Nutrient Release Dynamics Associated with Native and Invasive Leaf Litter Decomposition: A Mesocosm Experiment
Abstract
1. Introduction
2. Materials and Methods
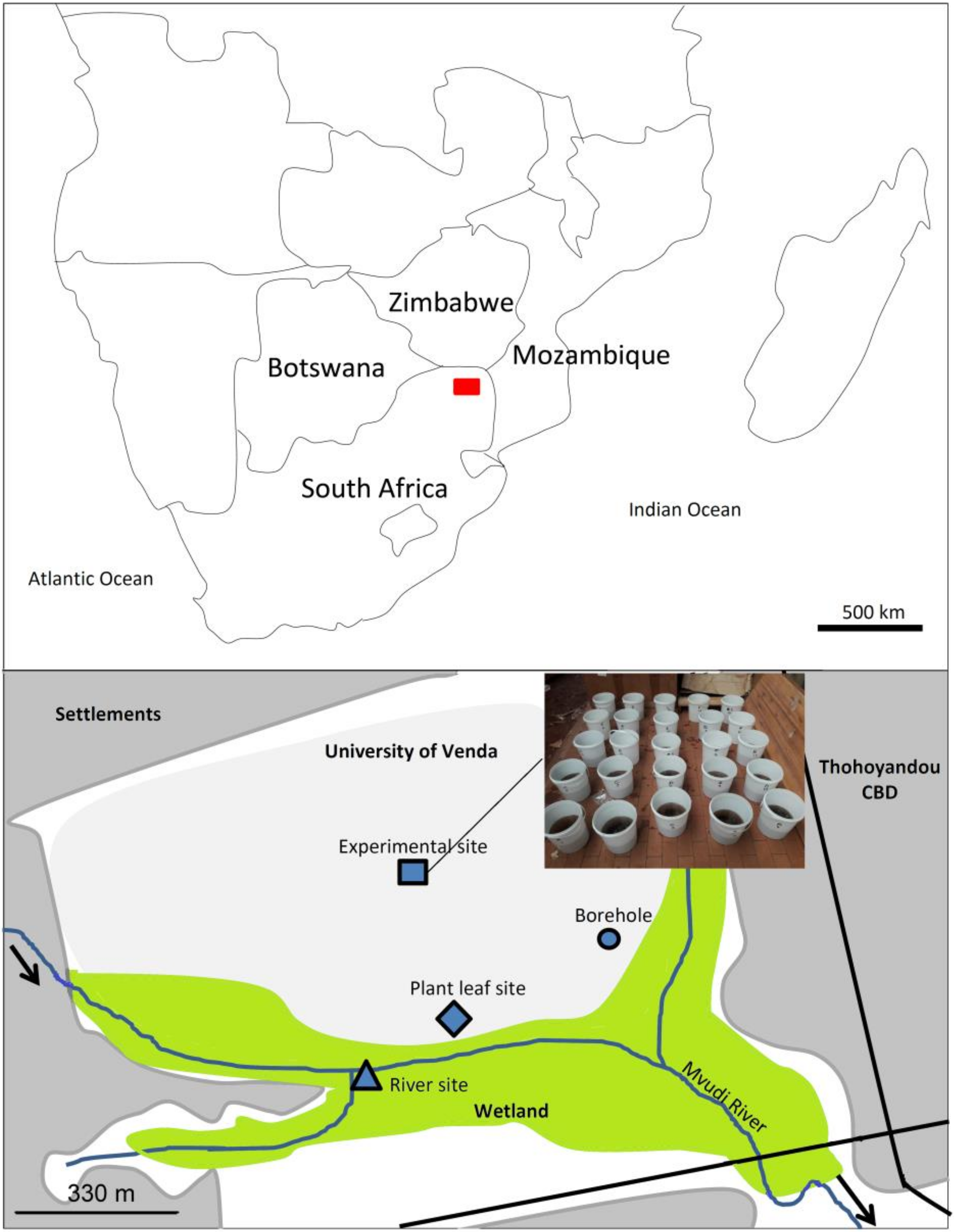
2.1. Study Area
2.2. Experimental Design
2.3. Sampling and Analyses
2.4. Statistical Analyses
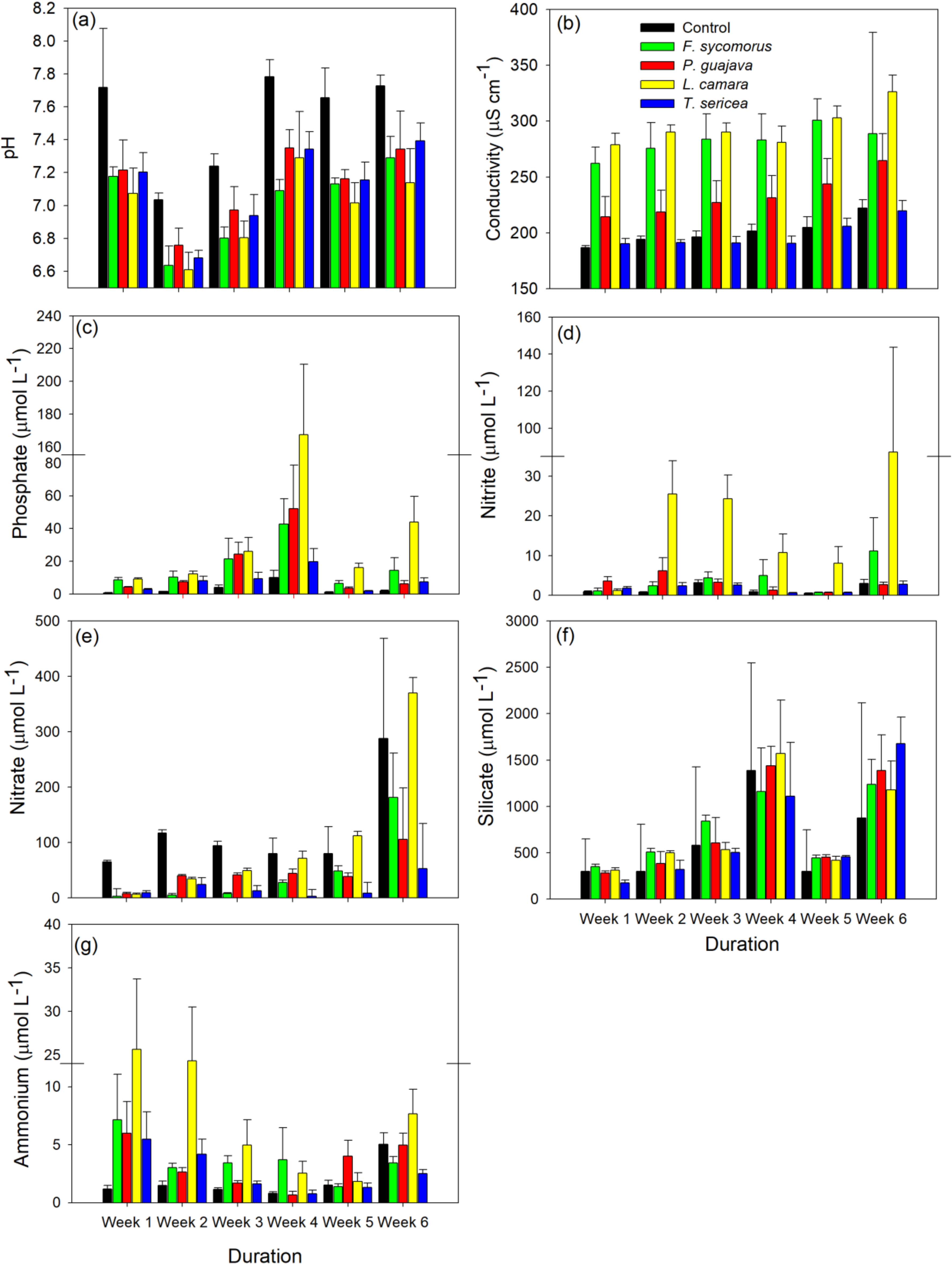
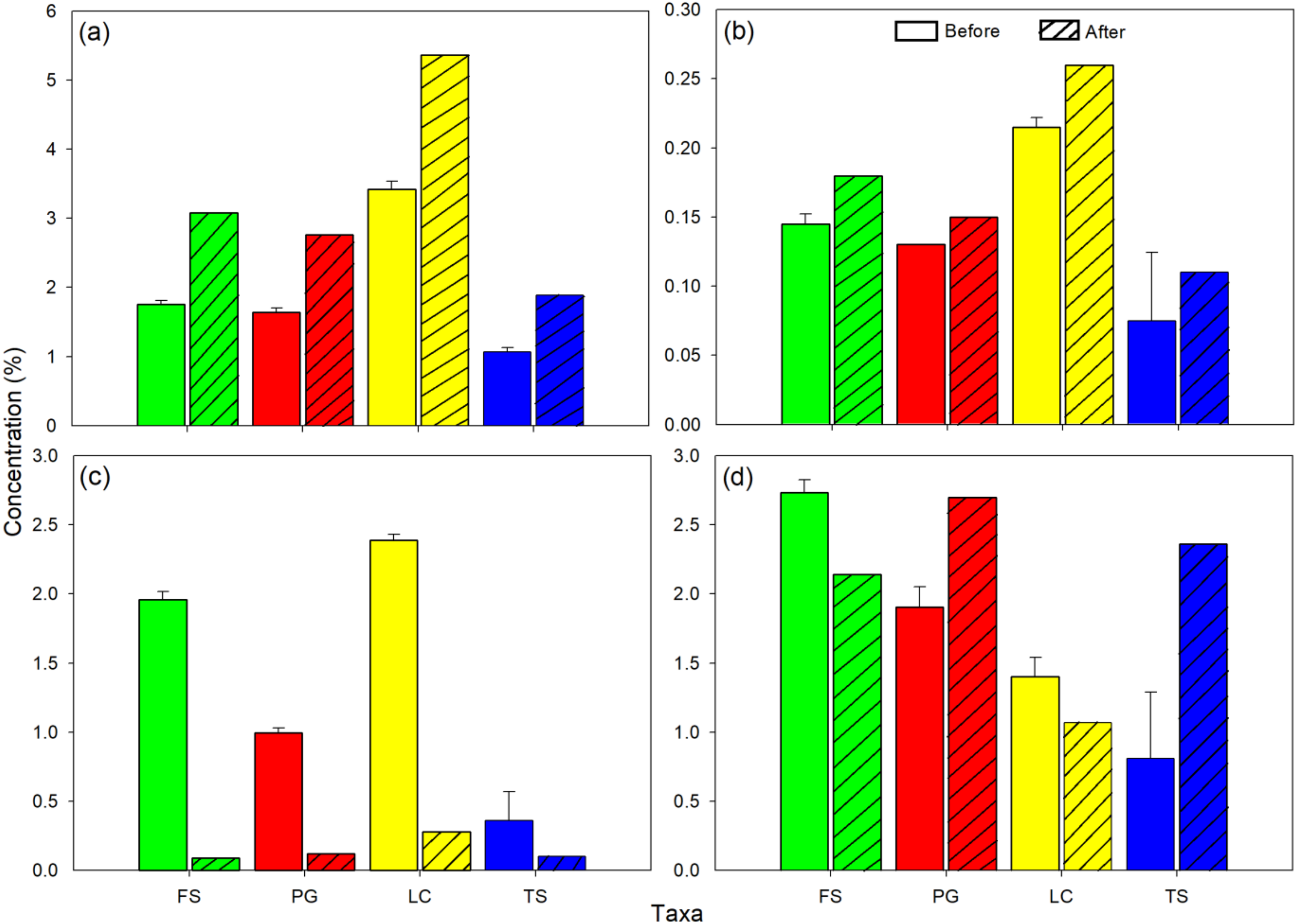
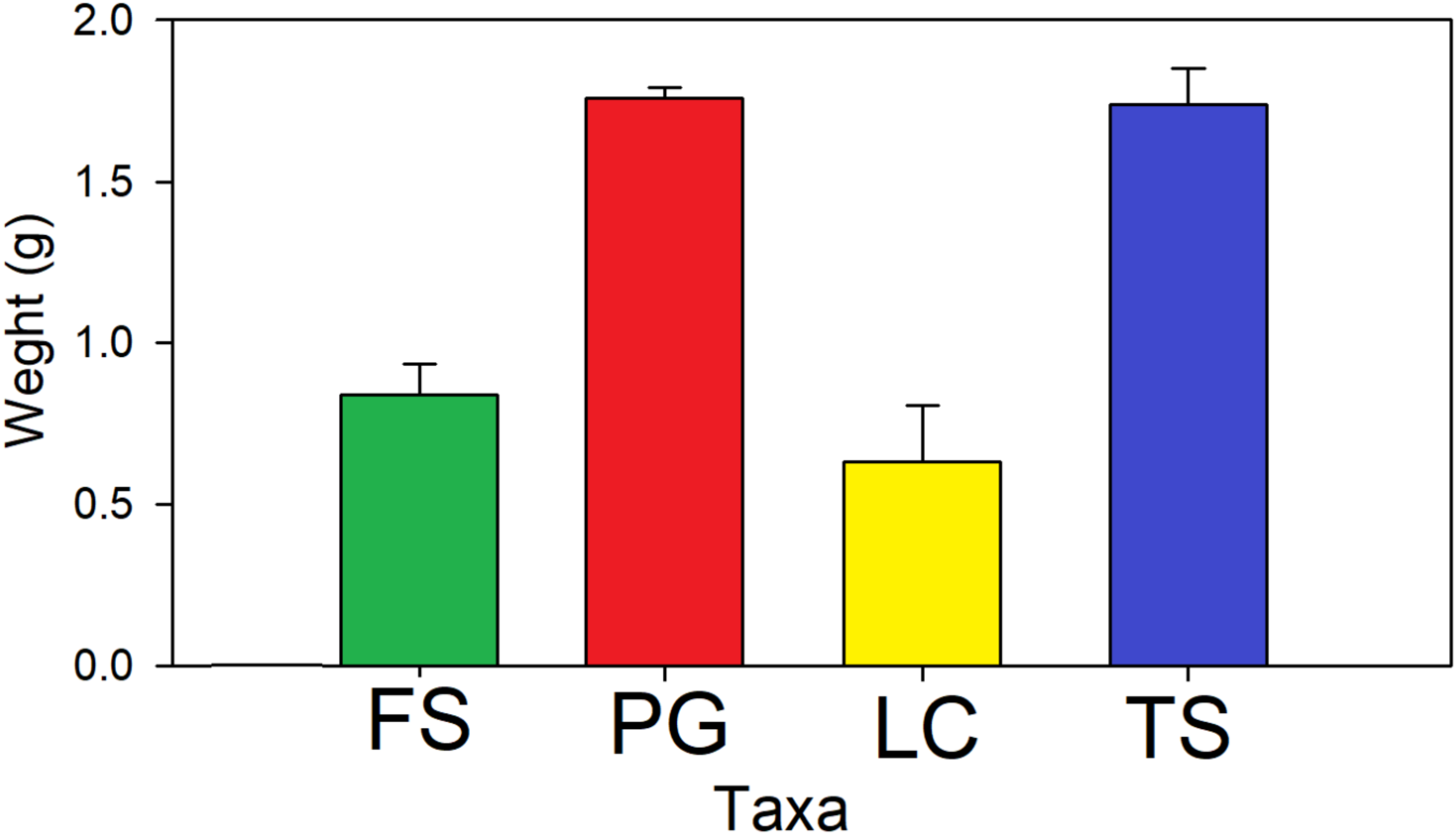
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vogt, K.A.; Grier, C.C.; Vogt, D.J. Production, turnover, and nutrient dynamics of above—And belowground detritus of world forests. Adv. Ecol. Res. 1986, 15, 303–377. [Google Scholar]
- DeGasparro, S.L.; Beresford, D.V.; Prater, C.; Frost, P.C. Leaf litter decomposition in boreal lakes: Variable mass loss and nutrient release ratios across a geographic gradient. Hydrobiologia 2020, 847, 819–830. [Google Scholar] [CrossRef]
- Aber, J.D.; Melillo, J.M. Nitrogen immobilization in decaying hardwood leaf litter as a function of initial nitrogen and lignin content. Can. J. Bot. 1982, 60, 2263–2269. [Google Scholar] [CrossRef]
- Zhang, D.; Hui, D.; Luo, Y.; Zhou, G. Rates of litter decomposition in terrestrial ecosystems: Global patterns and controlling factors. J. Plant Ecol. 2008, 1, 85–93. [Google Scholar] [CrossRef]
- Bruder, A.; Schindler, M.H.; Moretti, M.S.; Gessner, M.O. Litter decomposition in a temperate and a tropical stream: The effects of species mixing, litter quality and shredders. Freshwater Biol. 2014, 59, 438–449. [Google Scholar] [CrossRef]
- Riggs, C.E.; Hobbie, S.E.; Cavender–Bares, J.; Savage, J.A.; Wei, X. Contrasting effects of plant species traits and moisture on the decomposition of multiple litter fractions. Oecologia 2015, 179, 573–584. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, S.; Jiang, H.; Yangzom, D.; Cheng, G.; Lu, X. Decomposition time, chemical traits and climatic factors determine litter–mixing effects on decomposition in an alpine steppe ecosystem in Northern Tibet. Plant Soil 2019, 1–13. [Google Scholar] [CrossRef]
- Krishna, M.P.; Mohan, M. Litter decomposition in forest ecosystems: A review. Energy Ecol. Environ. 2017, 2, 236–249. [Google Scholar] [CrossRef]
- Dilly, O.; Bartsch, S.; Rosenbrock, P.; Buscot, F.; Munch, J.C. Shifts in physiological capabilities of the microbiota during the decomposition of leaf litter in a black alder (Alnus glutinosa (Gaertn.) L.) forest. Soil Biol. Biochem. 2001, 33, 921–930. [Google Scholar] [CrossRef]
- Fernández, V.; Guzmán-Delgado, P.; Graça, J.; Santos, S.; Gil, L. Cuticle structure in relation to chemical composition: Re-assessing the prevailing model. Front. Plant Sci. 2016, 7, 427. [Google Scholar] [CrossRef]
- Wolfe, B.E.; Klironomos, J.N. Breaking new ground: Soil communities and exotic plant invasion. BioScience 2005, 55, 477–487. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Martin, C.E.; Falasco, M.R.; James, J.J. Variation in resource acquisition and utilization traits between native and invasive perennial forbs. Am. J. Bot. 2008, 95, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Leishman, M.R.; Thomson, V.P.; Cooke, J. Native and exotic invasive plants have fundamentally similar carbon capture strategies. J. Ecol. 2010, 98, 28–42. [Google Scholar] [CrossRef]
- Webster, J.R.; Benfield, E.F. Vascular plant breakdown in freshwater ecosystems. Ann. Rev. Ecol. Syst. 1986, 17, 567–594. [Google Scholar] [CrossRef]
- McGroddy, M.E.; Daufresne, T.; Hedin, L.O. Scaling of C: N: P stoichiometry in forests worldwide: Implications of terrestrial Redfield-type ratios. Ecology 2004, 85, 2390–2401. [Google Scholar] [CrossRef]
- Brinson, M.M. Decomposition and nutrient exchange of litter in an alluvial swamp forest. Ecology 1977, 58, 601–609. [Google Scholar] [CrossRef]
- Richardson, D.M.; Van Wilgen, B.W. Invasive alien plants in South Africa: How well do we understand the ecological impacts? Working for water. S. Afr. J. Sci. 2004, 100, 45–52. [Google Scholar]
- Vardien, W.; Richardson, D.M.; Foxcroft, L.C.; Thompson, G.D.; Wilson, J.R.U.; Le Roux, J.J. Invasion dynamics of Lantana camara L. (sensu lato) in South Africa. S. Afr. J. Bot. 2012, 81, 81–94. [Google Scholar] [CrossRef]
- Urquía, D.; Gutierrez, B.; Pozo, G.; Pozo, M.J.; Espín, A.; de Lourdes Torres, M. Psidium guajava in the Galapagos Islands: Population genetics and history of an invasive species. PLoS ONE 2019, 14, e0203737. [Google Scholar] [CrossRef]
- Ramaswami, G.; Sukumar, R. Lantana camara L. (Verbenaceae) invasion along streams in a heterogeneous landscape. J. Biosci. 2014, 39, 717–726. [Google Scholar] [CrossRef]
- Pothasin, P.; Compton, S.G.; Wangpakapattanawong, P. Riparian Ficus tree communities: The distribution and abundance of riparian fig trees in Northern Thailand. PLoS ONE 2014, 9, 108945. [Google Scholar] [CrossRef] [PubMed]
- Venter, F.; Venter, J.A. Making the Most of Indigenous Trees; Briza Publications: Pretoria, South Africa, 2015. [Google Scholar]
- Sunil, C.; Somashekar, R.K.; Nagaraja, B.C. Diversity and composition of riparian vegetation across forest and agroecosystem landscapes of River Cauvery, southern India. Trop. Ecol. 2016, 57, 343–354. [Google Scholar]
- Mutshekwa, T.; Cuthbert, R.N.; Wasserman, R.J.; Murungweni, F.M.; Dalu, T. Macroinvertebrate colonisation associated with native and invasive leaf litter decomposition. Knowl. Manag. Aquat. Ecosyst. 2019, 421, 32. [Google Scholar]
- Staaf, H. Plant nutrient changes in beech leaves during senescence as influenced by site characteristics. Acta Oecol. 1982, 3, 161–170. [Google Scholar]
- Cuthbert, R.N.; Dalu, T.; Mutshekwa, T.; Wasserman, R.J. Leaf inputs from invasive and native plants drive differential mosquito abundances. Sci. Total Environ. 2019, 689, 652–654. [Google Scholar] [CrossRef]
- Grasshoff, K.; Ehrhardt, M.; Kremling, K. Methods of Seawater Analysis, 2nd ed.; Verlag Chemie: Weinheim, Germany, 1983. [Google Scholar]
- Armstrong, F.A.J.; Sterns, C.R.; Strickland, J.D.H. The measurement of upwelling and subsequent biological processes by means of the Technicon AutoAnalyzer and associated equipment. Deep Sea Res. 1967, 14, 381–389. [Google Scholar]
- Campbell, C.A.; Plank, C.O. Preparation of plant tissues for laboratory analysis. In Handbook of Reference Methods for Plant Analysis; Kalra, Y.P., Ed.; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Miller, M.K. Decomposition of bulk metallic glasses. Mat. Sci. Eng. A Struct. 1998, 250, 133–140. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Lenth, R.V. Least-Squares Means: The R Package lsmeans. J. Stat. Softw. 2018, 69, 1–33. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Core Development Team: Vienna, Austria, 2018. [Google Scholar]
- McNeish, R.E.; Benbow, M.E.; McEwan, R.W. Riparian forest invasion by a terrestrial shrub (Lonicera maackii) impacts aquatic biota and organic matter processing in headwater streams. Biol. Invasions 2012, 14, 1881–1893. [Google Scholar] [CrossRef]
- George, B.; Brandon, C.; Erwin, M. Degradation Rates of Native versus Exotic Leaves in a Tributary of the Yellow River in Georgia. Am. J. Plant. Sci. 2017, 8, 1967–1976. [Google Scholar] [CrossRef][Green Version]
- Callaway, R.M.; Ridenour, W.M. Novel weapons: Invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004, 2, 436–443. [Google Scholar] [CrossRef]
- Abelho, M. From litterfall to breakdown in streams: A Review. Sci. World 2001, 1, 656–680. [Google Scholar] [CrossRef]
- Cornut, J.; Elger, A.; Lambrigot, D.; Mamonier, P.; Chauvet, E. Early stages of leaf decomposition are mediated by aquatic fungi in the hyporheic zone of woodland streams. Freshwater Biol. 2010, 55, 2541–2556. [Google Scholar] [CrossRef]
- Hladyz, S.; Gessner, M.O.; Giller, P.S.; Pozo, J.; Woodward, G. Resource quality and stoichiometric constraints on stream ecosystem functioning. Freshwater Biol. 2009, 54, 957–970. [Google Scholar] [CrossRef]
- Bottollier-Curtet, M.; Charcosset, J.; Planty-Tabacchi, A.; Tabacchi, E. Degradation of native and exotic riparian plant leaf litter in a floodplain pond. Freshwater Biol. 2011, 56, 1798–1810. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. From tropics to tundra: Global convergence in plant functioning. Proc. Natl Acad. Sci. USA 1997, 94, 13730–13734. [Google Scholar] [CrossRef]
- Ong, C.S.; Juan, J.C.; Yule, C.M. The contribution of leaching to nutrient release from leaf litter of two emergent tree species in a Malaysian tropical peat swamp forest. Hydrobiologia 2017, 794, 125–137. [Google Scholar] [CrossRef]
- Cuassolo, F.; Navarro, M.B.; Balseiro, E.; Modenutti, B. Effect of light on particulate and dissolved organic matter production of native and exotic macrophyte species in Patagonia. Hydrobiologia 2016, 766, 29–42. [Google Scholar] [CrossRef]
- MacKenzie, R.A.; Wiegner, T.N.; Kinslow, F.; Cormier, N.; Strauch, A.M. Leaf-litter inputs from an invasive nitrogen-fixing tree influence organic-matter dynamics and nitrogen inputs in a Hawaiian river. Freshwater Sci. 2013, 32, 1036–1052. [Google Scholar] [CrossRef]
- Mineau, M.M.; Baxter, C.V.; Marcarelli, A.M.; Minshall, G.W. An invasive riparian tree reduces stream ecosystem efficiency via a recalcitrant organic matter subsidy. Ecology 2012, 93, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Allan, J.D.; Castillo, M.M. Stream Ecology. Structure and Function of Running Waters; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
| Parameter | Predictor | F-Value | p-Value |
|---|---|---|---|
| Temperature | Treatment | 1.52 | 0.23 |
| Week | 9155.33 | <0.001 | |
| Treatment:Week | 0.70 | 0.82 | |
| pH | Treatment | 42.03 | <0.001 |
| Week | 92.66 | <0.001 | |
| Treatment:Week | 1.15 | 0.31 | |
| Conductivity | Treatment | 69.99 | <0.001 |
| Week | 170.40 | <0.001 | |
| Treatment:Week | 2.70 | <0.001 | |
| Phosphate | Treatment | 30.51 | <0.001 |
| Week | 15.22 | <0.001 | |
| Treatment:Week | 1.00 | 0.47 | |
| Nitrite | Treatment | 23.33 | <0.001 |
| Week | 13.93 | <0.001 | |
| Treatment:Week | 1.96 | 0.01 | |
| Nitrate | Treatment | 18.18 | <0.001 |
| Week | 32.94 | <0.001 | |
| Treatment:Week | 4.91 | <0.001 | |
| Silicate | Treatment | 1.82 | 0.13 |
| Week | 31.80 | <0.001 | |
| Treatment:Week | 0.67 | 0.85 | |
| Ammonium | Treatment | 7.39 | 0.001 |
| Week | 11.11 | <0.001 | |
| Treatment:Week | 2.47 | 0.002 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutshekwa, T.; Cuthbert, R.N.; Wasserman, R.J.; Murungweni, F.M.; Dalu, T. Nutrient Release Dynamics Associated with Native and Invasive Leaf Litter Decomposition: A Mesocosm Experiment. Water 2020, 12, 2350. https://doi.org/10.3390/w12092350
Mutshekwa T, Cuthbert RN, Wasserman RJ, Murungweni FM, Dalu T. Nutrient Release Dynamics Associated with Native and Invasive Leaf Litter Decomposition: A Mesocosm Experiment. Water. 2020; 12(9):2350. https://doi.org/10.3390/w12092350
Chicago/Turabian StyleMutshekwa, Thendo, Ross N. Cuthbert, Ryan J. Wasserman, Florence M. Murungweni, and Tatenda Dalu. 2020. "Nutrient Release Dynamics Associated with Native and Invasive Leaf Litter Decomposition: A Mesocosm Experiment" Water 12, no. 9: 2350. https://doi.org/10.3390/w12092350
APA StyleMutshekwa, T., Cuthbert, R. N., Wasserman, R. J., Murungweni, F. M., & Dalu, T. (2020). Nutrient Release Dynamics Associated with Native and Invasive Leaf Litter Decomposition: A Mesocosm Experiment. Water, 12(9), 2350. https://doi.org/10.3390/w12092350







