Effect of Solar Canals on Evaporation, Water Quality, and Power Production: An Optimization Study
Abstract
1. Introduction
2. Site Description
3. Mathematical Modeling
3.1. Evaporation Model
3.2. Solar Energy Model
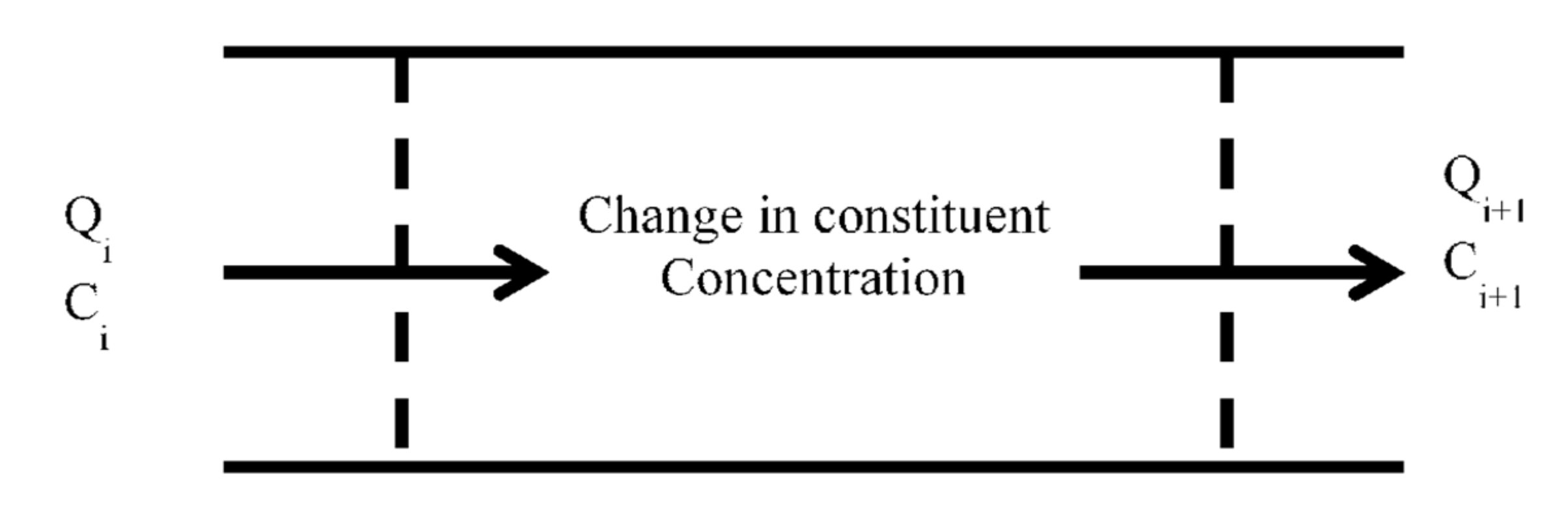
3.3. Water Quality Model
3.3.1. Water Quality Variables
- Dissolved oxygen
- Algae and nutrients
- pH and alkalinity
3.3.2. Initial Concentrations
3.3.3. Water Quality Standards
3.3.4. Model Validation
3.4. Optimization Model
4. Results
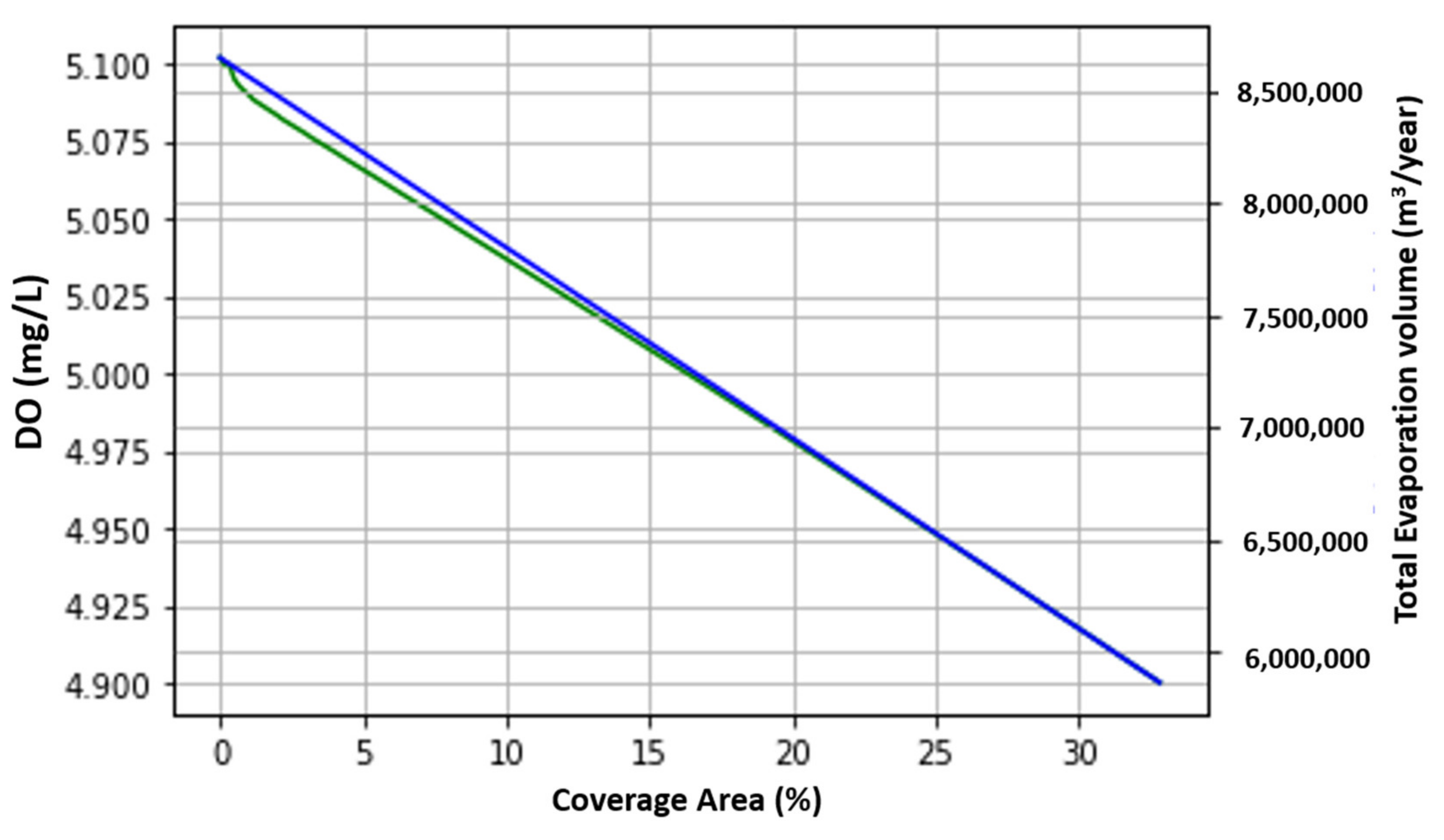
4.1. Evaporation Volume
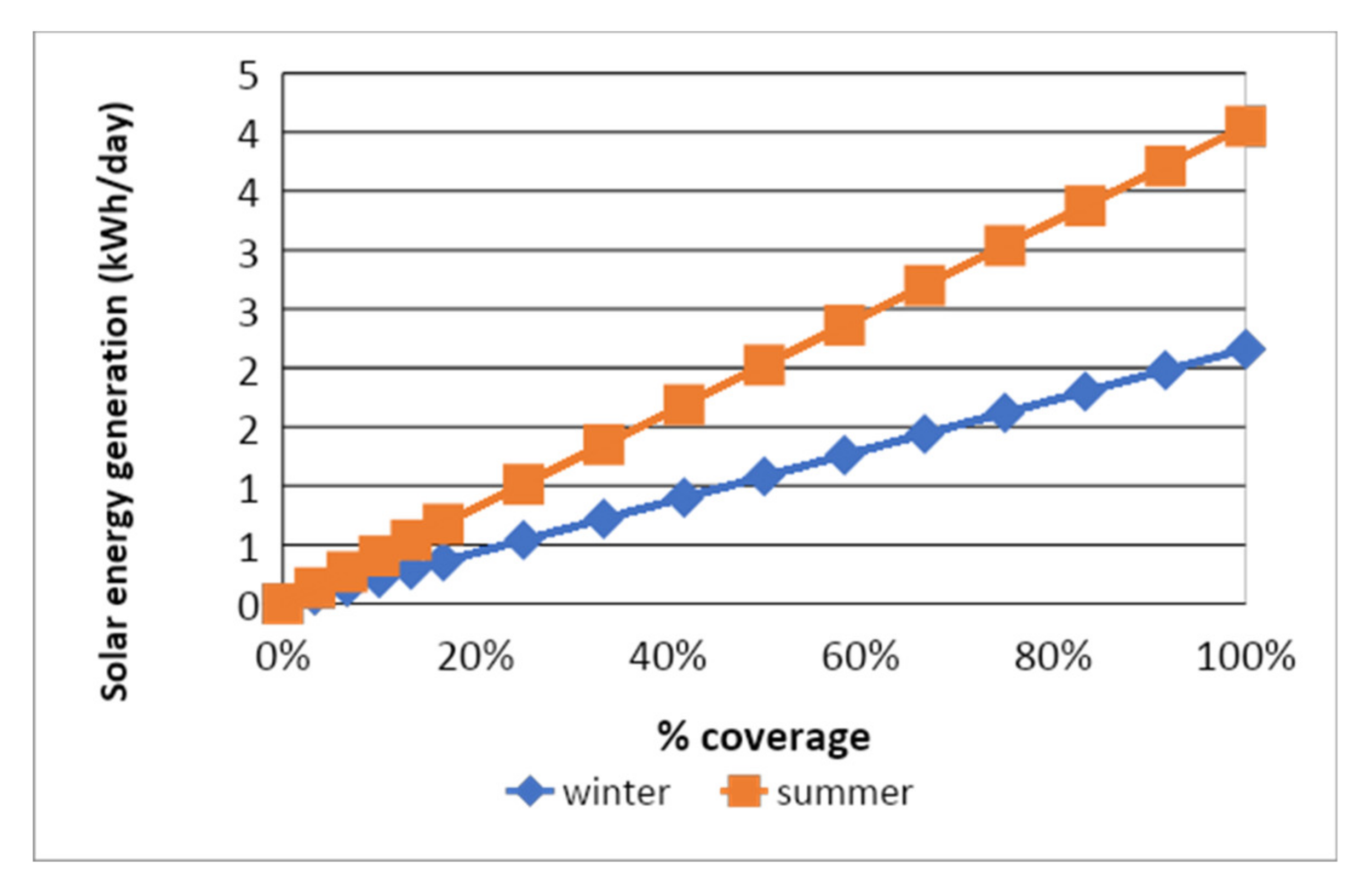
4.2. Solar Energy Generation
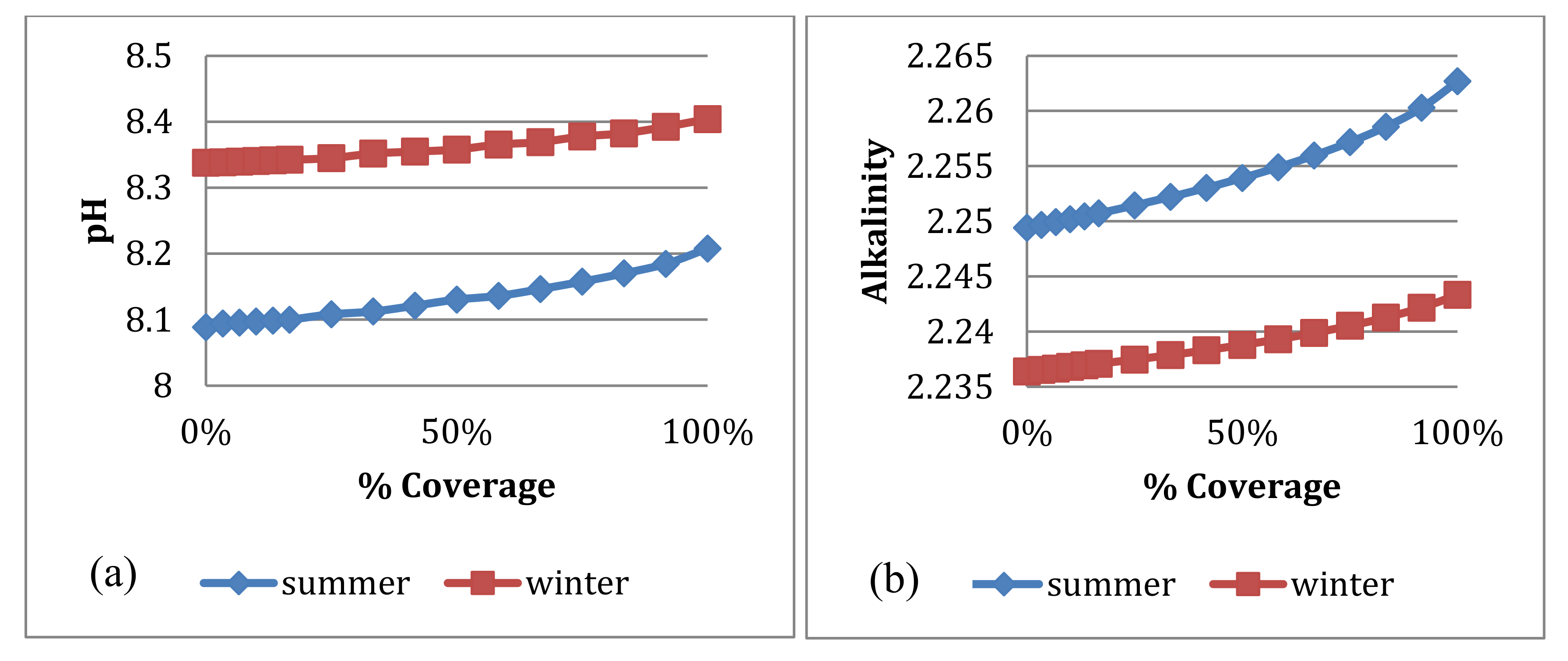
4.3. Water Quality Simulation
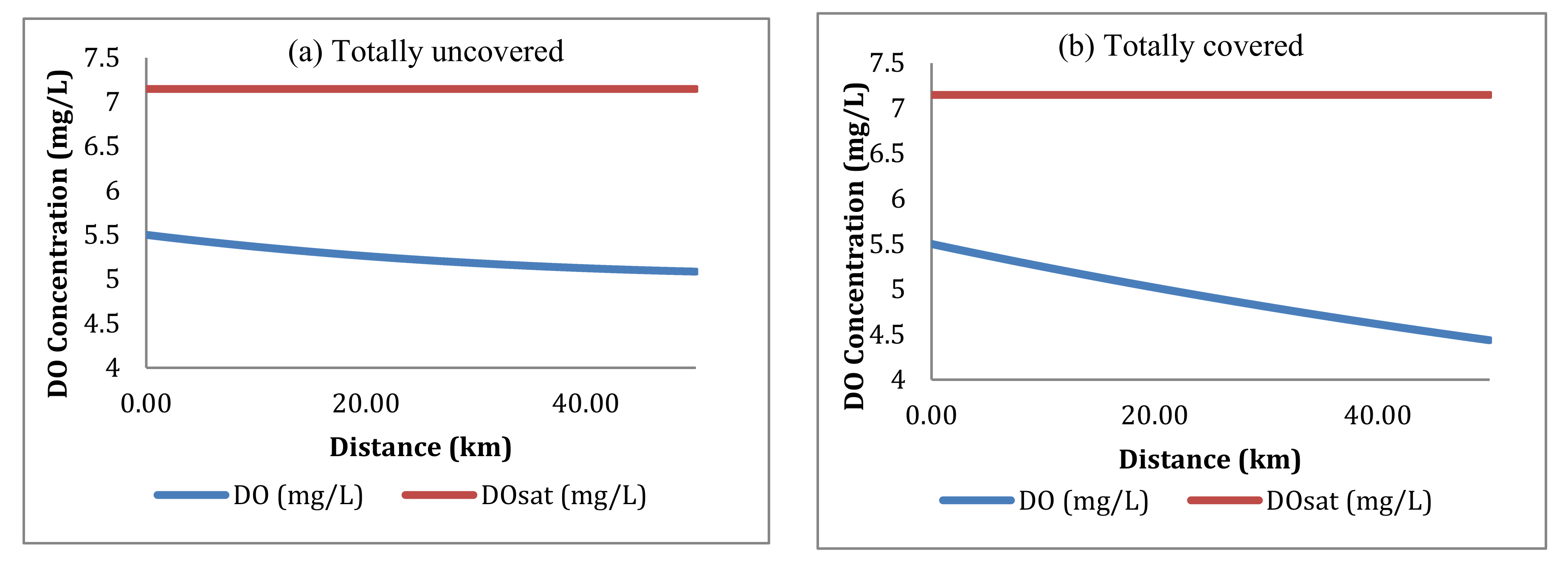
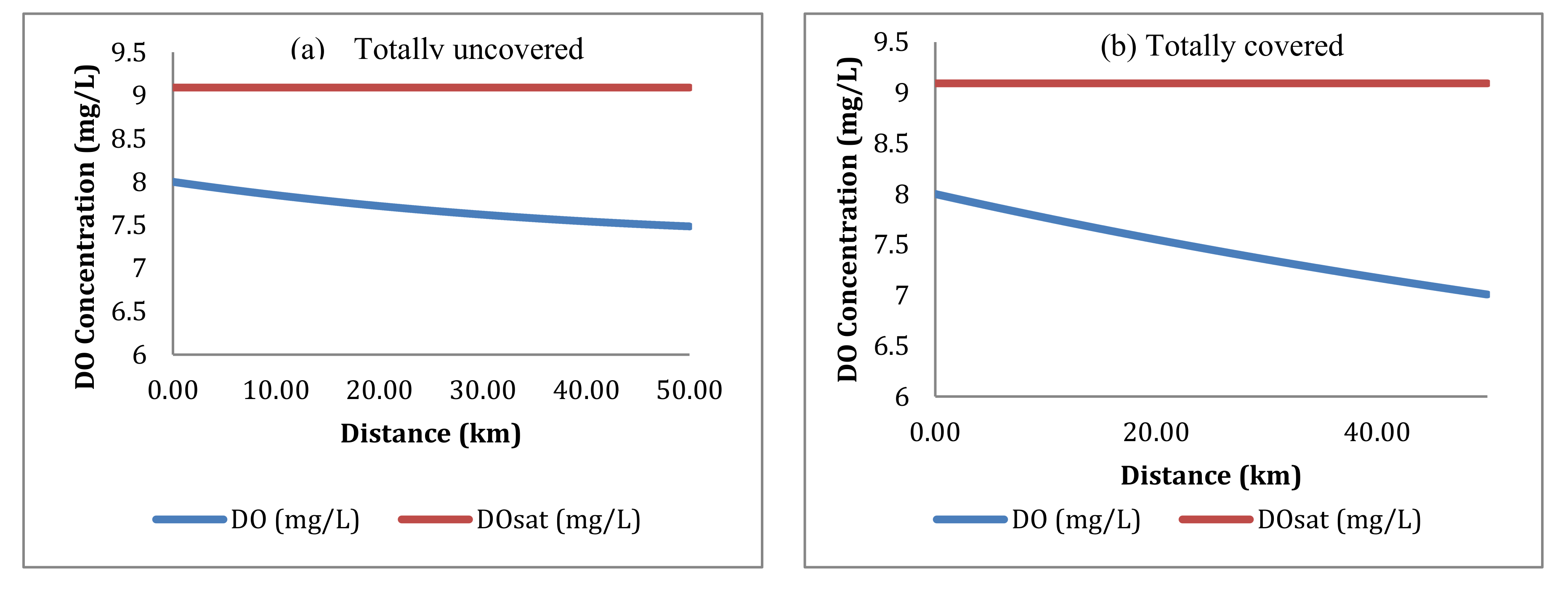
4.3.1. Dissolved Oxygen
4.3.2. Algae and Nutrients
4.3.3. pH and Alkalinity
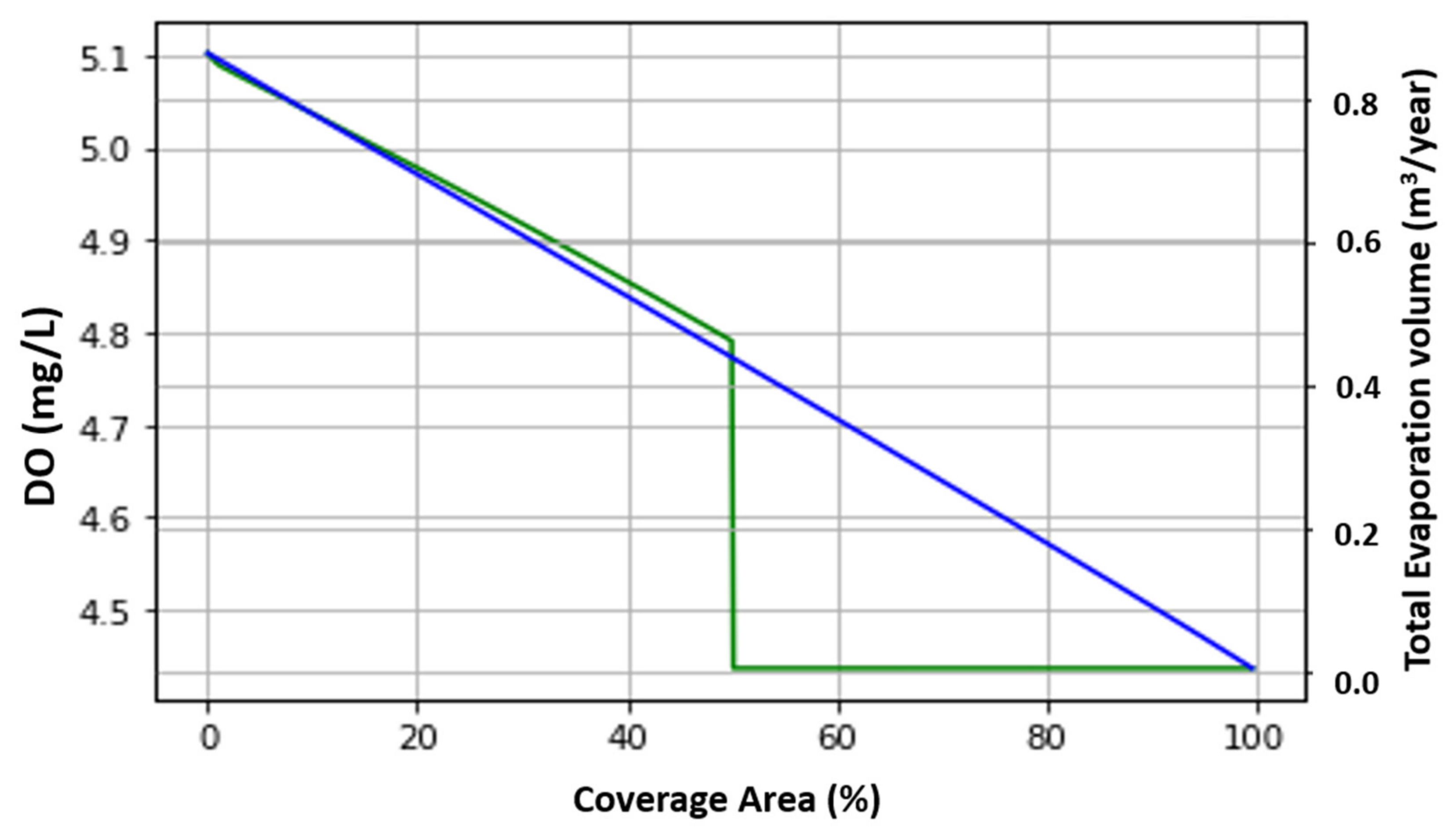
4.4. Optimized Canal Coverage
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IEA. International Energy Agency, World Energy Outlook; IEA: Paris, France, 2010; ISBN 978-92-64-08625-8. Available online: https://webstore.iea.org/world-energy-outlook-2010 (accessed on 10 March 2020).
- Shouman, E.R.; Khattab, N.M. Future economic of concentrating solar power (CSP) for electricity generation in Egypt. Renew. Sustain. Energy Rev. 2015, 41, 1119–1127. [Google Scholar] [CrossRef]
- WWAP. World Water Assessment Programme; UNESCO: Paris, France, 2018; Available online: http://www.unesco.org/new/en/natural-sciences/environment/water/wwap/ (accessed on 10 March 2020).
- Kumar, M.; Kumar, A. Experimental validation of performance and degradation study of canal-top photovoltaic system. Appl. Energy 2018, 243, 102–118. [Google Scholar] [CrossRef]
- Ranjbaran, P.; Yousefi, H.; Gharehpetian, G.B.; Astaraei, F.R. A review on floating photovoltaic (FPV)power generation units. Renew. Sustain. Energy Rev. 2018, 110, 332–347. [Google Scholar] [CrossRef]
- Trapani, K.; Redón Santafé, M. A review of floating photovoltaic installations: 2007–2013. Prog. Photovolt. Res. Appl. 2015, 23, 524–532. [Google Scholar] [CrossRef]
- Colmenar-Santos, A.; Buendia-Esparcia, Á.; de Palacio-Rodríguez, C.; Borge-Diez, D. Water canal use for the implementation and efficiency optimization of photovoltaic facilities: Tajo-Segura transfer scenario. Sol. Energy 2016, 126, 168–194. [Google Scholar] [CrossRef]
- Tina, G.M.; Cazzaniga, R.; Rosa-clot, M.; Rosa-clot, P. Geographic and technical floating. Therm. Sci. 2018, 22, 831–841. [Google Scholar] [CrossRef]
- Ferrer-Gisbert, C.; Ferrán-Gozálvez, J.J.; Redón-Santafé, M.; Ferrer-Gisbert, P.; Sánchez-Romero, F.J.; Torregrosa-Soler, J.B. A new photovoltaic floating cover system for water reservoirs. Renew. Energy 2013, 60, 63–70. [Google Scholar] [CrossRef]
- Santafé, M.; Soler, J.B.; Romero, F.J.; Gisbert, P.S.; Gozálvez, J.J.; Gisbert, C.M. Theoretical and experimental analysis of a floating photovoltaic cover for water irrigation reservoirs. Energy 2014, 67, 246–255. [Google Scholar] [CrossRef]
- El Baradei, S.A.; AlSadeq, M. Optimum coverage of irrigation canals to minimize evaporation and maximize dissolved oxygen concentration: Case study of Toshka. Egypt. Int. J. Environ. Sci. Technol. 2018, 16, 4223–4230. [Google Scholar] [CrossRef]
- Wahby, S.W. The Toshka project of Egypt: A multidisciplinary engineering education case study. In Proceedings of the American Society for Engineering Education Annual Conference & Exposition, Montreal, QC, Canada, 16–19 June 2002. [Google Scholar]
- Valiantzas, J.D. Simplified versions for the Penman evaporation equation using routine weather data. J. Hydrol. 2006, 331, 690–702. [Google Scholar] [CrossRef]
- Penman, H.L. Natural evaporation from open water, bare soil and grass. Proc. R. Soc. London A 1948, 193, 120–145. [Google Scholar] [CrossRef]
- Shuttleworth, J.W. Infiltration and soil water movement. In Handbook of Hydrology; Maidment, D.R., Ed.; McGraw-Hill: New York, NY, USA, 1992; pp. 4.1–4.53. [Google Scholar]
- Dingman, S.L. Physical Hydrology; Prentice Hall: Englewood Cliffs, NJ, USA, 1994; p. 646. [Google Scholar]
- Monteith, J.L. Evaporation and the Environment. In The State and Movement of Water in Living Organisms; Fogg, G.E., Ed.; Cambridge University Press: London, UK, 1965; pp. 205–234. [Google Scholar]
- WUO, Weather Underground Organization. 2018. Available online: www.wunderground.com (accessed on 15 February 2017).
- NASA, National Aeronautics and Space Administration, 2016. Available online: https://power.larc.nasa.gov (accessed on 17 February 2017).
- Egyptian Code for the Reuse of Treated Wastewater in the Agriculture Field; Code number ECP 501; National Research Center: Cairo, Egypt, 2005.
- Stiff, M.J.; Cartwright, N.G.; Crane, R.I. Environmental Quality Standards for Dissolved Oxygen; BS 12 4UD; National Rivers Authority Rivers House Waterside Drive Almonds bury Bristol: Almondsbury, UK, 1992. [Google Scholar]
- Streeter, H.W.; Phelps, E.B. A Study of the Pollution and Natural Purification of the Ohio River, Factors Concerned in the Phenomena of Oxidation and Reaeration; Public Health Bulletin no. 146; U.S. Department of Health, Education and Welfare, Public Health Service: Washington, DC, USA, 1958.
- Zhenli, H. Mechanisms and assessment of water eutrophication. J. Zhejiang Univ. 2008. [Google Scholar] [CrossRef]
- Hussian, A.M.; Napiórkowska-Krzebietke, A.; Toufeek, M.E.; Abd El-Monem, A.M.; Morsi, H.H. Phytoplankton response to changes of physicochemical variables in Lake Nasser. Egypt. J. Elem. 2015, 20, 855–871. [Google Scholar] [CrossRef]
- Chapra, S.C.; Pelletier, G.J. QUAL2K: A Modelling Framework for Simulating River and Stream Water Quality: Documentation and User’s Manual; Tufts University: Medford, MA, USA, 2003. [Google Scholar]
- Rutherford, J.C.; Scarsbrook, M.R.; Broekhuizen, N. Grazer control of stream algae: Modeling temperature and flood effects. J. Environ. Eng. 2000, 126, 331–339. [Google Scholar] [CrossRef][Green Version]
- Michaelis, L.; Menten, M.L. Die Kinetik der Invertinwirkung. Biochem. Z. 1913, 49, 333–369. [Google Scholar]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture, FAO Irrigation and Drainage Paper; 29 Rev. 1; FAO: Rome, Italy, 1994. [Google Scholar]
- Chapra, S.C.; Pelletier, G.J.; Tao, H. QUAL2K: A Modelling Framework for Simulating River and Stream Water Quality; Version 2.11: Documentation and user’s manual; Tufts University: Medford, MA, USA, 2008. [Google Scholar]
- Chapra, S.C. Canale, Numerical Methods for Engineers, 4th ed.; McGraw-Hill: New York, NY, USA, 2002. [Google Scholar]
- Kuhlman, D. A Python Book: Beginning Python. Advanced Python Exercises. 2009. Available online: https://web.archive.org/web/20130513070515/http://cutter.rexx.com/~dkuhlman/python_book_01.txt (accessed on 12 September 2018).
- Appelo, C.A.J. Geochemistry, Groundwater and Pollution; A.A. Balkema: Rotterdam, The Netherlands, 1999. [Google Scholar]
- Toufeek, M.F.; Korium, M.A. Physicochemical characteristics of water quality in Lake Nasser water. National Institute of Oceanography and Fishier, Egypt. Glob. J. Environ. Res. 2009, 46, 141–148. [Google Scholar]
- Chapra, S.C. Surface Water Quality Modelling; McGraw-Hill: New York, NY, USA, 1997; pp. 393–396. ISBN 0-07-011364-5. [Google Scholar]
- Hadgu, L.T.; Nyadawa, M.O.; Mwangi, J.K.; Kibetu, P.M.; Mehari, B.B. Application of Water Quality Model QUAL2K to Model the Dispersion of Pollutants in River Ndarugu, Kenya. Comput. Water Energy Environ. Eng. 2014, 3, 162–169. [Google Scholar] [CrossRef]
- Fang, X.; Jianying Zhang, J.; Yingxu Chen, Y.; Xu, X. QUAL2K Model Used in the Water Quality Assessment of Qiantang River. China Water Environ. Res. 2008, 80, 2125–2133. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.; Willems, P. Sensitivity and uncertainty analysis for river water quality modelling. In Proceedings of the Eleventh International Water Technology Conference, IWTC11, Sharm El-Sheikh, Egypt, 1 December 2007. [Google Scholar] [CrossRef]



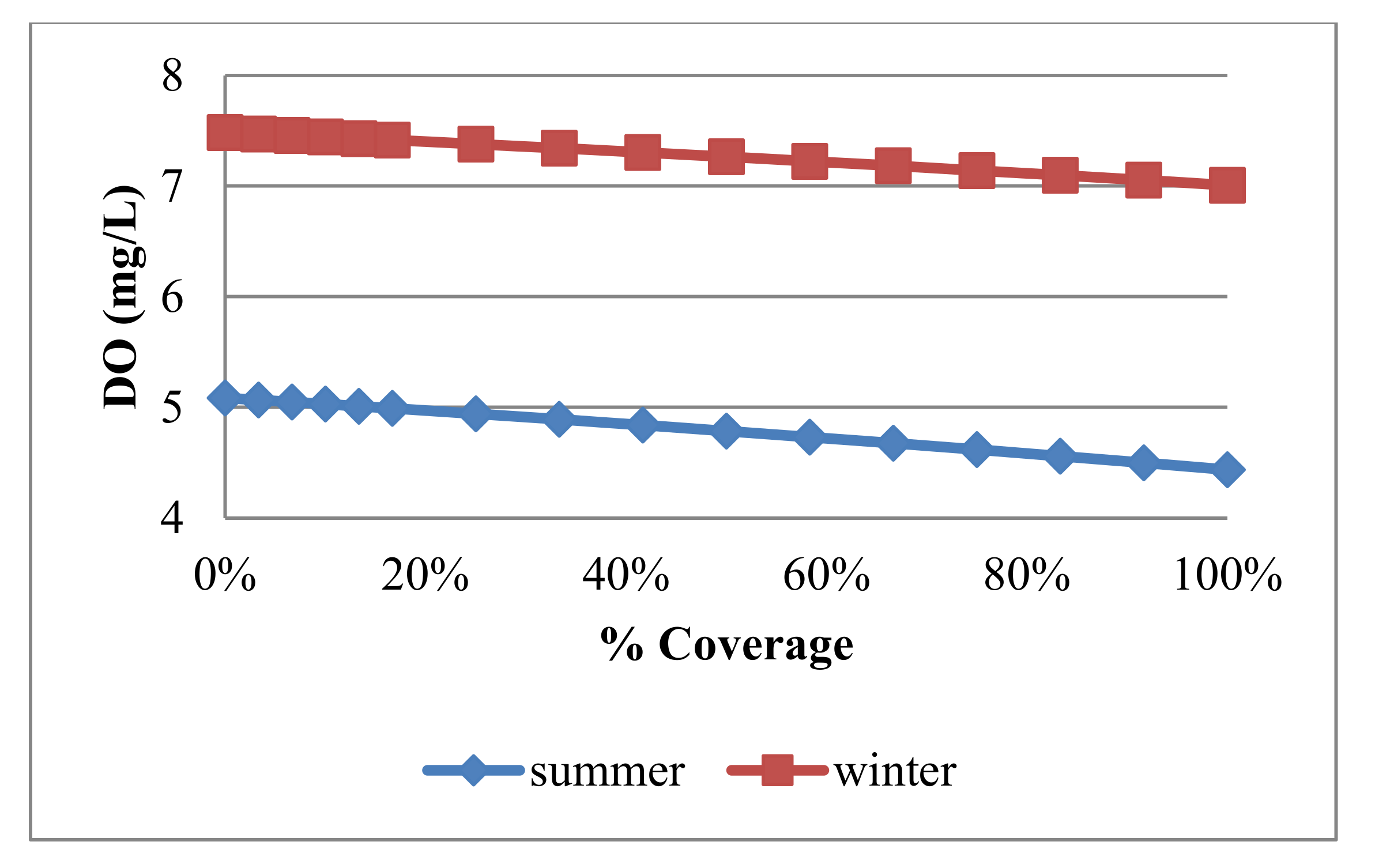
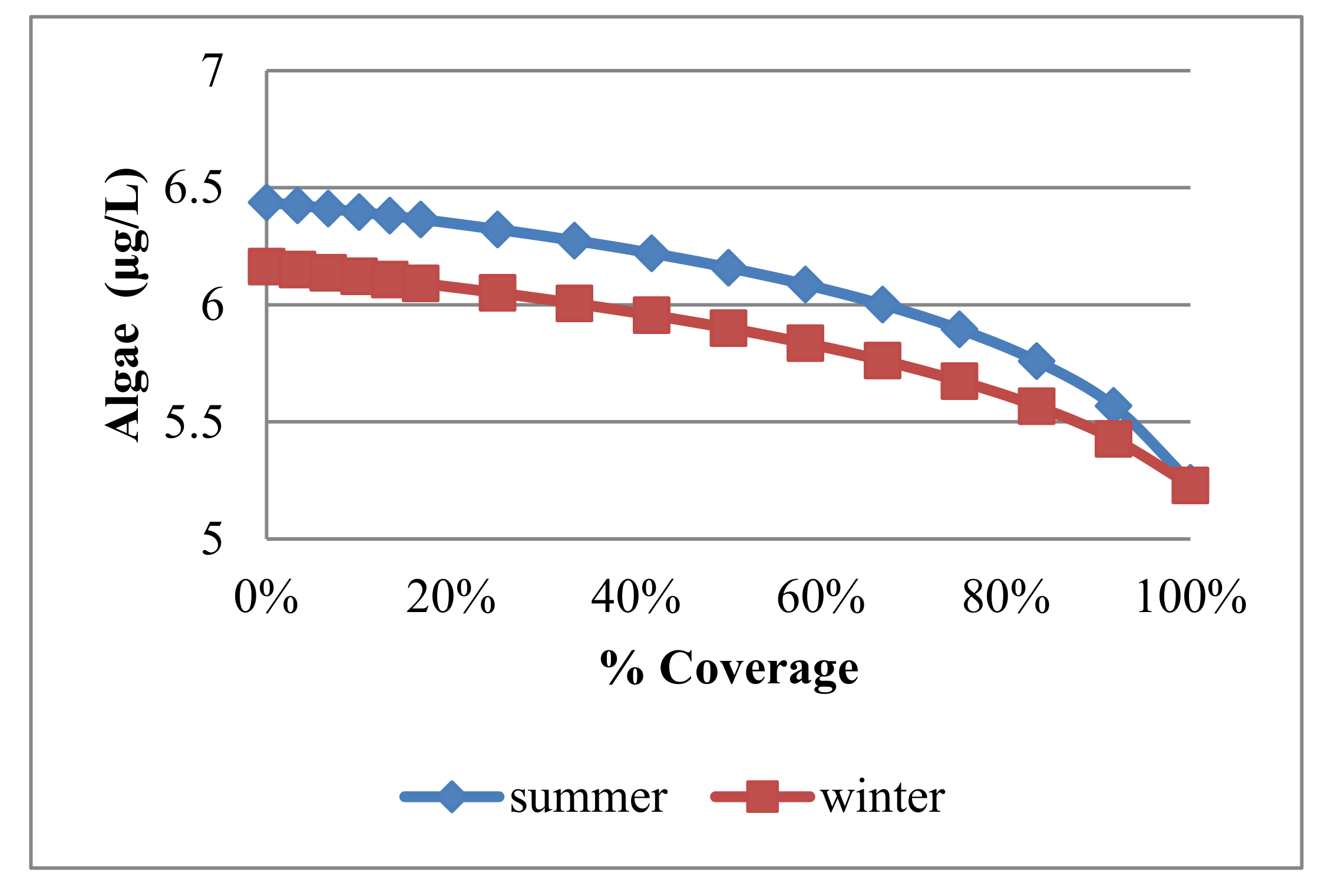
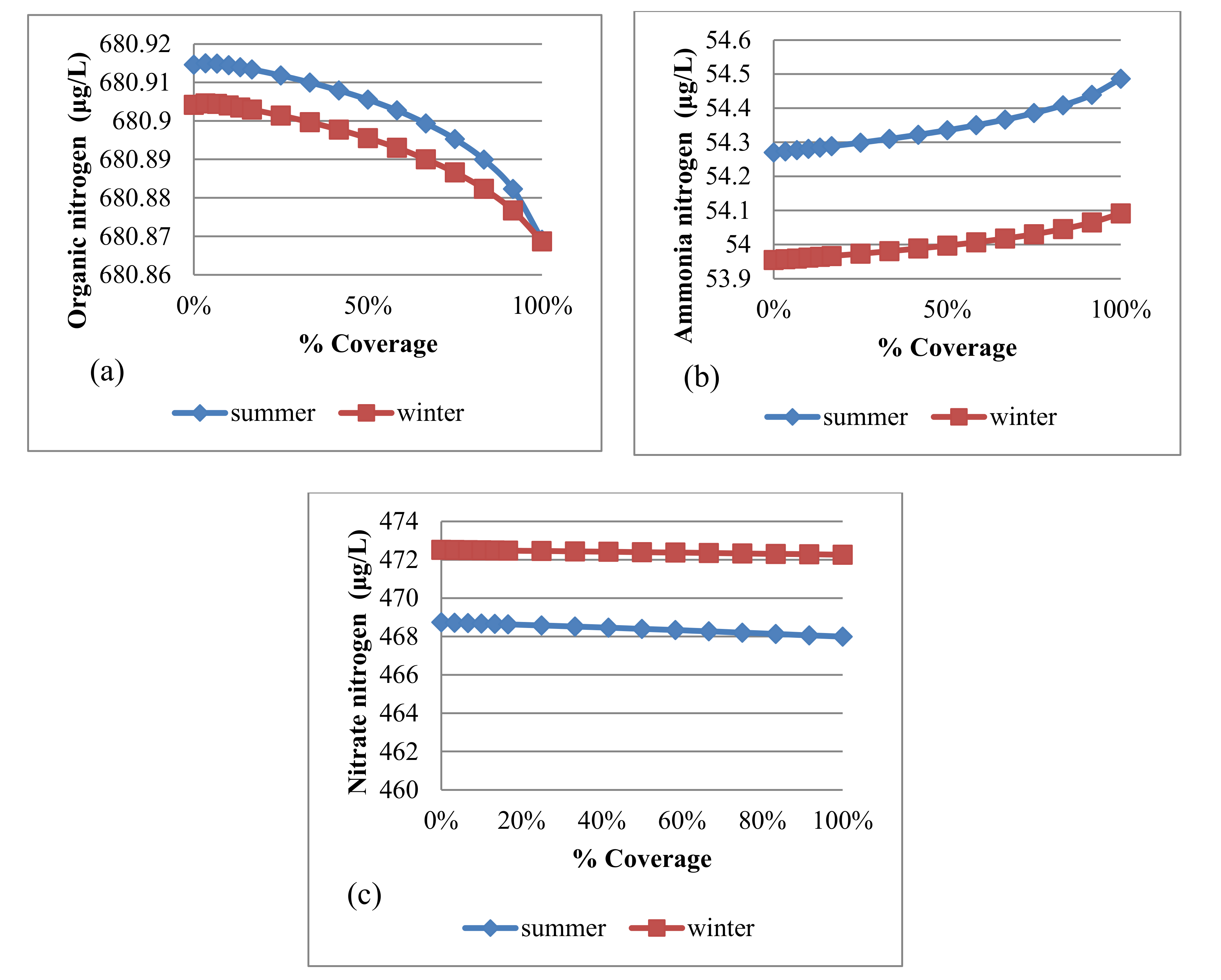
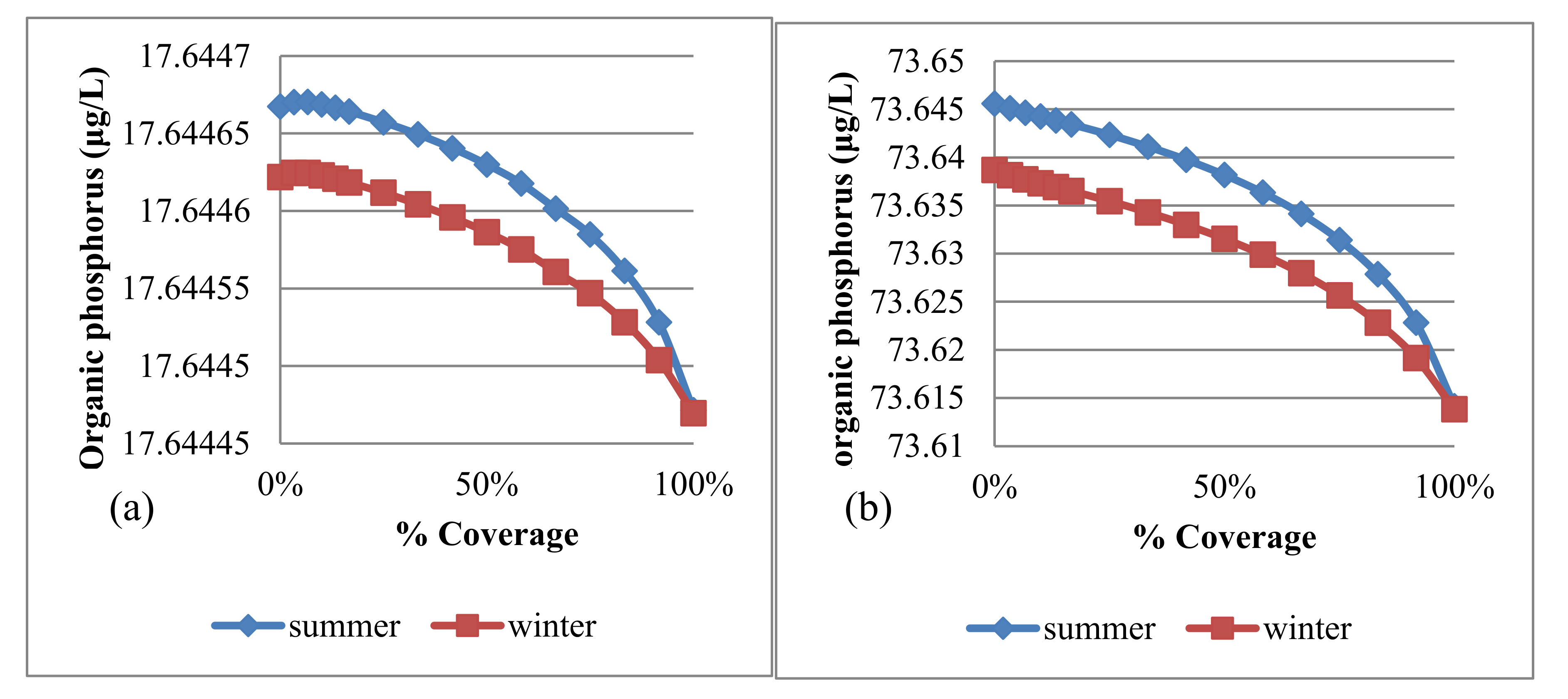
| Trial No. | No. of Panel Rows | Uncovered Area (m2) | Covered Area (m2) | % Covering |
|---|---|---|---|---|
| 0 | 0 | 2,700,000 | 0 | 0% |
| 1 | 1042 | 2,610,000 | 90,000 | 3% |
| 2 | 2083 | 2,520,000 | 180,000 | 7% |
| 3 | 3125 | 2,430,000 | 270,000 | 10% |
| 4 | 4167 | 2,340,000 | 360,000 | 13% |
| 5 | 5208 | 2,250,000 | 450,000 | 17% |
| 6 | 7813 | 2,025,000 | 675,000 | 25% |
| 7 | 10,417 | 1,800,000 | 900,000 | 33% |
| 8 | 13,021 | 1,575,000 | 1,125,000 | 42% |
| 9 | 15,625 | 1,350,000 | 1,350,000 | 50% |
| 10 | 18,229 | 1,125,000 | 1,575,000 | 58% |
| 11 | 20,833 | 900,000 | 1,800,000 | 67% |
| 12 | 23,438 | 675,000 | 2,025,000 | 75% |
| 13 | 26,042 | 450,000 | 2,250,000 | 83% |
| 14 | 28,646 | 225,000 | 2,475,000 | 92% |
| 15 | 31,250 | 0 | 2,700,000 | 100% |
| Water Quality Variable | Initial Value | Unit |
|---|---|---|
| DOsummer | 5.5 | mg/L |
| DOwinter | 8 | mg/L |
| BODsummer | 3 | mg/L |
| BODwinter | 2.8 | mg/L |
| ap | 6.338 | μg/L |
| no | 700 | μgN/L |
| na | 50.533 | μgN/L |
| nn | 469 | μgN/L |
| po | 18.15 | μgP/L |
| pi | 73.46 | μgP/L |
| pHsummer | 7.7 | – |
| pHwinter | 8.85 | – |
| Alkalinity | 2.24 | meq./L |
| Parameter | Value | unit |
|---|---|---|
| DOirrigation | 4 | mg/L |
| DOfreshwater | 5 | mg/L |
| Total Nitrogen | 5 | mg/L |
| Total Phosphorus | 0−2 | mg/L |
| pH | 6.5−8.4 | - |
| Water Quality variables | Constructed Model Outputs | Reference Value | %Error |
|---|---|---|---|
| DO | 7.50 | 7.50 | 0.00 |
| ap | 6.44 | 6.43 | 0.13 |
| na | 54.27 | 53.39 | 1.65 |
| no | 469.27 | 471.26 | 0.42 |
| Pi | 73.65 | 73.67 | 0.03 |
| Po | 17.64 | 18.04 | 2.19 |
| pH | 6.50 | 6.62 | 1.86 |
| Alk | 2.25 | 2.22 | 1.33 |
| Case # | Initial Values | Condition | DO (mg/L) | Evaporation (m3/year) | Covering % | Power Generation in Winter (kWh/day) | Power Generation in Summer (kWh/day) | |
|---|---|---|---|---|---|---|---|---|
| DO (mg/L) | BOD (mg/L) | |||||||
| a | 5.5 | 3 | 5 ± 20% | 4.9 | 5,846,884 | 32.8 | 720,000 | 1,350,000 |
| b | 4 ± 20% | 4.79 | 4,391,269 | 50 | 1,080,000 | 2,025,000 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baradei, S.E.; Sadeq, M.A. Effect of Solar Canals on Evaporation, Water Quality, and Power Production: An Optimization Study. Water 2020, 12, 2103. https://doi.org/10.3390/w12082103
Baradei SE, Sadeq MA. Effect of Solar Canals on Evaporation, Water Quality, and Power Production: An Optimization Study. Water. 2020; 12(8):2103. https://doi.org/10.3390/w12082103
Chicago/Turabian StyleBaradei, Sherine El, and Mai Al Sadeq. 2020. "Effect of Solar Canals on Evaporation, Water Quality, and Power Production: An Optimization Study" Water 12, no. 8: 2103. https://doi.org/10.3390/w12082103
APA StyleBaradei, S. E., & Sadeq, M. A. (2020). Effect of Solar Canals on Evaporation, Water Quality, and Power Production: An Optimization Study. Water, 12(8), 2103. https://doi.org/10.3390/w12082103




