Seasonal and Diurnal Variation of Air/Water Exchange of Gaseous Mercury in a Southern Reservoir Lake (Cane Creek Lake, Tennessee, USA)
Abstract
:1. Introduction
| Site | Time | Mean Flux (ng m−2 h−1) | S.D. | Reference |
|---|---|---|---|---|
| River, Knobesholm, southwestern Sweden | August, 1999 | 11.1 | 11.8 | [9] |
| Nova Scotia (Canada) | Summer, 2002 | 3.8 | 2.6 | [10] |
| St. Lawrence River (Canada) | Summer, 1998 | 2.9 | – | [12] |
| Michigan (USA) | Summer, 1996 | 3.4 | 3.5 | [14] |
| Lake Superior (USA) | Summer, 1997 | 2.5 | 0.5 | [14] |
| Everglades (FL, USA) | Summer, 1997 | 2.7 | 5.6 | [23] |
| St. Lawrence River (Canada) | Summer, 1995 | 0.33 | 0.25 | [24] |
| Bay Saint Franscois wetlands (Canada) | May, 2000 | 0.92 | 1.02 | [25] |
| Bay Saint Franscois wetlands (Canada) | June–July, 2003 | 0.32–0.77 | 0.37–0.60 | [25] |
| Swedish Lakes | Summer, 1988 | 7.9 | 4.4 | [26] |
| Kejimkujik National Park, Nova Scotia (Canada), high DOC lake | 5.4 | – | [27] | |
| Kejimkujik National Park, Nova Scotia (Canada), low DOC lake | 1.1 | – | [27] | |
| Cane Creek Lake (Cookeville, TN, USA) | Summer, 2003 | 1.2 | 0.6 | This study |
| Cane Creek Lake (Cookeville, TN, USA) | Fall, 2003 | 0.6 | 0.2 | This study |
| Cane Creek Lake (Cookeville, TN, USA) | Winter, 2004 | 0.8 | 0.2 | This study |
2. Site and Methods
2.1. Site Description and Field Study Period
2.2. Field Measurement Methods
3. Results and Discussion
3.1. Daily Magnitude and Variation of Hg Air/Water Exchange Flux
3.2. Seasonal Trends of Hg Air/Water Exchange Flux
3.3. Effect of Solar Radiation on Hg Air/Water Exchange
3.4. Effect of Wind on Hg Air/Water Exchange
3.5. Comparison of Effects of Solar Radiation and Wind Speed on Hg Air/Water Exchange
3.6. Calculation of Hg Fluxes Using the Two-Thin-Film Theory
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mason, R.P.; Fitzgerald, W.F.; Morel, F.M.M. The biogeochemical cycling of elemental mercury: Anthropogenic influences. Geochim. Cosmochim. Acta 1994, 58, 3191–3198. [Google Scholar] [CrossRef]
- Schroeder, W.H.; Munthe, J. Atmospheric Mercury—An Overview. Atmos. Environ. 1998, 32, 809–822. [Google Scholar] [CrossRef]
- Sommar, J.; Osterwalder, S.; Zhu, W. Recent advances in understanding and measurement of Hg in the environment: Surface-atmosphere exchange of gaseous elemental mercury (Hg0). Sci. Total Environ. 2020, 721, 137648. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, X.; Miller, D.R.; Helble, J.J.; Carley, R.J. Formulation of bi-directional atmosphere-surface exchanges of elemental mercury. Atmos. Environ. 1999, 33, 4345–4355. [Google Scholar] [CrossRef]
- Amyot, M.; Mierle, G.; Lean, D.R.; McQueen, D.J. Sunlight-induced formation of dissolved gaseous mercury in lake waters. Environ. Sci. Technol. 1994, 28, 2366–2371. [Google Scholar] [CrossRef]
- Amyot, M.; Lean, D.; Mierle, G. Photochemical formation of volatile mercury in high arctic lakes. Environ. Toxicol. Chem. 16, 2054–2063. [CrossRef]
- Amyot, M.; Mierle, G.; Lean, D.; McQueen, D.J. Effects of solar radiation on the formation of dissolved gaseous mercury in temperate lakes. Geochim. Cosmochim. Acta 1997, 61, 975–987. [Google Scholar] [CrossRef]
- Costa, M.; Liss, P.S. Photoreduction of mercury in sea water and its possible implications for Hg0 air/sea fluxes. Mar. Chem. 1999, 68, 87–95. [Google Scholar] [CrossRef]
- Gardfeldt, K.; Feng, X.; Sommar, J.; Lindqvist, O. Total gaseous mercury exchange between air and water at river and sea surface in Swedish coastal regions. Atmos. Environ. 2001, 35, 3027–3038. [Google Scholar] [CrossRef]
- O’Driscoll, N.J.; Beauchamp, S.; Siciliano, S.D.; Rencz, A.N.; Lean, D.R.S. Continuous analysis of dissolved gaseous mercury (DGM) and mercury flux in two freshwater lakes in Kejimkujik Park, Nova Scotia: Evaluating mercury flux models with quantitative data. Environ. Sci. Technol. 2003, 37, 2226–2235. [Google Scholar]
- Fitzgerald, W.F.; Mason, R.P.; Vandal, G.M. Atmospheric cycling and air-water exchange of mercury over mid-continental regions. Water Air Soil Poll. 1991, 56, 745–767. [Google Scholar] [CrossRef]
- Poissant, L.; Amyot, M.; Pilote, M.; Lean, D. Mercury water-air exchange over the upper St. Lawrence river and Lake Ontario. Environ. Sci. Technol. 2000, 34, 3069–3078. [Google Scholar] [CrossRef]
- Vandal, G.M.; Mason, R.P.; Fitzgerald, W.F. Cycling of volatile mercury in temperate lakes. Water Air Soil Poll. 1991, 56, 791–803. [Google Scholar] [CrossRef]
- Vette, A.F. Photochemical Influences on the Air-Water Exchange of Mercury. Ph.D. Thesis, University of Michigan, Ann Arbor, MI, USA, August 1998. [Google Scholar]
- Zhang, H.; Lindberg, S.E. Sunlight and iron (III)-induced photochemical production of dissolved gaseous mercury in freshwater. Environ. Sci. Technol. 2001, 35, 928–935. [Google Scholar] [CrossRef]
- Nriagu, J.O. Mechanistic steps in the photoreduction of mercury in natural waters. Sci. Total Environ. 1994, 154, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.F.; Stromberg, D.; Lindqvist, O. Influence of humic substances on photolysis of divalent mercury in aqueous solution. Water Air Soil Poll. 1995, 80, 789–798. [Google Scholar] [CrossRef]
- Zhang, H. Photochemical redox reactions of mercury. In Recent Developments in Mercury Science; Atwood, D.A., Ed.; Structure and Bonding series; Springer: Berlin, Germany, 2006; Volume 120, pp. 37–79. [Google Scholar]
- Dill, C.; Kuiken, T.; Zhang, H.; Ensor, M. Diurnal variation of dissolved gaseous mercury (DGM) levels in a southern reservoir lake (Tennessee, USA) in relation to solar radiation. Sci. Total Environ. 2006, 357, 176–193. [Google Scholar] [CrossRef]
- O’Driscoll, N.J.; Lean, D.R.S.; Loseto, L.L.; Carignan, R.; Siciliano, S.D. Effect of dissolved organic carbon on the photoproduction of dissolved gaseous mercury in lakes: Potential impacts of forestry. Environ. Sci. Technol. 2004, 38, 2664–2672. [Google Scholar] [CrossRef]
- Zhang, H.; Lindberg, S.E. Trends in dissolved gaseous mercury in the Tahquamenon river watershed and nearshore water of Whitefish bay in the Michigan Upper Peninsula. Water Air Soil Poll. 2002, 133, 381–391. [Google Scholar] [CrossRef]
- Zhang, H.; Dill, C.; Kuiken, T.; Ensor, M.; Crocker, W. Change of dissolved gaseous mercury (DGM) concentrations in a southern reservoir lake (Tennessee, USA) following seasonal variation of solar radiation. Environ. Sci. Technol. 2006, 40, 2114–2119. [Google Scholar] [CrossRef]
- Lindberg, S.; Zhang, H. Air/water exchange of mercury in the Everglades II: Measuring and modeling evasion of mercury from surface waters in the Everglades nutrient removal project. Sci. Total Environ. 2000, 259, 135–143. [Google Scholar] [CrossRef]
- Poissant, L.; Casimir, A. Water-air and soil-air exchange rate of total gaseous mercury measured at background sites. Atmos. Environ. 1998, 32, 883–893. [Google Scholar] [CrossRef]
- Zhang, H.H.; Poissant, L.; Xu, X.; Pilot, M.; Beauvais, C.; Amyot, M.; Garcia, E.; Laroulandie, L. Air-water gas exchange of mercury in the bay Sait Francois wetlands: Observation and model parameterization. J. Geophys. Res. Atmos. 2006, 111, D17307. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.F.; Munthe, J.; Schroeder, W.H.; Lindqvist, O. Vertical fluxes of volatile mercury over forest soil and lake surfaces in Sweden. Tellus B 1991, 43, 267–279. [Google Scholar] [CrossRef]
- Boudala, F.S.; Folkins, I.; Beauchamp, S.; Tordon, R.; Neima, J.; Johnson, B. Mercury flux measurement over air and water in Kejimkujik national park, Nova Scotia. Water Air Soil Poll. 2000, 122, 183–202. [Google Scholar] [CrossRef]
- Zhang, H.; Dill, C. Apparent rates of production and loss of dissolved gaseous mercury (DGM) in a southern reservoir lake (Tennessee, USA). Sci. Total Environ. 2008, 392, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Crocker, W.C. Air/water Exchange of Aquatic Gaseous Mercury in a Southern Reservoir Lake: Cane Creek Lake, Putman County, TN. Master’s Thesis, Tennessee Technological University, Cookeville, TN, USA, May 2005. [Google Scholar]
- Dill, C. Aquatic photochemokinetics of mercury in Cane Creek Lake, Putnam County, TN. Master’s Thesis, Tennessee Technological University, Cookeville, TN, USA, May 2004. [Google Scholar]
- Zhang, H.; Lindberg, S.; Barnett, M.; Vette, A.; Gustin, M. Dynamic flux chamber measurement of gaseous mercury emission fluxes over soils: I. Simulation of gaseous mercury emissions from soils using a two-resistance exchange interface model. Atmos. Environ. 2002, 36, 835–846. [Google Scholar] [CrossRef]
- Wangberg, I.; Schmolke, S.; Schager, P.; Munthe, J.; Ebinghaus, R.; Iverfeldt, A. Estimates of air-Sea exchange of mercury in the Baltic sea. Atmos. Environ. 2001, 35, 5477–5484. [Google Scholar] [CrossRef]
- Liss, P.S.; Slator, P.G. Flux of gases across the air-sea interface. Nature 1974, 247, 181–184. [Google Scholar] [CrossRef]
- Schroeder, W.H. Estimation of atmospheric input and evasion fluxes of mercury to and from the Great Lakes. In Global and Regional Mercury Cycles: Sources, Fluxes and Mass Balances; NATO ASI Series (Series 2: Environment); Baeyens, W., Ebinghaus, R., Vasiliev, O., Eds.; Springer: Dordrecht, The Netherlands, 1996; Volume 21, pp. 109–121. [Google Scholar]
- Achman, D.R.; Hornbuckle, K.C.; Eisenreich, S.J. Volatilization of polychlorinated biphenyls from Green Bay, Lake Michigan. Environ. Sci. Technol. 1993, 27, 75–87. [Google Scholar] [CrossRef]
- Siddiqui, M.H.K.; Loewen, M.R.; Richardson, C.; Asher, W.E.; Jessup, A.T. Turbulence generated by microscale breaking waves and its influence on air-water gas transfer. In Gas Transfer at Water Surfaces; Donelan, M.A., Drennan, W.M., Saltzman, E.S., Wanninkhof, R., Eds.; AGU: Washington, DC, USA, 2002; pp. 11–16. [Google Scholar]
- Sharif, A.; Tessier, E.; Bouchet, S.; Monperrus, M.; Pinaly, H.; Amouroux, D. Comparison of different air-water gas exchange models to determine gaseous mercury evasion from different European coastal lagoons and estuaries. Water Air Soil Poll. 2013, 224, 1606. [Google Scholar] [CrossRef]
- Kuss, J.; Holzmann, J.; Ludwig, R. An elemental mercury diffusion coefficient for natural waters determined by molecular dynamics simulation. Environ. Sci. Technol. 2009, 43, 3183–3186. [Google Scholar] [CrossRef] [PubMed]
- Kuss, J. Water-air gas exchange of elemental mercury: An experimentally determined mercury diffusion coefficient for Hg0 water-air flux calculations. Limnol. Oceanogr. 2014, 59, 1461–1467. [Google Scholar] [CrossRef]
- Lindberg, S.E.; Zhang, H.; Vette, A.F.; Gustin, M.S.; Barnett, M.O.; Kuiken, T. Dynamic fluxchamber measurement of gaseous mercury emission fluxes over soils: Part 2—Effect of flushing flow rate and verification of a two-resistance exchange interface simulation model. Atmos. Environ. 2002, 36, 847–859. [Google Scholar] [CrossRef]


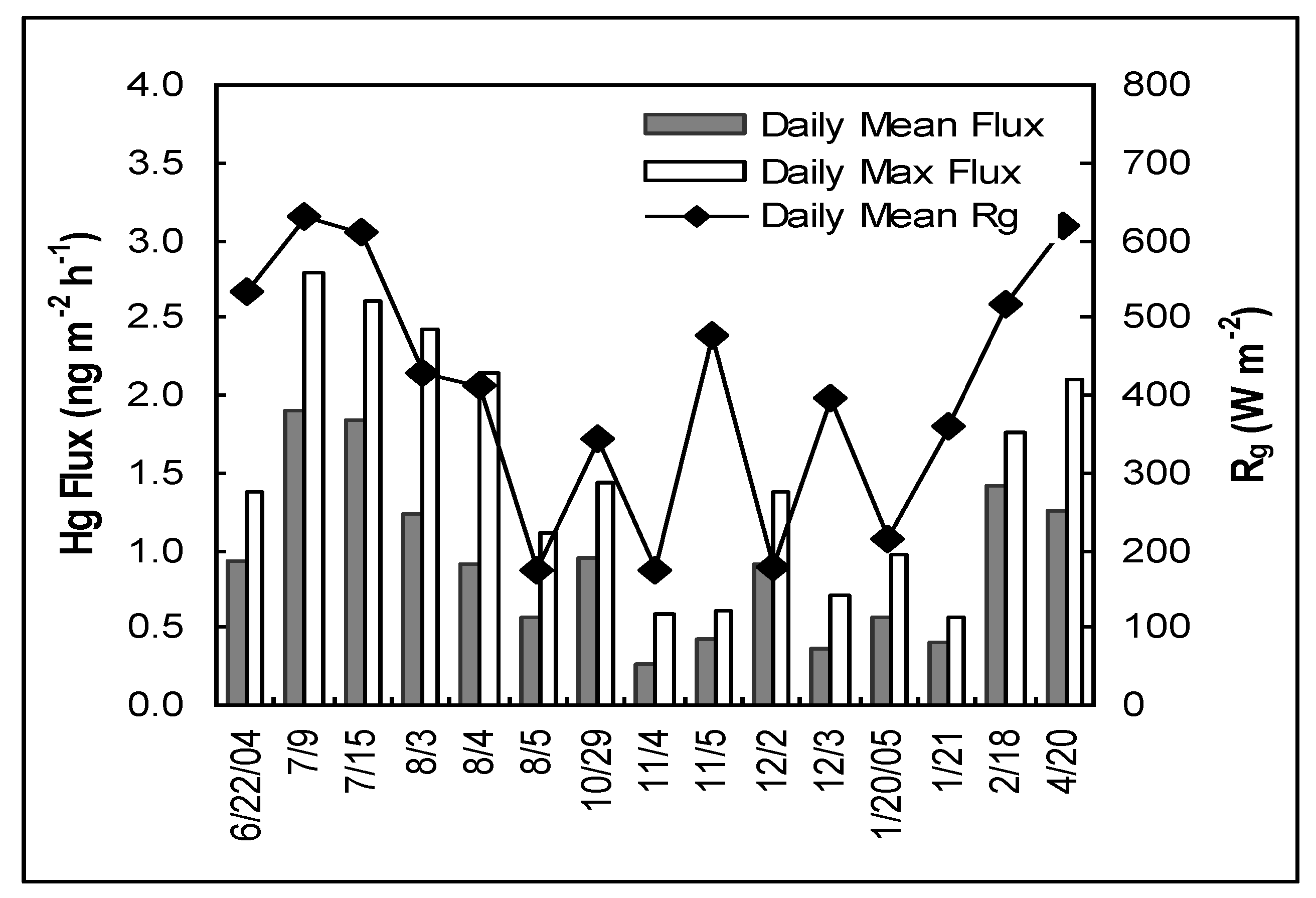
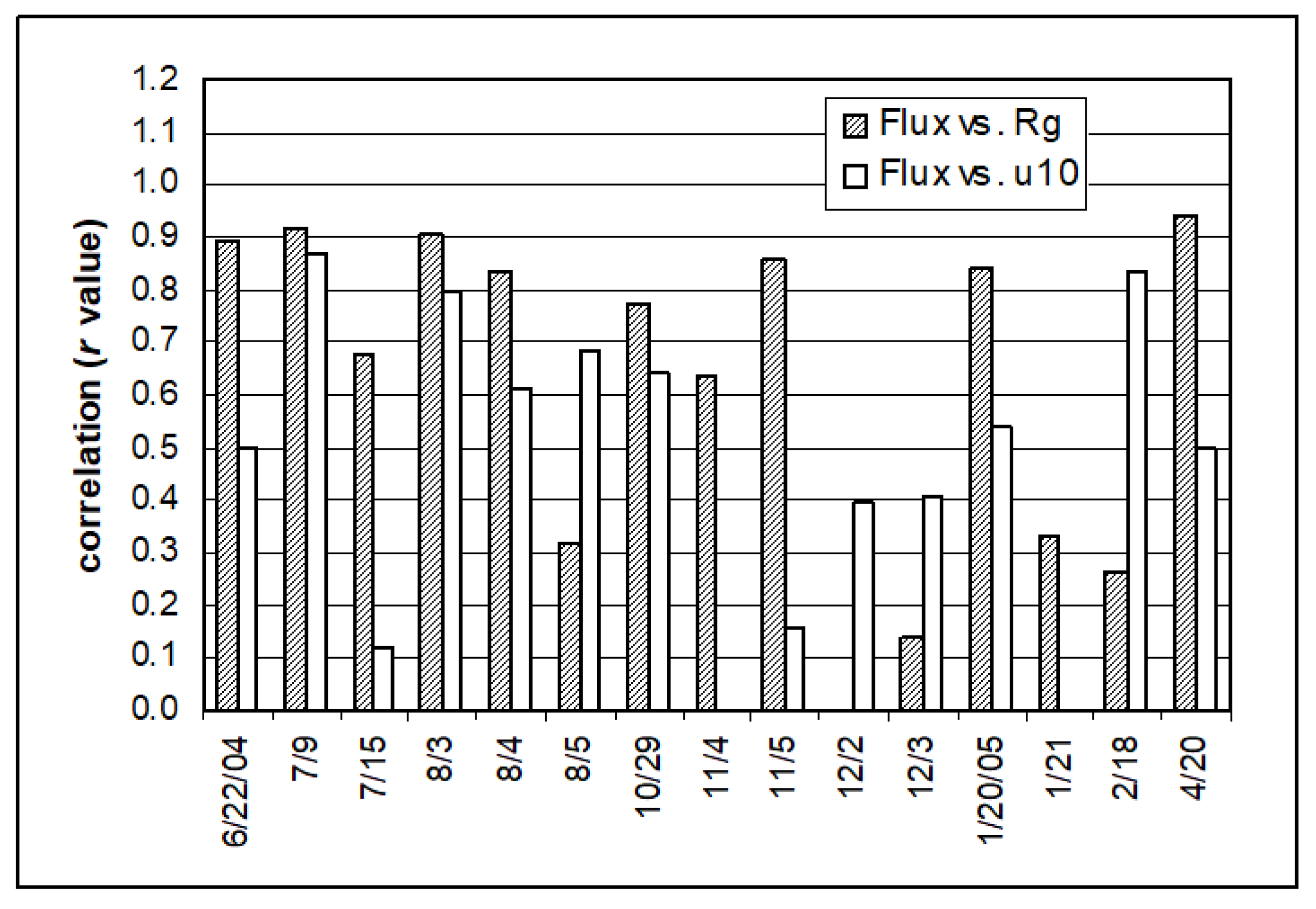



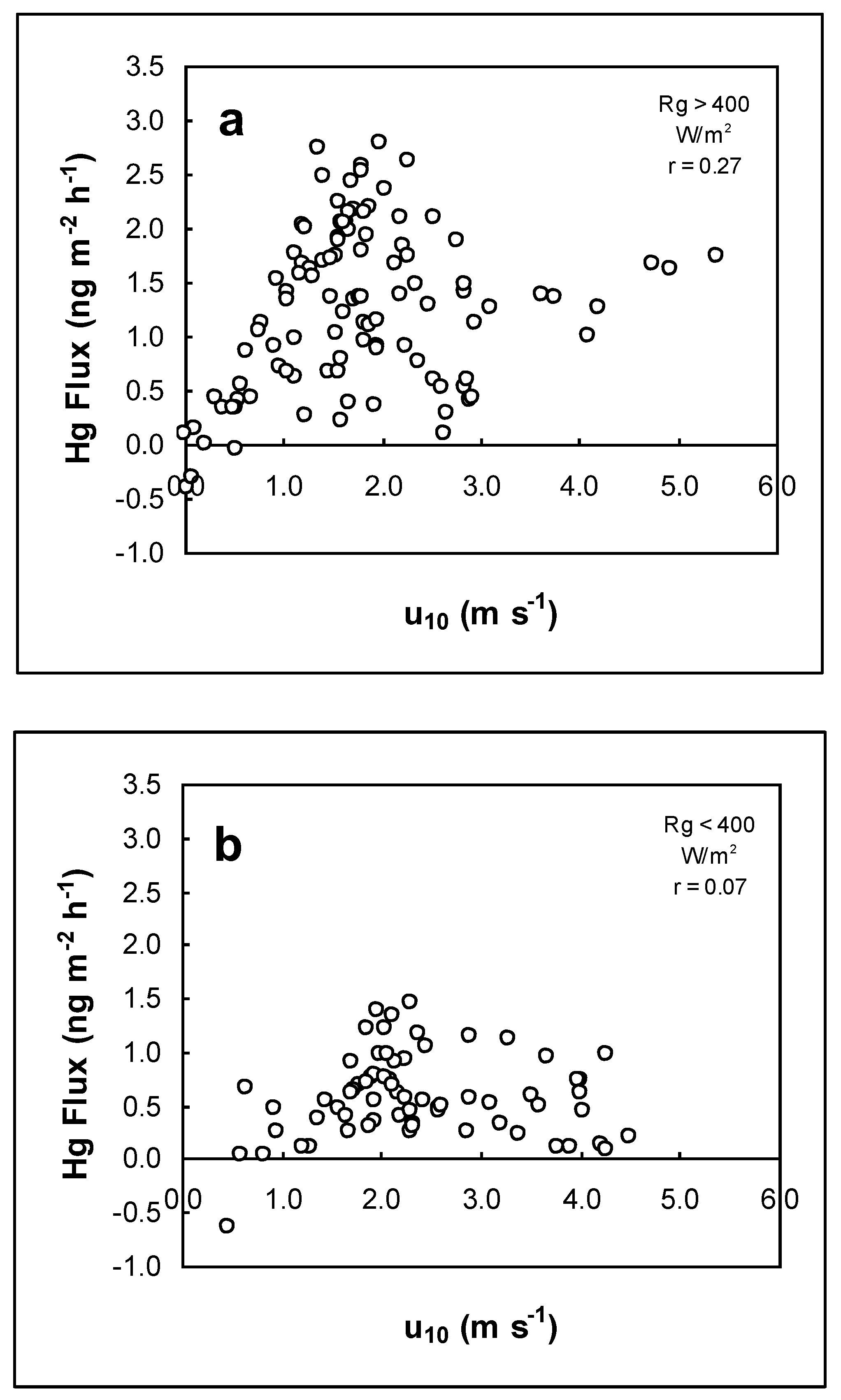
| Date | Flux (ng m−2 h−1) | Number of Flux | Rg (W m−2) | UVA (µW cm−2) | u10 (m s−1) | Tw (°C) | Ta (°C) | RH (%) |
|---|---|---|---|---|---|---|---|---|
| 22 June 2004 | 0.93 (0.27–1.37) | 10 | 533 (161–859) | 1224 (530–1860) | 1.6 (1.0–2.0) | 29.4 (28.0–30.0) | 26.4 (23.8–27.9) | 70 (63–80) |
| 9 July 2004 | 1.90 (0.42–2.79) | 13 | 630 (102–876) | 1430 (520–2150) | 1.4 (0.7–2.0) | 30.5 (29.0–32.0) | 29.7 (27.9–30.8) | 62 (56–82) |
| 15 July 2004 | 1.84 (0.56–2.61) | 14 | 611 (143–873) | 1211 (390–2210) | 1.7 (1.1–2.5) | 30.7 (29.0–32.0) | 26.0 (23.8–27.2) | 55 (49–72) |
| 3 August 2004 | 1.23 (0.32–2.43) | 14 | 427 (1–865) | 766 (0–1820) | 1.4 (0.4–2.2) | 30.8 (30.0–31.0) | 28.4 (22.9–30.7) | 65 (52–92) |
| 4 August 2004 | 0.92 (−0.40–2.14) | 22 | 414 (2–892) | 708 (0–1600) | 1.0 (0.0–4.1) | 30.4 (29.0–31.0) | 27.5 (19.3–31.6) | 72 (57–97) |
| 5 August 2004 | 0.57 (−0.63–1.12) | 16 | 176 (23–399) | 480 (80–1410) | 2.5 (0.5–4.3) | 28.8 (28.0–29.0) | 22.7 (21.3–24.3) | 89 (79–95) |
| 29 October 2004 | 0.95 (0.37–1.44) | 11 | 343 (120–628) | 272 (20–420) | 2.0 (1.4–2.9) | 21.4 (20.2–22.1) | 25.0 (22.4–26.2) | 67 (59–86) |
| 4 November 2004 | 0.27 (0.08–0.58) | 10 | 174 (62–270) | – | 3.8 (3.2–4.5) | 19.4 (19.0–19.4) | 14.5 (14.2–14.8) | 72 (68–75) |
| 5 November 2004 | 0.43 (0.10–0.61) | 9 | 477 (230–587) | 365 (60–580) | 2.6 (1.7–2.9) | 18.1 (17.5–18.3) | 12.9 (10.6–14.5) | 44 (33–60) |
| 2 December 2004 | 0.91 (0.61–1.37) | 8 | 179 (31–371) | – | 2.0 (1.7–2.5) | 10.7 (9.8–11.0) | 8.5 (6.4–10.6) | 57 (46–70) |
| 3 December 2004 | 0.36 (0.10–0.71) | 7 | 395 (42–534) | 164 (0–290) | 1.4 (0.9–1.9) | 9.8 (9.4–10.2) | 8.5 (6.7–10.2) | 51 (46–60) |
| 20 January 2005 | 0.56 (0.26–0.96) | 7 | 215 (58–311) | – | 2.0 (1.7–2.2) | 6.0 (5.8–6.2) | 7.8 (5.8–9.3) | 60 (55–68) |
| 21 January 2005 | 0.40 (0.25–0.57) | 10 | 360 (124–512) | 130 (80–150) | 2.5 (2.3–2.9) | 7.1 (6.6–7.4) | 7.2 (5.3–8.3) | 70 (64–80) |
| 18 February 2005 | 1.41 (1.13–1.75) | 9 | 518 (225–645) | – | 3.8 (1.6–5.4) | 9.7 (9.0–10.2) | 8.8 (5.8–10.5) | 39 (37–40) |
| 20 April 2005 | 1.26 (0.23–2.10) | 14 | 619 (114–860) | – | 2.3 (1.6–2.9) | 21.4 (19.0–32.2) | 23.7 (18.9–25.4) | 34 (24–50) |
| Flux ng m−2 h−1) | Rg (W m−2) | UVA (µW cm−2) | u10 (m s−1) | |
|---|---|---|---|---|
| summer | 1.2 (−0.6–2.8) | 453 (1–892) | 963 (0–2210) | 1.6 (0.0–4.3) |
| fall | 0.6 (0.08–1.4) | 327 (62–628) | 318 (20–580) | 2.8 (1.4–4.5) |
| winter | 0.7 (0.7–1.8) | 341 (31–645) | 147 (0–290) | 2.4 (0.9–5.4) |
| RMSE | |||||
|---|---|---|---|---|---|
| Date | Model 0 | Model 1 | Model 2 | Model 3 | Model 4 |
| 22 June 2004 | 3.5 | 1.1 | 1.0 | 1.0 | 115.1 |
| 9 July 2004 | 3.0 | 3.9 | 2.0 | 1.6 | 536.6 |
| 15 July 2004 | 2.9 | 1.1 | 2.2 | 2.1 | 109.4 |
| 3 August 2004 | 3.7 | 1.4 | 2.0 | 1.8 | 205.5 |
| 4 August 2004 | 2.3 | 1.2 | 1.4 | 1.3 | 175.2 |
| 5 August 2004 | 3.1 | 0.5 | 0.8 | 0.7 | 89.2 |
| 29 October 2004 | 3.8 | 0.9 | 1.1 | 1.0 | 122.1 |
| 5 November 2004 | 5.3 | 1.4 | 0.4 | 0.3 | 338.3 |
| 3 December 2004 | 2.9 | 3.3 | 0.4 | 0.3 | 165.4 |
| 21 January 2005 | 4.2 | 2.3 | 0.3 | 0.1 | 347.3 |
| Mean RMSE | 3.5 | 1.7 | 1.2 | 1.0 | 220.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crocker, W.C.; Zhang, H. Seasonal and Diurnal Variation of Air/Water Exchange of Gaseous Mercury in a Southern Reservoir Lake (Cane Creek Lake, Tennessee, USA). Water 2020, 12, 2102. https://doi.org/10.3390/w12082102
Crocker WC, Zhang H. Seasonal and Diurnal Variation of Air/Water Exchange of Gaseous Mercury in a Southern Reservoir Lake (Cane Creek Lake, Tennessee, USA). Water. 2020; 12(8):2102. https://doi.org/10.3390/w12082102
Chicago/Turabian StyleCrocker, William C., and Hong Zhang. 2020. "Seasonal and Diurnal Variation of Air/Water Exchange of Gaseous Mercury in a Southern Reservoir Lake (Cane Creek Lake, Tennessee, USA)" Water 12, no. 8: 2102. https://doi.org/10.3390/w12082102
APA StyleCrocker, W. C., & Zhang, H. (2020). Seasonal and Diurnal Variation of Air/Water Exchange of Gaseous Mercury in a Southern Reservoir Lake (Cane Creek Lake, Tennessee, USA). Water, 12(8), 2102. https://doi.org/10.3390/w12082102





