Abstract
Leaf litter contributes to the functioning of aquatic ecosystems through allochthonous inputs of carbon, nitrogen, and other elements. Here, we examine leaf litter nutrient inputs and decomposition associated with four plant species using a mesocosm approach. Native sycamore fig Ficus sycomorus L., and silver cluster–leaf Terminalia sericea Burch. ex DC. decomposition dynamics were compared to invasive tickberry Lantana camara L. and guava Psidium guajava L., whereby phosphate, nitrate, nitrite, silicate, and ammonium releases were quantified over time. Leaf inputs significantly reduced pH, with reductions most marked by invasive L. camara. Conductivity was heightened by all leaf input treatments, except native T. sericea. Leaf inputs significantly affected all nutrient levels monitored in the water over time, except for silicate. In particular, leaf litter from invasive L. camara drove significantly increased nutrient concentrations compared to other native plant species, whilst effects of invasive P. guajava were less statistically clear. The end weights of the leaf litter demonstrated decomposition differences among the species types, following a decreasing order of P. guajava > T. sericea > F. sycomorus > L. camara, further suggesting high organic inputs from invasive L. camara. The study results highlight that differential leaf litter decomposition rates of four plant species can play a significant role in nutrient release, in turn altering aquatic ecosystem productivity. However, these effects likely depend on species-specific differences, rather than between invasive–native species generally. Shifting terrestrial plant communities may alter aquatic community composition, but specific effects are likely associated with leaf traits.
1. Introduction
Plant litter decomposition is a key process in nutrient recycling, supporting primary production in many terrestrial and aquatic ecosystems [1,2]. Various studies e.g., [3,4,5,6] have demonstrated that plant litter decomposition is driven by abiotic and biotic processes. The breakdown by these abiotic and biotic processes facilitates nutrient release and subsequent bioavailability. Leaf litter is broken down by invertebrates and microorganisms. However, climatic factors such as temperature and rainfall are the strongest determining factors on litter mass loss [7]. Since litter decomposition involves several factors such as physical, chemical, and biological breakdown processes [8], changes in plant species in a landscape through, for example, biological invasions may alter leaf litter decomposition dynamics. In turn, this may have implications for nutrient cycling processes in environments receiving such allochthonous inputs, and have emergent effects on aquatic community structuring.
The plant litter quality also influences the decomposition process, as it decreases over time due to the loss of carbon and soluble compounds [8,9]. The nature and quality of plant litter is fundamentally driven by the plant community [8], with implications for nutrient dynamics within ecosystems and the adjacent receiving environments. Plant species are highly variable with regard to physical structure of leaves, and exhibit heterogeneity and specificity in chemical compound presence [10]. Globally, natural plant communities are increasingly changing given numerous pressures associated with human activities such as deforestation, soil erosion, and species augmentation. One major way in which plant communities are being altered is through alien plant species invasions [11]. While invasive plant species nutrient resource acquisition dynamics are prevalent in the literature [12,13], studies on their nutrient return dynamics are lacking in extensive geographic regions, such as southern Africa. In particular, terrestrial plant litter which ends up in aquatic environments acts as a key pathway for nutrient release and supply to aquatic biota. The leaf litter decomposition process is comprised of three phases: (i) leaching of components from the leaf litter; (ii) conditioning by microorganisms; (iii) fragmentation and consumption [14]. The biological degradation of litter is mostly influenced by microbial decomposers such as fungi and bacteria, which have significantly lower carbon (C) to nitrogen (N) ratios [15,16], indicated that the loss of dissolved organic matter from fallen leaf litter is an important indicator of nutrient release from these organic inputs. The leaf litter chemical composition, together with habitat characteristics and environmental abiotic and biotic factors, control the process of decomposition which drives nutrient release from the plant litter [15]. Here, we aim to assess such nutrient release dynamics using an ex situ mesocosms approach.
In the present study, we examine nutrient release (i.e., phosphate, nitrate, nitrite, silicate, ammonium) dynamics associated with the leaf litter decomposition process over time for two native (fig Ficus sycomorus, silver cluster–leaf Terminalia sericea) and two invasive (lantana/tickberry Lantana camara, guava Psidium guajava) terrestrial plants. Lantana camara and Psidium guajava are native to tropical central and southern America and are known to have a substantial negative impact on native plant species through competition and replacement [17,18,19]. Both species are recognized as invasive in South Africa (https://www.invasives.org.za/). These invasive species were selected because they have been reported to spread fast within the riparian zones, in turn threatening the abundance and diversity of native plant species and community stability of aquatic ecosystems. Ranaswanu and Sukumar [20] indicated that highest L. camara abundances are found in close proximity to aquatic ecosystems. Dominant native plant species were selected for comparison. Native F. sycomorus and T. sericea are both native to southern Africa and can naturally grow in the riparian zones and wetlands [21,22,23]. The focal species are known to coexist around aquatic ecosystems, with L. camara a deciduous shrub and P. guajava a semi-deciduous tree species. In turn, F. sycomorus and T. sericea are semi-deciduous and deciduous, respectively. We hypothesized that (i) nutrient (i.e., phosphate, nitrate, nitrite, silicate, ammonium) release rates would be higher in native plant leaves than invasive plant leaves, as decomposer communities may be better adapted to local plant species [24], and; (ii) native fig F. sycomorus and silver cluster–leaf T. sericea will decompose faster compared to invasive tickberry L. camara and guava P. guajava, with native communities less effective at breaking down litter from exogenous species. These decomposition processes are important to understand, as such differences in decomposition of leaf litter may have implications for aquatic food webs and community structuring.
2. Materials and Methods
2.1. Study Area
The study was conducted at the Department of Ecology and Resource Management, University of Venda, Limpopo province of South Africa (Figure 1). The area has a humid, subtropical climate and receives an average annual rainfall range of between 400 and 800 mm, with peak rainfall occurring between January and February. High temperatures (i.e., up to 40 °C) occur between October and March, with the cool-dry season temperatures ranging between 12 and 22 °C. The area soil type is loam which is red in color due to the presence of iron oxide. This iron oxide in the soil is a result of iron containing ultra-mafic and mafic parent rock, which was formed through physical and chemical weathering. Plant leaves were collected from a riparian zone of the wetland within the university campus perimeter, whereas borehole and river water were collected from a student resident construction borehole and a relatively pristine river site located in the wetland, respectively (Figure 1).
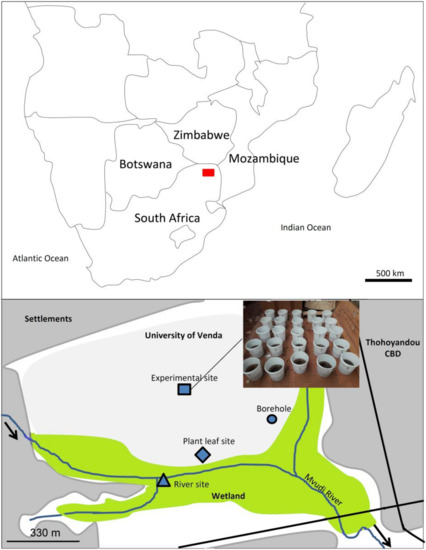
Figure 1.
Location of the experimental site within the University of Venda (South Africa), the plant leaves and water collection sites. The bottom map illustrates the zone shown in red on the upper map.
2.2. Experimental Design
Plant leaves of invasive i.e., L. camara and P. guajava and native i.e., F. sycomorus, and T. sericea were collected in November 2018 (hot-wet season) by hand from trees along Mvudi River riparian zone (22°58.967′ S 30°26.840′ E) in Thohoyandou, Limpopo province, South Africa, before being air dried at room temperature (range 27–30 °C). Only fresh plant leaves were selected in the field, given many plants reabsorb nutrients prior to senescence [25]. In March 2019 after drying the leaves for about 6 months, approximately 3 g of the leaves for each species was weighed and added into 5 L white polyethylene buckets, filled prior with 3.9 L of borehole + river water (70:30 borehole:river water ratio), with the water filtered through 63 µm mesh cloth to remove invertebrates (see [26]). The initial mean water temperature was 27.2 ± 0.2 °C (SD), conductivity 169.3 ± 4.0 µS cm−1, total dissolved solids 85.5 ± 2.6 mg L−1, and pH 6.6 ± 0.1. Buckets were regularly topped up to 3.9 L with filtered (GF/F filter 0.02 µm, Ø 47 mm) borehole water. The 25 5-L buckets (i.e., five replicates × four species, + five controls without leaves) were placed outside in a partially shaded area in the University of Venda campus in a randomized array for 6 weeks.
2.3. Sampling and Analyses
Water parameters (i.e., conductivity (µS cm−1), total dissolved solids (mg L−1), pH, and temperature (°C)) were measured every 7 days for 6 weeks using a portable handheld multi-parameter probe (PCTestr 35, Eutech/Oakton Instruments). Approximately 50 mL of water samples was collected from each treatment and replicated weekly for nutrient (ammonium, nitrite, nitrate, phosphates, silicate) analyses. The nutrients were analyzed at NRF SAEON Elwandle Node Coastal Biogeochemistry Laboratory using an Auto-Analyzer model AA3 segmented flow colorimetry (SEAL Analytical, Southampton, United Kingdom). Phosphates were analyzed using the colorimetric method and then read at 880 nm. The test range was 0–50 µg L−1. Silicate was analyzed using the reduction of silico-molybdate in acidic solution to molybdenum blue by ascorbic acid [27]. The test range was 0–41 µmol L−1. Nitrate and nitrite were analyzed based on a procedure where nitrate is reduced to nitrite by a copper-cadmium redactor column [28]. This method is based on the nitrate determination in Standard Methods and in the dissolved inorganic nitrogen standards for automatic nitrate measurements. The test range was 0–50 µmol L−1. Ammonium was based on the Berthelot reaction at 660 nm. The test range was 0–10 µmol L−1.
Before the experiment began, leaves (≈15–20 g) for each species were collected (n = 2), and again at the end of the experiment, and analyzed for nutrient (N, P) and metal (K, Ca) concentrations at a SANSAS accredited laboratory BEMLAB. Due to small leaf quantities at the end of the experiment, we combined the five replicates for each species to make one sample (≈4.5–10 g). After sampling, the leaf blades were washed with a Teepol solution, rinsed with de-ionized water and dried over night at 70 °C in an oven. The dried leaves were then milled and ashed at 480 °C, shaken up in a 50:50 32% HCl solution for extraction through filter paper [29,30]. The nutrient (total P, K, Ca) content of the extract was measured with a Varian ICP-OES optical emission spectrometer. Total N content of the ground leaves was determined through total combustion in a Leco N-analyzer.
2.4. Statistical Analyses
The effects of leaf treatment (five levels, including controls) and observation week (six levels), and their interaction, on important water parameters (pH, conductivity, and temperature) were examined using linear mixed effects models [31]. Individual containers were included as a random effect (intercept) to account for repeated measures over the experimental period. Leaf treatment and observation week effects on measured nutrients (phosphate, nitrate, nitrite, silicate, and ammonium) were analyzed similarly, following omission of missing records (n = 5). Type III analyses of variance with Satterthwaite’s method were used to infer effect sizes and significance levels of main effects [32]. End-weights of leaves following the experimental period were compared using one-way analysis of variance according to leaf species treatment (four levels). For all models, post-hoc Tukey comparisons were performed via estimated marginal means where effects were significant [33]. Normality and homoscedasticity of residuals was checked using diagnostic plots [34], with log10 transformations applied where necessary to meet model assumptions. All statistical analyses were performed using R v3.4.2 [35].
3. Results
Water temperature did not differ significantly among leaf treatments yet differed significantly over the monitoring period owing to daily temperature undulations (Table 1). This highlights that all water quality differences observed were independent of potentially confounding temperature effects. Leaf treatment and observation week had a significant effect on the pH and conductivity of water, with leaf effects on conductivity also differing significantly over time owing to a significant interaction term (Table 1). All leaf treatments drove significantly reduced pH compared to controls (all p < 0.001), whilst L. camara also caused significantly reduced pH compared to P. guajava (p = 0.04). Compared to week 1, pH levels were significantly reduced in the early monitoring period (weeks 2, 3; both p < 0.001), yet neutralized over time (weeks 4, 5, 6; all p > 0.05; Figure 2a). F. sycomorus, L. camara, and P. guajava always significantly increased conductivity relative to controls (all p < 0.05), whilst T. sericia treatments had no significant effect over the monitoring period (all p > 0.05). In turn, P. guajava leaf treatments displayed significantly reduced conductivity relative to F. sycomorus and L. camara (all p < 0.001). Conductivity increased significantly over time relative to week 1 (all p < 0.001; Figure 2b).

Table 1.
Linear mixed effects model results considering measured water parameters as a function of leaf treatment and week, and their interaction. F-values are discerned with Type III sums of squares via Satterthwaite’s method. Significant p-values are emboldened.
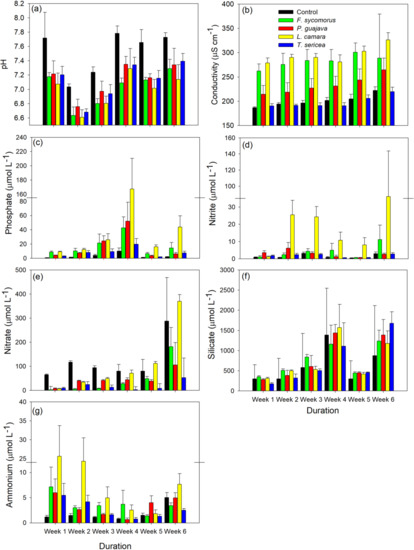
Figure 2.
Nutrient release concentrations (i.e., amount present in waterbody) (+standard deviation) of invasive Lantana camara (yellow) and Psidium guajava (red), and native Terminalia sericea (blue) and Ficus sycomorus (green), over a 6-week period: (a) pH, (b) conductivity, (c) phosphates, (d) nitrite, (e) nitrate, (f) silicate, and (g) ammonium.
Leaf treatment had a significant effect on all nutrient levels monitored in the water, except for silicate, and nutrient levels always differed significantly according to monitoring week (Table 1). Leaf inputs always significantly increased phosphate relative to controls (all p < 0.001). Further, leaf litter from invasive L. camara drove significantly increased phosphate compared to all other plant species overall (all p < 0.05; Figure 2c). Excepting week one, invasive L. camara also significantly increased nitrite levels relative to control groups (all p < 0.01), whilst effects of other plant inputs were not statistically clear (all p > 0.05). Nitrite levels following L. camara treatments were significantly higher than all other plant species overall over the monitoring period (all p < 0.001; Figure 2d). Contrastingly, leaf litter generally drove reductions in nitrate; yet, these reductions tended to become less pronounced compared to controls over time. At week 1, all plant inputs significantly reduced nitrate compared to controls (all p < 0.01), whilst only T. sericea treatments were significantly reduced at week 6 (p = 0.02). Nitrate levels also tended to be elevated following treatment with invasive L. camara and P. guajava compared to native F. sycomorus and T. sericea (Figure 2e), with nitrate levels following treatment with invasive L. camara significantly higher than native plants (both p < 0.01). Leaf inputs also tended to increase silicate levels overall, however, this effect was not statistically clear, with silicate levels only differing significantly over time (Table 1; Figure 2f). A significant interaction term indicated that ammonium levels responded differently over time according to leaf treatments, with increases relative to controls becoming less marked over the monitoring period (week 1, all p < 0.05 (excepting P. guajava); week 6, all p > 0.05). In particular, invasive L. camara significantly increased ammonium levels during the early monitoring stages relative to all other treatment groups (week 2: all p < 0.01; Figure 2g).
The leaf total N and P concentrations were higher at the end of the experiment for all leaf treatments, with highest and lowest concentrations being observed in invasive L. camara and indigenous T. sericea, respectively (Figure 3a,b). Invasive P. guajava and indigenous F. sycomorus had similar concentrations before and after the experiment. For K, the concentrations were high before the experiment, after which the concentrations significantly reduced at the end of the experiment; however, concentrations in T. sericea were relatively low (Figure 3c). Effects on Ca were generally different among species, with reductions over time shown in F. sycomorus and L. camara, and increases for P. guajava and T. sericea (Figure 3d).
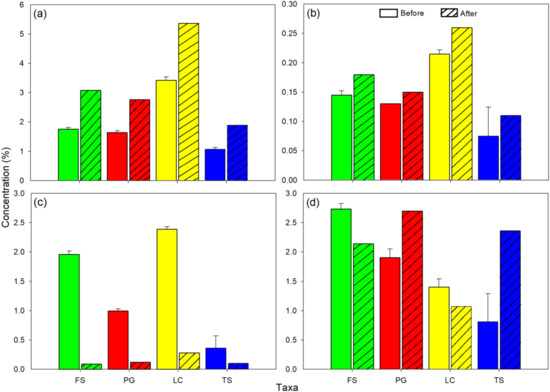
Figure 3.
Leaf nutrient concentrations (+standard deviation) of invasive L. camara (yellow) and P. guajava (red), and native T. sericea (blue) and F. sycomorus (green), before and after the experiment: (a) total N, (b) total P, (c) K, and (d) Ca.
Leaf weights at the end of the experiment differed significantly according to species (F3,16 = 14.08, p < 0.001) in comparison with the initial weights. Invasive L. camara (mass loss 78.9% ± 12.9%) and native F. sycomorus (mass loss 72.0% ± 7.3%) weights were reduced most, and significantly compared to native T. sericea (mass loss 42.0% ± 8.2%) and invasive P. guajava (mass loss 41.4% ± 2.6%) (all p < 0.05; Figure 4).
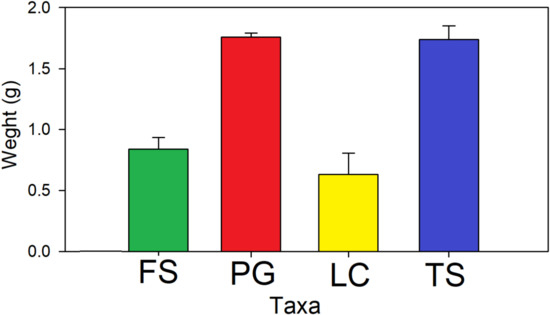
Figure 4.
Mean values (+standard deviation) of end-weights of invasive L. camara (LC; yellow) and P. guajava (PG; red) and native F. sycomorus (FS; green) and T. sericea (TS; blue). Initial weights for all species were 3.0 g of dried leaf material.
4. Discussion
Invasions of riparian ecosystems by exotic species could drive substantial changes to nutrient release, as properties of alien species may differ markedly to native analogues [36,37]. In this study, whilst we demonstrated that interspecific differences in leaf litter decomposition are important for allochthonous nutrient dynamics within freshwater environments, these differences were not conserved and thus generalizable according to alien-native statuses. Native decomposers have been previously posited to be more efficient in colonizing native leaf litter over alien leaves (but see [24]), owing to co-evolutionary backgrounds among species [38]. As such, higher nutrient release is to be generally expected from native species, at least in the short-term. However, leaf litter from the invasive L. camara generally drove greater nutrient concentrations than all other species, in contrast to what would be expected in respect to the importance of native decomposers [39,40]. P. guajava, however, was largely similar to the native F. sycamorus with regards to nutrient release, while the native T. sericea nutrient release was generally the slowest. Thus, nutrients in the invasive litter became readily available in water quickly which, in turn, could be taken up by aquatic biota. In relation to leaf litter decay, our results indicated that invasive L. camara and native F. sycomorus decomposed faster than invasive P. guajava and native T. sericea.
Both of our hypotheses were rejected, with differences in nutrient release and weights inconsistent across invader and native groupings. It was first hypothesized that native plant inputs would release higher nutrient concentration than invasive species, yet invasive L. camara drove higher nutrient concentrations than native F. sycomorus and T. sericea. L. camara had higher nutrient release concentrations (i.e., amount released into the waterbody) in every nutrient than native F. sycomorus and T. sericea. Effects of native P. guajava on nutrient concentrations were less pronounced, however. It was secondly hypothesized that native leaf types will decompose faster than invasive. However, invasive L. camara and native F. sycomorus decomposed faster than invasive P. guajava and native T. sericea, although L. camara generally decomposed faster compared to all leaf litter types. Hence, this hypothesis is also rejected, with interspecific differences between paired invasive and native species emergent. These differences are likely due to variations in leaf structure which facilitate more rapid decomposition, with this decomposition rate correlating tightly with nutrient release dynamics. Indeed, physical and chemical differences across leaf species have been found previously to be generally more important than origin [41,42].
The outcomes of this study suggest that certain invasive litter inputs into freshwater may contribute to dynamic interactions among invasion, invertebrate and microorganism colonization, and nutrient cycling [24]. Nutrient-rich leaf litter such as L. camara decomposes faster due to low concentrations of defensive compounds such as lignin [43]. Accordingly, organic inputs into ecosystems such as small streams by specific invasive leaf litter types influence nutrient status, potentially contributing to eutrophication and negatively affecting lentic and lotic ecosystems through, for example, sudden increases in primary productivity. Similarly, Ong et al. [44] highlighted that the litter leaching process of decomposition plays a vital role in nutrient release into the environment. Leaf litter decomposition had a significant effect on nutrient release throughout the experiment, however the timings of release between species varied over time. As such, experimental duration may also substantially alter observations of nutrient release.
A particularly important finding of the present study is the recorded decrease in nitrates over time, which could have been due to immobilization by the biofilm growing on the decomposing leaves, and this could explain an increase in total N within the leaves at the end of the experiment. In other studies, Cuassolo et al. [45] observed that the dissolved organic matter produced by the exotic species showed a low photoreactivity, and had more color than that produced by the native species similar our observations. Whereas, MacKenzie et al. [46] highlighted that the initial tannin content, leaf C:N and toughness were important intrinsic factors inhibiting leaf breakdown and fungal colonization, which might explain the low breakdown or decomposition rates of P. guajava and T. sericia. What the study findings show, similar to Mineau et al. [47], is that invasions by non-native plant species could alter resource fluxes in freshwater with consequences for whole-ecosystem functions.
Considering decomposition alone, L. camara and F. sycomorus leaves had the most significant weight losses compared to T. sericea and P. guajava. The pattern of leaf litter decomposition generally follows two phases i.e., leaching and microbial conditioning [48]. The initial stage could have resulted in a rapid loss of mass during the first 24–48 h of decomposition possibly due to leaching of the soluble compounds from the leaf, such as phenolics, carbohydrates, and amino acids. In the present study, the decay of leaf litter was first observed during the first 24–48 h of the experiment, with broken leaves accumulating and changing the color of the water (TM and TD, personal observation). Nevertheless, the context-dependency of such nutrient release among species requires further elucidation, considering the influence of other driving abiotic factors such as temperature.
In conclusion, we found that nutrient inputs proved to be a good decomposition indicator in our study, giving that high nutrient levels were often measured in L. camara and F. sycomorus and these species also decomposed faster than P. guajava and T. sericea. Further, the former two species drove the most marked changes to pH and conductivity, synonymous with greater decomposition rates. Whilst nutrient level inputs from one invasive plant were the highest of all four species investigated, this effect was not generalizable owing to differences at the invader–native species level. Further research is needed to investigate whether the decomposition of other native and invasive plant leaf litters not assessed in this study exhibit generalities in terms of decomposition and nutrient dynamics, as well as how this affects invasion success. Moreover, the effects of physical leaf characteristics on decomposition and nutrient dynamics should be investigated to further understand the nutrient release process.
Author Contributions
Conceptualization, R.J.W. and T.D.; Methodology, T.M., R.J.W. and T.D.; Formal Analysis, R.N.C. and T.D.; Investigation, T.M. and T.D.; Resources, T.D.; Data Curation, R.N.C. and T.D.; Writing—Original Draft Preparation, T.M., R.N.C. and T.D.; Writing—Review and Editing, T.M., R.N.C., R.J.W., F.M.M. and T.D.; Visualization, R.J.W. and T.D.; Supervision, R.J.W., F.M.M. and T.D.; Funding Acquisition, T.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Research Foundation of South Africa Thuthuka grant number 117700 and University of Venda Niche grant number SES/18/ERM/10, and the APC was funded by the University of Venda. Any opinions, findings, conclusions, or recommendations expressed in this material are those of the authors, and the NRF does not accept any liability in this regard. R.N.C. acknowledges funding from the Alexander von Humboldt Foundation.
Acknowledgments
Nutrient samples were analyzed at NRF SAEON Coastal Biogeochemistry Platform as part of the Department of Higher Education Science and Technology Shallow Marine and Coastal Research Infrastructure (SMCRI).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vogt, K.A.; Grier, C.C.; Vogt, D.J. Production, turnover, and nutrient dynamics of above—And belowground detritus of world forests. Adv. Ecol. Res. 1986, 15, 303–377. [Google Scholar]
- DeGasparro, S.L.; Beresford, D.V.; Prater, C.; Frost, P.C. Leaf litter decomposition in boreal lakes: Variable mass loss and nutrient release ratios across a geographic gradient. Hydrobiologia 2020, 847, 819–830. [Google Scholar] [CrossRef]
- Aber, J.D.; Melillo, J.M. Nitrogen immobilization in decaying hardwood leaf litter as a function of initial nitrogen and lignin content. Can. J. Bot. 1982, 60, 2263–2269. [Google Scholar] [CrossRef]
- Zhang, D.; Hui, D.; Luo, Y.; Zhou, G. Rates of litter decomposition in terrestrial ecosystems: Global patterns and controlling factors. J. Plant Ecol. 2008, 1, 85–93. [Google Scholar] [CrossRef]
- Bruder, A.; Schindler, M.H.; Moretti, M.S.; Gessner, M.O. Litter decomposition in a temperate and a tropical stream: The effects of species mixing, litter quality and shredders. Freshwater Biol. 2014, 59, 438–449. [Google Scholar] [CrossRef]
- Riggs, C.E.; Hobbie, S.E.; Cavender–Bares, J.; Savage, J.A.; Wei, X. Contrasting effects of plant species traits and moisture on the decomposition of multiple litter fractions. Oecologia 2015, 179, 573–584. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, S.; Jiang, H.; Yangzom, D.; Cheng, G.; Lu, X. Decomposition time, chemical traits and climatic factors determine litter–mixing effects on decomposition in an alpine steppe ecosystem in Northern Tibet. Plant Soil 2019, 1–13. [Google Scholar] [CrossRef]
- Krishna, M.P.; Mohan, M. Litter decomposition in forest ecosystems: A review. Energy Ecol. Environ. 2017, 2, 236–249. [Google Scholar] [CrossRef]
- Dilly, O.; Bartsch, S.; Rosenbrock, P.; Buscot, F.; Munch, J.C. Shifts in physiological capabilities of the microbiota during the decomposition of leaf litter in a black alder (Alnus glutinosa (Gaertn.) L.) forest. Soil Biol. Biochem. 2001, 33, 921–930. [Google Scholar] [CrossRef]
- Fernández, V.; Guzmán-Delgado, P.; Graça, J.; Santos, S.; Gil, L. Cuticle structure in relation to chemical composition: Re-assessing the prevailing model. Front. Plant Sci. 2016, 7, 427. [Google Scholar] [CrossRef]
- Wolfe, B.E.; Klironomos, J.N. Breaking new ground: Soil communities and exotic plant invasion. BioScience 2005, 55, 477–487. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Martin, C.E.; Falasco, M.R.; James, J.J. Variation in resource acquisition and utilization traits between native and invasive perennial forbs. Am. J. Bot. 2008, 95, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Leishman, M.R.; Thomson, V.P.; Cooke, J. Native and exotic invasive plants have fundamentally similar carbon capture strategies. J. Ecol. 2010, 98, 28–42. [Google Scholar] [CrossRef]
- Webster, J.R.; Benfield, E.F. Vascular plant breakdown in freshwater ecosystems. Ann. Rev. Ecol. Syst. 1986, 17, 567–594. [Google Scholar] [CrossRef]
- McGroddy, M.E.; Daufresne, T.; Hedin, L.O. Scaling of C: N: P stoichiometry in forests worldwide: Implications of terrestrial Redfield-type ratios. Ecology 2004, 85, 2390–2401. [Google Scholar] [CrossRef]
- Brinson, M.M. Decomposition and nutrient exchange of litter in an alluvial swamp forest. Ecology 1977, 58, 601–609. [Google Scholar] [CrossRef]
- Richardson, D.M.; Van Wilgen, B.W. Invasive alien plants in South Africa: How well do we understand the ecological impacts? Working for water. S. Afr. J. Sci. 2004, 100, 45–52. [Google Scholar]
- Vardien, W.; Richardson, D.M.; Foxcroft, L.C.; Thompson, G.D.; Wilson, J.R.U.; Le Roux, J.J. Invasion dynamics of Lantana camara L. (sensu lato) in South Africa. S. Afr. J. Bot. 2012, 81, 81–94. [Google Scholar] [CrossRef]
- Urquía, D.; Gutierrez, B.; Pozo, G.; Pozo, M.J.; Espín, A.; de Lourdes Torres, M. Psidium guajava in the Galapagos Islands: Population genetics and history of an invasive species. PLoS ONE 2019, 14, e0203737. [Google Scholar] [CrossRef]
- Ramaswami, G.; Sukumar, R. Lantana camara L. (Verbenaceae) invasion along streams in a heterogeneous landscape. J. Biosci. 2014, 39, 717–726. [Google Scholar] [CrossRef]
- Pothasin, P.; Compton, S.G.; Wangpakapattanawong, P. Riparian Ficus tree communities: The distribution and abundance of riparian fig trees in Northern Thailand. PLoS ONE 2014, 9, 108945. [Google Scholar] [CrossRef] [PubMed]
- Venter, F.; Venter, J.A. Making the Most of Indigenous Trees; Briza Publications: Pretoria, South Africa, 2015. [Google Scholar]
- Sunil, C.; Somashekar, R.K.; Nagaraja, B.C. Diversity and composition of riparian vegetation across forest and agroecosystem landscapes of River Cauvery, southern India. Trop. Ecol. 2016, 57, 343–354. [Google Scholar]
- Mutshekwa, T.; Cuthbert, R.N.; Wasserman, R.J.; Murungweni, F.M.; Dalu, T. Macroinvertebrate colonisation associated with native and invasive leaf litter decomposition. Knowl. Manag. Aquat. Ecosyst. 2019, 421, 32. [Google Scholar]
- Staaf, H. Plant nutrient changes in beech leaves during senescence as influenced by site characteristics. Acta Oecol. 1982, 3, 161–170. [Google Scholar]
- Cuthbert, R.N.; Dalu, T.; Mutshekwa, T.; Wasserman, R.J. Leaf inputs from invasive and native plants drive differential mosquito abundances. Sci. Total Environ. 2019, 689, 652–654. [Google Scholar] [CrossRef]
- Grasshoff, K.; Ehrhardt, M.; Kremling, K. Methods of Seawater Analysis, 2nd ed.; Verlag Chemie: Weinheim, Germany, 1983. [Google Scholar]
- Armstrong, F.A.J.; Sterns, C.R.; Strickland, J.D.H. The measurement of upwelling and subsequent biological processes by means of the Technicon AutoAnalyzer and associated equipment. Deep Sea Res. 1967, 14, 381–389. [Google Scholar]
- Campbell, C.A.; Plank, C.O. Preparation of plant tissues for laboratory analysis. In Handbook of Reference Methods for Plant Analysis; Kalra, Y.P., Ed.; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Miller, M.K. Decomposition of bulk metallic glasses. Mat. Sci. Eng. A Struct. 1998, 250, 133–140. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Lenth, R.V. Least-Squares Means: The R Package lsmeans. J. Stat. Softw. 2018, 69, 1–33. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Core Development Team: Vienna, Austria, 2018. [Google Scholar]
- McNeish, R.E.; Benbow, M.E.; McEwan, R.W. Riparian forest invasion by a terrestrial shrub (Lonicera maackii) impacts aquatic biota and organic matter processing in headwater streams. Biol. Invasions 2012, 14, 1881–1893. [Google Scholar] [CrossRef]
- George, B.; Brandon, C.; Erwin, M. Degradation Rates of Native versus Exotic Leaves in a Tributary of the Yellow River in Georgia. Am. J. Plant. Sci. 2017, 8, 1967–1976. [Google Scholar] [CrossRef][Green Version]
- Callaway, R.M.; Ridenour, W.M. Novel weapons: Invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004, 2, 436–443. [Google Scholar] [CrossRef]
- Abelho, M. From litterfall to breakdown in streams: A Review. Sci. World 2001, 1, 656–680. [Google Scholar] [CrossRef]
- Cornut, J.; Elger, A.; Lambrigot, D.; Mamonier, P.; Chauvet, E. Early stages of leaf decomposition are mediated by aquatic fungi in the hyporheic zone of woodland streams. Freshwater Biol. 2010, 55, 2541–2556. [Google Scholar] [CrossRef]
- Hladyz, S.; Gessner, M.O.; Giller, P.S.; Pozo, J.; Woodward, G. Resource quality and stoichiometric constraints on stream ecosystem functioning. Freshwater Biol. 2009, 54, 957–970. [Google Scholar] [CrossRef]
- Bottollier-Curtet, M.; Charcosset, J.; Planty-Tabacchi, A.; Tabacchi, E. Degradation of native and exotic riparian plant leaf litter in a floodplain pond. Freshwater Biol. 2011, 56, 1798–1810. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. From tropics to tundra: Global convergence in plant functioning. Proc. Natl Acad. Sci. USA 1997, 94, 13730–13734. [Google Scholar] [CrossRef]
- Ong, C.S.; Juan, J.C.; Yule, C.M. The contribution of leaching to nutrient release from leaf litter of two emergent tree species in a Malaysian tropical peat swamp forest. Hydrobiologia 2017, 794, 125–137. [Google Scholar] [CrossRef]
- Cuassolo, F.; Navarro, M.B.; Balseiro, E.; Modenutti, B. Effect of light on particulate and dissolved organic matter production of native and exotic macrophyte species in Patagonia. Hydrobiologia 2016, 766, 29–42. [Google Scholar] [CrossRef]
- MacKenzie, R.A.; Wiegner, T.N.; Kinslow, F.; Cormier, N.; Strauch, A.M. Leaf-litter inputs from an invasive nitrogen-fixing tree influence organic-matter dynamics and nitrogen inputs in a Hawaiian river. Freshwater Sci. 2013, 32, 1036–1052. [Google Scholar] [CrossRef]
- Mineau, M.M.; Baxter, C.V.; Marcarelli, A.M.; Minshall, G.W. An invasive riparian tree reduces stream ecosystem efficiency via a recalcitrant organic matter subsidy. Ecology 2012, 93, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Allan, J.D.; Castillo, M.M. Stream Ecology. Structure and Function of Running Waters; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).