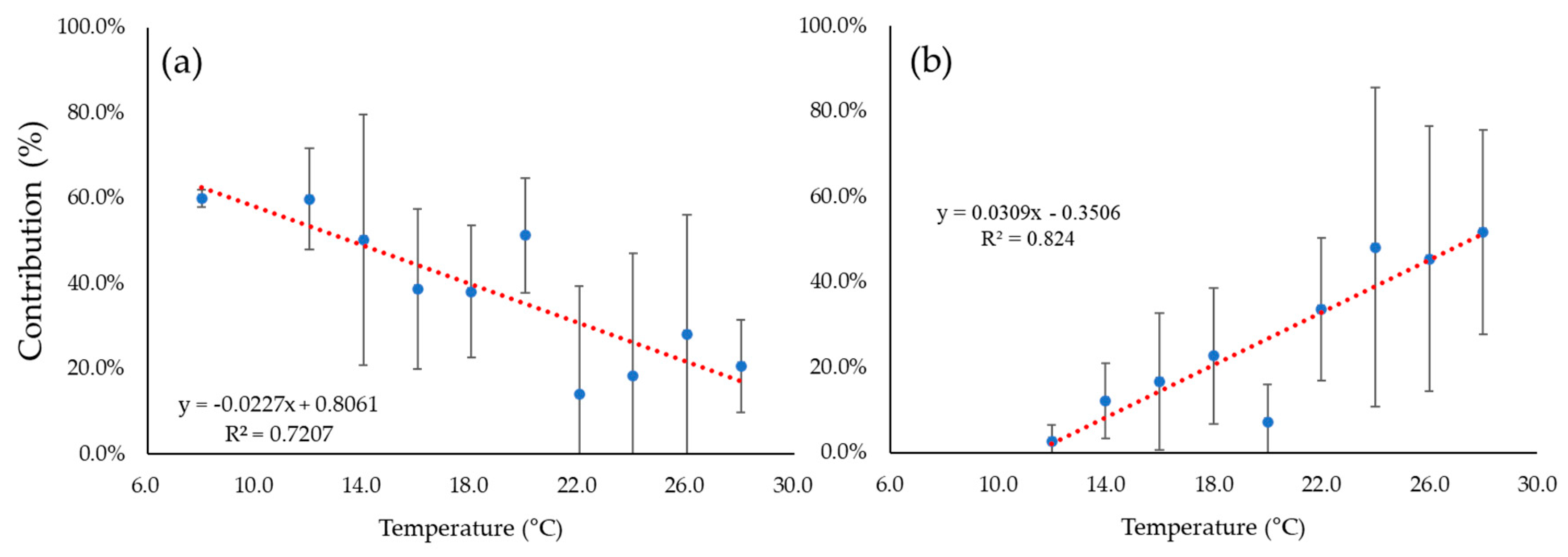
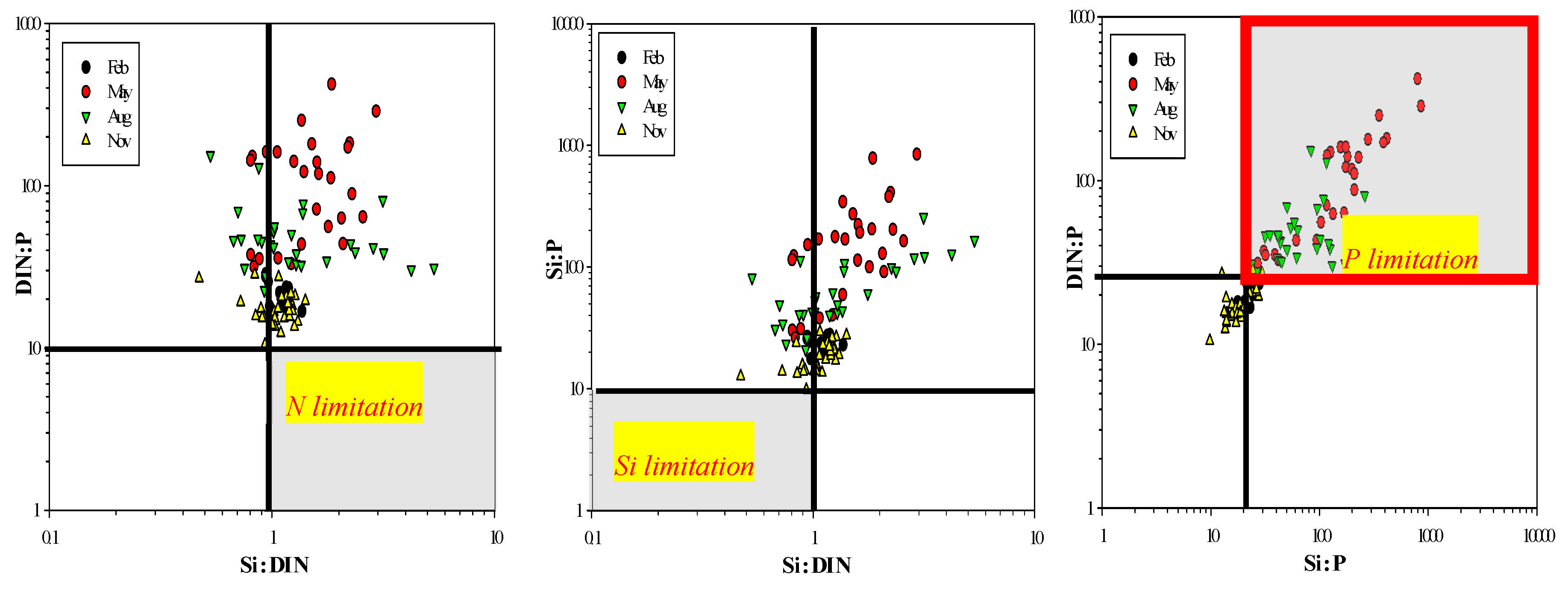
4. Discussion
5. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, W., Jr.; Sakshaug, E. Polar Phytoplankton. In Polar Oceanography; Academic: San Diego, CA, USA, 1990; pp. 477–525. [Google Scholar]
- Sommer, U. The impact of light intensity and daylength on silicate and nitrate competition among marine phytoplankton. Limnol. Oceanogr. 1994, 39, 1680–1688. [Google Scholar] [CrossRef]
- Hilligsøe, K.M.; Richardson, K.; Bendtsen, J.; Sørensen, L.-L.; Nielsen, T.G.; Lyngsgaard, M.M. Linking phytoplankton community size composition with temperature, plankton food web structure and sea–air CO2 flux. Deep Sea Res. Part I 2011, 58, 826–838. [Google Scholar]
- Kilham, P.; Hecky, R.E. Comparative ecology of marine and freshwater phytoplankton 1. Limnol. Oceanogr. 1988, 33, 776–795. [Google Scholar] [CrossRef]
- Domingues, R.B.; Galvao, H. Phytoplankton and environmental variability in a dam regulated temperate estuary. Hydrobiologia 2007, 586, 117–134. [Google Scholar] [CrossRef]
- Wu, N.; Schmalz, B.; Fohrer, N. Development and testing of a phytoplankton index of biotic integrity (P-IBI) for a German lowland river. Ecol. Indic. 2012, 13, 158–167. [Google Scholar] [CrossRef]
- Smayda, T. Biogeographical meaning; indicators. In Phytoplankton Manual; UNESCO: Paris, France, 1978; pp. 225–229. [Google Scholar]
- Soares, M.C.S.; Lobão, L.M.; Vidal, L.O.; Oyma, N.P.; Barros, N.O.; Cardoso, S.J.; Roland, F. Light microscopy in aquatic ecology: Methods for plankton communities studies. Methods Mol. Biol. 2011, 689, 215–227. [Google Scholar]
- Chisholm, S.W.; Olson, R.J.; Zettler, E.R.; Goericke, R.; Waterbury, J.B.; Welschmeyer, N.A. A novel free-living prochlorophyte abundant in the oceanic euphotic zone. Nature 1988, 334, 340. [Google Scholar] [CrossRef]
- Gieskes, W.; Kraay, G. Dominance of Cryptophyceae during the phytoplankton spring bloom in the central North Sea detected by HPLC analysis of pigments. Mar. Biol. 1983, 75, 179–185. [Google Scholar] [CrossRef]
- Simon, N.; Barlow, R.G.; Marie, D.; Partensky, F.; Vaulot, D. Characterization of oceanic photosynthetic picoeukaryotes by flow cytometry. J. Phycol. 1994, 30, 922–935. [Google Scholar] [CrossRef]
- Readman, J.; Devilla, R.; Tarran, G.; Llewellyn, C.; Fileman, T.; Easton, A.; Burkill, P.; Mantoura, R. Flow cytometry and pigment analyses as tools to investigate the toxicity of herbicides to natural phytoplankton communities. Mar. Environ. Res. 2004, 58, 353–358. [Google Scholar] [CrossRef]
- Campbell, L.; Nolla, H.; Vaulot, D. The importance of Prochlorococcus to community structure in the central North Pacific Ocean. Limnol. Oceanogr. 1994, 39, 954–961. [Google Scholar] [CrossRef]
- Partensky, F.; Blanchot, J.; Lantoine, F.; Neveux, J.; Marie, D. Vertical structure of picophytoplankton at different trophic sites of the tropical northeastern Atlantic Ocean. Deep Sea Res. Part I 1996, 43, 1191–1213. [Google Scholar] [CrossRef]
- Wright, S.; Jeffrey, S.; Mantoura, R.; Llewellyn, C.; Bjørnland, T.; Repeta, D.; Welschmeyer, N. Improved HPLC method for the analysis of chlorophylls and carotenoids from marine phytoplankton. Mar. Ecol. Prog. Ser. 1991, 77, 183–196. [Google Scholar] [CrossRef]
- Mackey, M.; Mackey, D.; Higgins, H.; Wright, S. CHEMTAX-a program for estimating class abundances from chemical markers: Application to HPLC measurements of phytoplankton. Mar. Ecol. Prog. Ser. 1996, 144, 265–283. [Google Scholar] [CrossRef]
- Furuya, K.; Kurita, K.; Odate, T. Distribution of phytoplankton in the East China Sea in the winter of 1993. J. Oceanol. 1996, 52, 323–333. [Google Scholar] [CrossRef]
- Jiang, Y.-Z.; Cheng, J.-H.; Li, S.-F. Temporal changes in the fish community resulting from a summer fishing moratorium in the northern East China Sea. Mar. Ecol. Prog. Ser. 2009, 387, 265–273. [Google Scholar] [CrossRef]
- Chen, C.-T.A. Distributions of nutrients in the East China Sea and the South China Sea connection. J. Oceanol. 2008, 64, 737–751. [Google Scholar] [CrossRef]
- Moriyasu, S. The Tsushima Current. In Kuroshio, Its Physical Aspects; Stommel, H., Yoshida, K., Eds.; University of Tokyo Press: Tokyo, Japan, 1972; pp. 353–369. [Google Scholar]
- Nitani, H. Beginning of the Kuroshio. In Kuroshio Physical Aspect of the Japan Current; University of Washington Press: Seattle, WA, USA, 1972; pp. 129–163. [Google Scholar]
- Seung, Y. Water masses and circulations around Korean Peninsula. J. Oceanol. Soc. Korea 1992, 27, 324–331. [Google Scholar]
- Qilong, Z.; Xuechuan, W.; Yuling, Y. Analysis of water masses in the south Yellow Sea in spring. Oceanol. Limnol. Sin. 1996, 27, 42–428. (In Chinese) [Google Scholar]
- Zhou, M.-J.; Shen, Z.-L.; Yu, R.-C. Responses of a coastal phytoplankton community to increased nutrient input from the Changjiang (Yangtze) River. Cont. Shelf Res. 2008, 28, 1483–1489. [Google Scholar] [CrossRef]
- Guo, C.; Liu, H.; Zheng, L.; Song, S.; Chen, B.; Huang, B. Seasonal and spatial patterns of picophytoplankton growth, grazing and distribution in the East China Sea. Biogeosciences 2014, 11, 1847–1862. [Google Scholar] [CrossRef]
- Furuya, K.; Hayashi, M.; Yabushita, Y.; Ishikawa, A. Phytoplankton dynamics in the East China Sea in spring and summer as revealed by HPLC-derived pigment signatures. Deep Sea Res. Part II 2003, 50, 367–387. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, W.; Landry, M.R.; Chiang, K.-P.; Wang, L.; Huang, B. Responses of phytoplankton communities to environmental variability in the East China Sea. Ecosystems 2016, 19, 832–849. [Google Scholar] [CrossRef]
- Guo, S.; Feng, Y.; Wang, L.; Dai, M.; Liu, Z.; Bai, Y.; Sun, J. Seasonal variation in the phytoplankton community of a continental-shelf sea: The East China Sea. Mar. Ecol. Prog. Ser. 2014, 516, 103–126. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, J.; Zhou, F.; Shou, L.; Chen, Q.; Tao, B.; Yan, X.; Wang, K. Controlling factors of summer phytoplankton community in the Changjiang (Yangtze River) Estuary and adjacent East China Sea shelf. Cont. Shelf Res. 2015, 101, 71–84. [Google Scholar] [CrossRef]
- Zhao, R.; Sun, J.; Bai, J. Phytoplankton assemblages in Yangtze River Estuary and its adjacent water in autumn 2006. Mar. Sci. 2010, 34, 32–39. [Google Scholar]
- He, Q.; Sun, J.; Luan, Q.-S.; Yu, Z.-M. Phytoplankton in Changjiang Estuary and adjacent waters in winter. Mar. Environ. Sci. 2009, 28, 360–365. [Google Scholar]
- Yoon, Y. Spatial distribution of phytoplankton community and red tide of dinoflagellate, Prorocentrum donghaience in the East China Sea during early summer. Korean J. Environ. Biol. 2003, 21, 132–141. [Google Scholar]
- Park, M.-O.; Kang, S.-W.; Lee, C.-I.; Choi, T.-S.; Lantoine, F. Structure of the phytoplanktonic communities in Jeju Strait and northern East China Sea and dinoflagellate blooms in spring 2004: Analysis of photosynthetic pigments. Sea 2008, 13, 27–41. [Google Scholar]
- Yoon, Y.-H.; Park, J.-S.; Soh, H.-Y.; Hwang, D.-J. On the marine environment and distribution of phytoplankton community in the northern East China Sea in early summer 2004. J. Korean Soc. Mar. Environ. Energy 2005, 8, 100–110. [Google Scholar]
- kim, D.; Choi, S.H.; Kim, K.H.; Shim, J.; Yoo, S.; Kim, C.H. Spatial and temporal variations in nutrient and chlorophyll-a concentrations in the northern East China Sea surrounding Cheju Island. Cont. Shelf Res. 2009, 29, 1426–1436. [Google Scholar] [CrossRef]
- Kang, J.J.; Lee, J.H.; Kim, H.C.; Lee, W.C.; Lee, D.; Jo, N.; Min, J.-O.; Lee, S.H. Monthly Variations of Phytoplankton Community in Geoje-Hansan Bay of the Southern Part of Korea Based on HPLC Pigment Analysis. J. Coast. Res. 2018, 85, 356–360. [Google Scholar] [CrossRef]
- Zapata, M.; Rodríguez, F.; Garrido, J.L. Separation of chlorophylls and carotenoids from marine phytoplankton: A new HPLC method using a reversed phase C8 column and pyridine-containing mobile phases. Mar. Ecol. Prog. Ser. 2000, 195, 29–45. [Google Scholar] [CrossRef]
- Lee, Y.-W.; Park, M.-O.; Im, Y.-S.; Kim, S.-S.; Kang, C.-K. Application of photosynthetic pigment analysis using a HPLC and CHEMTAX program to studies of phytoplankton community composition. Sea 2011, 16, 117–124. [Google Scholar] [CrossRef]
- Wolf, C.; Frickenhaus, S.; Kilias, E.S.; Peeken, I.; Metfies, K. Protist community composition in the Pacific sector of the Southern Ocean during austral summer 2010. Polar Biol. 2014, 37, 375–389. [Google Scholar] [CrossRef]
- Torrecilla, E.; Stramski, D.; Reynolds, R.A.; Millán-Núñez, E.; Piera, J. Cluster analysis of hyperspectral optical data for discriminating phytoplankton pigment assemblages in the open ocean. Remote Sens. Environ. 2011, 115, 2578–2593. [Google Scholar] [CrossRef]
- Wong, G.; Gong, G.; Liu, K.; Pai, S. ‘Excess nitrate’in the East China Sea. Estuar. Coast. Shelf Sci. 1998, 46, 411–418. [Google Scholar] [CrossRef][Green Version]
- Xu, Q.; Sukigara, C.; Goes, J.I.; do Rosario Gomes, H.; Zhu, Y.; Wang, S.; Shen, A.; de Raús Maúre, E.; Matsuno, T.; Yuji, W. Interannual changes in summer phytoplankton community composition in relation to water mass variability in the East China Sea. J. Oceanol. 2019, 75, 61–79. [Google Scholar] [CrossRef]
- Goes, J.I.; do Rosario Gomes, H.; Chekalyuk, A.M.; Carpenter, E.J.; Montoya, J.P.; Coles, V.J.; Yager, P.L.; Berelson, W.M.; Capone, D.G.; Foster, R.A. Influence of the Amazon River discharge on the biogeography of phytoplankton communities in the western tropical north Atlantic. Prog. Oceanogr. 2014, 120, 29–40. [Google Scholar] [CrossRef]
- Ter Braak, C.J.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power: Ithaca, NY, USA, 2002. [Google Scholar]
- Gong, G.-C.; Chen, Y.-L.L.; Liu, K.-K. Chemical hydrography and chlorophyll a distribution in the East China Sea in summer: Implications in nutrient dynamics. Cont. Shelf Res. 1996, 16, 1561–1590. [Google Scholar] [CrossRef]
- Chen, C.-T.A. Chemical and physical fronts in the Bohai, Yellow and East China seas. J. Mar. Sci. 2009, 78, 394–410. [Google Scholar] [CrossRef]
- Chen, C.; Beardsley, R.C.; Limeburner, R.; Kim, K. Comparison of winter and summer hydrographic observations in the Yellow and East China Seas and adjacent Kuroshio during 1986. Cont. Shelf Res. 1994, 14, 909–929. [Google Scholar] [CrossRef]
- Kusakabe, M. Hydrographic feature of the East China Sea. Bull. Coast. Oceanogr. 1998, 36, 5–17. [Google Scholar]
- Jang, S.-T.; Lee, J.-H.; Hong, C.-S. Mixing of Sea Waters in the Northern Part of the East China Sea in Summer. Sea 2007, 12, 390–399. [Google Scholar]
- Beardsley, R.; Limeburner, R.; Yu, H.; Cannon, G. Discharge of the Changjiang (Yangtze river) into the East China sea. Cont. Shelf Res. 1985, 4, 57–76. [Google Scholar] [CrossRef]
- Zuo-sheng, Y.; Milliman, J.D.; Fitzgerald, M.G. Transfer of water and sediment from the Yangtze River to the East China Sea, June 1980. Can. J. Fish. Aqua. Sci. 1983, 40, s72–s82. [Google Scholar] [CrossRef]
- Kim, D.; Shim, J.; Yoo, S. Seasonal variations in nutrients and chlorophyll-a concentrations in the northern East China Sea. Ocean Sci. J. 2006, 41, 125–137. [Google Scholar] [CrossRef]
- Justić, D.; Rabalais, N.N.; Turner, R.E.; Dortch, Q. Changes in nutrient structure of river-dominated coastal waters: Stoichiometric nutrient balance and its consequences. Estuar. Coast. Shelf Sci. 1995, 40, 339–356. [Google Scholar] [CrossRef]
- Harrison, P.; Hu, M.; Yang, Y.; Lu, X. Phosphate limitation in estuarine and coastal waters of China. J. Exp. Mar. Biol. Ecol. 1990, 140, 79–87. [Google Scholar] [CrossRef]
- Chen, Y.-L.L.; Lu, H.-B.; Shiah, F.-K.; Gong, G.; Liu, K.-K.; Kanda, J. New production and f-ratio on the continental shelf of the East China Sea: Comparisons between nitrate inputs from the subsurface Kuroshio Current and the Changjiang River. Estuar. Coast. Shelf Sci. 1999, 48, 59–75. [Google Scholar] [CrossRef]
- Gong, G.-C.; Wen, Y.-H.; Wang, B.-W.; Liu, G.-J. Seasonal variation of chlorophyll a concentration, primary production and environmental conditions in the subtropical East China Sea. Deep Sea Res. Part II 2003, 50, 1219–1236. [Google Scholar] [CrossRef]
- Hillebrand, H.; Steinert, G.; Boersma, M.; Malzahn, A.; Meunier, C.L.; Plum, C.; Ptacnik, R. Goldman revisited: Faster-growing phytoplankton has lower N: P and lower stoichiometric flexibility. Limnol. Oceanogr. 2013, 58, 2076–2088. [Google Scholar] [CrossRef]
- Li, J.; Glibert, P.M.; Zhou, M.; Lu, S.; Lu, D. Relationships between nitrogen and phosphorus forms and ratios and the development of dinoflagellate blooms in the East China Sea. Mar. Ecol. Prog. Ser. 2009, 383, 11–26. [Google Scholar] [CrossRef]
- Hampton, S.E.; Gray, D.K.; Izmest’eva, L.R.; Moore, M.V.; Ozersky, T. The rise and fall of plankton: Long-term changes in the vertical distribution of algae and grazers in Lake Baikal, Siberia. PLoS ONE 2014, 9, e88920. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D. Resource competition between plankton algae: An experimental and theoretical approach. Ecology 1977, 58, 338–348. [Google Scholar] [CrossRef]
- Tilman, D. Resource Competition and Community Structure; Princeton University Press: Princeton, NJ, USA, 1982. [Google Scholar]
- Tilman, D.; Kilham, S.S.; Kilham, P. Phytoplankton community ecology: The role of limiting nutrients. Annu. Rev. Ecol. Syst. 1982, 13, 349–372. [Google Scholar] [CrossRef]
- Smith, R.E.; Kalff, J. Size dependence of growth rate, respiratory electron transport system activity and chemical composition in marine diatoms in the laboratory. J. Phycol. 1982, 18, 275–284. [Google Scholar] [CrossRef]
- Fogg, G.E. Review Lecture-Picoplankton. Proc. R. Soc. Lond. B Biol. Sci. 1986, 228, 1–30. [Google Scholar]
- Raven, J. The twelfth Tansley Lecture. Small is beautiful: The picophytoplankton. Funct. Ecol. 1998, 12, 503–513. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W.; Czygan, F.-C.; Schreiber, U.; Lange, O.L. Differences in the capacity for radiationless energy dissipation in the photochemical apparatus of green and blue-green algal lichens associated with differences in carotenoid composition. Planta 1990, 180, 582–589. [Google Scholar] [CrossRef]
- Descy, J.-P.; Sarmento, H.; Higgins, H.W. Variability of phytoplankton pigment ratios across aquatic environments. Eur. J. Phycol. 2009, 44, 319–330. [Google Scholar] [CrossRef]
- Claustre, H.; Kerhervé, P.; Marty, J.-C.; Prieur, L. Phytoplankton photoadaptation related to some frontal physical processes. J. Mar. Syst. 1994, 5, 251–265. [Google Scholar] [CrossRef]
- Mur, R.; Skulberg, O.; Utkilen, H. Cyanobacteria in the environment. In Toxic Cyanobacteria in Water; Chorus, I., Batram, J., Eds.; E&FN Spon: London, UK, 1999; pp. 15–40. [Google Scholar]
- Peperzak, L. Climate change and harmful algal blooms in the North Sea. Acta Oecol. 2003, 24, S139–S144. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Dusenberry, J.; Olson, R.; Chisholm, S. Field observations of oceanic mixed layer dynamics and picophytoplankton photoacclimation. J. Mar. Syst. 2000, 24, 221–232. [Google Scholar] [CrossRef]
- Moore, L.R.; Goericke, R.; Chisholm, S.W. Comparative physiology of Synechococcus and Prochlorococcus: Influence of light and temperature on growth, pigments, fluorescence and absorptive properties. Mar. Ecol. Prog. Ser. 1995, 116, 259–275. [Google Scholar] [CrossRef]
- Goldman, J.C.; McGillicuddy, D.J.J. Effect of large marine diatoms growing at low light on episodic new production. Limnol. Oceanogr. 2003, 48, 1176–1182. [Google Scholar] [CrossRef]
- Chakraborty, S.; Lohrenz, S.E. Phytoplankton community structure in the river-influenced continental margin of the northern Gulf of Mexico. Mar. Ecol. Prog. Ser. 2015, 521, 31–47. [Google Scholar] [CrossRef]
- Anglès, S.; Jordi, A.; Henrichs, D.W.; Campbell, L. Influence of coastal upwelling and river discharge on the phytoplankton community composition in the northwestern Gulf of Mexico. Prog. Oceanogr. 2019, 173, 26–36. [Google Scholar] [CrossRef]
- Shi, W.; Wang, M. Satellite views of the bohai sea, yellow sea, and East China Sea. Prog. Oceanogr. 2012, 104, 30–45. [Google Scholar] [CrossRef]
- Reid, P.; Lancelot, C.; Gieskes, W.; Hagmeier, E.; Weichart, G. Phytoplankton of the North Sea and its dynamics: A review. Neth. J. Sea Res. 1990, 26, 295–331. [Google Scholar] [CrossRef]
- Johnson, F.A. Green, bluegreen and diatom algae: Taxonomic differences in competitive ability for phosphorus, silicon and nitrogen. Arch. Hydrobiol. 1986, 106, 473485. [Google Scholar]
- Hassen, M.B.; Drira, Z.; Hamza, A.; Ayadi, H.; Akrout, F.; Issaoui, H. Summer phytoplankton pigments and community composition related to water mass properties in the Gulf of Gabes. Estuar. Coast. Shelf Sci. 2008, 77, 645–656. [Google Scholar] [CrossRef]
- Lagus, A.; Suomela, J.; Weithoff, G.; Heikkilä, K.; Helminen, H.; Sipura, J. Species-specific differences in phytoplankton responses to N and P enrichments and the N: P ratio in the Archipelago Sea, northern Baltic Sea. J. Plankton Res. 2004, 26, 779–798. [Google Scholar] [CrossRef]
- Kanoshina, I.; Lips, U.; Leppänen, J.-M. The influence of weather conditions (temperature and wind) on cyanobacterial bloom development in the Gulf of Finland (Baltic Sea). Harmful Algae 2003, 2, 29–41. [Google Scholar] [CrossRef]
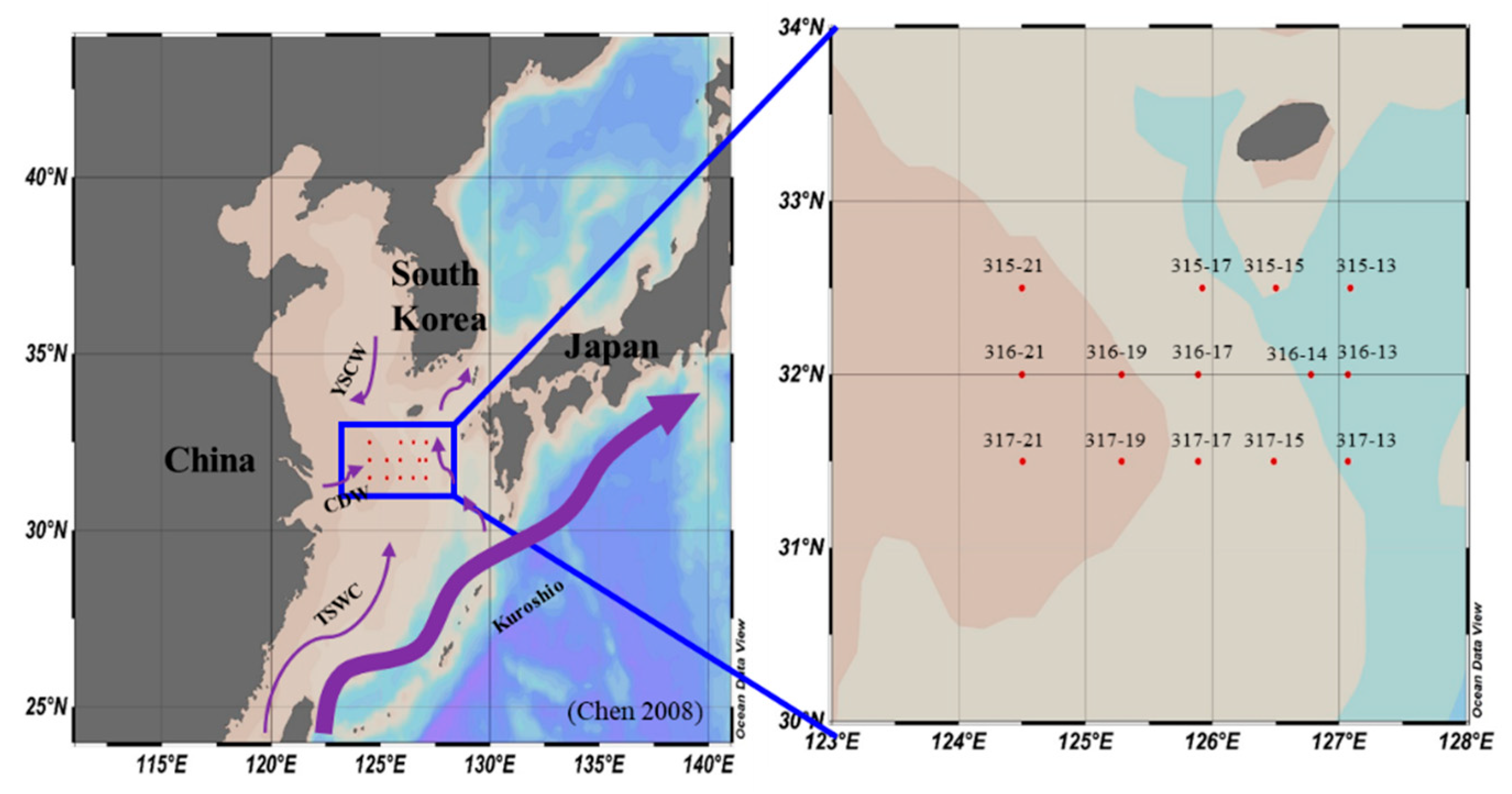
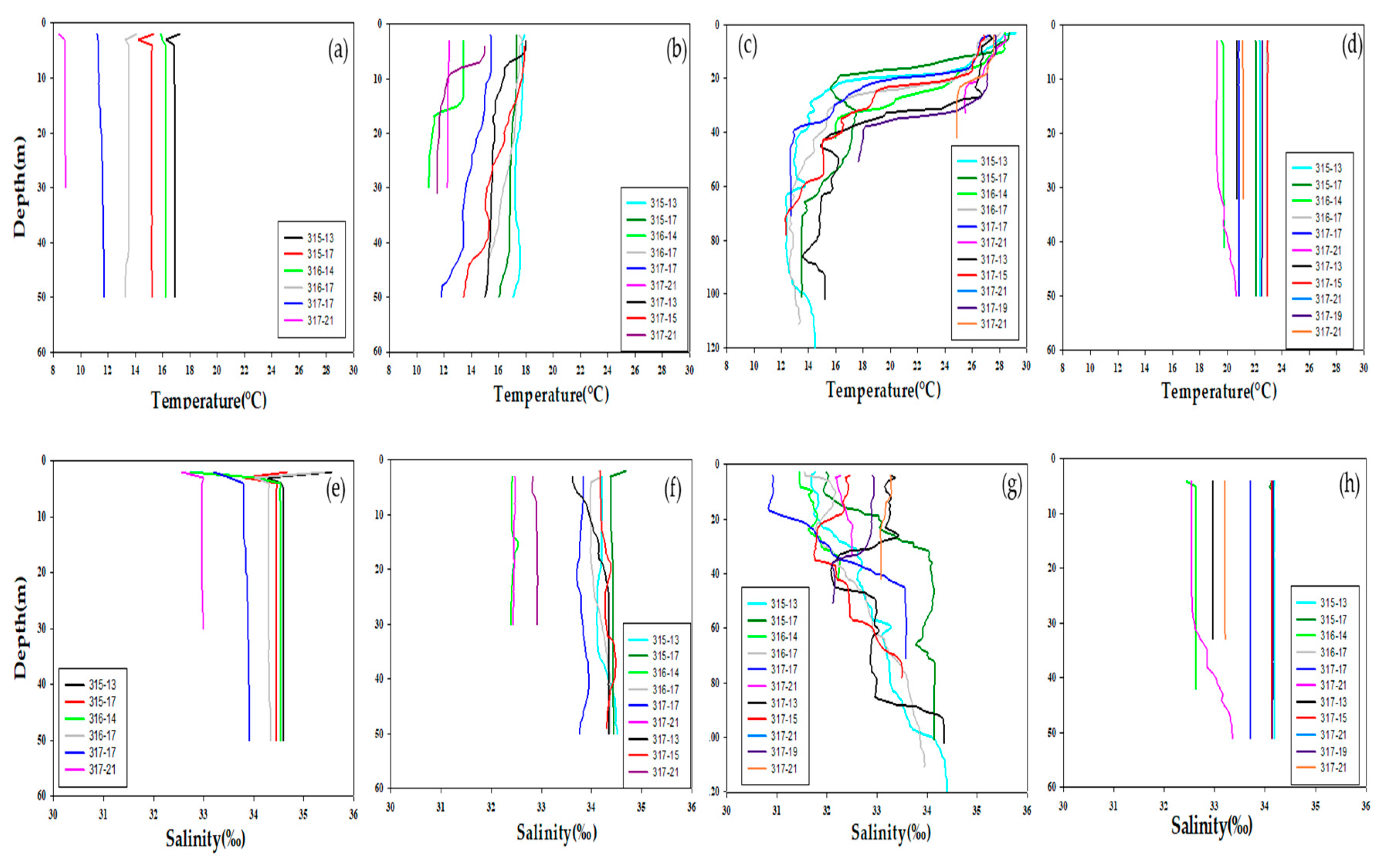



| Date | Station | Latitude | Longitude | Euphotic Depth (m) |
|---|---|---|---|---|
| February | 315-13 | 32.5 | 127.0 | 19 |
| 315-17 | 32.5 | 125.9 | 5 | |
| 316-14 | 32.0 | 126.8 | 19 | |
| 316-17 | 32.0 | 125.9 | 14 | |
| 317-17 | 31.5 | 125.9 | 19 | |
| 317-21 | 31.5 | 124.5 | 5 | |
| May | 315-13 | 32.5 | 127.0 | 27 |
| 315-15 | 32.5 | 126.5 | 38 | |
| 315-21 | 32.5 | 124.5 | 11 | |
| 316-13 | 32 | 127.0 | 27 | |
| 316-17 | 32 | 125.9 | 27 | |
| 316-21 | 32 | 124.5 | 5 | |
| 317-13 | 31.5 | 127.0 | 16 | |
| 317-15 | 31.5 | 126.5 | 33 | |
| 317-21 | 31.5 | 124.5 | 14 | |
| August | 315-13 | 32.5 | 127.0 | 41 |
| 315-17 | 32.5 | 125.9 | 46 | |
| 315-21 | 32.5 | 124.5 | 22 | |
| 316-13 | 32 | 127.0 | 41 | |
| 316-17 | 32 | 125.9 | 27 | |
| 316-21 | 32 | 124.5 | 30 | |
| 317-13 | 31.5 | 127.0 | 35 | |
| 317-15 | 31.5 | 126.5 | 54 | |
| 317-19 | 31.5 | 125.3 | 35 | |
| 317-21 | 31.5 | 124.5 | 19 | |
| October | 315-13 | 32.5 | 127.0 | 41 |
| 315-15 | 32.5 | 126.5 | 41 | |
| 315-21 | 32.5 | 124.5 | 8 | |
| 316-13 | 32 | 127.0 | 33 | |
| 316-17 | 32 | 125.9 | 27 | |
| 316-19 | 32 | 125.3 | 8 | |
| 316-21 | 32 | 124.5 | 5 | |
| 317-13 | 31.5 | 127.0 | 49 | |
| 317-15 | 31.5 | 126.5 | 49 | |
| 317-21 | 31.5 | 124.5 | 5 |
| Station | Light (%) | NH4 | NO2 | NO3 | DIN | PO4 | SiO2 | |
|---|---|---|---|---|---|---|---|---|
| February | 315-13 | 100% | 0.84 | 0.40 | 4.81 | 6.04 | 0.33 | 7.15 |
| 30% | 0.92 | 0.36 | 4.01 | 5.29 | 0.29 | 6.27 | ||
| 1% | 1.01 | 0.39 | 5.07 | 6.48 | 0.32 | 7.11 | ||
| 315-17 | 100% | 0.80 | 0.10 | 10.66 | 11.56 | 0.60 | 13.67 | |
| 30% | 0.93 | 0.08 | 5.07 | 6.08 | 0.33 | 6.79 | ||
| 1% | 0.90 | 0.08 | 4.92 | 5.90 | 0.35 | 8.07 | ||
| 316-14 | 100% | 0.93 | 0.09 | 8.31 | 9.33 | 0.33 | 8.76 | |
| 30% | 0.91 | 0.09 | 7.95 | 8.95 | 0.35 | 8.65 | ||
| 1% | 0.91 | 0.10 | 8.06 | 9.07 | 0.33 | 8.50 | ||
| 316-17 | 100% | 0.85 | 0.28 | 5.10 | 6.23 | 0.29 | 6.96 | |
| 30% | 0.73 | 0.27 | 4.79 | 5.78 | 0.29 | 6.83 | ||
| 1% | 1.05 | 0.24 | 4.66 | 5.95 | 0.29 | 6.60 | ||
| 317-17 | 100% | 1.01 | 0.18 | 7.87 | 9.07 | 0.41 | 9.82 | |
| 30% | 0.80 | 0.15 | 5.24 | 6.19 | 0.34 | 6.04 | ||
| 1% | 0.79 | 0.11 | 7.80 | 8.70 | 0.41 | 9.97 | ||
| 317-21 | 100% | 0.81 | 0.09 | 11.55 | 12.46 | 0.53 | 14.46 | |
| 30% | 0.78 | 0.10 | 12.34 | 13.22 | 0.61 | 15.37 | ||
| 1% | 0.82 | 0.10 | 13.19 | 14.11 | 0.60 | 16.77 | ||
| average | 0.88 | 0.18 | 7.30 | 8.36 | 0.39 | 9.32 | ||
| S.D | 0.09 | 0.11 | 2.94 | 2.84 | 0.11 | 3.40 | ||
| May | 315-13 | 100% | 1.58 | 0.09 | 1.98 | 3.66 | 0.02 | 3.45 |
| 30% | 1.51 | 0.05 | 1.17 | 2.74 | 0.02 | 3.44 | ||
| 1% | 1.56 | 0.06 | 1.31 | 2.93 | 0.02 | 4.68 | ||
| 315-15 | 100% | 1.59 | 0.06 | 3.23 | 4.88 | 0.03 | 3.99 | |
| 30% | 1.49 | 0.04 | 1.43 | 2.95 | 0.02 | 4.11 | ||
| 1% | 1.56 | 0.05 | 1.29 | 2.89 | 0.04 | 4.59 | ||
| 315-21 | 100% | 1.64 | 0.23 | 4.47 | 6.34 | 0.11 | 11.36 | |
| 30% | 1.65 | 0.21 | 3.68 | 5.54 | 0.13 | 11.54 | ||
| 1% | 1.52 | 0.16 | 3.51 | 5.19 | 0.08 | 10.68 | ||
| 316-13 | 100% | 1.96 | 0.07 | 3.43 | 5.46 | 0.03 | 5.78 | |
| 30% | 1.60 | 0.05 | 1.04 | 2.68 | 0.02 | 4.36 | ||
| 1% | 1.77 | 0.19 | 4.51 | 6.47 | 0.18 | 6.91 | ||
| 316-17 | 100% | 2.14 | 0.06 | 1.41 | 3.61 | 0.03 | 6.64 | |
| 30% | 1.61 | 0.07 | 1.11 | 2.79 | 0.04 | 7.15 | ||
| 1% | 1.54 | 0.04 | 1.30 | 2.88 | 0.03 | 6.59 | ||
| 316-21 | 100% | 1.53 | 0.51 | 10.23 | 12.26 | 0.33 | 9.86 | |
| 30% | 1.44 | 0.48 | 8.90 | 10.82 | 0.30 | 9.50 | ||
| 1% | 1.47 | 0.44 | 7.51 | 9.43 | 0.30 | 7.83 | ||
| 317-13 | 100% | 2.65 | 0.30 | 1.69 | 4.64 | 0.03 | 3.72 | |
| 30% | 1.55 | 0.06 | 1.06 | 2.66 | 0.01 | 5.95 | ||
| 1% | 1.60 | 0.06 | 0.85 | 2.51 | 0.01 | 5.52 | ||
| 317-15 | 100% | 1.55 | 0.05 | 1.27 | 2.86 | 0.01 | 3.89 | |
| 30% | 1.57 | 0.05 | 1.01 | 2.63 | 0.01 | 3.97 | ||
| 1% | 1.57 | 0.26 | 2.61 | 4.44 | 0.10 | 6.03 | ||
| 317-21 | 100% | 2.06 | 0.10 | 2.61 | 4.78 | 0.01 | 8.87 | |
| 30% | 1.72 | 0.08 | 1.45 | 3.26 | 0.01 | 9.58 | ||
| 1% | 1.58 | 0.45 | 8.12 | 10.16 | 0.31 | 12.41 | ||
| average | 1.67 | 0.16 | 3.04 | 4.87 | 0.08 | 6.75 | ||
| S.D | 0.26 | 0.15 | 2.66 | 2.76 | 0.10 | 2.79 | ||
| Station | Light (%) | NH4 | NO2 | NO3 | DIN | PO4 | SiO2 | |
| August | 315-13 | 100% | 0.41 | 0.06 | 1.49 | 1.97 | 0.06 | 8.31 |
| 30% | 0.41 | 0.09 | 1.99 | 2.50 | 0.06 | 7.94 | ||
| 1% | 0.44 | 0.09 | 14.14 | 14.67 | 0.53 | 13.75 | ||
| 315-17 | 100% | 1.46 | 0.09 | 1.41 | 2.97 | 0.07 | 8.48 | |
| 30% | 0.40 | 0.05 | 1.16 | 1.61 | 0.05 | 8.62 | ||
| 1% | 0.36 | 0.09 | 8.45 | 8.90 | 0.49 | 10.75 | ||
| 315-21 | 100% | 0.80 | 0.14 | 2.18 | 3.13 | 0.07 | 7.07 | |
| 30% | 0.80 | 0.11 | 2.00 | 2.92 | 0.07 | 6.91 | ||
| 1% | 1.17 | 1.66 | 5.68 | 8.51 | 0.11 | 11.79 | ||
| 316-13 | 100% | 0.54 | 0.06 | 2.04 | 2.63 | 0.03 | 8.29 | |
| 30% | 2.76 | 0.07 | 0.96 | 3.78 | 0.03 | 3.31 | ||
| 1% | 0.43 | 0.06 | 9.11 | 9.59 | 0.42 | 8.88 | ||
| 316-17 | 100% | 0.72 | 0.20 | 2.96 | 3.88 | 0.08 | 2.61 | |
| 30% | 0.61 | 0.17 | 3.05 | 3.83 | 0.05 | 2.70 | ||
| 1% | 0.74 | 0.29 | 13.84 | 14.86 | 0.34 | 14.71 | ||
| 316-21 | 100% | 1.29 | 0.05 | 1.54 | 2.88 | 0.06 | 2.10 | |
| 30% | 0.56 | 0.06 | 1.68 | 2.31 | 0.07 | 2.74 | ||
| 1% | 0.74 | 1.26 | 4.06 | 6.06 | 0.18 | 7.80 | ||
| 317-13 | 100% | 0.58 | 0.04 | 3.35 | 3.96 | 0.06 | 5.45 | |
| 30% | 3.15 | 0.04 | 0.79 | 3.98 | 0.03 | 2.11 | ||
| 1% | 0.49 | 0.45 | 10.73 | 11.67 | 0.28 | 11.89 | ||
| 317-15 | 100% | 1.30 | 0.06 | 1.56 | 2.93 | 0.06 | 3.59 | |
| 30% | 1.02 | 0.06 | 1.96 | 3.04 | 0.09 | 4.12 | ||
| 1% | 3.71 | 0.06 | 9.46 | 13.23 | 0.43 | 9.97 | ||
| 317-19 | 100% | 0.67 | 0.05 | 1.63 | 2.35 | 0.05 | 2.12 | |
| 30% | 0.64 | 0.05 | 1.85 | 2.54 | 0.05 | 2.59 | ||
| 1% | 1.11 | 0.69 | 6.50 | 8.30 | 0.15 | 8.53 | ||
| 317-21 | 100% | 1.54 | 0.04 | 1.16 | 2.74 | 0.06 | 2.38 | |
| 30% | 0.40 | 0.04 | 1.68 | 2.11 | 0.06 | 3.73 | ||
| 1% | 0.56 | 0.10 | 2.30 | 2.96 | 0.08 | 3.81 | ||
| average | 0.99 | 0.21 | 4.02 | 5.23 | 0.14 | 6.57 | ||
| S.D | 0.83 | 0.37 | 3.88 | 4.00 | 0.15 | 3.76 | ||
| October | 315-13 | 100% | 0.66 | 0.15 | 1.79 | 2.61 | 0.18 | 2.72 |
| 30% | 0.74 | 0.10 | 1.38 | 2.22 | 0.13 | 1.99 | ||
| 1% | 0.79 | 0.14 | 1.18 | 2.11 | 0.17 | 2.32 | ||
| 315-15 | 100% | 0.49 | 0.48 | 2.66 | 3.62 | 0.22 | 3.71 | |
| 30% | 0.63 | 0.40 | 1.88 | 2.90 | 0.17 | 3.09 | ||
| 1% | 0.73 | 0.47 | 2.32 | 3.51 | 0.23 | 3.63 | ||
| 315-21 | 100% | 2.07 | 0.13 | 13.73 | 15.93 | 0.56 | 13.36 | |
| 30% | 0.70 | 0.11 | 10.35 | 11.16 | 0.57 | 15.78 | ||
| 1% | 0.62 | 0.12 | 10.91 | 11.65 | 0.56 | 14.81 | ||
| 316-13 | 100% | 1.04 | 0.21 | 2.42 | 3.67 | 0.19 | 2.66 | |
| 30% | 0.60 | 0.07 | 1.93 | 2.60 | 0.10 | 2.79 | ||
| 1% | 0.74 | 0.11 | 1.41 | 2.27 | 0.17 | 2.35 | ||
| 316-17 | 100% | 0.60 | 0.49 | 5.38 | 6.47 | 0.36 | 7.99 | |
| 30% | 0.54 | 0.52 | 4.62 | 5.68 | 0.39 | 7.44 | ||
| 1% | 0.60 | 0.54 | 4.34 | 5.49 | 0.41 | 6.93 | ||
| 316-19 | 100% | 1.24 | 0.14 | 8.70 | 10.07 | 0.49 | 11.69 | |
| 30% | 0.78 | 0.12 | 9.28 | 10.18 | 0.47 | 12.39 | ||
| 1% | 0.56 | 0.11 | 7.79 | 8.47 | 0.50 | 10.50 | ||
| 316-21 | 100% | 0.87 | 0.07 | 6.30 | 7.24 | 0.47 | 8.25 | |
| 30% | 0.75 | 0.11 | 8.29 | 9.15 | 0.59 | 10.99 | ||
| 1% | 0.89 | 0.11 | 9.28 | 10.28 | 0.55 | 12.24 | ||
| 317-13 | 100% | 1.10 | 0.18 | 4.74 | 6.02 | 0.22 | 2.84 | |
| 30% | 0.66 | 0.09 | 1.81 | 2.55 | 0.17 | 2.35 | ||
| 1% | 0.71 | 0.11 | 1.47 | 2.29 | 0.22 | 2.13 | ||
| 317-15 | 100% | 0.88 | 0.16 | 2.27 | 3.30 | 0.22 | 2.99 | |
| 30% | 0.85 | 0.15 | 1.90 | 2.90 | 0.21 | 2.91 | ||
| 1% | 1.39 | 0.21 | 2.00 | 3.60 | 0.23 | 3.04 | ||
| 317-21 | 100% | 1.05 | 0.14 | 9.23 | 10.41 | 0.51 | 11.52 | |
| 30% | 0.65 | 0.12 | 8.16 | 8.93 | 0.53 | 10.68 | ||
| 1% | 0.65 | 0.12 | 8.70 | 9.48 | 0.51 | 11.18 | ||
| average | 0.82 | 0.20 | 5.21 | 6.22 | 0.34 | 6.91 | ||
| S.D | 0.32 | 0.15 | 3.67 | 3.75 | 0.17 | 4.59 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Youn, S.-H.; Oh, H.J.; Kang, J.J.; Lee, J.H.; Lee, D.; Kim, K.; Jang, H.K.; Lee, J.; Lee, S.H. Spatiotemporal Variation in Phytoplankton Community Driven by Environmental Factors in the Northern East China Sea. Water 2020, 12, 2695. https://doi.org/10.3390/w12102695
Kim Y, Youn S-H, Oh HJ, Kang JJ, Lee JH, Lee D, Kim K, Jang HK, Lee J, Lee SH. Spatiotemporal Variation in Phytoplankton Community Driven by Environmental Factors in the Northern East China Sea. Water. 2020; 12(10):2695. https://doi.org/10.3390/w12102695
Chicago/Turabian StyleKim, Yejin, Seok-Hyun Youn, Hyun Ju Oh, Jae Joong Kang, Jae Hyung Lee, Dabin Lee, Kwanwoo Kim, Hyo Keun Jang, Junbeom Lee, and Sang Heon Lee. 2020. "Spatiotemporal Variation in Phytoplankton Community Driven by Environmental Factors in the Northern East China Sea" Water 12, no. 10: 2695. https://doi.org/10.3390/w12102695
APA StyleKim, Y., Youn, S.-H., Oh, H. J., Kang, J. J., Lee, J. H., Lee, D., Kim, K., Jang, H. K., Lee, J., & Lee, S. H. (2020). Spatiotemporal Variation in Phytoplankton Community Driven by Environmental Factors in the Northern East China Sea. Water, 12(10), 2695. https://doi.org/10.3390/w12102695








