Adsorption Mechanisms and Characteristics of Hg2+ Removal by Different Fractions of Biochar
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Biochar Fractions
2.2. Physicochemical Properties of Biochar Fractions
2.3. Determination of Oxygen-Containing Functional Groups on Biochar Surface
2.4. Adsorption Experiment and Characterization before and after Hg2+ Adsorption
3. Results and Discussion
3.1. Physicochemical Properties of Biochar and Analysis of Surface Functional Groups
3.2. FTIR Analysis of Biochar Fractions before and after Hg2+ Adsorption
3.3. XPS Analysis of Biochar Fractions before and after Hg2+ Adsorption
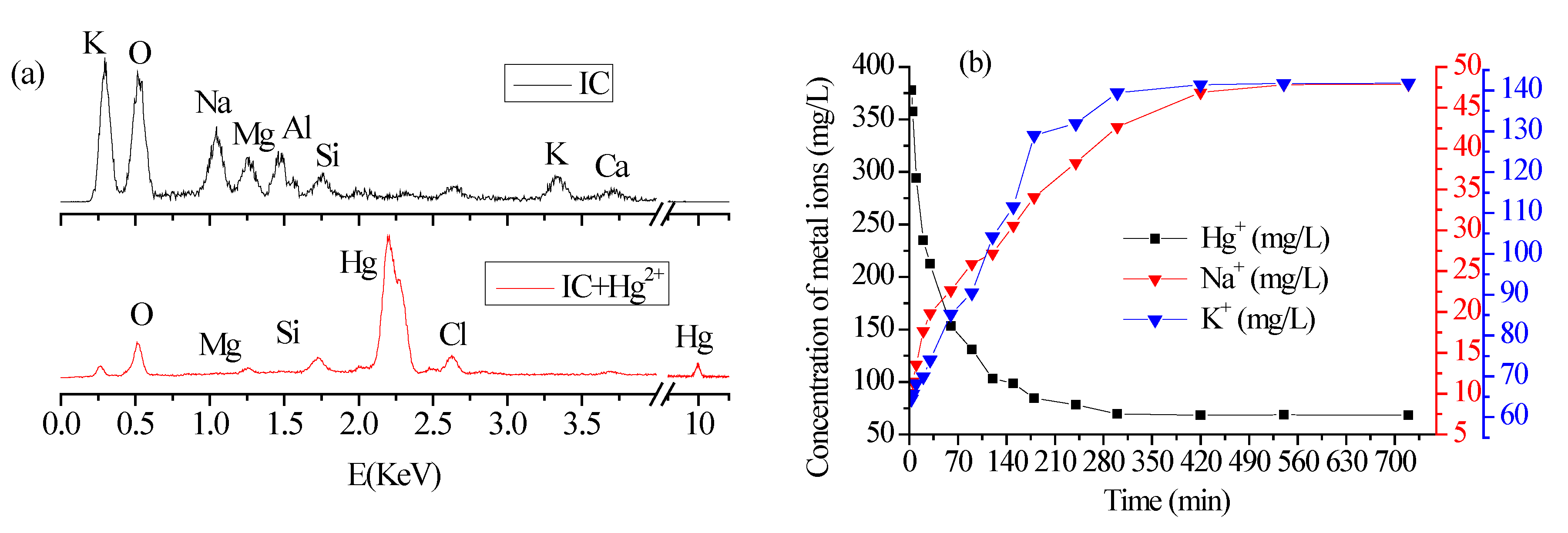
3.4. EDS Analysis of Biochar Fractions before and after Hg2+ Adsorption and Leaching Experiments
3.5. Adsorption Mechanisms
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lyu, H.H.; Xia, S.Y.; Tang, J.C.; Zhang, Y.R.; Cao, B.; Shen, B.X. Thiol-Modified biochar synthesized by a facile ball-milling method for enhanced sorption of inorganic Hg2+ and organic CH3Hg+. J. Haz. Mater. 2019, 384, 121357. [Google Scholar] [CrossRef] [PubMed]
- Inbaraj, B.S.; Wang, J.; Lu, J.; Siao, F.; Chen, B. Adsorption of toxic mercury(II) by an extracellular biopolymer poly(γ-glutamic acid). Bioresour. Technol. 2009, 100, 200–207. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Peng, S.C.; Lu, P.P.; Chen, T.H.; Yang, Y. Mercury Removal from Aqueous Solutions Using Modified Pyrite: A Column Experiment. Minerals 2019, 10, 43. [Google Scholar] [CrossRef]
- Mahmudov, R.; Huang, C.P. Selective adsorption of oxyanions on activated carbon exemplified by Filtrasorb 400 (F400). Sep. Purif. Technol. 2011, 77, 294–300. [Google Scholar] [CrossRef]
- Wang, T.; Wu, J.W.; Zhang, Y.S.; Liu, J.; Sui, Z.F.; Zhang, H.C.; Chen, W.Y.; Norris, P.; Pan, W.P. Increasing the chlorine active sites in the micropores of biochar for improved mercury adsorption. Fuel 2018, 229, 60–67. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Yang, W.; Xu, W.; Liu, Y.X. Removal of elemental mercury by bio-chars derived from seaweed impregnated with potassium iodine. Environ. Sci. Health 2018, 339, 468–478. [Google Scholar] [CrossRef]
- Xu, Y.L. Thermodynamic Properties of Biochar Preparation and Sorption Characteristics and Mechanisms of Cadmium onto Biochars; Zhejiang University: Hangzhou, China, 2013. [Google Scholar]
- Zabihi, M.; Ahmadpour, A.; Haghighi, A.A. Removal of mercury from water by carbonaceous sorbents derived from walnut shell. J. Hazard. Mater. 2008, 167, 230–236. [Google Scholar] [CrossRef]
- Cox, M.; El-Shafey, E.I.; Pichugin, A.A.; Appleton, Q. Removal of mercury (II) from aqueous solution on a carbonaceous sorbent prepared from flax shive. J. Chem. Technol. Biotechnol. 2000, 75, 427–435. [Google Scholar] [CrossRef]
- Minaxi, B.; Lohani Amarika, S.; Rupainwar, D.C.; Dhar, D.N. Studies on efficiency of guava (Psidium guajava) bark as bioadsorbent for removal of Hg(II) from aqueous solutions. J. Hazard. Mater. 2008, 159, 626–629. [Google Scholar] [CrossRef]
- Stephen Inbaraj, B.; Sulochana, N. Mercury adsorption on a carbon sorbent derived from fruit shell of Terminalia catappa. J. Hazard. Mater. 2005, 133, 283–290. [Google Scholar] [CrossRef]
- El-Shafey, E.I. Removal of Zn(II) and Hg(II) from aqueous solution on a carbonaceous sorbent chemically prepared from rice husk. J. Hazard. Mater. 2009, 175, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Khoramzadeh, E.; Nasernejad, B.; Halladj, R. Mercury biosorption from aqueous solutions by Sugarcane Bagasse. J. Taiwan Inst. Chem. Eng. 2013, 44, 266–269. [Google Scholar] [CrossRef]
- Johari, K.; Saman, N.; Song, S.T.; Chin, C.S.; Kong, H.; Mat, H. Adsorption enhancement of elemental mercury by various surface modified coconut husk as eco-friendly low-cost adsorbents. Int. Biodeter. Biodegr. 2016, 109, 45–52. [Google Scholar] [CrossRef]
- Xue, Y.W.; Gao, B.; Yao, Y.; Inyang, M.D.; Zhang, M.; Zimmerman, A.R.; Ro, K.S. Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueous heavy metals: Batch and column tests. Chem. Eng. J. 2012, 200, 673–680. [Google Scholar] [CrossRef]
- Li, H.B.; Dong, X.L.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Kong, H.; He, J.; Gao, Y.; Wu, H.; Zhu, X. Cosorption of phenanthrene and mercury(II) from aqueous solution by soybean stalk-based biochar. J. Agric. Food. Chem. 2011, 59, 12116–12123. [Google Scholar] [CrossRef]
- Das, S.K.; Das, A.R.; Guha, A.K. A study on the adsorption mechanism of mercury on Aspergillus versicolor biomass. Environ. Sci. Technol. 2007, 41, 8281–8287. [Google Scholar] [CrossRef]
- Kílıç, M.; Keskin, M.E.; Mazlum, S.; Mazlum, N. Hg(II) and Pb(II) adsorption on activated sludge biomass: Effective biosorption mechanism. Int. J. Miner. Process. 2008, 87, 1–8. [Google Scholar] [CrossRef]
- Dong, X.L.; Ma, L.Q.; Zhu, Y.J.; Li, Y.C.; Gu, B. Mechanistic investigation of mercury sorption by Brazilian pepper biochars of different pyrolytic temperatures based on X-ray photoelectron spectroscopy and flow calorimetry. Environ. Sci. Technol. 2013, 47, 12156–12164. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; Becker-Hapak, M.K.; Hosea, J.M.; Darnall, D.W. Effect of chemical modification of algal carboxyl groups on metal ion binding. Environ. Sci. Technol. 1990, 24, 1372–1378. [Google Scholar] [CrossRef]
- Chen, J.P.; Yang, L. Study of a heavy metal biosorption onto raw and chemically modified Sargassum sp. via spectroscopic and modeling analysis. Langmuir 2006, 22, 8906–8914. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.L.; Zhang, W.H.; Yang, Y.X.; Huang, X.; Wang, S.; Qiu, R. Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res. 2012, 46, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Liu, G.C.; Zheng, H.; Li, F.M.; Ngo, H.H.; Guo, W.S.; Liu, C.; Chen, L.; Xing, B.S. Investigating the mechanisms of biochar’s removal of lead from solution. Bioresour. Technol. 2015, 177, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Ofomaja, A.E.; Naidoo, E.B.; Modise, S.J. Removal of copper(II) from aqueous solution by pine and base modified pine cone powder as biosorbent. J. Hazard. Mater. 2009, 168, 909–917. [Google Scholar] [CrossRef]
- Boehm, H.P. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Goertzen, S.L.; Thériault, K.D.; Oickle, A.M.; Tarasuk, A.C.; Andreas, H.A. Standardization of the Boehm titration. Part, I. CO2 expulsion and endpoint determination. Carbon 2010, 48, 1252–1261. [Google Scholar] [CrossRef]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, A.; Se, S.M.; Dimin, M.F.; Juoi, J.M.; Mohd Husin, M.H.; Mitan, N.M.M. Influence of heating temperature and holding time on biochars derived from rubber wood sawdust via slow pyrolysis. J. Anal. Appl. Pyrol. 2014, 107, 31–39. [Google Scholar] [CrossRef]
- Sari, A.; Tuzen, M. Removal of mercury (II) from aqueous solution using moss (Drepanocladus revolvens) biomass: Equilibrium, thermodynamic and kinetic studies. J. Hazard. Mater. 2009, 171, 500–507. [Google Scholar] [CrossRef]
- Jia, M.Y.; Wang, F.; Bian, Y.R.; Jin, X.; Song, Y.; Kengara, F.O.; Xu, R.K.; Jiang, X. Effects of pH and metal ions on oxytetracycline sorption to maize-straw-derived biochar. Bioresour. Technol. 2013, 136, 87–93. [Google Scholar] [CrossRef]
- Tüzün, İ.; Bayramoğlu, G.; Yalçın, E.; Başaran, G.; Celik, G.; Arica, M.Y. Equilibrium and kinetic studies on biosorption of Hg(II), Cd(II) and Pb(II) ions onto microalgae Chlamydomonas reinhardtii. J. Environ. Manag. 2005, 77, 85–92. [Google Scholar] [CrossRef]
- Walcarius, A.; Delacôte, C. Mercury(II) binding to thiol-functionalized mesoporoussilicas: Critical effect of pH and sorbent properties on capacity and selectivity. Anal. Chim. Acta 2005, 547, 3–13. [Google Scholar] [CrossRef]
- Thy, P.; Jenkins, B.M.; Grundvig, S.; Shiraki, R.; Lesher, C. High temperature elemental losses and mineralogical changes in common biomass ashes. Fuel 2006, 85, 783–795. [Google Scholar] [CrossRef]
- Yardim, M.F.; Budinova, T.; Ekinci, E.; Petrov, N.; Razvigorova, M.; Minkova, V. Removal of mercury(II) from aqueous solution by activated carbon obtained from furfural. Chemosphere 2003, 52, 835–841. [Google Scholar] [CrossRef]
- Dougherty, D.A. The cation -π interaction. Acc. Chem. Res. 2012, 46, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Yang, G.D.; Zeng, G.M.; Cai, Y.; Li, S.S.; Zhou, Y.Y.; Pang, Y.; Liu, Y.Y.; Zhang, Y.; Luna, B. Synergistic effect of iron doped ordered mesoporous carbon on adsorption-coupled reduction of hexavalent chromium and the relative mechanism study. Chem. Eng. J. 2014, 239, 114–122. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Y.; Wang, H.; Lu, W.; Zhou, Z.; Zhang, Y.; Ren, L. Influence of pyrolysis temperature on characteristics and heavy metal adsorptive performance of biochar derived from municipal sewage sludge. Bioresour. Technol. 2014, 164, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Lammers, K.; Arbuckle-Keil, G.; Dighton, J. FT-IR study of the changes in carbohydrate chemistry of three New Jersey pine barrens leaf litters during simulated control burning. Soil Biol. Biochem. 2009, 41, 340–347. [Google Scholar] [CrossRef]
- Herrero, R.; Lodeiro, P.; Rey-Castro, C.; Vilariño, T.; Sastre de Vicente, M.E. Removal of inorganic mercury from aqueous solutions by biomass of the marine macroalga Cystoseira baccata. Water Res. 2005, 39, 3199–3210. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Ju, M.T.; Liu, L.; Zheng, K. Mercury adsorption to aged biochar and its management in China. Environ. Sci. Pollut. Res. 2019, 26, 4867–4877. [Google Scholar] [CrossRef]
- Goyal, M.; Bhagat, M.; Dhawan, R. Removal of mercury from water by fixed bed activated carbon columns. J. Hazard. Mater. 2009, 171, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Carro, L.; Anagnostopoulos, V.; Lodeiro, P.; Barriada, J.L.; Herrero, R.; Sastre de Vicente, M.E. A dynamic proof of mercury elimination from solution through a combined sorption-reduction process. Bioresour. Technol. 2010, 101, 8969–8974. [Google Scholar] [CrossRef] [PubMed]
- Pulido-Novicio, L.; Kurimoto, Y.; Aoyama, M.; Seki, K.; Doi, S.; Hata, T.; Ishihara, S.; Imamura, Y. Adsorption of mercury by sugi wood carbonized at 1000 °C. J. Wood Sci. 2001, 47, 159–162. [Google Scholar] [CrossRef]
- Park, J.H.; Wang, J.J.; Zhou, B.; Mikhael, J.E.R.; DeLaune, R.D. Removing mercury from aqueous solution using sulfurized biochar and associated mechanisms. Environ. Pollut. 2018, 244, 627–635. [Google Scholar] [CrossRef]
- Bond, A.M.; Miao, W.; Raston, C.L. Mercury(II) immobilized on carbon nanotubes: Synthesis, characterization, and redox properties. Langmuir 2000, 16, 6004–6012. [Google Scholar] [CrossRef]
- Behra, P.; Bonnissel-Gissinger, P.; Alnot, M.; Revel, R.; Ehrhardt, J.J. XPS and XAS study of the sorption of Hg(II) onto pyrite. Langmuir 2001, 17, 3970–3979. [Google Scholar] [CrossRef]
- Manivannan, S.; Park, S.; Jeong, J.; Kim, K. Aggregation-Free optical and colorimetric detection of Hg(II) with M13 bacteriophage-templated Au nanowires. Biosens. Bioelectron. 2020, 161, 112237. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Deng, B.L.; Chen, H.; Wang, X.R.; Zheng, J.Z. Removal of aqueous Hg(II) by polyaniline: Sorption characteristics and mechanisms. Environ. Sci. Technol. 2009, 43, 5223–5228. [Google Scholar] [CrossRef]
- Hyland, M.M.; Jean, G.E.; Bancroft, G.M. XPS and AES studies of Hg(II) sorption and desorption reactions on sulphide minerals. Pergamon 1990, 54, 1957–1967. [Google Scholar] [CrossRef]
- Xu, X.Y.; Ariette, S.; Xu, N.; Cao, X.D. Comparison of the characteristics and mechanisms of Hg(II) sorption by biochars and activated carbon. J. Colloid Interf. Sci. 2016, 463, 55–60. [Google Scholar] [CrossRef]
- Carro, L.; Barriada, J.L.; Herrero, R.; Sastre de Vicente, M.E. Adsorptive behavior of mercury on algal biomass: Competition with divalent cations and organic compounds. J. Hazard. Mater. 2011, 192, 284–291. [Google Scholar] [CrossRef] [PubMed]


| Biochar | pHpzc | pH | pH after Adsorption | H/C | Carboxyl | Lactone Group | Phenolic Hydroxyl | Acid Functional Groups | Basic Functional Groups |
|---|---|---|---|---|---|---|---|---|---|
| RC | 9.3 | 9.5 | 7.6 | 0.036 | 0.370 | 0.050 | 0.125 | 0.545 | 0.970 |
| IC | 10.4 | 10.9 | 8.6 | 0.314 | 0.105 | 0.210 | 0.080 | 0.395 | 1.390 |
| OC | 3.3 | 3.6 | 3.3 | 0.043 | 0.495 | 0.090 | 0.355 | 0.940 | 0.285 |
| BHC | 8.2 | 8.4 | 6.4 | 0.017 | 0.400 | 0.190 | 0.015 | 0.605 | 0.905 |
| BCC | 8.1 | 8.3 | 6.5 | 0.018 | 0.180 | 0.195 | 0.175 | 0.550 | 0.895 |
| Element | BE(eV) a | PA(%) b of RC | PA(%) b of IC | PA(%) b of OC | PA(%) b of BHC | PA(%) b of BCC | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BS c | AS d | BS | AS | BS | AS | BS | AS | BS | AS | ||
| C1s(C=C) | 284.7 | 54.0% | 49.7% | 55.8% | 51.2% | 50.6% | 52.1% | 61.4% | 55.5% | 60.7% | 52.8% |
| C1s(C–O) | 285.6 | 25.7% | 36.7% | 14.0% | 27.1% | 12.9% | 24.1% | 5.7% | 25.4% | 7.5% | 23.6% |
| C1s(C=O) | 287.1 | 8.0% | 7.7% | 22.2% | 18.6% | 16.4% | 15.1% | 22.1% | 13.6% | 27.4% | 19.7% |
| C1s(–COO) | 288.8 | 12.3% | 5.9% | 8.0% | 3.1% | 18.1% | 8.6% | 10.8% | 5.5% | 4.5% | 4.0% |
| O1s(C=O) | 531.7 | 25.5% | 33.2% | 41.2% | 38.1% | 27.4% | 37.7% | 51.5% | 38.5% | 53.4% | 37.5% |
| O1s(hydroxyl) | 532.4 | 23.5% | 18.8% | 15.5% | 11.7% | 25.5% | 16.9% | 7.4% | 7.2% | 21.7% | 14.6% |
| O1s(C–O) | 533.6 | 25.2% | 31.7% | 21.3% | 36.9% | 17.0% | 28.3% | 15.1% | 35.1% | 19.8% | 40.9% |
| O1s(COO) | 534.6 | 25.8% | 16.3% | 21.0% | 13.3% | 30.2% | 17.2% | 26.1% | 19.3% | 5.1% | 5.0% |
| Biochars | Mass Percentage (%) | Adsorption Quantity of Hg2+ (mg/g) | Adsorption Mechanism | Adsorption Contribution Rate (%) |
|---|---|---|---|---|
| RC | 100.0 | 75.56 | Complexation with –COOH and –OH | 68.9 |
| Reduction reaction | 25.2 | |||
| IC | 22.4 | 92.63 | Complexation with –COOH and –OH | 39.8 |
| Reduction reaction | 36.4 | |||
| Cation exchange | 41.2 | |||
| OC | 77.6 | 70.57 | Complexation with –COOH and –OH | 71.6 |
| Reduction reaction | 13.0 | |||
| BHC | 87.3 | 66.30 | Complexation with –COOH | 23.3 |
| Reduction reaction | 54.6 | |||
| BCC | 81.3 | 61.13 | Complexation with –OH | 28.3 |
| Reduction reaction | 54.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Li, M.; Liu, A.; Jiang, M.; Niu, X.; Liu, X. Adsorption Mechanisms and Characteristics of Hg2+ Removal by Different Fractions of Biochar. Water 2020, 12, 2105. https://doi.org/10.3390/w12082105
Guo X, Li M, Liu A, Jiang M, Niu X, Liu X. Adsorption Mechanisms and Characteristics of Hg2+ Removal by Different Fractions of Biochar. Water. 2020; 12(8):2105. https://doi.org/10.3390/w12082105
Chicago/Turabian StyleGuo, Xiaoli, Menghong Li, Aijv Liu, Man Jiang, Xiaoyin Niu, and Xinpeng Liu. 2020. "Adsorption Mechanisms and Characteristics of Hg2+ Removal by Different Fractions of Biochar" Water 12, no. 8: 2105. https://doi.org/10.3390/w12082105
APA StyleGuo, X., Li, M., Liu, A., Jiang, M., Niu, X., & Liu, X. (2020). Adsorption Mechanisms and Characteristics of Hg2+ Removal by Different Fractions of Biochar. Water, 12(8), 2105. https://doi.org/10.3390/w12082105




