Reduction of Nutrient Leaching Potential in Coarse-Textured Soil by Using Biochar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Soil Samples and Preparation of Biochars
2.2. Preparations of Leaching Column
2.3. Soil Column Incubation and Leaching
2.4. Analytical Methods
2.5. Statistical Analysis
3. Results
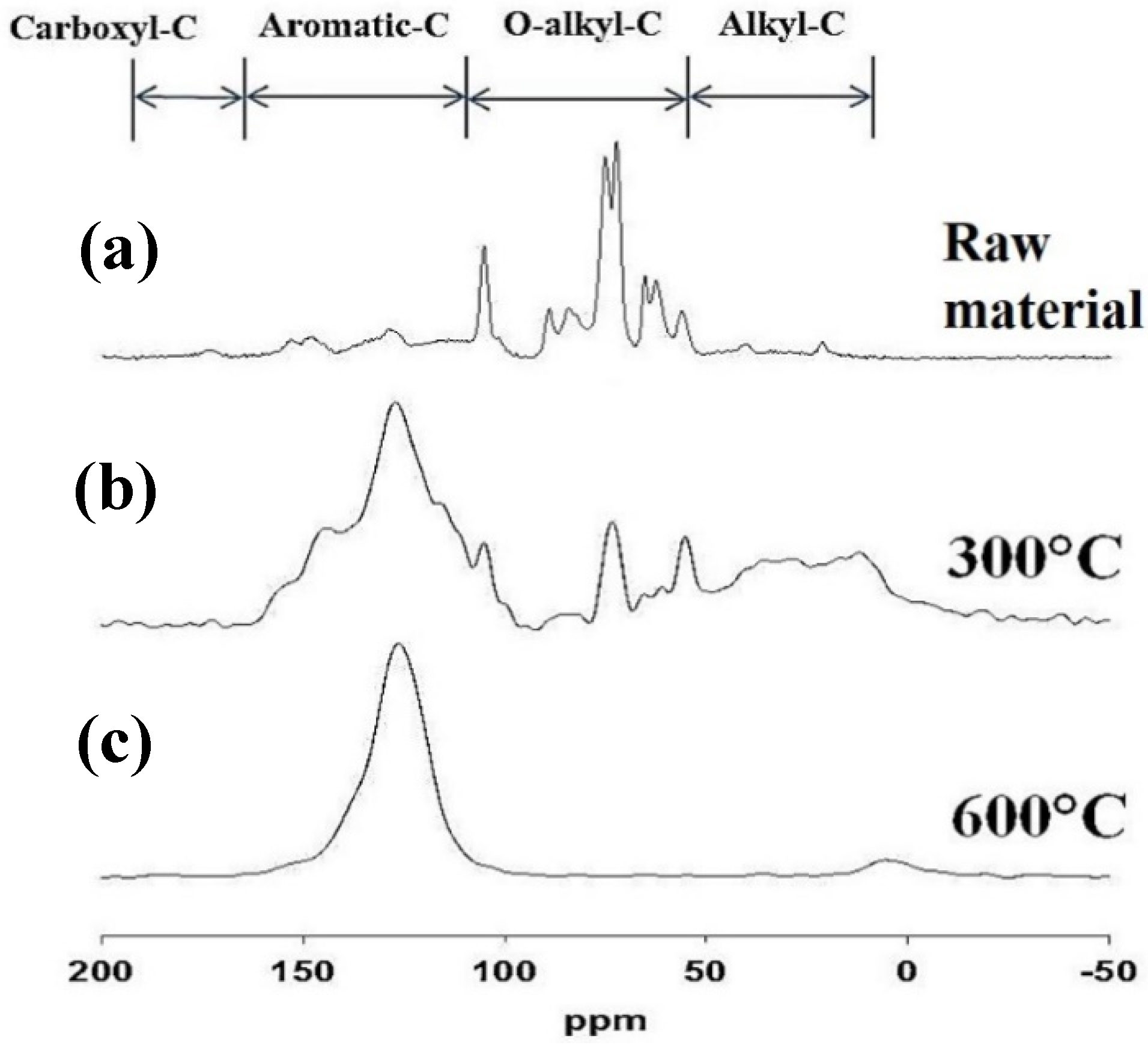
3.1. Properties of the Soil and Biochar Materials
3.2. Soil Physicochemical Properties
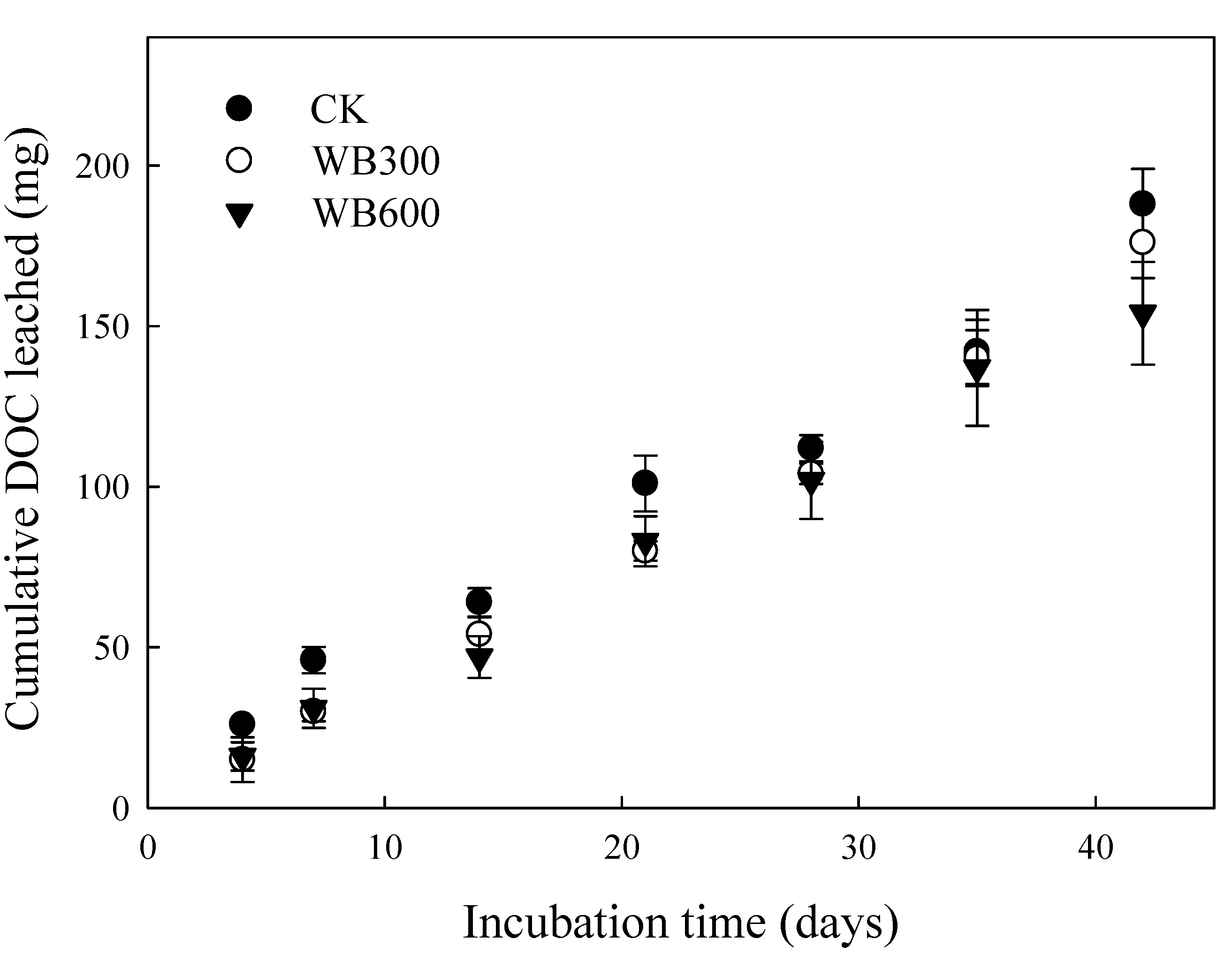
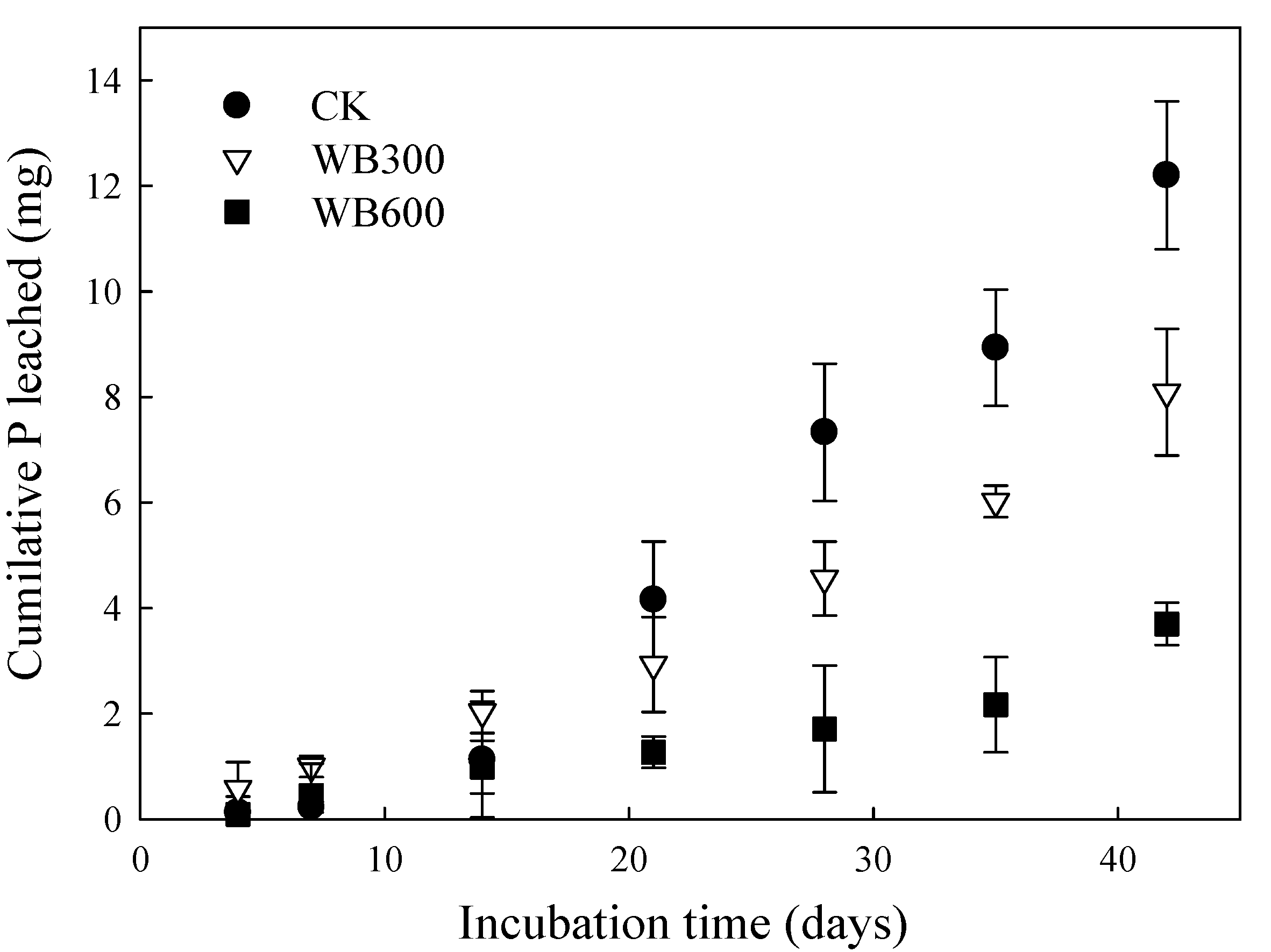
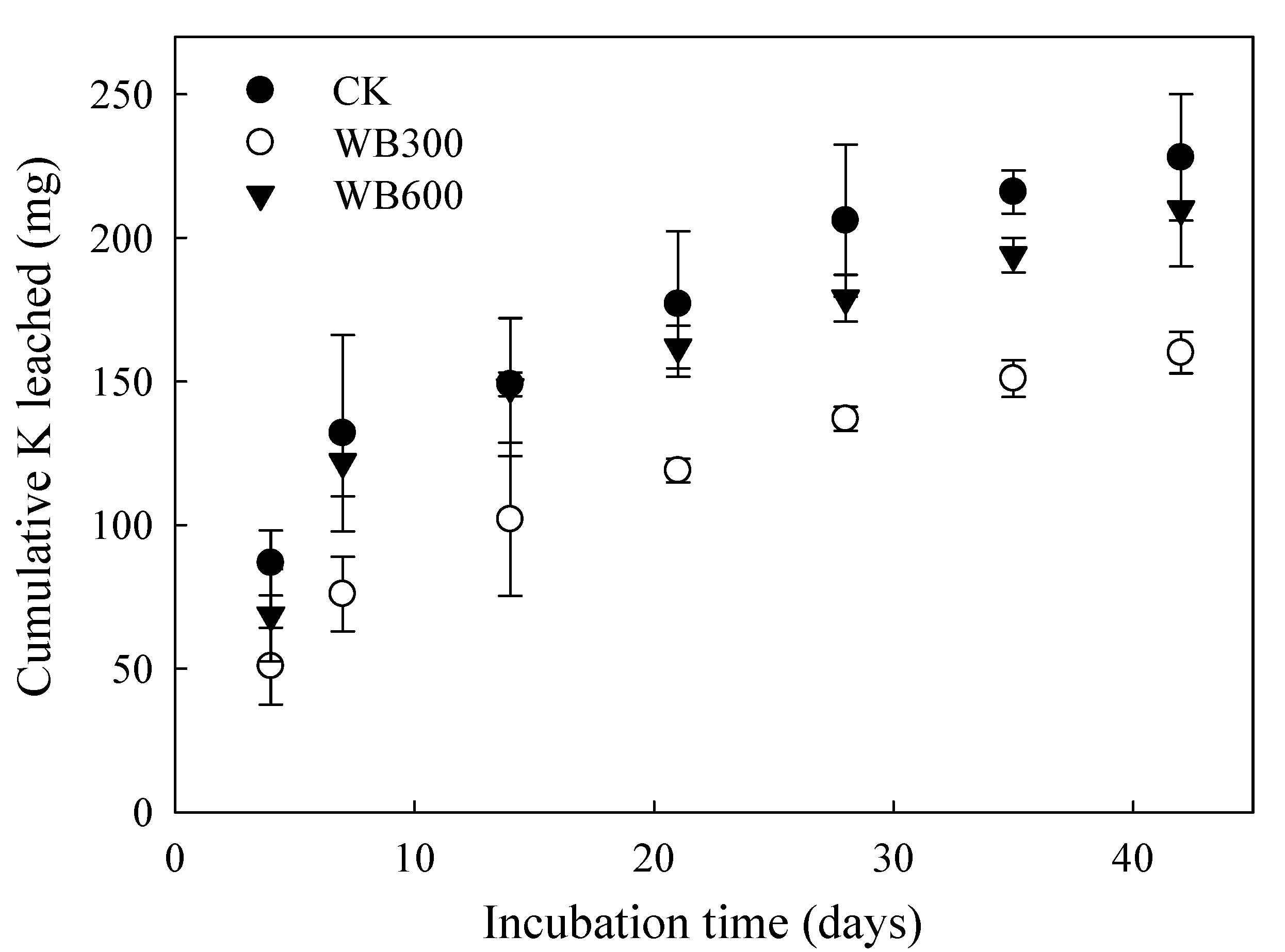
3.3. Properties of Leachate
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robertson, M. Sustainability Principles and Practice, 2nd ed.; Taylor & Francis: London, UK, 2017. [Google Scholar]
- Jordan, C.F.; Herrera, R. Tropical rain forests: Are nutrients really critical? Am. Nat. 1981, 117, 167–180. [Google Scholar] [CrossRef]
- Laird, D.; Fleming, P.; Wang, B.; Horton, R.; Karlen, D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef] [Green Version]
- O’Green, A.T.; Budd, R.; Gan, J.; Manynard, J.J.; Parikh, S.J.; Dahlgren, R.A. Mitigating nonpoint source pollution in agriculture with constructed and restored wetlands. Adv. Agron. 2010, 108, 1–76. [Google Scholar]
- Lal, R. Tillage and agricultural sustainability. Soil Tillage Res. 1991, 20, 133–146. [Google Scholar] [CrossRef]
- Lu, K.; Yang, X.; Shen, J.; Robinson, B.; Huang, H.; Liu, D.; Bolan, N.; Pei, J.; Wang, H. Effect of bamboo and rice straw biochars on the bioavailability of Cd, Cu, Pb and Zn to Sedum plumbizincicola. Agric. Ecosyst. Environ. 2014, 191, 124–132. [Google Scholar] [CrossRef]
- Jien, S.H.; Wang, C.C.; Lee, C.H.; Lee, T.Y. Stabilization of organic matter by biochar application in compost-amended soils with contrasting pH values and textures. Sustainability 2015, 7, 13317–13333. [Google Scholar] [CrossRef] [Green Version]
- Busscher, W.J.; Novak, J.M.; Evans, D.E.; Watts, D.W.; Niandou, M.; Ahmedna, M. Influence of pecan biochar on physical properties of a Norfolk loamy sand. Soil Sci. 2010, 175, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Kammann, C.I.; Linsel, S.; Gößling, J.W.; Koyro, H.W. Influence of biochar on drought tolerance of Chenopodium quinoa Willd and on soil–plant relations. Plant Soil 2011, 345, 195–210. [Google Scholar] [CrossRef]
- Basso, A.S.; Miguez, F.E.; Laird, D.A.; Horton, R.; Westgate, M. Assessing potential of biochar for increasing water-holding capacity of sandy soils. Gcb Bioenergy 2013, 5, 132–143. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.H.; Xu, R.K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef]
- Vaccari, F.; Baronti, S.; Lugato, E.; Genesio, L.; Castaldi, S.; Fornasier, F.; Miglietta, F. Biochar as a strategy to sequester carbon and increase yield in durum wheat. Eur. J. Agron. 2011, 34, 231–238. [Google Scholar] [CrossRef]
- Jien, S.H.; Wang, C.S. Effects of biochar on soil properties and erosion potential in a highly weathered soil. Catena 2013, 110, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Wang, J.; Xu, H.; Zhou, C.; Wang, S.; Xing, G. Effects of crop-straw biochar on crop growth and soil fertility over a wheat-millet rotation in soils of China. Soil Use Manag. 2014, 30, 311–319. [Google Scholar] [CrossRef]
- Clough, T.J.; Condron, L.M.; Kammann, C.; Müller, C. A review of biochar and soil nitrogen dynamics. Agronomy 2013, 3, 275–293. [Google Scholar] [CrossRef] [Green Version]
- Ventura, M.; Sorrenti, G.; Panzacchi, P.; George, E.; Tonon, G. Biochar reduces short-term nitrate leaching from a horizon in an apple orchard. J. Environ. Qual. 2013, 42, 76–82. [Google Scholar] [CrossRef]
- Laird, D.A. The charcoal vision: A win–win–win scenario for simultaneously producing bioenergy, permanently sequestering carbon, while improving soil and water quality. Agron. J. 2008, 100, 178–181. [Google Scholar]
- Gaunt, J.L.; Lehmann, L. Energy balance and emissions associated with biochar sequestration and pyrolysis bioenergy production. Environ. Sci. Technol. 2008, 42, 4152–4158. [Google Scholar] [CrossRef]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Stewart, C.E.; Cotrufo, M.F. Biochar and nitrogen fertilizer alters soil nitrogen dynamics and greenhouse gas fluxes from two temperate soils. J. Environ. Qual. 2012, 41, 1361–1370. [Google Scholar] [CrossRef] [Green Version]
- Stewart, C.E.; Zheng, J.; Botte, J.; Cotrufo, M.F. Co-generated fast pyrolysis biochar mitigates green-house gas emissions and increases carbon sequestration in temperate soils. GCB Bioenergy 2013, 5, 153–164. [Google Scholar] [CrossRef]
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal responses to biochar in soil–concepts and mechanisms. Plant Soil 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Jin, H. Characterization of Microbial Life Colonizing Biochar and Biochar-Amended Soils; Cornell University: New York, NY, USA, 2010. [Google Scholar]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Lehmann, J.; da Silva, J.P., Jr.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Major, J.; Rondon, M.; Molina, D.; Riha, S.J.; Lehmann, J. Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol. Plant Soil 2010, 333, 117–128. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zimmerman, A.R. Organic carbon and nutrient release from a range of laboratory-produced biochars and biochar–soil mixtures. Geoderma 2013, 193, 122–130. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A.R. Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef]
- Lo, C.H. Manual of Fertilizer Application, 6th ed.; Agriculture and Food Agency, Council of Agriculture, Executive Yuan: Taipei, Taiwan, 2005. [Google Scholar]
- Yoo, G.Y.; Kim, H.J.; Chen, J.J.; Kim, Y.S. Effects of biochar addition on nitrogen leaching and soil structure following fertilizer application to rice paddy soil. Soil Sci. Soc. Am. J. 2013, 78, 852–860. [Google Scholar] [CrossRef]
- Blake, G.R.; Hartge, K.H. Bulk density. In Methods of Soil Analysis, Part 1. Physical and Mineralogical Methods; Klut, A., Ed.; ASA and SSSA: Madison, WI, USA, 1986; Agronomy monograph No. 9; pp. 363–375. [Google Scholar]
- McLean, E. Soil pH and lime requirement. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; ASA and SSSA: Madison, WI, USA, 1982; pp. 199–224. [Google Scholar]
- Rhoades, J. 1982. Cation exchange capacity. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; ASA and SSSA: Madison, WI, USA, 1982; pp. 149–157. [Google Scholar]
- Gee, G.W.; Bauder, J.W.; Klute, A. Particle-size analysis. In Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods; Klut, A., Ed.; ASA and SSSA: Madison, WI, USA, 1986; Agronomy monograph No. 9; pp. 383–411. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. In Methods of Soil Analysis: Soil Science Society of America Book Series 5 Part 3—Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Eds.; ASA and SSSA: Madison, WI, USA, 1996; pp. 1201–1229. [Google Scholar]
- Nelson, D.W.; Sommers, L.E.; Sparks, D.; Page, A.; Helmke, P.; Loeppert, R.; Soltanpour, P.; Tabatabai, M.; Johnston, C.; Sumner, M. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Soil Science Society of America Book Series 5 Part 3—Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Eds.; ASA and SSSA: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Bray, R.H.; Kurtz, L. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Mulvaney, R.L. Nitrogen-Inorganic forms. In Methods of Soil Analysis: Soil Science Society of America Book Series 5 Part 3—Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Eds.; ASA and SSSA: Madison, WI, USA, 1996; pp. 1123–1184. [Google Scholar]
- Jien, S.H.; Chen, W.C.; Ok, Y.S.; Awad, Y.M.; Liao, C.S. Short-term biochar application induced variation of C and N mineralization in a compost-added rural soil. Environ. Sci. Pollut. Res. 2018. [Google Scholar] [CrossRef]
- Lee, C.H.; Wang, C.C.; Lin, H.H.; Lee, S.S.; Tsang, D.C.W.; Jien, S.H.; Ok, Y.S. In situ biochar application conserves water and nutrients while simultaneously mitigating the erosion of an Fe-oxide-enriched tropical soil. Sci. Total Environ. 2018, 619, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, B.P.; Cowie, A.L. Characterisation and evaluation of biochars for their application as a soil amendment. Soil Res. 2010, 48, 516–525. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zimmerman, A.R.; Harris, W. Surface chemistry variations among a series of laboratory-produced biochars. Geoderma 2011, 163, 247–255. [Google Scholar] [CrossRef]
- Kasozi, G.N.; Zimmerman, A.R.; Kizza, P.N.; Gao, B. Catechol and Humic Acid Sorption onto a Range of LaboratoryProduced Black Carbons (Biochars). Environ. Sci. Technol. 2010, 44, 6189–6195. [Google Scholar] [CrossRef] [PubMed]
- Steiner, C.; Glaser, B.; Teixeira, W.G.; Lehmann, J.; Blum, W.E.H.; Zech, W. Nitrogen retention and plant uptake on a highly weathered central Amazonian Ferralsol amended with compost and charcoal. J. Plant Nutr. Soil Sci. 2008, 171, 893–899. [Google Scholar] [CrossRef]
- Lentz, R.D.; Ippolito, J.A. Biochar and manure affect calcareous soil and corn silage nutrient concentrations and uptake. J. Environ. Qual. 2011, 41, 1033–1043. [Google Scholar] [CrossRef]
- Hagemann, N.; Kammann, C.; Schmidt, H.P.; Kappler, A. Nitrate capture and slow release in biochar amended compost and soil. PLoS ONE 2017, 12, e171214. [Google Scholar] [CrossRef]





| Properties | Leachate | Soil | |
|---|---|---|---|
| Leachate Volume | VL | ✓ | |
| pH | pH | ✓ | |
| Bulk Density | DB | ✓ | |
| Organic Carbon | OC | ✓ | ✓ |
| Ammonium Nitrogen | NH4+-N | ✓ | ✓ |
| Nitrate-Nitrogen | NO3−-N | ✓ | ✓ |
| Available Phosphorus | Ava. P | ✓ | ✓ |
| Exchangeable Potassium | Ex. K | ✓ | ✓ |
| Properties | Soil | Wood Biochar (WB) | |
|---|---|---|---|
| 300 °C (WB300) | 600 °C (WB600) | ||
| pH | 6.2 | 6.5 | 10.4 |
| EC (dS m−1) | 0.35 | - | - |
| Sand (%) | 72.5 | - | - |
| Silt (%) | 18.1 | - | - |
| Clay (%) | 9.4 | - | - |
| Texture | SL | - | - |
| DB (g cm−3) | 1.44 | - | - |
| DP (g cm−3) | 2.69 | - | - |
| Porosity (%) | 42.8 | - | - |
| CEC (cmol(+)/kg) | 10.2 | 55.1 | 20.4 |
| OC (%) | 0.33 | 6.8 | 2.0 |
| TC (%) | - | 69.0 | 79.5 |
| H (%) | - | 4.5 | 2.9 |
| O (%) | - | 24.7 | 14.6 |
| N (%) | - | 0.92 | 0.82 |
| H/C | - | 0.06 | 0.04 |
| O/C | - | 0.36 | 0.18 |
| NH4+-N (mg/kg) | 82.4 | 56.7 | 41.7 |
| NO3−-N (mg/kg) | 131 | 548 | 341 |
| Ava. P (mg/kg) | 6.63 | 5.29 | 3.30 |
| Ex. K (mg/kg) | 259 | 449 | 323 |
| Properties | Day | Treatments | ||
|---|---|---|---|---|
| CK | WB300 | WB600 | ||
| pH | 0 | 6.1 ± 0.1 a | 6.2 ± 0.1 a | 6.5 ± 0.1 b |
| 42 | 4.6 ± 0.1 a | 5.2 ± 0.2 b | 5.8 ± 0.1 c | |
| DB (g cm−3) | 42 | 1.11 ± 0.24 a | 1.09 ± 0.11 a | 1.05 ± 0.10 a |
| SOC (%) | 0 | 0.33 ± 0.05 a | 0.46 ± 0.03 b | 0.36 ± 0.05 a |
| 42 | 0.21 ± 0.05 a | 0.39 ± 0.05 b | 0.31 ± 0.07 a,b | |
| NH4+-N (mg kg−1) | 0 | 206 ± 6.28 a | 205 ± 6.16 a | 205 ± 6.35 a |
| 42 | 5.87 ± 1.19 a | 12.7 ± 1.15 b | 12.7 ± 1.21 b | |
| NO3−-N (mg kg−1) | 0 | 131 ± 8.35 a | 138 ± 8.98 a | 135 ± 8.14 a |
| 42 | 7.52 ± 1.14 a | 33.0 ± 1.31 c | 28.0 ± 3.63 b | |
| Inorganic N (mg kg−1) | 0 | 336 ± 8.61 a | 343 ± 9.60 a | 339 ± 8.38 a |
| 42 | 13.4 ± 0.82 a | 45.7 ± 0.11 c | 40.7 ± 3.42 b | |
| Ava. P (mg kg−1) | 0 | 19.6 ± 0.27 a | 19.6 ± 0.26 a | 19.8 ± 0.28 a |
| 42 | 4.08 ± 0.45 a | 5.10 ± 0.28 b | 5.36 ± 0.75 b | |
| Ex. K (mg kg−1) | 0 | 488 ± 46.2 a | 457 ± 30.6 a | 497 ± 45.2 a |
| 42 | 302 ± 3.02 a | 315 ± 0.24 b | 351 ± 0.13 c | |
| Treatments | Volume of the Leachate (mL) | ||||||
|---|---|---|---|---|---|---|---|
| Incubation Time (Days) | |||||||
| 4 | 7 | 14 | 21 | 28 | 35 | 42 | |
| CK | 530 ± 45 b | 539 ± 55 | 579 ± 39 | 598 ± 287 | 625 ± 21 | 624 ± 82 | 633 ± 08 b |
| WB300 | 378 ± 63 a | 459 ± 53 | 539 ± 23 | 577 ± 18 | 600 ± 22 | 598 ± 08 | 597 ± 11 a |
| WB600 | 377 ± 05 a | 426 ± 55 | 488 ± 110 | 525 ± 64 | 570 ± 05 | 598 ± 10 | 578 ± 24 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, Y.-L.; Lee, C.-H.; Jien, S.-H. Reduction of Nutrient Leaching Potential in Coarse-Textured Soil by Using Biochar. Water 2020, 12, 2012. https://doi.org/10.3390/w12072012
Kuo Y-L, Lee C-H, Jien S-H. Reduction of Nutrient Leaching Potential in Coarse-Textured Soil by Using Biochar. Water. 2020; 12(7):2012. https://doi.org/10.3390/w12072012
Chicago/Turabian StyleKuo, Yu-Lin, Chia-Hisng Lee, and Shih-Hao Jien. 2020. "Reduction of Nutrient Leaching Potential in Coarse-Textured Soil by Using Biochar" Water 12, no. 7: 2012. https://doi.org/10.3390/w12072012
APA StyleKuo, Y.-L., Lee, C.-H., & Jien, S.-H. (2020). Reduction of Nutrient Leaching Potential in Coarse-Textured Soil by Using Biochar. Water, 12(7), 2012. https://doi.org/10.3390/w12072012





