Abstract
One in six couples experience fertility problems. Environmental factors may affect reproductive health; however, evidence is lacking regarding drinking water nitrates and outcomes of male and female fertility. The aim of this study was to investigate if exposure to nitrates in drinking water is associated with adverse reproductive outcomes in humans, and animals of fertile age. We conducted a systematic literature search and included case-control studies, cohort studies, and randomized control trials reporting on the association between drinking water nitrate exposure of men, women, or animals and adverse reproductive outcomes, specified as: Semen quality parameters, time to pregnancy (TTP), pregnancy rates, assisted reproductive technologies (ART), and spontaneous abortion. Findings were reported in a narrative synthesis. A total of 12 studies were included. The only human study included reported a decrease in spontaneous abortion at any detectable nitrate level. Overall, the 11 included animal studies support a potential negative effect on semen quality parameters but report equivocal results on TTP and number of offspring produced, and higher risk of spontaneous abortion. In conclusion, animal studies indicate possible effects on semen quality parameters and spontaneous abortion. However, with a few studies, including some with methodological limitations and small sample sizes, caution must be applied when interpreting these results.
1. Introduction
The toxicology impact on human health for short and long-term exposure of nitrate is complex and not fully explored. Nitrates occur in both drinking water, food and medicine [1]. The nitrate ion NO3− can undergo transformation to the more potent nitrite (NO2−) [2] and to the N-nitroso compound (NOC) that are known to be carcinogenic in animals [3] and possibly in humans too [4]. Furthermore, nitrates can pass the placental blood barrier, thus exposing the fetus in utero [5]. The best evidence for acute and chronic adverse effects of nitrate ingestion via drinking water is its connection with methemoglobinemia, colorectal cancer, thyroid disease, and neural tube defects [4,6,7]. Nitrate drinking water contamination is a global issue, especially in areas with agricultural pressure, and 2–3% of the population in U.S. and Europe might be exposed to levels exceeding the maximum contaminant level (MCL) for drinking water nitrites and nitrates (3 mg/L and 50 mg/L respectively) set by the World Health Organization (WHO) [1,8]. A study of 11 EU countries estimated that 6.5% of the population were exposed to nitrate levels above 25 mg/L nitrate (ranging from 2.0% in the UK to 16.2% in Denmark) [9]. Less data exists from other parts of the world; nonetheless high levels of drinking water nitrate have been reported in India and The Gaza Strip [7]. In areas where nitrate levels in drinking water exceed 50 mg/L, it is estimated to be the most important contributor to nitrate ingestion, but usually it accounts for less than 14% [1]. Nitrate is an important water pollutant, originating from agricultural and other human sources. Depending on the source of nitrate and type of drinking water (ground water or surface water), nitrate concentrations in drinking water vary with season and type of agriculture conducted in the area [1].
The above-mentioned potential genotoxic, teratogenic and carcinogenic properties of nitrate underline the necessity of considering whether environmental aspects, like drinking water nitrates, could explain some of the unknown etiologies of infertility. Approximately 30% of all pregnancies end in spontaneous abortions [10], and approximately one in six couples worldwide experience fertility problems in their reproductive age [11,12]. Part of this is due to low semen quality, to which the etiology is poorly understood. These factors may lead to a prolonged waiting time to pregnancy (TTP) and an increasing need for medically assisted reproduction, which is a burden for the individual and society.
However, only a strikingly low number of studies on possible adverse effects of nitrate and fertility exists. Animal studies have revealed possible adverse effects; in one study, increased spontaneous abortion rate was present in cattle feeding on pastures with high level nitrates [13]. Another showed spontaneous abortion in cattle when ingesting nitrate containing capsules [6]. This suggests that drinking water nitrates might be related to an increased risk of subfecundity and infertility. Furthermore, studies have suggested associations between drinking water nitrates and other adverse reproductive outcomes, e.g., stillbirth, preterm birth, low birth weight, small for gestational age (SGA) and most convincing birth defects of the central nervous system [7].
The association between drinking water nitrates and human health has previously been reviewed [3,6,7,14,15]. A systematic focus on reproductive health outcomes including human male and female studies as well as animal studies is, however, absent.
Therefore, the aim of this study was to investigate whether exposure to nitrates in drinking water is associated with adverse reproductive outcomes in men, women, or animals of fertile age.
2. Methods
We performed at systematic search of literature describing associations between exposure to nitrates in drinking water with adverse reproductive outcomes in men, women, and animals of fertile age. The outcomes assessed were measures of subfecundity and infertility (e.g., semen quality parameters, TTP, pregnancy rates, use of assisted reproductive technologies (ART)), and spontaneous abortion, which could be an indirect measurement of infertility. The included studies were case-control studies, cohort studies, and randomized control trials (RCT).
This systematic review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [16], a protocol and a flowchart was made accordingly hereto. Elaborated reasons for exclusion are available in Supplementary Material.
2.1. Search Strategy and Study Selection
A systematic computerized literature search was conducted on 2 May 2019 using the databases PubMed/Medline, Embase, and The Cochrane Library. The search strategy was developed and conducted by two authors (H.S.C., N.H.E.) in cooperation with a medical librarian.
To identify potentially relevant studies’ keywords, i.e., medical subject headings (MeSH), and Emtree terms were used. Additionally, a search using free text terms was conducted to include new, non-indexed literature. The search was made with a restriction to the English language but no limit on year of publication. Furthermore, the bibliographies of the included studies were hand searched and other studies citing the included studies were searched for in Scopus in order to include additional relevant studies.
From the searches, all retrieved studies were screened by title and abstract for eligibility individually by two authors (H.S.C., N.H.E.), reaching consensus by discussion including a third reviewer (U.B.K.) if necessary. Studies potentially eligible for inclusion were retrieved and read in full text by two authors (H.S.C., N.H.E.) to ensure they met the following inclusion criteria:
- Studies containing a relevant population:
- Women of fertile age (15–51 years)
- Men of fertile age (15–60 years)
- Animal population
- Studies reporting a numerical exposure range for drinking water nitrate
- Studies containing a relevant control group
- Studies investigating at least one of the outcomes:
- Subfecundity or fertility (TTP, pregnancy rates, assisted reproductive technology treatment (ART))
- Spontaneous abortion
- Semen quality
- Original studies
- Studies with one of the following designs:
- Case-control study
- Cohort study
- Randomized control trials (RCT) study
Studies not meeting the criteria mentioned above were excluded. Agreement was reached through discussion by two authors (H.S.C., N.H.E.) and, if necessary, a third author was consulted.
The computerized literature search was repeated on 25 November 2019 and no new literature was deemed eligible for inclusion.
2.2. Data Extraction and Quality Assessment
All included studies were read in full text. To ensure a standardized procedure, data was extracted using an a priori specified data extraction form, available in Supplementary Material. The quality was assessed by the Newcastle-Ottawa Scale (NOS) [17] for human studies and the Risk of Bias (RoB) tool by Systematic Review Centre for Laboratory animal Experimentation (SYRCLE) [18] for animal studies. The assessment was performed separately by two authors (H.S.C., N.H.E.), reaching agreement by discussion and involving a third author (U.B.K.) if necessary.
SYRCLE’s RoB tool is based on the Cochrane Collaboration RoB tool [19] and focuses on aspects of risk of bias that are relevant when considering animal studies. All the included animal studies were assessed a score of high, unclear or low risk of bias according to the ten items of SYRCLE’s RoB tool.
To ensure standardized scoring, an explanatory form was made for NOS. “Maternal age” was chosen to be the most important covariate in human studies. Studies were allocated a score between 0 and 9. The studies with a score of 7 or above were considered high quality studies and they were observed separately to see if they had any impact on the conclusion of this systematic review.
No core outcome set (COS) was available for the outcomes in this review. No meta-analyses were made. Regarding Aschengrau et al. 1989 [20], the concentration reported in the article was corrected from mg/L to mg-N/L after personal communication. Apart from the above mentioned, no authors, investigators and alike were contacted to obtain missing information, nor were protocols for included studies obtained.
3. Results
A total number of 144 potentially relevant studies were found through database and bibliography searches. Of these, 12 were found eligible for inclusion in the review (Figure 1).
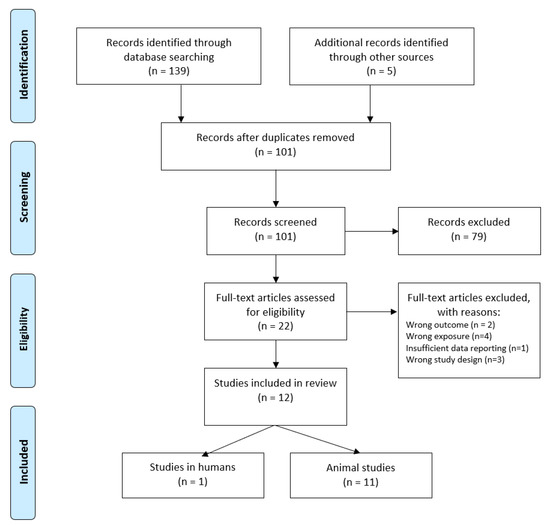
Figure 1.
Flowchart of literature search and identification.
Study characteristics, results, and the assigned score from quality assessment are presented for the human studies in women (Table 1), animal studies in females (Table 2) and animal studies in males (Table 3). The reported nitrate values and forms represent the original from the included studies; hence, no conversion was made except for Aschengrau et al. 1989 [20].

Table 1.
Studies investigating adverse reproductive outcomes in women included in the systematic review.

Table 2.
Animal studies investigating adverse reproductive outcomes in females included in the systematic review.

Table 3.
Animal studies investigating adverse reproductive outcomes in males included in the systematic review.
3.1. Studies Reporting on Human Outcomes
One human study reporting on female outcomes [20] was included. This American case-control study compromised 286 case subjects and 1391 control subjects. It reported on spontaneous abortion up to 28 weeks of gestation and found a decreased risk of spontaneous abortion with an adjusted OR (95% CI) of 0.5 (0.2, 0.9) at any detectable nitrate level (0.1–5.5 mg/L) and a crude OR (95% CI) of 1.1 (0.8, 1.6) at any detectable nitrite level (0.01–0.03 mg/L). Comparison was made with undetectable levels and measured in public water supply. No studies investigating TTP, pregnancy rates, impact on ART-results, or male reproductive outcomes in humans were found.
3.2. Studies Reporting on Animal Outcomes
3.2.1. Studies on Female Animals
Five of the included studies reported female outcomes [21,22,23,24,25]. Three of these studies [21,22,25] reported on longer days to litter (comparable to extended TTP) and/or fewer offspring produced (comparable to pregnancy rate). Anderson et al. 1978 [21] reported on this in mice exposed to 1000 ppm sodium nitrate in drinking water compared to controls. In the study by Anderson et al. 1985 [22], mice exposed to 184 and 1840 ppm sodium nitrate had a lower number of litters compared to controls not being exposed to sodium nitrate. Sleight and Atallah 1968 [25] investigated on guinea pigs exposed to potassium nitrate or potassium nitrite compared to controls. They found a poor reproductive performance at an exposure of 30,000 ppm potassium nitrate, no live births at 5000 or 10,000 ppm potassium nitrite, and overall a dose-response relationship. Higher rates of spontaneous abortion/fetal death or increased apoptosis in the embryo were reported in three studies [21,23,25]. All the animal studies showed an inverse association between nitrate levels and minimum one outcome in at least one exposure group, primarily the highest exposure groups. In one study [23], an increased apoptosis in mice embryos was seen at low doses not expected to be harmful to humans.
3.2.2. Studies on Male Animals
Eight studies [24,25,26,27,28,29,30,31] reported on male outcomes. The outcomes were reported differently across the studies with direct and indirect measurements of semen parameters. Possible negative effects on semen parameters were suggested in all included studies except for one [25], which reported no association as an indirect measurement, e.g., conception in guinea pigs was seen at all levels of exposure but this was not specified in detail as the female outcomes were the focus of this study.
Two studies studied rats. One [24] reported on lower sperm motility and lower epididymis weight, the other [26] reported significant reductions of e.g., sperm count, sperm motility, testicular enzymes, and testis weight.
Five studies studied mice. Expression of laminin α5 (a glycoprotein) in testicles was studied in two of these studies and changes were observed in the seminiferous epithelium [27] and in total level in testicular tissue but not in the extracellular matrix (ECM) [28]. One study in mice [29] found weaker expression of fibronectin in testis. Two studies [24,31] found no differences in mice organ weights (e.g., testis and epididymis) but both of these studies found a reduced sperm motility and one [31] also reduced sperm count, increased total abnormal sperm and declined activity of testicular enzymes. One study [30] on rabbits revealed an inverse association between nitrate exposure and e.g., testosterone levels, sperm parameters and number of offspring.
3.3. Quality of Included Studies
The study in humans [20] obtained a NOS score of 9 and was therefore considered a high quality study.
The quality of the animal studies was varied when evaluated with SYRCLE’s RoB tool, and were mainly low quality, as the score “unclear risk of bias” was dominant, with all rated studies assigned this score in five out of ten score-items due to weak reporting. A score of “high risk of bias” was given once to two studies [23,30].
Further details on quality assessment can be found in the Supplementary Material.
4. Discussion
This systematic review reports findings in human and animal studies on the potential influence of nitrate in drinking water in relation to adverse reproductive outcomes. It reveals that only few studies have been conducted in this field and highlights the complexity in evaluating the exposure of nitrates. Animal studies support a possible association between exposure to drinking water nitrates and semen quality parameters and spontaneous abortion. Only one study investigating human outcomes was found, reporting a lowered risk of spontaneous abortions in women exposed to drinking water nitrates.
4.1. Strengths and Limitations
This review is the first to systematically review studies on the potential effects of exposure to drinking water nitrates and adverse reproductive outcomes with focus on fertility measures. Despite the comprehensive literature search, there might be incomplete retrieval of data due to publication bias and the English language restriction. This problem was partly addressed as additional literature was searched for in the bibliographies of included studies.
The lack of consensus on the definition of spontaneous abortion in the included studies may be another limitation. UpToDate defines spontaneous abortion as fetal loss up to 20 weeks of gestation [32], in Denmark the limit is set at 22 weeks of gestation [33] and the study by Aschengrau et al. 1989 set 28 weeks of gestation as the limit in 1989 [20]. The 28-week limit is consistent with a change of definition over time when considering the older date of the study [20]. The different limits in gestational age demonstrate that there may be an overlap between the outcome spontaneous abortion and perinatal outcomes like stillbirth, which were not evaluated in this review. Similarly, there was no defined cut-off available in the animal studies to separate spontaneous abortion from perinatal outcomes.
4.2. Studies Reporting on Human Outcomes
Overall, the only human study was from 1989, thus revealing a lack of focus on this area. The study [20] showed an OR below 1 for spontaneous abortion at any detectable nitrate level, thus pointing to a possible beneficial effect. This aligns with the possible beneficial effect of nitrates on blood pressure [1]. The nitrate levels reported in this study are low compared to the maximum contaminant level (MCL) for drinking water nitrites and nitrates 3 mg/L and 50 mg/L respectively set by the World Health Organization (WHO) [1]. The study was high quality (NOS score of 9), but limitations were still present due to e.g., the high complexity of evaluation of nitrate exposure.
It can be questioned whether the composition in the public water taps reflected the actual composition of the drinking water ingested by the women, as the amount of home water intake, and respectively bottled water intake, was not considered and these factors vary between individuals [34]. Furthermore, water samples close to the pregnancy outcome were not always available, and it can be discussed whether the nitrate levels in groundwater were stable over time [35].
Aschengrau et al. 1989 [20] did not report details on other nitrate sources (e.g., food or medicine), nor did the study account for the endogenous nitrosation of nitrates. A follow-up study from 2019 suggested an association between increased risk of stillbirth and use of nitrosable drugs of which some are very commonly used during pregnancy [36].
Aschengrau et al. 1989 [20] adjusted for some other water contaminants, but still it is possible that unmeasured water contaminants could explain some of the results.
No studies were found to report on the important human outcomes: Subfecundity or infertility (specified as extended TTP), lower pregnancy rates, and use of ART, spontaneous abortion, or semen quality parameters.
Several studies did not qualify for inclusion in this systematic review. These, however, have indications of the possible influence of nitrate on fertility. A review on studies on tap water indicated some correlation with spontaneous abortion [37], but the exposure of nitrates was not specified. A case-report [38] from a U.S. community reported a cluster of spontaneous abortions in women exposed to high drinking water nitrate levels. In contrast, a cross-sectional study [39] showed higher infant mortality but no higher rate of spontaneous abortion or stillbirths in mothers living in high-nitrate areas in West Africa. Similarly, an ecological study [40] from the U.S. including 30,980 infants and fetuses showed no association between drinking water nitrates and fetal mortality.
Extensive evidence exists on the association between drinking water nitrates and thyroid disease, development of infant methemoglobinemia (an affection of oxygen transportation) and fetal malformations. This is also what WHO primarily aims to avoid with the MCL for nitrates [1]. The above mentioned health aspects and underlying mechanisms could also be indirectly related to infertility and fetal death [25,38,41,42,43]. In line with this, a U.S. study of 25 women [44] showed a possible relationship between high maternal methemoglobinemia level and spontaneous abortion in the first trimester.
4.3. Studies Reporting on Animal Outcomes
The animal studies reported different outcomes and scored by SYRCLE’s RoB tool, they were of varying quality, indicating low quality. The risk of bias was classified as “unclear” according to several parameters in SYRCLE’s RoB tool. This was mainly due to a lack of reporting on or actual blinding of investigators and caretakers or randomization. These factors might be overlooked as important factors due to the similarity in appearance that is often present in the animals studied. In general, there seems to be a lack of consensus and tradition on how to report animal studies and what to include. The Navigation Guide Methodology [45] and The REFLECT statement [46] was made to address this issue and introduce new methods for environmental health research, but still needs further implementation.
The animals showed equivocal results on days to litter and number of offspring produced and negative results on spontaneous abortion and fetal death. Overall, a negative association between drinking water nitrates and semen quality parameters in animal studies was found. Nonetheless, none of the studies were described as blinded, which could lead to an overestimation of the potential effect on the semen quality parameters, because the investigators might be biased. Furthermore, assessing the effect in the animal studies is challenged by the heterogenicity regarding reported outcomes and animal species.
The advantages of the animal studies are the more exact diet and water consumption, the relatively similar animals and controlled environment which make it less complex to evaluate the actual nitrate exposure. This also makes it possible to study other aspects of nitrate exposure as done in some of the included animal studies. Anderson et al. 1978 [21] showed a lower number of offspring and a higher number of infertile female mice in the group exposed to the drug imipramine (a tricyclic antidepressant) together with nitrate compared to nitrate exposure alone. Another included animal study [22] showed similar litter sizes in groups exposed to nitrate alone or in combination with the nitrosable drug cimetidine. Furthermore, Attia et al. 2013 [30] showed some reversing effect of adding antioxidants and probiotics to the nitrate contaminated water, pointing to the importance of taking into considerations the amount and type of food consumed.
Evaluating drinking water nitrates without taking into consideration other drinking water contaminants might be problematic. The included study on mice embryos [23] accounted for this by making an exposure group with ammonium nitrate alone and one with a mixture of groundwater contaminants; both exposures resulting in significantly reduced cell numbers in embryos.
Among studies excluded from this review, one assimilated a realistic mixture of groundwater contamination and results on female fertility in rats and mice were equivocal [47]. Another study, showed indication of a protective effect of nitrate in drinking water on semen parameters in diabetic mice [48], while two other studies [49,50] showed no association between nitrate in drinking water and litter size or fertility.
4.4. Comparisons: Studies in Humans and Animals
Animal studies are indicators of possible mechanisms in humans, but many challenges exist when transferring these results to actual effects in humans. Animal studies will often be conducted with a high exposure over a shorter period (acute toxic dose)—which is also the case for the studies included in this review. As opposed to this, humans are often exposed to a lower exposure for a longer period. Considering the difference in exposure dose and time, the mechanisms might differ. Furthermore, one cannot directly transfer study results from one species of animal to another, nor from animals to humans or from humans living in different exposure settings (i.e., external validity). Given these issues, the MCL set by WHO has been questioned [51]. Thus, in future research these factors should be considered.
5. Conclusions
In conclusion, this systematic review showed that equivocal and scarce evidence of the impact of nitrates in drinking water on adverse reproductive outcomes exists. Only one study in humans was found, showing lower rate of spontaneous abortion at higher exposure levels. No studies were found reporting on the important human outcomes subfecundity, infertility, and semen parameters. The included animal studies reported inverse associations between nitrate exposure and semen parameters and equivocal results regarding female fertility. However, studies were scored to be of low quality.
Large high-quality epidemiological studies are needed to investigate the possible effects suggested by the animal studies. These studies should account for the complexity of evaluating nitrate exposure and include the outcome measures spontaneous abortion, extended TTP and semen quality parameters.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4441/12/8/2287/s1, Table S1: Characteristics and details on quality assessment of studies on human outcomes according to Newcastle Ottawa Scale, Table S2: Results for quality assessment of animal studies using SYRCLE’s Risk of Bias tool.
Author Contributions
Conceptualization, H.S.C., N.H.E., I.M.B., J.S., C.H.R.-H., and U.B.K.; methodology, H.S.C., N.H.E., I.M.B., J.L., B.B. and U.B.K.; validation, H.S.C. and N.H.E.; formal analysis, H.S.C., N.H.E.; investigation, H.S.C., N.H.E.; data curation, H.S.C., N.H.E.; writing—original draft preparation, H.S.C., N.H.E., I.M.B. and U.B.K.; writing—review and editing, H.S.C., N.H.E., I.M.B., J.L., J.S., C.H.R.-H., B.B., and U.B.K.; visualization, H.S.C.; supervision, U.B.K.; project administration, H.S.C., N.H.E, U.B.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
B.B. reports personal fees from Merck (lecture for fertility staff in offspring health following ART), outside the submitted work. This had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- WHO (World Health Organization) Nitrate and nitrite in drinking-water: Background document for development of WHO guidelines for drinking-water quality. Available online: https://www.who.int/water_sanitation_health/dwq/chemicals/nitratenitrite2ndadd.pdf (accessed on 4 March 2020).
- Keshari, V.; Adeeb, B.; Simmons, A.E.; Simmons, T.W.; Diep, C.Q. Zebrafish as a model to assess the teratogenic potential of nitrite. J. Vis. Exp. 2016, 11, e53615. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.H.; deKok, T.M.; Levallois, P.; Brender, J.; Gulis, G.; Nolan, B.T.; VanDerslice, J. Workgroup report: Drinking-water nitrate and health - Recent findings and research needs. Environ. Health Perspect. 2005, 113, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Schullehner, J.; Hansen, B.; Thygesen, M.; Pedersen, C.B.; Sigsgaard, T. Nitrate in drinking water and colorectal cancer risk: A nationwide population-based cohort study. Int. J. Cancer 2018, 143, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Bruning-Fann, C.S.; Kaneene, J.B. The effects of nitrate, nitrite and N-nitroso compounds on human health: A review. Vet. Hum. Toxicol. 1993, 35, 521–538. [Google Scholar] [PubMed]
- Manassaram, D.M.; Backer, L.C.; Moll, D.M. A review of nitrates in drinking water: Maternal exposure and adverse reproductive and developmental outcomes. Environ. Health Perspect. 2006, 114, 320–327. [Google Scholar] [CrossRef]
- Ward, M.H.; Jones, R.R.; Brender, J.D.; de Kok, T.M.; Weyer, P.J.; Nolan, B.T.; Villanueva, C.M.; van Breda, S.G. Drinking water nitrate and human health: An updated review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef]
- van Grinsven, H.J.M.; Ward, M.H.; Benjamin, N.; de Kok, T.M. Does the evidence about health risks associated with nitrate ingestion warrant an increase of the nitrate standard for drinking water? Environ. Heal. A Glob. Access Sci. Source 2006, 5, 26. [Google Scholar] [CrossRef]
- Van Grinsven, H.J.; Rabl, A.; De Kok, T.M. Estimation of incidence and social cost of colon cancer due to nitrate in drinking water in the EU: A tentative cost-benefit assessment. Environ. Health A Glob. Access Sci. Source 2010, 9, 1–12. [Google Scholar] [CrossRef]
- Weselak, M.; Arbuckle, T.E.; Walker, M.C.; Krewski, D. The influence of the environment and other exogenous agents on spontaneous abortion risk. J. Toxicol. Environ. Heal. Part B Crit. Rev. 2008, 11, 221–241. [Google Scholar] [CrossRef]
- Slama, R.; Hansen, O.K.H.; Ducot, B.; Bohet, A.; Sorensen, D.; Giorgis Allemand, L.; Eijkemans, M.J.C.; Rosetta, L.; Thalabard, J.C.; Keiding, N.; et al. Estimation of the frequency of involuntary infertility on a nation-wide basis. Hum. Reprod. 2012, 27, 1489–1498. [Google Scholar] [CrossRef]
- Thoma, M.E.; McLain, A.C.; Louis, J.F.; King, R.B.; Trumble, A.C.; Sundaram, R.; Buck Louis, G.M. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil. Steril. 2013, 99, 1324–1331.e1. [Google Scholar] [CrossRef] [PubMed]
- Sund, J.M.; Wright, M.J.; Simon, J. Weeds containing nitrates cause abortion in cattle. Agron. J. 1957, 49, 278–279. [Google Scholar] [CrossRef]
- Fan, A.M.; Steinberg, V.E. Health implications of nitrate and nitrite in drinking water: An update on methemoglobinemia occurrence and reproductive and developmental toxicity. Regul. Toxicol. Pharmacol. 1996, 23, 35–43. [Google Scholar] [CrossRef]
- Fan, A.M.; Willhite, C.C.; Book, S.A. Evaluation of the Nitrate Drinking Water Standard with Reference to Infant Methemoglobinemia and Potential Reproductive Toxicity. Regul. Toxicol. Pharmacol. 1987, 7, 135–148. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1–e28. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugweel, P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 30 May 2019).
- Hooijmans, C.R. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 1–19. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, 1–8. [Google Scholar] [CrossRef]
- Aschengrau, A.; Zierler, S.; Cohen, A. Quality of community drinking water and the occurrence of spontaneous abortion. Arch. Environ. Health 1989, 44, 283–290. [Google Scholar] [CrossRef]
- Anderson, L.M.; Giner-Sorolla, A.; Ebeling, D.; Budinger, J.M. Effects of imipramine, nitrite, and dimethylnitrosamine on reproduction in mice. Res. Commun. Chem. Pathol. Pharmacol. 1978, 19, 311–327. [Google Scholar]
- Anderson, L.M.; Giner-Sorolla, A.; Haller, I.M.; Budinger, J.M. Effects of cimetidine, nitrite, cimetidine plus nitrite, and nitrosocimetidine on tumors in mice following transplacental plus chronic lifetime exposure. Cancer Res. 1985, 45, 3561–3566. [Google Scholar]
- Greenlee, A.R.; Ellis, T.M.; Berg, R.L. Low-dose agrochemicals and lawn-care pesticides induce developmental toxicity in murine preimplantation embryos. Environ. Health Perspect. 2004, 112, 703–709. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program Toxicology and carcinogenesis studies of sodium nitrite (CAS NO. 7632-00-0) in F344/N rats and B6C3F1 mice (drinking water studies). Natl. Toxicol. Program Tech. Rep. Ser. 2001, 495, 7–273.
- Sleight, S.D.; Atallah, O.A. Reproduction in the Guinea Pig as Affected by Chronic Administration of Potassium Nitrate and Potassium Nitrite. Toxicol. Appl. Pharmacol. 1968, 12, 179–185. [Google Scholar] [CrossRef]
- Aly, H.A.A.; Mansour, A.M.; Abo-Salem, O.M.; Abd-Ellah, H.F.; Abdel-Naim, A.B. Potential testicular toxicity of sodium nitrate in adult rats. Food Chem. Toxicol. 2010, 48, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.; Nikravesh, M.R.; Fazel, A.; Jalali, M.; Nabavi, A.S. Evaluation of the laminin α5 expression in mice testicular parenchyma following an exposure to water contaminated with sodium nitrite. Int. J. Adv. Biotechnol. Res. 2016, 7, 513–522. [Google Scholar]
- Amini, S.; Nikravesh, M.R.; Jalali, M.; Fazel, A.; Nabavi, A.S. Effects of oral administration of sodium nitrite on laminin expression in mice testicular interstitium: An immunohistochemical study. Biomed. Res. 2017, 28, 8671–8676. [Google Scholar]
- Amini, S.; Nikravesh, M.R.; Jalali, M.; Fazel, A.; Nabavi, A.S. Effect of chronic sodium nitrite administration on the expression of fibronectin in interstitial tissue of mice testis: An immunohistochemical study. Biomed. Res. 2018, 29, 91–95. [Google Scholar] [CrossRef]
- Attia, Y.A.; Abd El Hamid, E.A.; Ismaiel, A.M.; El-Nagar, A. The detoxication of nitrate by two antioxidants or a probiotic, and the effects on blood and seminal plasma profiles and reproductive function of New Zealand White rabbit bucks. Animal 2013, 7, 591–601. [Google Scholar] [CrossRef]
- Pant, N.; Srivastava, S.P. Testicular and spermatotoxic effect of nitrate in mice. Hum. Exp. Toxicol. 2002, 37–41. [Google Scholar] [CrossRef]
- Prager, S.; Micks, E.; Dalton, V.K. Pregnancy loss (miscarriage): Risk factors, etiology, clinical manifestations, and diagnostic evaluation – UpToDate. Available online: https://www.uptodate.com/contents/pregnancy-loss-miscarriage-risk-factors-etiology-clinical-manifestations-and-diagnostic-evaluation (accessed on 16 November 2019).
- Laegehaandbogen 2. trimester abort eller dødfødsel. Available online: https://www.sundhed.dk/sundhedsfaglig/laegehaandbogen/obstetrik/tilstande-og-sygdomme/komplikationer-i-svangerskabet/anden-trimester-abort-eller-doedfoedsel/ (accessed on 16 November 2019).
- Smith, R.B.; Toledano, M.B.; Wright, J.; Raynor, P.; Nieuwenhuijsen, M.J. Tap water use amongst pregnant women in a multi-ethnic cohort. Environ. Health A Glob. Access Sci. Source 2009, 8, S7. [Google Scholar] [CrossRef]
- Ruckart, P.Z.; Henderson, A.K.; Black, M.L.; Flanders, W.D. Are nitrate levels in groundwater stable over time? J. Expo. Sci. Environ. Epidemiol. 2008, 18, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, A.M.L.; Liew, Z.; Riis, A.H.; Stayner, L.T.; Ramlau-Hansen, C.H.; Sigsgaard, T.; Olsen, J. Nitrosatable drug exposure during pregnancy and risk of stillbirth. Pharmacoepidemiol. Drug Saf. 2019, 28, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Swan, S.H.; Neutra, R.R.; Wrensch, M.; Hertz-picciotto, I.; Windham, G.C.; Fenster, L.; Epstein, D.M.; Deane, M. Is Drinking Water Related to Spontaneous Abortion? Reviewing the Evidence from the California Department of Health Services Studies. Epidemiology 1992, 3, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.; Steele, G.; Isiorho, S. Spontaneous abortions possibly related to ingestion of nitrate-contaminated well water--LaGrange County, Indiana, 1991–1994. MMWR. Morb. Mortal. Wkly. Rep. 1996, 45, 569–572. [Google Scholar]
- Super, M.; Heese, H.D.V.; MacKenzie, D., Dempster; Du Plessis, J.; Ferreira, J.J. An epidemiological study of well-water nitrates in a group of south west african/namibian infants. Water Res. 1981, 15, 1265–1270. [Google Scholar] [CrossRef]
- Gelperin, A.; Moses, V.K.; Bridger, C. Relationship of high nitrate community water supply to infant and fetal mortality. IMJ. Ill. Med. J. 1975, 147, 155–157, 186. [Google Scholar]
- Scragg, R.K.; Dorsch, M.M.; McMichael, A.J.; Baghurst, P.A. Birth defects and household water supply. Epidemiological studies in the Mount Gambier region of South Australia. Med. J. Aust. 1982, 2, 577–579. [Google Scholar] [CrossRef]
- Dorsch, M.M.; Scragg, R.K.; McMichael, A.J.; Baghurst, P.A.; Dyer, K.F. Congenital malformations and maternal drinking water supply in rural South Australia: A case-control study. Am. J. Epidemiol. 1984, 119, 473–486. [Google Scholar] [CrossRef]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef]
- Schmitz, J.T. Methemoglobinemia - A Cause of Abortions? Preliminary Report. Obstet. Gynecol. 1961, 17, 413–415. [Google Scholar]
- Woodruff, T.J.; Sutton, P. The navigation guide systematic review methodology: A rigorous and transparent method for translating environmental health science into better health outcomes. Environ. Health Perspect. 2014, 122, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Sargeant, J.M.; O’Connor, A.M.; Gardner, I.A.; Dickson, J.S.; Torrence, M.E.; Dohoo, I.R.; Lefebvre, S.L.; Morley, P.S.; Ramirez, A.; Snedeker, K. The REFLECT statement: Reporting guidelines for randomized controlled trials in livestock and food safety: Explanation and elaboration. J. Food Prot. 2010, 73, 579–603. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.K.; Chapin, R.E.; Gulati, D.K.; George, J.D.; Price, C.J.; Marr, M.C.; Myers, C.B.; Barnes, L.H.; Fail, P.A.; Grizzle, T.B.; et al. Assessment of the reproductive and developmental toxicity of pesticide/fertilizer mixtures based on confirmed pesticide contamination in California and Iowa groundwater. Fundam. Appl. Toxicol. 1994, 22, 605–621. [Google Scholar] [CrossRef] [PubMed]
- Keyhanmanesh, R.; Hamidian, G.; Alipour, M.R.; Ranjbar, M.; Oghbaei, H. Protective effects of sodium nitrate against testicular apoptosis and spermatogenesis impairments in streptozotocin-induced diabetic male rats. Life Sci. 2018, 211, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, M. [Influence of nitrates on reproduction in domestic mammals]. Contracept. Fertil. Sex. 1993, 21, 642–647. [Google Scholar]
- Bruning-Fann, C.S.; Kaneene, J.B.; Lloyd, J.W.; Stein, A.D.; Thacker, B.; Hurd, H.S. Associations between drinking-water nitrate and the productivity and health of farrowing swine. Prev. Vet. Med. 1996, 26, 33–46. [Google Scholar] [CrossRef]
- Frisbie, S.H.; Mitchell, E.J.; Sarkar, B. Urgent need to reevaluate the latest World Health Organization guidelines for toxic inorganic substances in drinking water. Environ. Health 2015, 14, 1–15. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).