Optimization of Combined Submerged Macrophyte Planting Conditions for Inhibiting Algae by Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Cultivation
2.1.1. Algae
2.1.2. Submerged Macrophytes
2.1.3. Tested Water
2.2. Experimental Method
- (1)
- The single planting density of H. verticillata and M. spicatum was set as 0, 5, 10, 15 and 20 g/L to determine the suitable combined planting density range. The planting density was set according to previous studies, it had reported significant allelopathic effects on algae with a plant density of 1–10 g, or 14.38 g wet weight/L [19,32], so the tested planting density was 0–20 g/L;
- (2)
- The two submerged macrophytes were combined and planted according to the planting experimental scheme. It was obtained using the CCD unit in the Design Expert software. The scheme was set based on the suitable combined planting density range;
- (3)
- To verify the reliability of the model and the synergistic algal-inhibiting effect of the plant assemblage, the following was applied: single planting groups EG1 (H. verticillata = x + y g/L) and EG2 (M. spicatum = x + y g/L) and an optimum combined planting group, EG3, acquired from the results of CCD model analysis (H. verticillata = x g/L, M. spicatum = y g/L).
2.3. Data Analysis
2.3.1. Kinetic Analysis of Algal Growth
2.3.2. Macrophyte Growth Analysis
2.3.3. Response Surface Methodology
3. Results
3.1. Effect of Different Planting Densities
3.1.1. Growth Characteristics of Algae
3.1.2. Growth Characteristics of Submerged Macrophytes
3.2. Optimization of Algal Suppression Using Combined Plants
3.2.1. Design of CCD and Associated Results
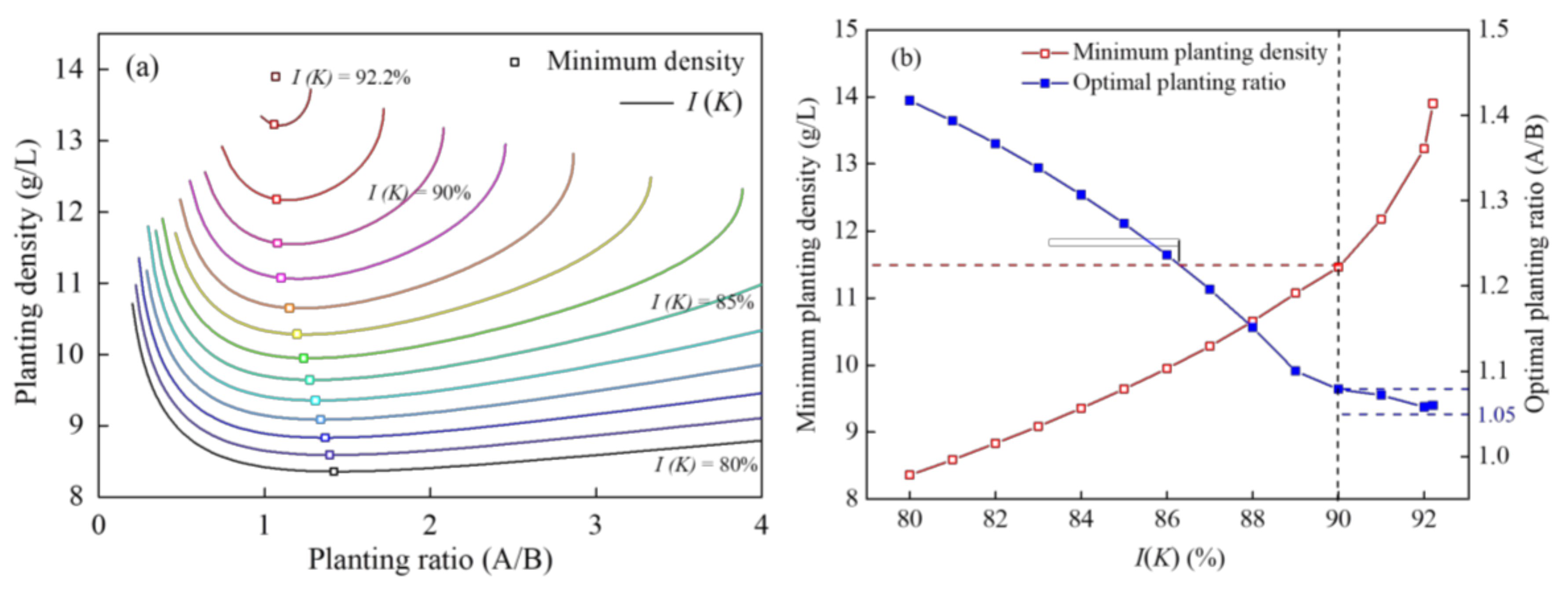
3.2.2. Analysis of I (K) in Different Treatments Based on the CCD Model
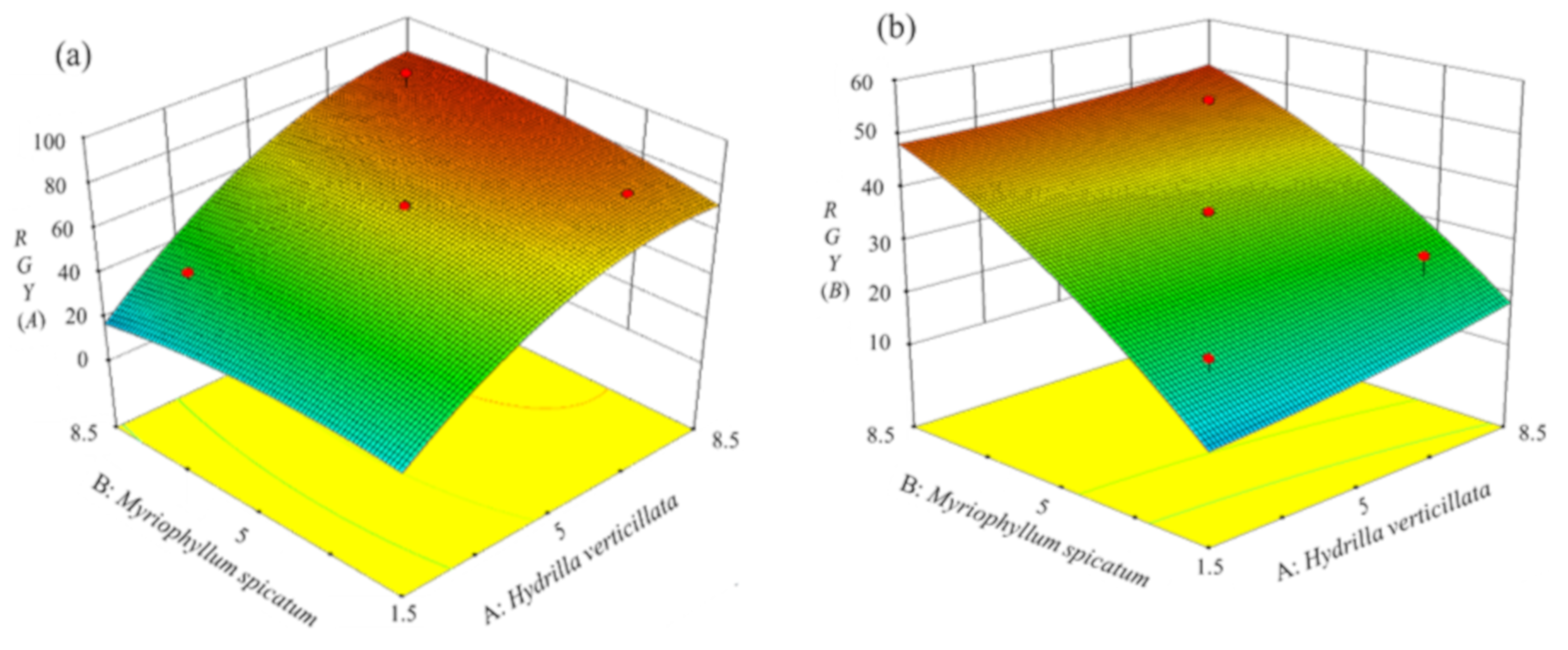
3.2.3. Analysis of RGY in Different Treatments Based on the CCD Model
3.3. Verification Test of the CCD Model
4. Discussion
4.1. Synergistic Effect of Combined Submerged Macrophyte Planting
4.2. Stability of Combined Planting
4.3. Optimization of Plant Combination in Practical Application
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sun, R.; Chen, L. How can urban water bodies be designed for climate adaptation? Landsc. Urban Plan 2012, 105, 27–33. [Google Scholar] [CrossRef]
- The United Nations. World Water Development Report: Leaving No One Behind; The United Nations: Geneva, Switzerland, 2019. [Google Scholar]
- Wei, D.; Tan, Z.; Du, Y. A Biological Safety Evaluation on Reclaimed Water Reused as Scenic Water Using a Bioassay Battery. Environ. J. Sci. 2019, 23, 1611–1618. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, Z.; Jia, H.; Huang, X. Factors Influencing Water Quality Indices in a Typical Urban River Originated with Reclaimed Water. Front. Environ. Sci. Eng. 2017, 11, 8. [Google Scholar] [CrossRef]
- Hampel, J.J.; McCarthy, M.J.; Gardner, W.S. Nitrification and Ammonium Dynamics in Taihu Lake, China: Seasonal Competition for Ammonium between Nitrifiers and Cyanobacteria. Biogeosciences 2018, 15, 733–748. [Google Scholar] [CrossRef]
- Paerl, H.W.; Scott, J.T.; Mccarthy, M.J. It Takes Two to Tango: When and Where Dual Nutrient (N & P) Reductions are Needed to Protect Lakes and Downstream Ecosystems. Environ. Sci. Technol. 2016, 50, 10805–10813. [Google Scholar] [CrossRef] [PubMed]
- Huisman, J.; Codd, G.A.; Paerl, H.W. Cyanobacterial Blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Lau, S.; Lane, S.N. Nutrient and Grazing Factors in Relation to Phytoplankton Level in a Eutrophic Shallow Lake: The Effect Of Low Macrophyte Abundance. Water Res. 2002, 36, 3593–3601. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G. Harmful Cyanobacterial Blooms: Causes, Consequences, and Controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef]
- Nakai, S.; Zou, G.; Okuda, T.; Nishijima, W.; Hosomi, M.; Okada, M. Polyphenols and Fatty Acids Responsible for Anti-Cyanobacterial Allelopathic Effects of Submerged Macrophyte, Myriophyllum Spicatum. Water Sci. Technol. 2012, 66, 993. [Google Scholar] [CrossRef]
- Wu, Q.; Hu, Y.; Li, S.; Peng, S.; Zhao, H. Microbial Mechanisms of Using Enhanced Ecological Floating Beds for Eutrophic Water Improvement. Bioresour. Technol. 2016, 211, 451–456. [Google Scholar] [CrossRef]
- Espinosaortiz, E.J.; Rene, E.R.; Pakshirajan, K.; Van Hullebusch, E.D.; Lens, P.N. Fungal Pelleted Reactors in Wastewater Treatment: Applications and Perspectives. Chem. Eng. J. 2016, 283, 553–571. [Google Scholar] [CrossRef]
- Sun, R.; Sun, P.; Zhang, J.; Esquivel-Elizondo, S.; Wu, Y. Microorganisms-Based Methods for Harmful Algal Blooms Control: A Review. Bioresour. Technol. 2018, 248, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, M. Ecology of shallow lakes. In Population & Community Biology; Springer Science & Business Media: Berlin, Germany, 1997. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, B.; Wang, J.; Gao, Y.; Wu, Z. Study on the Mechanism of Allelopathic Influence on Cyanobacteria and Chlorophytes by Submerged Macrophyte (Myriophyllum Spicatum) and its Secretion. Aquat. Toxicol. 2010, 98, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hua, M.; Yu, Y.; Zhang, M.; Xian, Q.-M.; Yin, D.-Q. Evaluating the Effects of Allelochemical Ferulic Acid on Microcystis Aeruginosa by Pulse-Amplitude-Modulated (Pam) Fluorometry and Flow Cytometry. Chemosphere 2016, 147, 264–271. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, J.; Yang, S. The Effect of Pyrogallic Acid on Growth, Oxidative Stress, and Gene Expression in Cylindrospermopsis Raciborskii (Cyanobacteria). Ecotoxicology 2012, 22, 271–278. [Google Scholar] [CrossRef]
- Mohamed, Z.A. Macrophytes-Cyanobacteria Allelopathic Interactions and their Implications for Water Resources Management-A Review. Limnology 2017, 63, 122–132. [Google Scholar] [CrossRef]
- Hilt, S.; Gross, E.M. Can Allelopathically Active Submerged Macrophytes Stabilise Clear-Water States in Shallow Lakes? Basic Appl. Ecol. 2008, 9, 422–432. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z.H. Research Progress on the Use of Plant Allelopathy in Agriculture and the Physiological and Ecological Mechanisms of Allelopathy. Front. Plant Sci. 2015, 6, 1697. [Google Scholar] [CrossRef]
- Zuo, S.P.; Wan, K.; Ma, S.; Ye, L.T. Combined Allelopathic Potential of Aquatic Plants Species to Control Algae. Allelopath. J. 2014, 34, 315–323. [Google Scholar]
- Rojo, C.; Segura, M.; Rodrigo, M.A. The Allelopathic Capacity of Submerged Macrophytes Shapes the Microalgal Assemblages from a Recently Restored Coastal Wetland. Ecol. Eng. 2013, 58, 149–155. [Google Scholar] [CrossRef]
- Schamphelaere, K.; Vasconcelos, F.M.; Heijerick, D.G.; Tack, F.M.; Delbeke, K.; Allen, H.E.; Janssen, C.R. Development and Field Validation of a Predictive Copper Toxicity Model for the Green Alga Pseudokirchneriella Subcapitata. Environ. Toxicol. Chem. 2003, 22, 2454–2465. [Google Scholar] [CrossRef] [PubMed]
- Arazo, R.O.; Genuino, D.A.D.; De Luna, M.D.G.; Capareda, S.C. Bio-Oil Production from Dry Sewage Sludge by Fast Pyrolysis in An Electrically-Heated Fluidized Bed Reactor. Sustain. Environ. Res. 2017, 27, 7–14. [Google Scholar] [CrossRef]
- Dastkhoon, M.; Ghaedi, M.; Asfaram, A.; Arabi, M.; Ostovan, A.; Goudarzi, A. Cu@Sns/Sno2 Nanoparticles as Novel Sorbent for Dispersive Micro Solid Phase Extraction of Atorvastatin in Human Plasma and Urine Samples by High-Performance Liquid Chromatography with Uv Detection: Application of Central Composite Design (CCD). Ultrason. Sonochem. 2017, 36, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Asfaram, A.; Ghaedi, M.; Yousefi, F.; Dastkhoon, M. Experimental Design and Modeling of Ultrasound Assisted Simultaneous Adsorption of Cationic Dyes Onto Zns: Mn-Nps-Ac From Binary Mixture. Ultrason. Sonochem. 2016, 33, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Techer, D.; Fontaine, P.; Personne, A.; Viot, S.; Thomas, M. Allelopathic Potential and Ecotoxicity Evaluation of Gallic and Nonanoic Acids to Prevent Cyanobacterial Growth in Lentic Systems: A Preliminary Mesocosm Study. Sci. Total. Environ. 2016, 547, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.T.; Zheng, C.Y.; Hu, W.; Xu, W.-W.; Wang, H.-F. The Allelopathy and Allelopathic Mechanism of Phenolic Acids on Toxic Microcystis Aeruginosa. J. Appl. Phycol. 2010, 22, 71–77. [Google Scholar] [CrossRef]
- Zuo, S.P.; Zhou, S.; Ye, L.; Ma, S. Synergistic and Antagonistic Interactions among Five Allelochemicals with Antialgal Effects on Bloom-Forming, Microcystis Aeruginosa. Ecol. Eng. 2016, 97, 486–492. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, S.; Luo, P.; Zhuang, X.; Chen, X.; Wu, J. Purification and Reuse of Non-Point Source Wastewater via, Myriophyllum -Based Integrative Biotechnology: A Review. Bioresour. Technol. 2018, 248, 3–11. [Google Scholar] [CrossRef]
- He, Y.; Zhou, Q.H.; Liu, B.Y.; Cheng, L.; Tian, Y.; Zhang, Y.-Y.; Wu, Z.-B. Programmed Cell Death in the Cyanobacterium Microcystis Aeruginosainduced by Allelopathic Effect of Submerged Macrophyte Myriophyllum Spicatumin Co-culture . Environ. Boil. Fishes 2016, 28, 2805–2814. [Google Scholar] [CrossRef]
- Korner, S.; Nicklisch, A. Allelopathic Growth Inhibition of Selected Phytoplankton Species by Submerged Macrophyes. J. Phycol. 2002, 38, 862–871. [Google Scholar] [CrossRef]
- Wang, J.W.; Yu, D.; Xiong, W.; Han, Y.-Q. Above- And Belowground Competition between Two Submersed Macrophytes. Hydrobiology 2008, 607, 113–122. [Google Scholar] [CrossRef]
- Park, Y.T.; Lee, H.; Yun, H.S.; Song, K.-G.; Yeom, S.-H.; Choia, J. Removal of Metal from Acid Mine Drainage Using a Hybrid System Including a Pipes Inserted Microalgae Reactor. Bioresour. Technol. 2013, 150, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Kong, W.; Yang, Z.; Yu, H.; Li, F. Combination of Logistic and Modified Monod Functions to Study Microcystis Aeruginosa Growth Stimulated by Fish Feed. Ecotoxicol. Environ. Saf. 2019, 167, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Guria, C.; Maiti, S.K.; Banerjee, C.; Shukla, P. Carbon Bio-Fixation, Effect of Physicochemical Factors and Carbon Supply Strategies by Nannochloropsis Sp. Using Flue Gas and Fertilizer. Biomass Bioenergy 2019, 125, 95–104. [Google Scholar] [CrossRef]
- Priyadharshini, S.; Bakthavatsalam, A.K. Optimization of Phenol Degradation by the Microalga Chlorella Pyrenoidosa Using Plackett-Burman Design and Response Surface Methodology. Bioresour. Technol. 2016, 207, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Barrow, J.L.; Beisner, B.E.; Giles, R.; Domaizon, I.; Gregory-Eaves, I. Macrophytes Moderate the Taxonomic and Functional Composition of Phytoplankton Assemblages during a Nutrient Loading Experiment. Freshw. Boil. 2019, 64, 1369–1381. [Google Scholar] [CrossRef]
- Blindow, I.; Hargeby, A.; Andersson, G. Seasonal Changes of Mechanisms Maintaining Clear Water in a Shallow Lake with Abundant Chara Vegetation. Aquat. Bot. 2002, 72, 315–334. [Google Scholar] [CrossRef]
- Mony, C.; Koschnick, T.J.; Haller, W.T.; Muller, S. Competition between Two Invasive Hydrocharitaceae (Hydrilla Verticillata (l.f.) (Royle) and Egeria Densa (Planch)) as Influenced by Sediment Fertility and Season. Aquat. Bot. 2007, 86, 236–242. [Google Scholar] [CrossRef]
- Gao, Y.N.; Ge, F.J.; Zhang, L.P.; He, Y.; Lu, Z.Y.; Zhang, Y.Y.; Liu, B.Y.; Zhou, Q.H.; Wu, Z.B. Enhanced Toxicity to the Cyanobacterium Microcystis Aeruginosa by Low-Dosage Repeated Exposure to the Allelochemical N-Phenyl-1-Naphthylamine. Chemosphere 2017, 174, 732–738. [Google Scholar] [CrossRef]
- Kayombo, S.; Mbwette, T.; Katima, J.; Jorgensen, S. Effects of Substrate Concentrations on the Growth of Heterotrophic Bacteria and Algae in Secondary Facultative Ponds. Water Res. 2003, 37, 2937–2943. [Google Scholar] [CrossRef]
- Phillips, G.; Willby, N.; Moss, B. Submerged Macrophyte Decline in Shallow Lakes; What have We Learnt in the Last Forty Years? Aquat. Bot. 2016, 135, 37–45. [Google Scholar] [CrossRef]
- Schindler, D.W.; Hecky, R.E.; Findlay, D.L.; Stainton, M.P.; Parker, B.R.; Paterson, M.J.; Beaty, K.G.; Lyng, M.; Kasian, S.E.M. Eutrophication of Lakes Cannot be Controlled by Reducing Nitrogen Input: Results of a 37-Year Whole-Ecosystem Experiment. Proc. Natl. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef]
- Barrat-Segretain, M.H. Biomass Allocation in Three Macrophyte Species in Relation to the Disturbance Level of Their Habitat. Freshw. Boil. 2001, 46, 935–945. [Google Scholar] [CrossRef]
- Bornette, G.; Puijalon, S. Response of Aquatic Plants to Abiotic Factors: A Review. Aquat. Sci. 2010, 73, 1–14. [Google Scholar] [CrossRef]
- Richardson, R.J. Aquatic Plant Management and the Impact of Emerging Herbicide Resistance Issues. Weed Technol. 2008, 22, 8–15. [Google Scholar] [CrossRef]
- Kuiper, J.J.; Kuiper, J.J.; Verhofstad, M.J.; Louwers, E.L.; Bakker, E.S.; Brederveld, R.J.; van Gerven, L.P.; Janssen, A.B.; de Klein, J.J.; Mooij, W.M. Mowing Submerged Macrophytes in Shallow Lakes with Alternative Stable States: Battling the Good Guys? Environ. Manag. 2017, 59, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Verhofstad, M.; Poelen, M.; Van Kempen, M.; Bakker, E.S.; Smolders, A. Finding the Harvesting Frequency to Maximize Nutrient Removal in a Constructed Wetland Dominated by Submerged Aquatic Plants. Ecol. Eng. 2017, 106, 423–430. [Google Scholar] [CrossRef]




| Number | Name | Percentage of Abundance (%) | Number | Name | Percentage of Abundance (%) |
|---|---|---|---|---|---|
| Cyanophyta | 61.2 | 10 | Ulothrix | 0.25 | |
| 1 | Microcystis | 58.54 | 11 | Crucigenia | 0.19 |
| 2 | Anabaena | 0.74 | 12 | Cosmarium | 0.14 |
| 3 | Aphanizomenon | 1.59 | 13 | Chlamydomonas | 0.10 |
| 4 | Dactylococcopsis | 0.23 | Bacillariophyta | 9.73 | |
| 5 | Chroococcus | 0.097 | 1 | Melosira | 4.79 |
| Chlorophyta | 27.5 | 2 | Cyclotella | 4.39 | |
| 1 | Scenedesmus | 20.73 | 3 | Navicula | 0.34 |
| 2 | Pediastrum | 1.64 | 4 | Navicula | 0.22 |
| 3 | Oocystis | 2.08 | Pyrrophyta | 0.37 | |
| 4 | Chodatela | 0.10 | 1 | Ceratium | 0.16 |
| 5 | Strurastrum | 0.25 | 2 | Peridinium | 0.21 |
| 6 | Schroederia | 0.07 | Cryptophyta | 1.2 | |
| 7 | Selenastrum | 1.74 | 1 | Cryptomonas | 0.94 |
| 8 | Closterium | 0.17 | 2 | Chroomonas | 0.26 |
| 9 | Tetraedron | 0.05 | |||
| Number | Factors | Response Variable | |||||
|---|---|---|---|---|---|---|---|
| Coded Level | Data Level | ||||||
| A | B | A | B | I (K)% | RGY (A)% | RGY (B)% | |
| 1 | −1 | −1 | 2.5 | 2.5 | 61.42 | 38.7 | 21.2 |
| 2 | 1 | −1 | 7.5 | 2.5 | 84.89 | 77.02 | 27.2 |
| 3 | −1 | 1 | 2.5 | 7.5 | 82.01 | 41.5 | 43.67 |
| 4 | 1 | 1 | 7.5 | 7.5 | 91.45 | 89.77 | 47.2 |
| 5 | −α | 0 | 1.46 | 5 | 65.86 | 25.67 | 26.84 |
| 6 | α | 0 | 8.54 | 5 | 92.16 | 83.86 | 39.04 |
| 7 | 0 | −α | 5 | 1.46 | 68.79 | 65.63 | 7.06 |
| 8 | 0 | α | 5 | 8.54 | 90.66 | 66.54 | 53.4 |
| 9 | 0 | 0 | 5 | 5 | 85.98 | 71.63 | 35.59 |
| 10 | 0 | 0 | 5 | 5 | 86.14 | 67.7 | 34.9 |
| 11 | 0 | 0 | 5 | 5 | 85.42 | 69 | 34 |
| 12 | 0 | 0 | 5 | 5 | 85.69 | 67.1 | 32.16 |
| 13 | 0 | 0 | 5 | 5 | 87.13 | 71.5 | 35.72 |
| Theoretical Value (%) | Verified Value (%) | |
|---|---|---|
| I(K) | 92.20 | 91.45 ± 1.60 |
| RGY(A) | 80.88 | 81.05 ± 1.88 |
| RGY(B) | 44.08 | 42.80 ± 2.96 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Yu, J.; Guo, F.; Pan, G.; Zhang, L.; Hu, H.; Lu, Y.; Dao, G. Optimization of Combined Submerged Macrophyte Planting Conditions for Inhibiting Algae by Response Surface Methodology. Water 2020, 12, 2093. https://doi.org/10.3390/w12082093
Wang S, Yu J, Guo F, Pan G, Zhang L, Hu H, Lu Y, Dao G. Optimization of Combined Submerged Macrophyte Planting Conditions for Inhibiting Algae by Response Surface Methodology. Water. 2020; 12(8):2093. https://doi.org/10.3390/w12082093
Chicago/Turabian StyleWang, Shengnan, Junxia Yu, Fang Guo, Guoqiang Pan, Lin Zhang, Hongying Hu, Yifeng Lu, and Guohua Dao. 2020. "Optimization of Combined Submerged Macrophyte Planting Conditions for Inhibiting Algae by Response Surface Methodology" Water 12, no. 8: 2093. https://doi.org/10.3390/w12082093
APA StyleWang, S., Yu, J., Guo, F., Pan, G., Zhang, L., Hu, H., Lu, Y., & Dao, G. (2020). Optimization of Combined Submerged Macrophyte Planting Conditions for Inhibiting Algae by Response Surface Methodology. Water, 12(8), 2093. https://doi.org/10.3390/w12082093




