Ecosystem-Based Restoration to Mitigate Eutrophication: A Case Study in a Shallow Lake
Abstract
1. Introduction
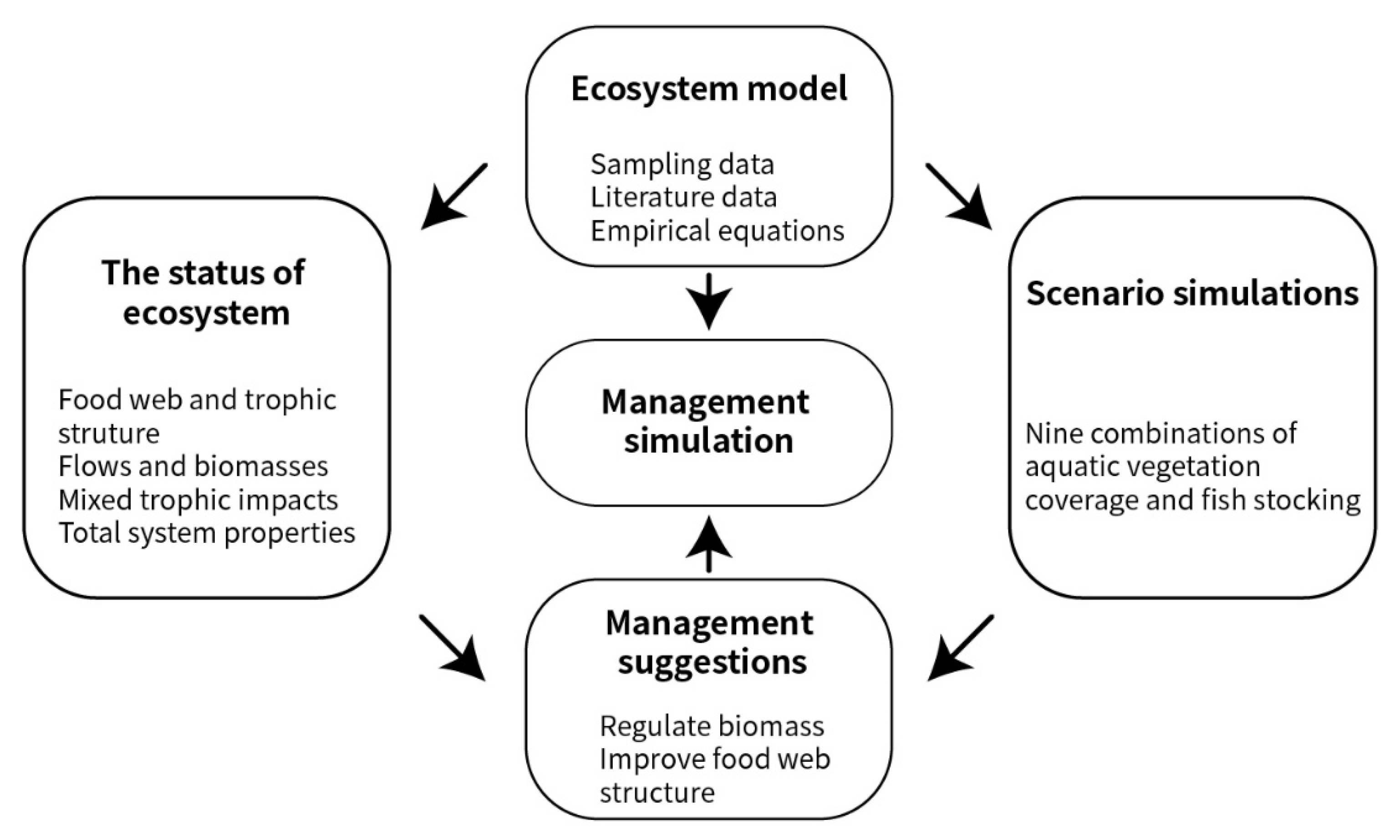
2. Materials and Methods
2.1. Study Area
2.2. Modeling the Lake Datong Ecosystem
2.2.1. Ecosystem Model
2.2.2. Functional Groups
2.2.3. Input Parameters
2.2.4. Model Balancing and Uncertainty
2.3. Scenario Simulations
2.4. Management Suggestions
2.5. Management Simulation
3. Results and Discussion
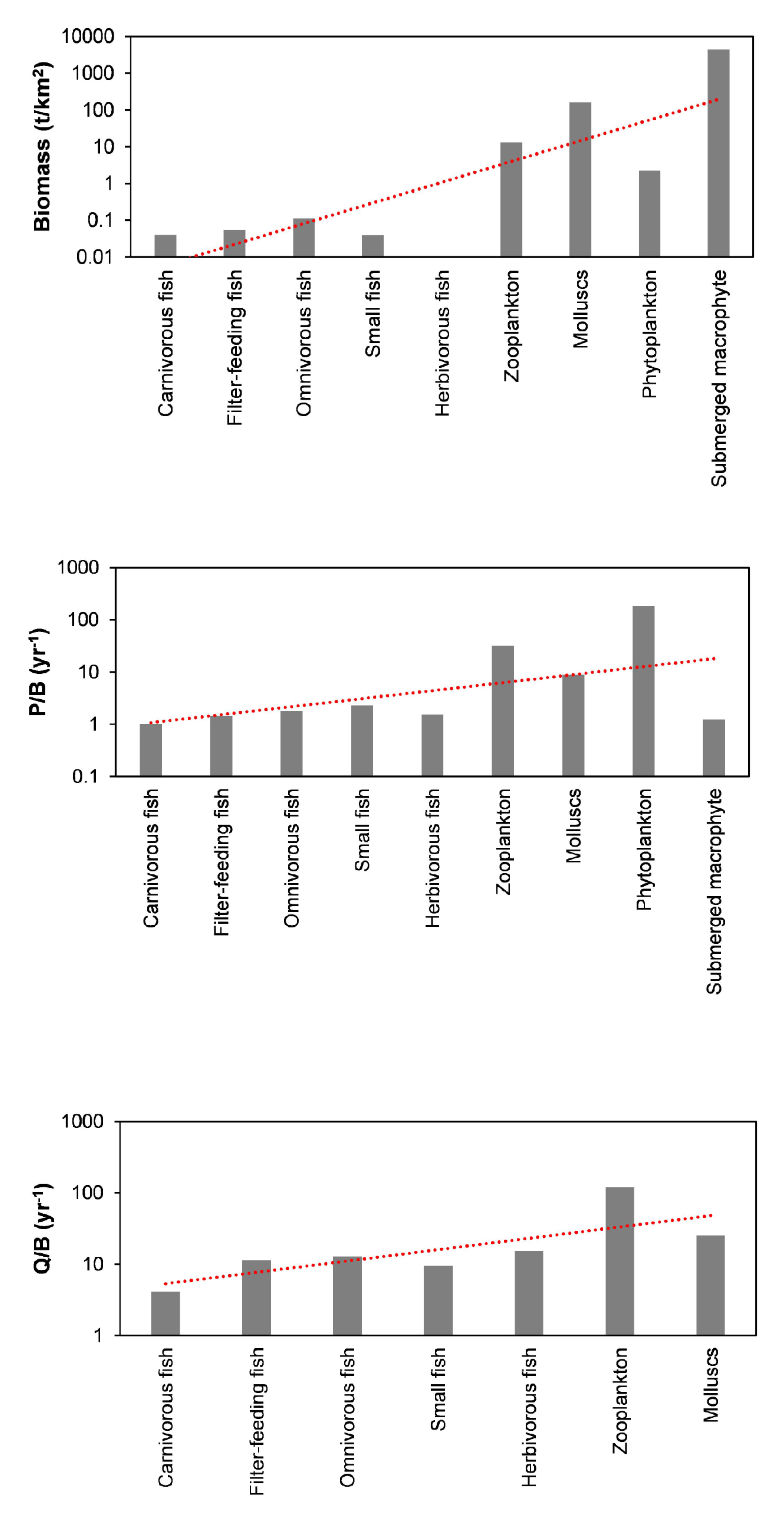
3.1. PREBAL and Uncertainty
3.2. Status of the Lake Datong Ecosystem
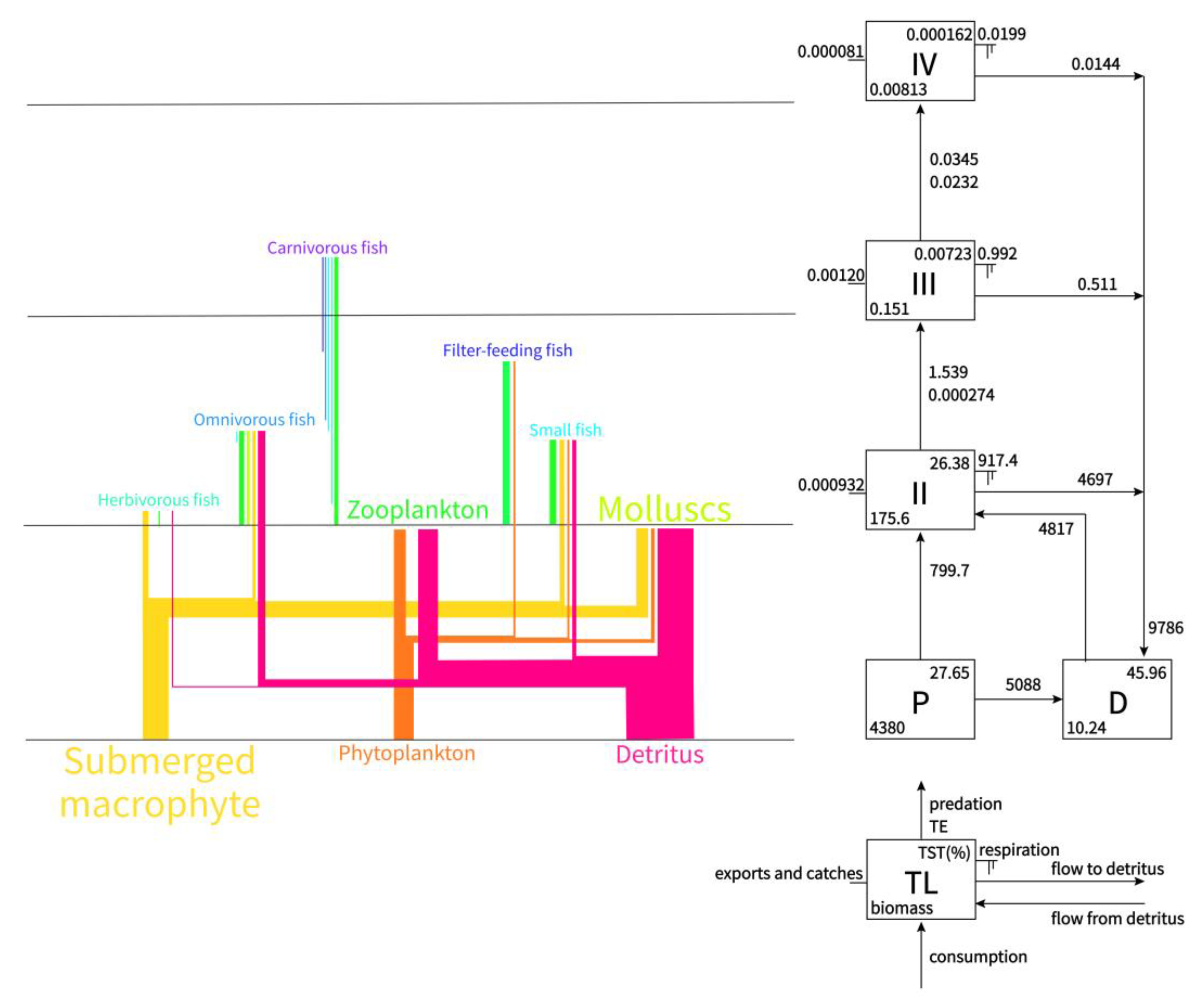
3.2.1. Food Web and Trophic Structure
3.2.2. Flows and Biomasses
3.2.3. Mixed Trophic Impacts
3.2.4. Total Properties of the Ecosystem
3.3. Scenario Simulations
3.4. Management Suggestions
3.4.1. Regulate Biomass
3.4.2. Improve Food Web Structure
3.5. Management Simulation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| No. | Functional Group | Composition |
|---|---|---|
| 1 | Carnivorous fish | Siniperca chuatsi, Culter alburnus, Culter mongolicus, Erythroculter dabryi, Cultrichthys erythropterus, Coilia brachygnathus |
| 2 | Filter-feeding fish | Aristichthys nobilis, Hypophthalmichthys molitrix |
| 3 | Omnivorous fish | Pelteobagrus fulvidraco, Cyprinus carpio, Carassius auratus |
| 4 | Small fish | Paracanthobrama guichenoti, Acheilognathus macropterus, Squalidus argentatus, Sarcocheilichthys sinensis, Saurogobio dabryi, Rhodeus sinensis, Hemibarbus maculatus, Sarcocheilichthys nigripinnis, Hyporhamphus intermedius, Pseudorasbora parva, Hemiculter leucisculus, Hemiculter bleekeri, Mastacembelus aculeatus |
| 5 | Herbivorous fish | Parabramis pekinensis, Megalobrama amblycephala |
| 6 | Zooplankton | Protozoans, Rorifers, Cladoceras, Copepodas |
| 7 | Molluscs | Cristaria plicata, Unio douglasiae, Lanceolaria gladiola, Arconaia lanceolate, Hyriopsis cumingii, Cristaria plicata, Bellamya sp. |
| 8 | Phytoplankton | Chlorophytes, Bacillariophytes, Cyanophytes, Euglenophytes, Cryptophytes, Dinophytes, Chrysophytes |
| 9 | Submerged macrophyte | Hydrilla verticillata, Myriophyllum spicatum, Ceratophyllum demersum, Vallisneria natans |
| 10 | Detritus | Detritus |
| No. | Groups | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|---|
| 1 | Carnivorous fish | 0.03 | ||||||
| 2 | Filter-feeding fish | 0.076 | ||||||
| 3 | Omnivorous fish | 0.175 | ||||||
| 4 | Small fish | 0.077 | 0.004 | |||||
| 5 | Herbivorous fish | 0.053 | ||||||
| 6 | Zooplankton | 0.589 | 0.937 | 0.293 | 0.420 | 0.170 | 0.004 | |
| 7 | Molluscs | 0.152 | ||||||
| 8 | Phytoplankton | 0.063 | 0.120 | 0.200 | 0.01 | |||
| 9 | Submerged macrophyte | 0.131 | 0.200 | 0.796 | 0.11 | |||
| 10 | Detritus | 0.420 | 0.260 | 0.034 | 0.796 | 0.88 | ||
| 11 | Sum | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
References
- Dodds, W. Freshwater Ecology: Concepts and Environmental Applications; Elsevier Science: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Frumin, G.T.; Gildeeva, I.M. Eutrophication of water bodies—A global environmental problem. Russ. J. Gen. Chem. 2014, 84, 2483–2488. [Google Scholar] [CrossRef]
- Khan, F.A.; Ansari, A.A. Eutrophication: An ecological vision. Bot. Rev. 2005, 71, 449–482. [Google Scholar] [CrossRef]
- Mooij, W.M.; Hülsmann, S.; De Senerpont Domis, L.N.; Nolet, B.A.; Bodelier, P.L.E.; Boers, P.C.M.; Pires, L.M.D.; Gons, H.J.; Ibelings, B.W.; Noordhuis, R.; et al. The impact of climate change on lakes in the Netherlands: A review. Aquat. Ecol. 2005, 39, 381–400. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Bhagowati, B.; Ahamad, K.U. A review on lake eutrophication dynamics and recent developments in lake. Ecohydrol. Hydrobiol. 2019, 19, 155–166. [Google Scholar] [CrossRef]
- Söderström, M.; Nylund, K.; Järnberg, U.; Lithner, G.; Rosén, G.; Kylin, H. Seasonal Variations of DDT Compounds and PCB in a Eutrophic and an Oligotrophic Lake in Relation to Algal Biomass. AMBIO 2000, 29, 230–237. [Google Scholar] [CrossRef]
- Smith, V.H.; Schindler, D.W. Eutrophication science: Where do we go from here? Trends Ecol. Evol. 2009, 24, 201–207. [Google Scholar] [CrossRef]
- Hosper, S.H. Biomanipulation, new perspectives for restoration of shallow, eutrophic lakes in The Netherlands. Hydrobiol. Bull. 1989, 23, 5–10. [Google Scholar] [CrossRef]
- Wang, Z.; Li, D.; Qin, H.; Li, Y. An integrated method for removal of harmful cyanobacterial blooms in eutrophic lakes. Environ. Pollut. 2012, 160, 34–41. [Google Scholar] [CrossRef]
- Shan, M.; Wang, Y.; Xue, S. Study on bioremediation of eutrophic lake. J. Environ. Sci. 2009, 21, S16–S18. [Google Scholar] [CrossRef]
- Hilt, S.; Gross, E.M.; Hupfer, M.; Morscheid, H.; Mählmann, J.; Melzer, A.; Poltz, J.; Sandrock, S.; Scharf, E.-M.; Schneider, S.; et al. Restoration of submerged vegetation in shallow eutrophic lakes—A guideline and state of the art in Germany. Limnologica 2006, 36, 155–171. [Google Scholar] [CrossRef]
- Wang, G.; Cheng, X. Lake Eutrophication Control in Technology, Theory and Application. J. Lake Sci. 2002, 14, 273–282. [Google Scholar]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling eutrophication: Nitrogen and phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Dhote, S.; Dixit, S. Water quality improvement through macrophytes—A review. Environ. Monit. Assess. 2009, 152, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Krot, Y. The use of higher aquatic plants in biotechnologies of surface water and wastewater treatment. Hydrobiol. J. 2006, 42, 44–55. [Google Scholar] [CrossRef]
- Nina, C.; Jonathan, C.; Stuart, F.; Cathleen, W. Vascular plants as engineers of oxygen in aquatic systems. Bioscience 2006, 56, 219–225. [Google Scholar]
- Reddy, K.R.; DeBusk, T.A. State-of-the-art utilization of aquatic plants in water pollution control. Water Sci. Technol. 1987, 19, 61–79. [Google Scholar] [CrossRef]
- Zhao, F.L.; Xi, S.; Yang, X.E.; Yang, W.D.; Li, J.J.; Gu, B.H.; He, Z.L. Purifying eutrophic river waters with integrated floating island systems. Ecol. Eng. 2012, 40, 53–60. [Google Scholar] [CrossRef]
- Scheffer, M. Ecology of Shallow Lakes; Springer: New York, NY, USA, 1997. [Google Scholar]
- Li, W.C. Ecological restoration of shallow, eutrophic lakes-experimental studies on the recovery of aquatic vegetation in Wuli Lake. J. Lake Sci. 1996, 8, 1–10. [Google Scholar]
- Zhang, Y.L.; Liu, X.H.; Qin, B.Q.; Shi, K.; Deng, J.M.; Zhou, Y.Q. Aquatic vegetation in response to increased eutrophication and degraded light climate in Eastern Lake Taihu: Implications for lake ecological restoration. Sci. Rep. 2016, 6, 23867. [Google Scholar] [CrossRef]
- Nakamura, K.; Tockner, K.; Amano, K. River and wetland restoration: Lessons from Japan. Bioscience 2006, 56, 419–429. [Google Scholar] [CrossRef]
- Sayah, S.M.; Boillat, J.-L.; Schleiss, A. The use of soft shore protection measures in shallow lakes: Research methodology and case study. Limnologica 2004, 34, 65–74. [Google Scholar] [CrossRef]
- Keddy, P.A. Wetland Ecology: Principles and Conservation; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Chambers, P.A.; DeWreede, R.E.; Irlandi, E.A.; Vandermeulen, H. Management issues in aquatic macrophyte ecology: A Canadian perspective. Can. J. Bot. 1999, 77, 471–487. [Google Scholar] [CrossRef]
- Li, D.L.; Zhang, T.; Yu, J.B.; Mao, X.W.; Wang, H.Q.; Chen, K.J.; Liu, A.M.; Li, Z.J.; Xiao, T.Y. Temporal and spatial distributional patterns of mollusca in a typical aquacultural lake—Datong Lake. Acta Hydrobiol. Sin. 2011, 35, 946–954. [Google Scholar]
- Wu, K. The Evolutional Characteristics of Water Environment and Total Amount Control of Pollutant in Lake Datong Basin Based on GIS. Master’s Thesis, Hunan University of Science and Technology, Xiangtan, China, 2015. [Google Scholar]
- Jian, Y.X.; Wang, J.B.; He, G.Q.; Chen, J.K. A comparative study on aquatic plant diversity and its long-term changes in the three lakes of dongtinghu district in china. Acta Hydrobiol. Sin. 2002, 2, 160–167. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Plagányi, É.E.; Food and Agriculture Organization of the United Nations. Models for an Ecosystem Approach to Fisheries; Food and Agriculture Organization of the United Nations: Rome, Italy, 2007. [Google Scholar]
- Heymans, J.J.; Coll, M.; Libralato, S.; Morissette, L.; Christensen, V. Global patterns in ecological indicators of marine food webs: A modelling approach. PLoS ONE 2014, 9, e95845. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.-C.; Adlerstein, S.; Rutherford, E. The relative impacts of nutrient loads and invasive species on a Great Lakes food web: An Ecopath with Ecosim analysis. J. Great Lakes Res. 2014, 40, 35–52. [Google Scholar] [CrossRef]
- Christensen, V.; Walters, C.J. Ecopath with Ecosim: Methods, capabilities and limitations. Ecol. Model. 2004, 172, 109–139. [Google Scholar] [CrossRef]
- Christensen, V.; Walters, C.J.; Pauly, D.; Forrest, R. Ecopath with Ecosim Version 6: User Guide; University of British Columbia Fisheries Centre: Vancouver, BC, Canada, 2008. [Google Scholar]
- Li, C.-H.; Xian, Y.; Ye, C.; Wang, Y.-H.; Wei, W.-W.; Xi, H.-Y.; Zheng, B.-H. Wetland ecosystem status and restoration using the Ecopath with Ecosim (EWE) model. Sci. Total Environ. 2019, 658, 305–314. [Google Scholar] [CrossRef]
- Palomares, M.L.D.; Pauly, D. Predicting food consumption of fish populations as functions of mortality, food type, morphometrics, temperature and salinity. Mar. Freshw. Res. 1998, 49, 447–453. [Google Scholar] [CrossRef]
- Von Bertalanffy, L. A quantitative throry of organic growth (inquiries on growth laws. II). Hum. Biol. 1938, 10, 181–213. [Google Scholar]
- Guo, C.B.; Ye, S.W.; Lek, S.; Liu, J.S.; Zhang, T.L.; Yuan, J.; Li, Z.J. The need for improved fishery management in a shallow macrophytic lake in the Yangtze River basin: Evidence from the food web structure and ecosystem analysis. Ecol. Model. 2013, 267, 138–147. [Google Scholar] [CrossRef]
- Li, Y.K.; Liu, E.S.; Wang, H.; Gong, Y. Analysis on the ecosystem structure and function of Lake Taihu based on ecopath model. Chin. J. Appl. Ecol. 2014, 25, 2033–2040. [Google Scholar]
- Wang, Y.Y.; Kao, Y.-C.; Zhou, Y.M.; Zhang, H.; Yu, X.B.; Lei, G.C. Can water level management, stock enhancement, and fishery restriction offset negative effects of hydrological changes on the four major Chinese carps in China’s largest freshwater lake? Ecol. Model. 2019, 403, 1–10. [Google Scholar] [CrossRef]
- Pauly, D.; Bartz, M.L.S.; Palomares, M.L.D. Improved construction, parametrization and interpretation of steady-state ecosystem models. In Trophic Models of Aquatic Ecosystems; Christensen, V., Pauly, D., Eds.; ICLARM: Penang, Malaysia, 1993. [Google Scholar]
- Link, J.S. Adding rigor to ecological network models by evaluating a set of pre-balance diagnostics: A plea for PREBAL. Ecol. Model. 2010, 221, 1580–1591. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Walters, C. Ecopath, Ecosim, and Ecospace as tools for evaluating ecosystem impact of fisheries. ICES J. Mar. Sci. 2000, 57, 697–706. [Google Scholar] [CrossRef]
- Morissette, L.; Hammill, M.O.; Savenkoff, C. The trophic role of marine mammals in the northern Gulf of ST. Lawrence. Mar. Mamm. Sci. 2006, 22, 74–103. [Google Scholar] [CrossRef]
- Lindeman, R.L. The trophic-dynamic aspect of ecology. Ecology 1942, 23, 399–417. [Google Scholar] [CrossRef]
- Li, Y.K.; Chen, Y.; Song, B.; Olson, D.; Yu, N.; Chen, L.Q. Ecosystem structure and functioning of Lake Taihu (China) and the impacts of fishing. Fish. Res. 2009, 95, 309–324. [Google Scholar] [CrossRef]
- Christensen, V.; Pauly, D.; The International Center for Living Aquatic Resources Management; Immigration and Customs Enforcement. Trophic Models of Aquatic Ecosystems; International Center for Living Aquatic Resources Management: Penang, Malaysia, 1993.
- Odum, E.P. The strategy of ecosystem development. Science 1969, 164, 262–270. [Google Scholar] [CrossRef]
- Odum, E.P. Fundamental of Ecology; Saunders: Philadelphia, PA, USA, 1971. [Google Scholar]
- Christensen, V. Ecosystem maturity—Towards quantification. Ecol. Model. 1995, 77, 3–32. [Google Scholar] [CrossRef]
- Finn, J.T. Measures of ecosystem structure and function derived from analysis of flows. J. Theor. Biol. 1976, 56, 363–380. [Google Scholar] [CrossRef]
- Findlay, S.E.G.; Nieder, W.C.; Blair, E.A.; Fischer, D.T. Multi-scale controls on water quality effects of submerged aquatic vegetation in the tidal freshwater Hudson River. Ecosystems 2006, 9, 84–96. [Google Scholar] [CrossRef]
- Meerhoff, M.; Clemente, J.; Teixeira de Mello, F.; Iglesias, C.; Pedersen, A.; Jeppesen, E. Can warm climate-related structure of littoral predator assemblies weaken clear water state in shallow lakes? Glob. Chang. Biol. 2007, 13, 1888–1897. [Google Scholar] [CrossRef]
- Yamaki, A.; Yamamuro, M. Floating-leaved and emergent vegetation as habitat for fishes in a eutrophic temperate lake without submerged vegetation. Limnology 2013, 14, 257–268. [Google Scholar] [CrossRef]
- Qiu, D.R.; Wu, Z.B.; Liu, B.Y.; Deng, J.Q.; Fu, G.P.; He, F. The restoration of aquatic macrophytes for improving water quality in a hypertrophic shallow lake in Hubei Province, China. Ecol. Eng. 2001, 18, 147–156. [Google Scholar] [CrossRef]
- Webster, J.R.; Benfield, E.F. Vascular plant breakdown in freshwater ecosystems. Annu. Rev. Ecol. Syst. 1986, 17, 567–594. [Google Scholar] [CrossRef]
- Environmental Protection Agency Office of Water Planning and Standards. Lake Restoration, Proceedings of the National Conference, Minneapolis, MN, USA, 22–24 August 1978; U.S. Environmental Protection Agency, Office of Water Planning and Standards: Minneapolis, USA, 1979.
- Dall Armellina, A.A.; Bezic, C.R.; Gajardo, O.A. Submerged macrophyte control with herbivorous fish in irrigation channels of semiarid Argentina. Hydrobiologia 1999, 415, 265–269. [Google Scholar] [CrossRef]
- Colle, D.E.; Shireman, J.V. Coefficients of condition for largemouth bass, bluegill, and redear sunfish in hydrilla-infested lakes. Trans. Am. Fish. Soc. 1980, 109, 521–531. [Google Scholar] [CrossRef]
- Polis, G.A.; Power, M.E.; Huxel, G.R. Food Webs at the Landscape Level; University of Chicago Press: Chicago, IL, USA, 2004. [Google Scholar]
- Shapiro, J.; Lamarra, V.; Lynch, M. Biomanipulation: An ecosystem approach to lake restoration. In Water Quality Management through Biological Control; Brezonik, P.L., Fox, J.L., Eds.; University of Florida: Gainesville, FL, USA, 2013. [Google Scholar]
- Carey, M.P.; Wahl, D.H. Fish diversity as a determinant of ecosystem properties across multiple trophic levels. Oikos 2011, 120, 84–94. [Google Scholar] [CrossRef]
- Hargrave, C.W. Effects of fish species richness and assemblage composition on stream ecosystem function. Ecol. Freshw. Fish. 2009, 18, 24–32. [Google Scholar] [CrossRef]
- Allan, J.D.; Abell, R.; Hogan, Z.; Revenga, C.; Taylor, B.W.; Welcomme, R.L.; Winemiller, K. Overfishing of inland waters. Bioscience 2005, 55, 1041–1051. [Google Scholar] [CrossRef]
- Myers, R.A.; Rosenberg, A.A.; Mace, P.M.; Barrowman, N.; Restrepo, V.R. In search of thresholds for recruitment overfishing. ICES J. Mar. Sci. 1994, 51, 191–205. [Google Scholar] [CrossRef]
- Gardner, M.R.; Ashby, W.R. Connectance of large dynamic (cybernetic) systems: Critical values for stability. Nature 1970, 228, 784. [Google Scholar] [CrossRef] [PubMed]
- Gann, G.D.; McDonald, T.; Walder, B.; Aronson, J.; Nelson, C.R.; Jonson, J.; Hallett, J.G.; Eisenberg, C.; Guariguata, M.R.; Liu, J.; et al. International principles and standards for the practice of ecological restoration, 2nd Ed. Restor. Ecol. 2019, 27, S1–S46. [Google Scholar] [CrossRef]
- Carignan, R.; Kalff, J. Phosphorus release by submerged macrophytes: Significance to epiphyton and phytoplankton. Limnol. Oceanogr. 1982, 27, 419–427. [Google Scholar] [CrossRef]
- Landers, D.H. Effects of naturally senescing aquatic macrophytes on nutrient chemistry and chlorophyll a of surrounding waters. Limnol. Oceanogr. 1982, 27, 428–439. [Google Scholar] [CrossRef]


| Parameters | Unit | Average | Maximum | Minimum | Standard Deviation |
|---|---|---|---|---|---|
| Chlorophyll a | mg/L | 0.014 | 0.042 | 0.001 | 0.014 |
| Total phosphorus | mg/L | 0.121 | 0.184 | 0.078 | 0.034 |
| Total nitrogen | mg/L | 0.98 | 1.75 | 0.41 | 0.44 |
| Secchi depth | cm | 39 | 86 | 11 | 19 |
| Chemical oxygen demand (Mn) | mg/L | 5.3 | 6.5 | 4.3 | 0.6 |
| Groups | Trophic Level | Biomass (t/km2) | P/B (year−1) | Q/B (year−1) | EE | P/Q |
|---|---|---|---|---|---|---|
| Carnivorous fish | 3.232 | 0.040 | 1.020 | 4.156 | 0.132 | 0.245 |
| Filter-feeding fish | 2.941 | 0.054 | 1.440 | 11.47 | 0.167 | 0.126 |
| Omnivorous fish | 2.452 | 0.112 | 1.800 | 12.88 | 0.151 | 0.140 |
| Small fish | 2.422 | 0.040 | 2.300 | 9.408 | 0.211 | 0.244 |
| Herbivorous fish | 2.171 | 0.010 | 1.550 | 15.31 | 0.576 | 0.101 |
| Zooplankton | 2.004 | 12.98 | 31.69 | 120.0 | 0.019 | 0.264 |
| Molluscs | 2.000 | 162.6 | 8.830 | 25.00 | 0.000 | 0.353 |
| Phytoplankton | 1.000 | 2.245 | 185 | 0.848 | ||
| Submerged macrophyte | 1.000 | 4378 | 1.25 | 0.082 | ||
| Detritus | 1.000 | 10.24 | 0.492 |
| Trophic Level | Total Throughput | Throughput from Primary Producers | Throughput from Detritus | Biomass |
|---|---|---|---|---|
| IV | 0.0345 | 0.00659 | 0.0279 | 0.00813 |
| III | 1.539 | 0.302 | 1.237 | 0.151 |
| II | 5616 | 799.7 | 4817 | 175.6 |
| I | 15,674 | 5888 | 9786 | 4380 |
| Sum | 21,292 | 6688 | 14604 | 4556 |
| Source | Trophic Level | ||
|---|---|---|---|
| II | III | IV | |
| Primary producer | 0.0378 | 2.265 | 0.388 |
| Detritus | 0.0257 | 0.332 | 0.379 |
| All flows | 0.0274 | 2.319 | 0.381 |
| From primary producers | 0.321 | ||
| From detritus | 0.283 | ||
| Total | 0.289 | ||
| Parameters | Value | Units |
|---|---|---|
| Sum of all consumption | 5624.34 | t·km−2·a−1 |
| Sum of all exports | 4969.63 | t·km−2·a−1 |
| Sum of all respiratory flows | 918.42 | t·km−2·a−1 |
| Sum of all flows into detritus | 9786.15 | t·km−2·a−1 |
| Total system throughput | 21,298.54 | t·km−2·a−1 |
| Sum of all production | 7735.19 | t·km−2·a−1 |
| Calculated total net primary production | 5888.05 | t·km−2·a−1 |
| Total primary production/total respiration (TPP/TR) | 6.411 | - |
| Net system production | 4969.63 | t·km−2·a−1 |
| Total primary production/total biomass (TPP/TB) | 1.292 | - |
| Total biomass (excluding detritus) | 4556.28 | t·km−2·a−1 |
| Total transfer efficiency (Total TE) | 0.289 | % |
| Connectance Index (CI) | 0.325 | - |
| System Omnivory Index (SOI) | 0.038 | - |
| Finn’s Cycling Index (FCI) | 28.84 | % of total throughput |
| Finn’s Mean Path Length (FMPL) | 3.617 | - |
| Ecopath pedigree index | 0.459 | - |
| Parameters | Before the Management Simulation | After the Management Simulation | Units |
|---|---|---|---|
| Total primary production/total respiration (TPP/TR) | 6.411 | 1.401 | - |
| Total primary production/total biomass (TPP/TB) | 1.292 | 1.410 | - |
| Total transfer efficiency (Total TE) | 0.289 | 3.515 | % |
| Connectance Index (CI) | 0.325 | 0.323 | - |
| System Omnivory Index (SOI) | 0.038 | 0.121 | - |
| Finn’s Cycling Index (FCI) | 26.84 | 59.19 | % of total throughput |
| Finn’s Mean Path Length (FMPL) | 3.617 | 8.427 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, K.; Wu, Y.; Li, C.; Xu, J.; Zhang, M. Ecosystem-Based Restoration to Mitigate Eutrophication: A Case Study in a Shallow Lake. Water 2020, 12, 2141. https://doi.org/10.3390/w12082141
Zhu K, Wu Y, Li C, Xu J, Zhang M. Ecosystem-Based Restoration to Mitigate Eutrophication: A Case Study in a Shallow Lake. Water. 2020; 12(8):2141. https://doi.org/10.3390/w12082141
Chicago/Turabian StyleZhu, Konghao, Yao Wu, Chunhua Li, Jun Xu, and Min Zhang. 2020. "Ecosystem-Based Restoration to Mitigate Eutrophication: A Case Study in a Shallow Lake" Water 12, no. 8: 2141. https://doi.org/10.3390/w12082141
APA StyleZhu, K., Wu, Y., Li, C., Xu, J., & Zhang, M. (2020). Ecosystem-Based Restoration to Mitigate Eutrophication: A Case Study in a Shallow Lake. Water, 12(8), 2141. https://doi.org/10.3390/w12082141





