A Dual Source of Phosphorus to Lake Sediments Indicated by Distribution, Content, and Speciation: Inle Lake (Southern Shan State, Myanmar)
Abstract
1. Introduction
2. Study Area
3. Materials and Methods
- Inorganic-P (hereafter referred to as IP), extracted using 1 M HCl;
- Organic-P (hereafter referred to as OP), extracted on the same aliquot of IP, after calcination at 450 °C, using 1 M HCl;
- NaOH-extractable P (also indicated as P bound to Al, Fe and Mn oxy-hydroxides, hereafter referred to as Fe-P), extracted using 1 M NaOH;
- HCl-extractable P (also indicated as calcium-bound P, hereafter referred to as Ca-P), extracted on the same sample aliquot of the previous fraction, using 1 M HCl.
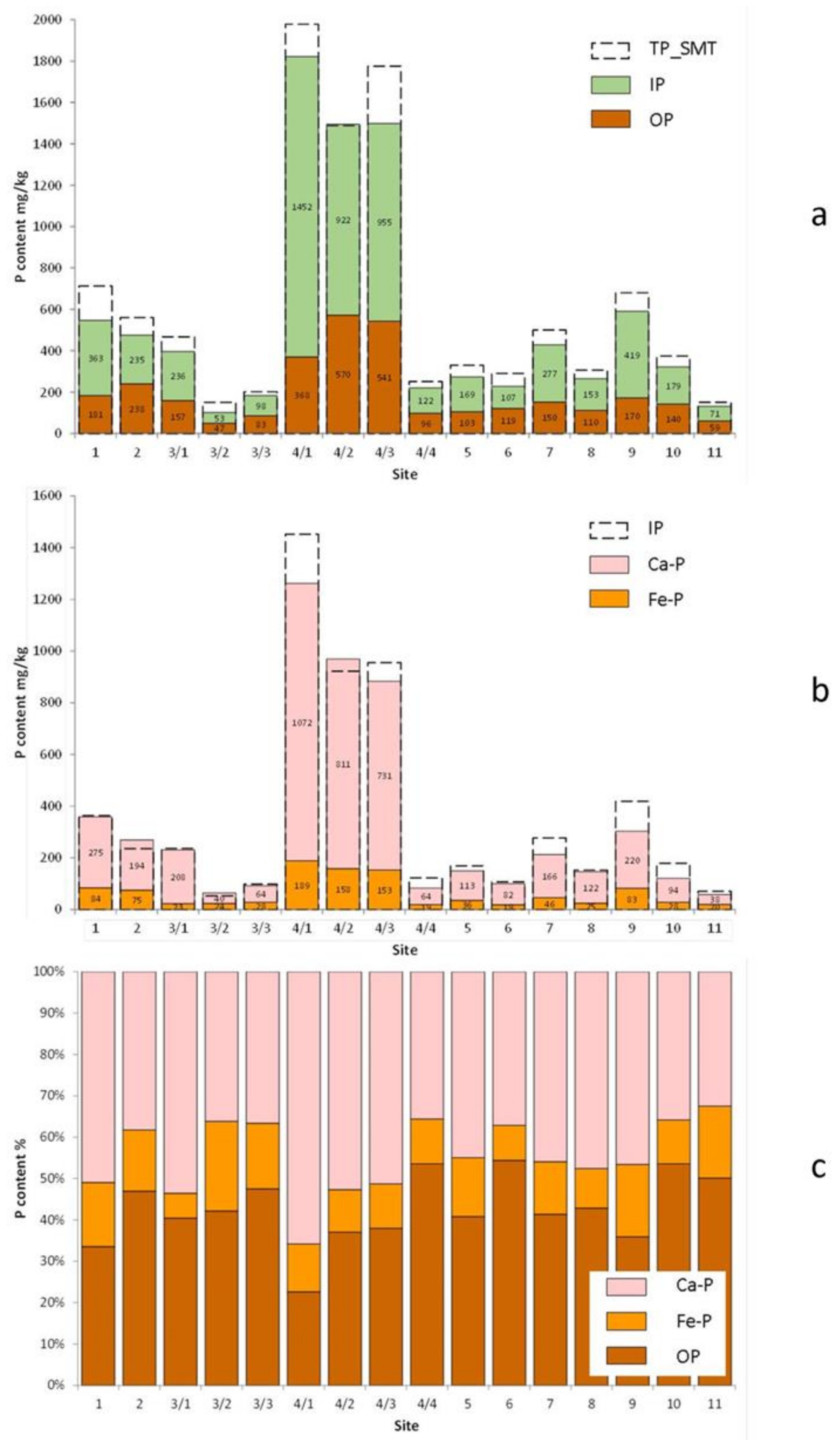
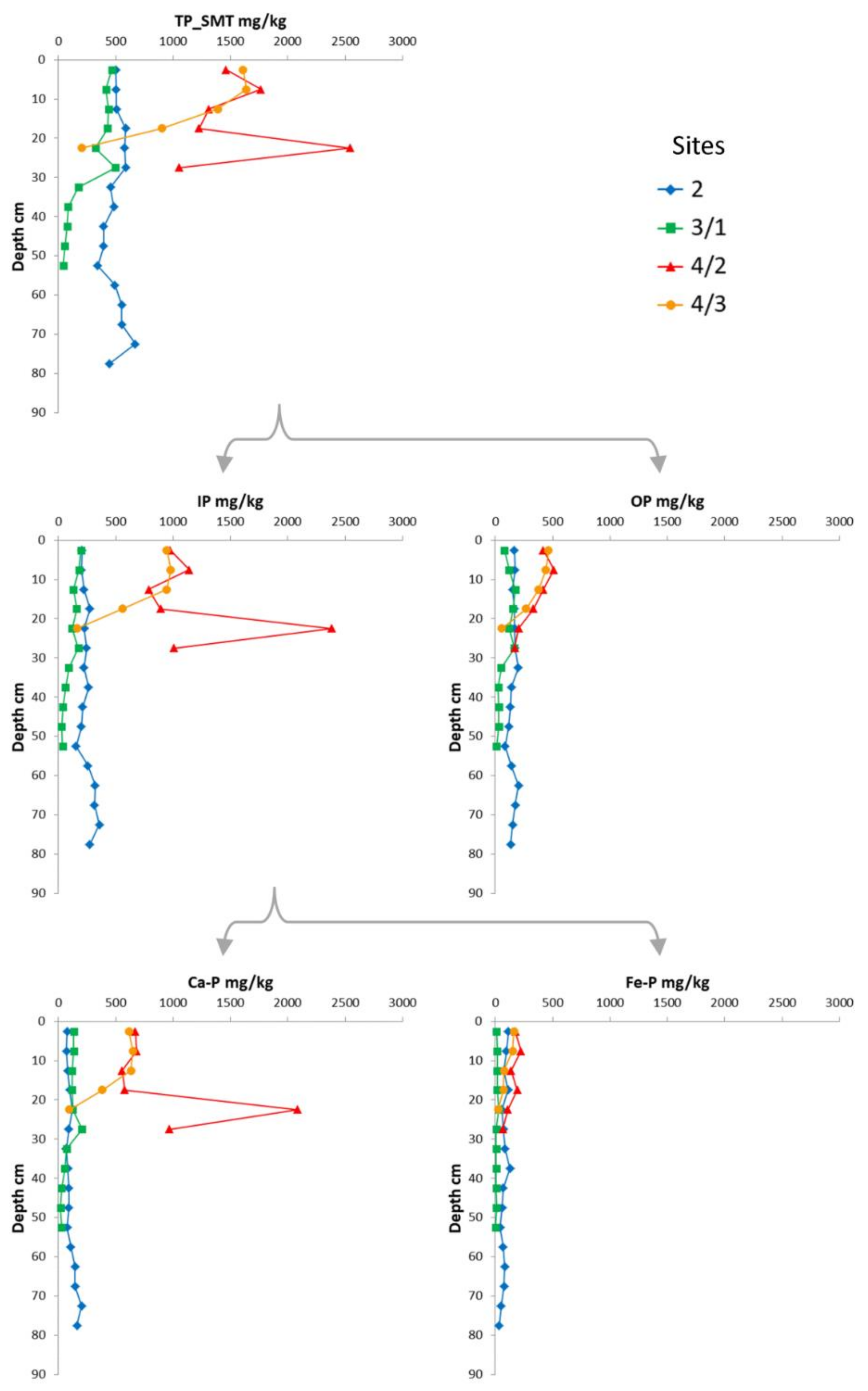
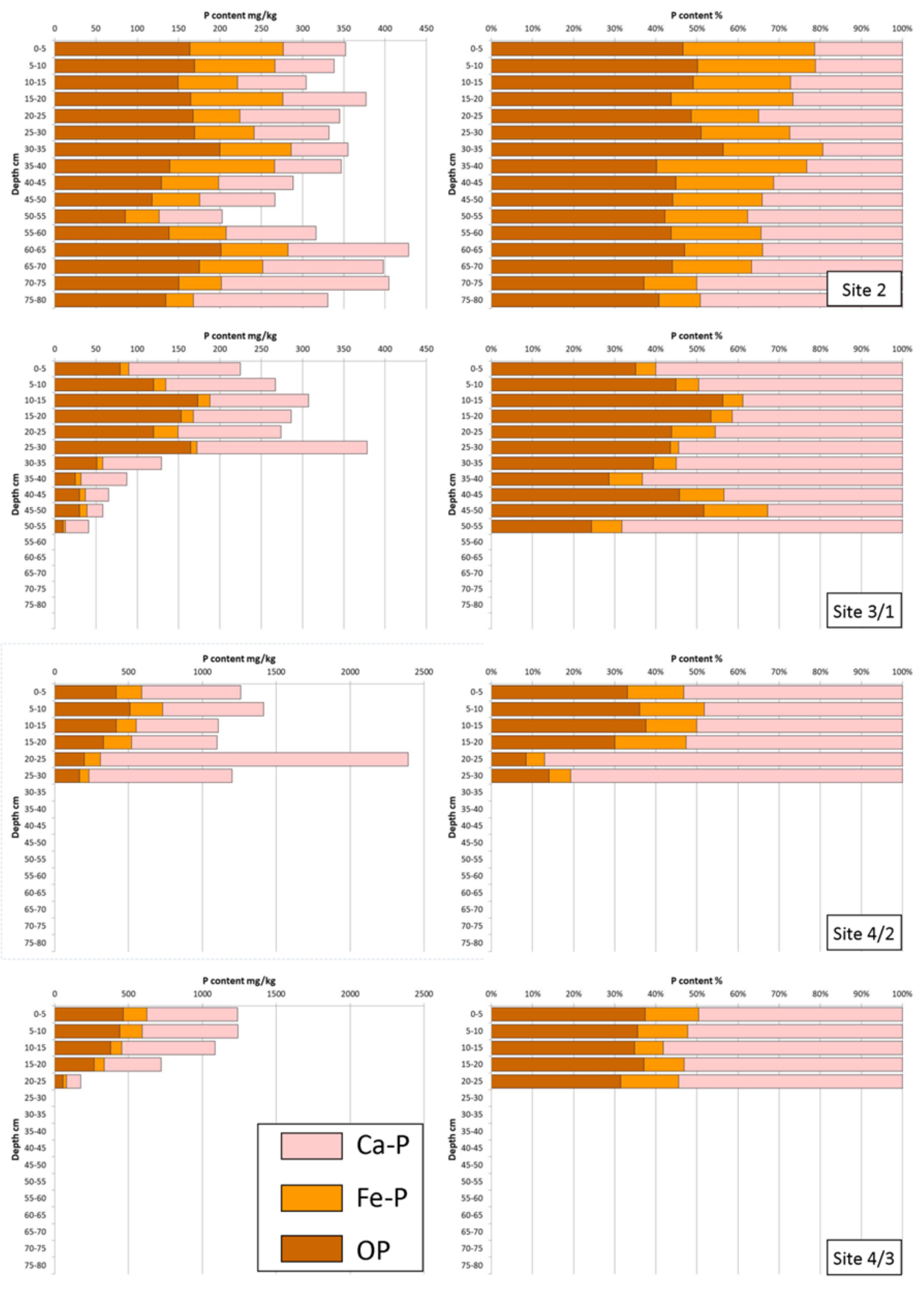
4. Results
5. Discussion
5.1. Identification of P Bearing Phases
5.2. Phosphorus Distribution in Lake Sediments
5.3. Characterisation of the Anthropogenic Phosphorus Inputs
5.4. Characterisation of the Dual Phosphorus Sources
- Factor 1 (F1), accounting for 60% of the total variance, is composed of the high positive loading for silicates (Kaolinite, Mica/Illite, and Quartz) and numerous elements (Ba, Be, Li, Na, K, Rb, Cs, Al, Fe, Mn, Co, Cr, Cu, Ni, Pb, V, Zn, Tl, Bi, Sn, As, Ag, Sc, Ti, Y, Zr, La, Ce, Hf, and Th). This factor reflects the binding of these elements to allogenic minerals. It is also characterised by the high negative correlation with Calcite content. The strongly bipolar character of the sediment mineralogy reflects the antithesis between detrital input and endogenic Calcite precipitation (see also the discussion in [37]). High factor scores for F1 are observed at or near inflow water (sites 1 and 2), indicating a detrital origin of these elements and minerals contributed to the lake by the erosion of the rocks and soil in the watershed.
- Factor 2 (F2) resulting from the analysis explains 10% of the total variance and is mostly composed of the positive relation of P, U, Cd, Mo, and Sb. This factor can be considered as the “P pollution factor” due to the association of this element to other trace elements that are commonly found in phosphate fertilisers [70,71,72]. High scores of F2 are recorded at sites 4/1, 4/2, and 4/3, in the floating gardens area, further supporting this interpretation.
- Factor 3 (F3), accounting for 8% of the total variance, is associated with the positive loadings for W, Nb, and Ta and the negative loading for Dolomite. These elements are classified as High Field Strength Elements (HFSE) and have similar geochemical behaviour. This factor is not clearly attributable to a natural or an anthropogenic process. Dolomite displays a low communality value (0.300), indicating that the factors do not adequately explain its variance. The attribution to this factor is uncertain, partly due to the relatively low number of samples showing the presence of this mineral [63].
- Factor 4 (F4), accounting for 6% of the total variance, is characterised by the negative loading for Ca, Mg, and Sr. This factor can be assumed as the carbonate pole as indicated by the excellent correlation of F4 with Mg and Sr, while the lower correlation with Ca could be due to the fact that concentrations of these elements above 50% could not be adequately assessed with the analytical method used.
- Factor 5 (F5), accounting for 4% of the total variance, is associated with the negative loading for Aragonite and Hematite and may be related to authigenic minerals of a biogenic origin [37].
- Factor 6 (F6), accounting for 2% of the total variance, is composed of Au and S. This factor cannot provide significant information as the two elements are only present in very small amounts which are close to the detecting limit.
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Granlund, K.; Räike, A.; Ekholm, P.; Rankinen, K.; Rekolainen, S. Assessment of water protection targets for agricultural nutrient loading in Finland. J. Hydrol. 2005, 304, 251–260. [Google Scholar] [CrossRef]
- Dokulil, M.T.; Teubner, K. Eutrophication and Climate Change: Present Situation and Future Scenarios. In Eutrophication: Causes, Consequences and Control; Springer: Dordrecht, The Netherlands, 2010; pp. 1–16. [Google Scholar]
- Prepas, E.E.; Charette, T.; Prepas, E.E.; Charette, T. Worldwide Eutrophication of Water Bodies: Causes, Concerns, Controls. TrGeo 2003, 9, 612. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Moss, B.; Jeppesen, E.; Søndergaard, M.; Lauridsen, T.L.; Liu, Z. Nitrogen, macrophytes, shallow lakes and nutrient limitation: Resolution of a current controversy? Hydrobiologia 2013, 710, 3–21. [Google Scholar] [CrossRef]
- Boström, B.; Pettersson, K. Different patterns of phosphorus release from lake sediments in laboratory experiments. Hydrobiologia 1982, 91–92, 415–429. [Google Scholar] [CrossRef]
- Kim, L.H.; Choi, E.; Gil, K.I.; Stenstrom, M.K. Phosphorus release rates from sediments and pollutant characteristics in Han River, Seoul, Korea. Sci. Total Environ. 2004, 321, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Macintosh, K.A.; Mayer, B.K.; McDowell, R.W.; Powers, S.M.; Baker, L.A.; Boyer, T.H.; Rittmann, B.E. Managing Diffuse Phosphorus at the Source versus at the Sink. Environ. Sci. Technol. 2018, 52, 11995–12009. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.T.; Zhu, X.D.; Sun, X.; Song, X.W. Identifying external nutrient reduction requirements and potential in the hypereutrophic Lake Taihu Basin, China. Environ. Sci. Pollut. Res. 2018, 25, 10014–10028. [Google Scholar] [CrossRef]
- Moore, P.A.; Reddy, K.R.; Fisher, M.M. Phosphorus Flux between Sediment and Overlying Water in Lake Okeechobee, Florida: Spatial and Temporal Variations. J. Environ. Qual. 1998, 27, 1428–1439. [Google Scholar] [CrossRef]
- Egemose, S.; de Vicente, I.; Reitzel, K.; Flindt, M.R.; Andersen, F.; Lauridsen, T.L.; Søndergaard, M.; Jeppesen, E.; Jensen, H.S. Changed cycling of P, N, Si, and DOC in danish lake nordborg after aluminum treatment. Can. J. Fish. Aquat. Sci. 2011, 68, 842–856. [Google Scholar] [CrossRef]
- Palmer-Felgate, E.J.; Mortimer, R.J.G.; Krom, M.D.; Jarvie, H.P.; Williams, R.J.; Spraggs, R.E.; Stratford, C.J. Internal loading of phosphorus in a sedimentation pond of a treatment wetland: Effect of a phytoplankton crash. Sci. Total Environ. 2011, 409, 2222–2232. [Google Scholar] [CrossRef]
- Orihel, D.M.; Baulch, H.M.; Casson, N.J.; North, R.L.; Parsons, C.T.; Seckar, D.C.M.; Venkiteswaran, J.J. Internal phosphorus loading in Canadian fresh waters: A critical review and data analysis. Can. J. Fish. Aquat. Sci. 2017, 74, 2005–2029. [Google Scholar] [CrossRef]
- Vollenweider, R.A. Advances in defining critical loading levels of phosphorous in lake eutrofication Mem. Ist. Ital. Idrobiol. 1976, 33, 53–83. [Google Scholar]
- Jeppesen, E.; Søndergaard, M.; Jensen, J.P.; Havens, K.E.; Anneville, O.; Carvalho, L.; Coveney, M.F.; Deneke, R.; Dokulil, M.T.; Foy, B.; et al. Lake responses to reduced nutrient loading - An analysis of contemporary long-term data from 35 case studies. Freshw. Biol. 2005, 50, 1747–1771. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Internal phosphorus loading in shallow Danish lakes. Hydrobiologia 1999, 408/409, 145–152. [Google Scholar] [CrossRef]
- Scheffer, M. Multiplicity of stable states in freshwater systems. Hydrobiologia 1990, 200–201, 475–486. [Google Scholar] [CrossRef]
- Scheffer, M.; Hosper, S.H.; Meijer, M.L.; Moss, B.; Jeppesen, E. Alternative equilibria in shallow lakes. Trends Ecol. Evol. 1993, 8, 275–279. [Google Scholar] [CrossRef]
- Scheffer, M.; Carpenter, S.; Foley, J.A.; Folke, C.; Walker, B. Catastrophic shifts in ecosystems. Nature 2001, 413, 591–596. [Google Scholar] [CrossRef]
- Andersen, T.K.; Nielsen, A.; Jeppesen, E.; Hu, F.; Bolding, K.; Liu, Z.; Søndergaard, M.; Johansson, L.S.; Trolle, D. Predicting ecosystem state changes in shallow lakes using an aquatic ecosystem model: Lake Hinge, Denmark, an example. Ecol. Appl. 2020, eap.2160. [Google Scholar] [CrossRef]
- Scheffer, M.; Van Nes, E.H. Shallow lakes theory revisited: Various alternative regimes driven by climate, nutrients, depth and lake size. In Proceedings of the Hydrobiologia; Springer: Berlin/Heidelberg, Germany, 2007; Volume 584, pp. 455–466. [Google Scholar]
- Viaroli, P.; Bartoli, M.; Giordani, G.; Naldi, M.; Orfanidis, S.; Zaldivar, J.M. Community shifts, alternative stable states, biogeochemical controls and feedbacks in eutrophic coastal lagoons: A brief overview. In Proceedings of the Aquatic Conservation: Marine and Freshwater Ecosystems; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; Volume 18, pp. S105–S117. [Google Scholar]
- Sirota, J.; Baiser, B.; Gotelli, N.J.; Ellison, A.M. Organic-matter loading determines regime shifts and alternative states in an aquatic ecosystem. Proc. Natl. Acad. Sci. USA 2013, 110, 7742–7747. [Google Scholar] [CrossRef]
- Perrone, U.; Facchinelli, A.; Sacchi, E. Phosphorus dynamics in a small eutrophic Italian lake. Water Air. Soil Pollut. 2008, 189, 335–351. [Google Scholar] [CrossRef]
- Şahin, Y.; Demirak, A.; Keskin, F. Phosphorus fractions and its potential release in the sediments of Koycegiz Lake, Turkey. Ponds 2012, 6, 139–153. [Google Scholar]
- Bassi, N.; Kumar, M.D.; Sharma, A.; Pardha-Saradhi, P. Status of wetlands in India: A review of extent, ecosystem benefits, threats and management strategies. J. Hydrol. Reg. Stud. 2014, 2, 1–19. [Google Scholar] [CrossRef]
- Butkus, S.; Myint, S. Pesticide Use Limits for Protection of Human Health in Inle Lake (Myanmar) Watershed; Living Earth Institute: Olympia, WA, USA, 2001. [Google Scholar]
- Michalon, M.; Gunnell, Y.; Lejot, J.; Mialhe, F.; Aung, T. Accelerated degradation of Lake Inle (Myanmar): A baseline study for environmentalists and developers. L. Degrad. Dev. 2019, 30, 928–941. [Google Scholar] [CrossRef]
- Inlay Lake Ramsar Site|Ramsar Sites Information Service. Available online: https://rsis.ramsar.org/ris/2356 (accessed on 10 June 2020).
- Akaishi, F.; Satake, M.; Otaki, M.; Tominaga, N. Surface water quality and information about the environment surrounding Inle Lake in Myanmar. Limnology 2006, 7, 57–62. [Google Scholar] [CrossRef]
- Lwin, Z.; Sharma, M.P. Environmental Management of the Inle Lake in Myanmar. Hydro Nepal J. Water Energy Environ. 2012, 11, 57–60. [Google Scholar] [CrossRef]
- Mjelde, M.; Ballot, A.; Swe, T.; Eriksen, T.E.; Nesheim, I.; Aung, T.T. Integrated Water Resources Management in Myanmar. Assessing ecological status in Inlay Lake. Preliminary report Report n. 7163-2017, Norwegian Institute for Water Research 2017; p. 76. Available online: https://niva.brage.unit.no/niva-xmlui/handle/11250/2449892 (accessed on 13 July 2020).
- Re, V.; Thin, M.M.; Setti, M.; Comizzoli, S.; Sacchi, E. Present status and future criticalities evidenced by an integrated assessment of water resources quality at catchment scale: The case of Inle Lake (Southern Shan state, Myanmar). Appl. Geochem. 2018, 92, 82–93. [Google Scholar] [CrossRef]
- Koschel, R.; Bemmdorf, J.; Proft, G.; Recknagel, F. Calcite precipitation as a natural control mechanism of eutrophication. Arch. für Hydrobiol. 1983, 98, 380–408. [Google Scholar]
- Thin, M.M.; Sacchi, E.; Setti, M. Hydrological processes at Inle Lake (Southern Shan State, Myanmar) inferred from hydrochemical, mineralogical and isotopic data. Isotopes Environ. Health Stud. 2016, 52, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Hope, G.; van der Kaars, S.; Finn, J.; Moe, K.Z. Holocene environmental change at Inle Lake, Shan State, Myanmar, and its implications for the regional development of agriculture. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 523, 18–29. [Google Scholar] [CrossRef]
- Thin, M.M.; Setti, M.; Sacchi, E.; Re, V.; Riccardi, M.P.; Allais, E. Mineralogical and geochemical characterisation of alkaline lake sediments to trace origin, depositional processes, and anthropogenic impacts: Inle Lake (Southern Shan State, Myanmar). Environ. Earth Sci. 2020, 79, 1–18. [Google Scholar] [CrossRef]
- Rainfall Radar and Weather Nyaungshwe. 14 day forecast Nyaungshwe. Available online: http://www.meteovista.co.uk/Asia/Myanmar/Nyaungshwe/3408406 (accessed on 10 June 2020).
- Tun, Z.W. Seasonal Variations of Air Temperature and Rainfall of Naung Shwe Township; Myanmar Agricultural Service: Naung Shwe, Myanmar, 2014. [Google Scholar]
- Su, M.; Jassby, A.D. Inle: A large Myanmar lake in transition. Lakes Reserv. Res. Manag. 2000, 5, 49–54. [Google Scholar] [CrossRef]
- Sidle, R.C.; Ziegler, A.D.; Vogler, J.B. Contemporary changes in open water surface area of Lake Inle, Myanmar. Sustain. Sci. 2007, 2, 55–65. [Google Scholar] [CrossRef]
- Okamoto, I. Coping and adaptation against decreasing fish resources: Case study of fishermen in Lake Inle, Myanmar. IDE Discuss. Pap. 2012, 329, 1–28. [Google Scholar]
- Government of Myanmar. Long Term Restoration and Conservation Plan for Inle Lake; Government of Myanmar: Naypyidaw, Myanmar, 2014. [Google Scholar]
- Htwe, T.N.; Kywe, M.; Buerkert, A.; Brinkmann, K. Transformation processes in farming systems and surrounding areas of Inle Lake, Myanmar, during the last 40 years. J. Land Use Sci. 2015, 10, 205–223. [Google Scholar] [CrossRef]
- Metcalfe, I. Kyi Pyar Aung Late Tournaisian conodonts from the Taungnyo Group near Loi Kaw, Myanmar (Burma): Implications for Shan Plateau stratigraphy and evolution of the Gondwana-derived Sibumasu Terrane. Gondwana Res. 2014, 26, 1159–1172. [Google Scholar] [CrossRef]
- Oo, T.; Hlaing, T.; Htay, N. Permian of Myanmar. J. Asian Earth Sci. 2002, 20, 683–689. [Google Scholar] [CrossRef]
- Bertrand, G.; Rangin, C. Tectonics of the western margin of the Shan plateau (central Myanmar): Implication for the India-Indochin oblique convergence since the Oligocene. J. Asian Earth Sci. 2003, 21, 1139–1157. [Google Scholar] [CrossRef]
- Robinson, R.A.J.; Brezina, C.A.; Parrish, R.R.; Horstwood, M.S.A.; Nay, W.O.; Bird, M.I.; Myint, T.; Walters, A.S.; Oliver, G.J.H.; Zaw, K. Large rivers and orogens: The evolution of the Yarlung Tsangpo-Irrawaddy system and the eastern Himalayan syntaxis. Gondwana Res. 2014, 26, 112–121. [Google Scholar] [CrossRef]
- Wang, Y.; Sieh, K.; Tun, S.T.; Lai, K.Y.; Myint, T. Active tectonics and earthquake potential of the Myanmar region. J. Geophys. Res. Solid Earth 2014, 119, 3767–3822. [Google Scholar] [CrossRef]
- Crosetto, S.; Watkinson, I.M.; Min, S.; Gori, S.; Falcucci, E. Nwai Le Ngal Evidence of Quaternary and recent activity along the Kyaukkyan Fault, Myanmar. J. Asian Earth Sci. 2018, 156, 207–225. [Google Scholar] [CrossRef]
- Htwe, T.N.; Brinkmann, K.; Buerkert, A. Spatio-temporal assessment of soil erosion risk in different agricultural zones of the Inle Lake region, southern Shan State, Myanmar. Environ. Monit. Assess. 2015, 187, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ma, T. Floating Island Agriculture (Ye-Chan) of Inle Lake. Ph.D. Thesis, University of Yangon, Yangon, Myanmar, 1996. [Google Scholar]
- Buijtendijk, H.; Tschunkert, K. Hotel industry expansion and sustainable development: A case study of Inle Lake, Myanmar. Res. Hosp. Manag. 2016, 6, 9–23. [Google Scholar] [CrossRef]
- Pradhan, N.; Habib, H.; Venkatappa, M.; Ebbers, T.; Duboz, R.; Shipin, O. Framework tool for a rapid cumulative effects assessment: Case of a prominent wetland in Myanmar. Environ. Monit. Assess. 2015, 187, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Allais, E. Studio Geochimico Delle Acque e dei Sedimenti di Alcuni Laghi Piemontesi al Fine di una Ricostruzione Paleoclimatica e Paleoambientale Regionale; University of Torino: Torino, Italy, 2001. [Google Scholar]
- Thin, M.M. Environmental Applications of Physical Nuclear and Geochemical Techniques: Origin, Dynamics and Impact of Phosphates and Heavy Metals in Cultivated Areas and at Inle Lake, Myanmar; University of Mandalay: Mandalay, Myanmar, 2015. [Google Scholar]
- Boyle, J. A comparison of two methods for estimating the organic matter content of sediments. J. Paleolimnol. 2004, 31, 125–127. [Google Scholar]
- Ruban, V.; López-Sánchez, J.F.; Pardo, P.; Rauret, G.; Muntau, H.; Quevauviller, P. Selection and evaluation of sequential extraction procedures for the determination of phosphorus forms in lake sediment. J. Environ. Monit. 1999, 1, 51–56. [Google Scholar] [CrossRef]
- Ruban, V.; López-Sánchez, J.F.; Pardo, P.; Rauret, G.; Muntau, H.; Quevauviller, P. Harmonized protocol and certified reference material for the determination of extractable contents of phosphorus in freshwater sediments - A synthesis of recent works. Anal. Bioanal. Chem. 2001, 370, 224–228. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Li, H.; Morrison, R.J. Sequential extraction procedures for the determination of phosphorus forms in sediment. Limnology 2013, 14, 147–157. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. Test Methods for Phosphorous in Water D 515-88, 474–483. 1988. Available online: http://www.astm.org/cgi-bin/resolver.cgi?D515 (accessed on 13 July 2020).
- Thin, M.M.; Sacchi, E.; Setti, M. Geochemical and Mineralogical Composition of Grab and Core Sediments from Inle Lake (Southern Shan State, Myanmar). Available online: https://doi.pangaea.de/10.1594/PANGAEA.908284 (accessed on 11 June 2020).
- Chatfield, C.; Collins, A.J. Introduction to Multivariate Analysis; Chapman and Hall: London, UK, 1980; ISBN 9780412160301. [Google Scholar]
- SPSS Inc. Released 2007. SPSS for Windows, Version 16.0; SPSS Inc.: Chicago, IL, USA, 2007. [Google Scholar]
- Tarvainen, T.; Reeder, S.; Albanese, S. Database Management and Map Production. In Geochemical Atlas of Europe. Part 1—Background Information, Methodology and Maps; Salminen, R., Ed.; Geological Survey of Finland: Espoo, Finland, 2005; ISBN 951-690-921-3. [Google Scholar]
- Kaiser, H.F. The varimax criterion for analytic rotation in factor analysis. Psychometrika 1958, 23, 187–200. [Google Scholar] [CrossRef]
- Singh, B.R.; Krogstad, T.; Shivay, Y.S.; Shivakumar, B.G.; Bakkegard, M. Phosphorus fractionation and sorption in P-enriched soils of Norway. Nutr. Cycl. Agroecosyst. 2005, 73, 245–256. [Google Scholar] [CrossRef]
- Golterman, H.L. The role of the ironhydroxide-phosphate-sulphide system in the phosphate exchange between sediments and overlying water. Hydrobiologia 1995, 297, 43–54. [Google Scholar] [CrossRef]
- Lottermoser, B.G. Trace metal enrichment in sugarcane soils due to the long-term application of fertilisers, North Queensland, Australia: Geochemical and Pb, Sr, and U isotopic compositions. Soil Res. 2009, 47, 311–320. [Google Scholar] [CrossRef]
- Niu, L.; Yang, F.; Xu, C.; Yang, H.; Liu, W. Status of metal accumulation in farmland soils across China: From distribution to risk assessment. Environ. Pollut. 2013, 176, 55–62. [Google Scholar] [CrossRef]
- Azzi, V.; Kazpard, V.; Lartiges, B.; Kobeissi, A.; Kanso, A.; El Samrani, A.G. Trace Metals in Phosphate Fertilizers Used in Eastern Mediterranean Countries. CLEAN—Soil Air Water 2017, 45. [Google Scholar] [CrossRef]
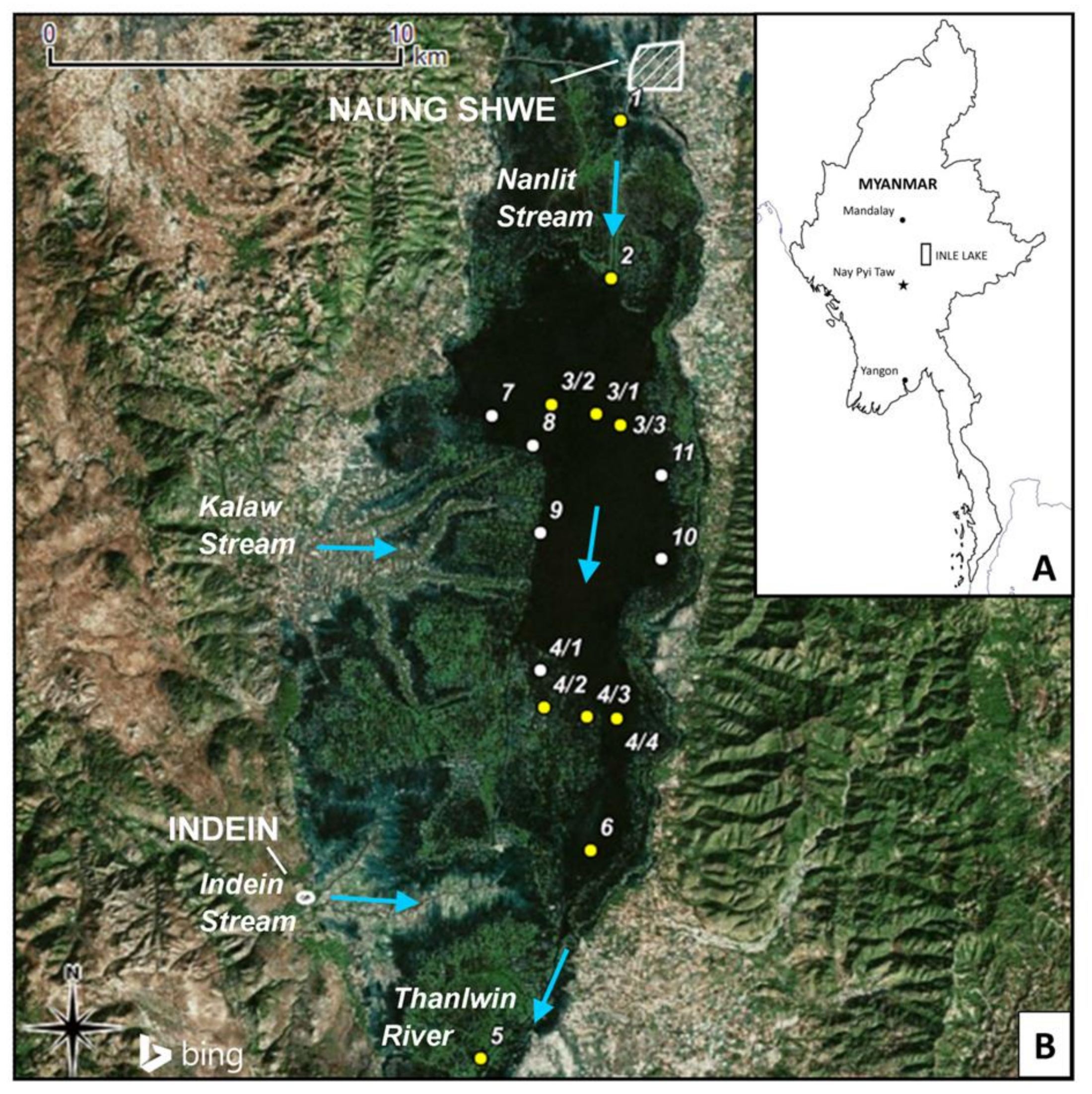
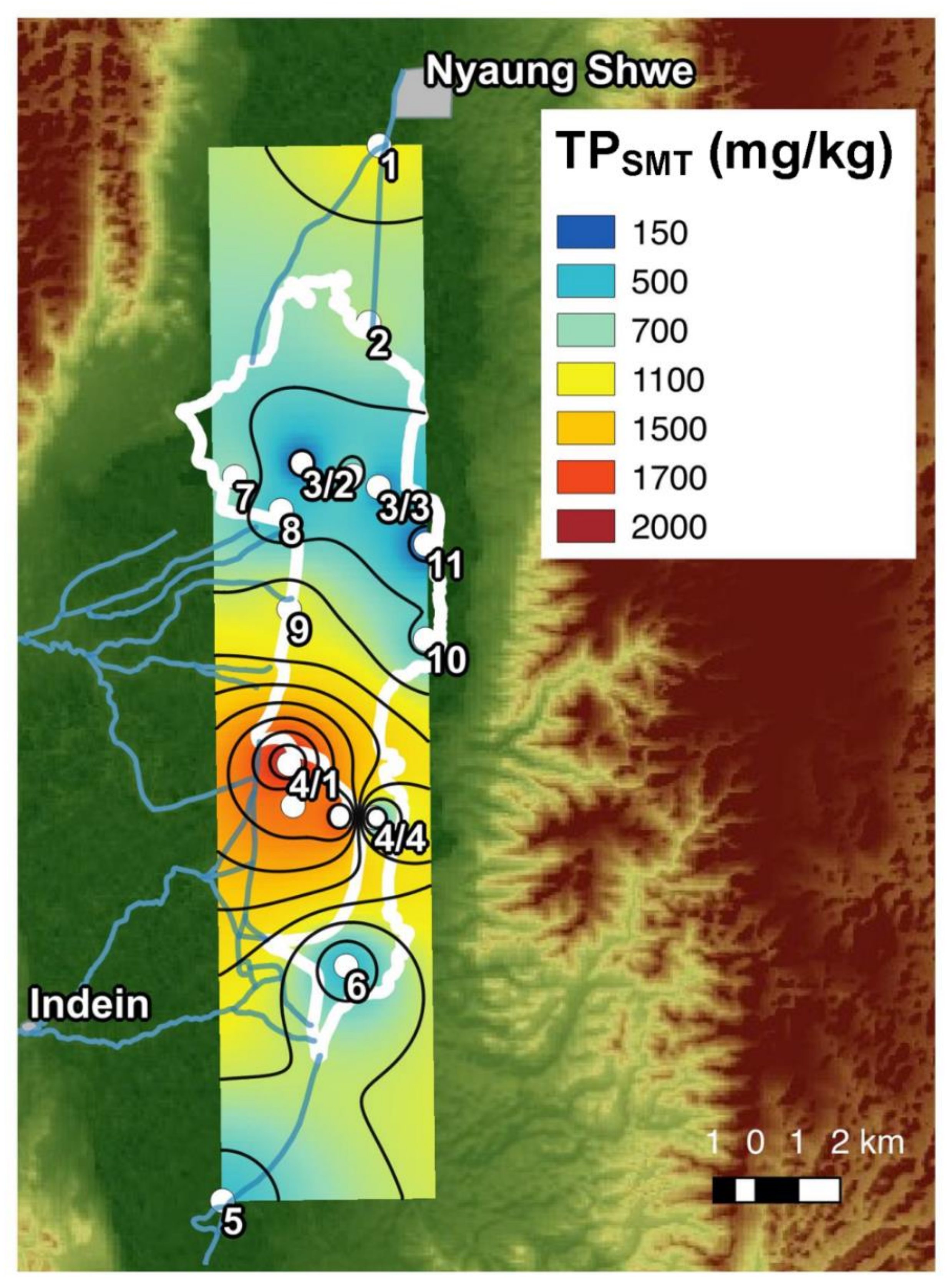
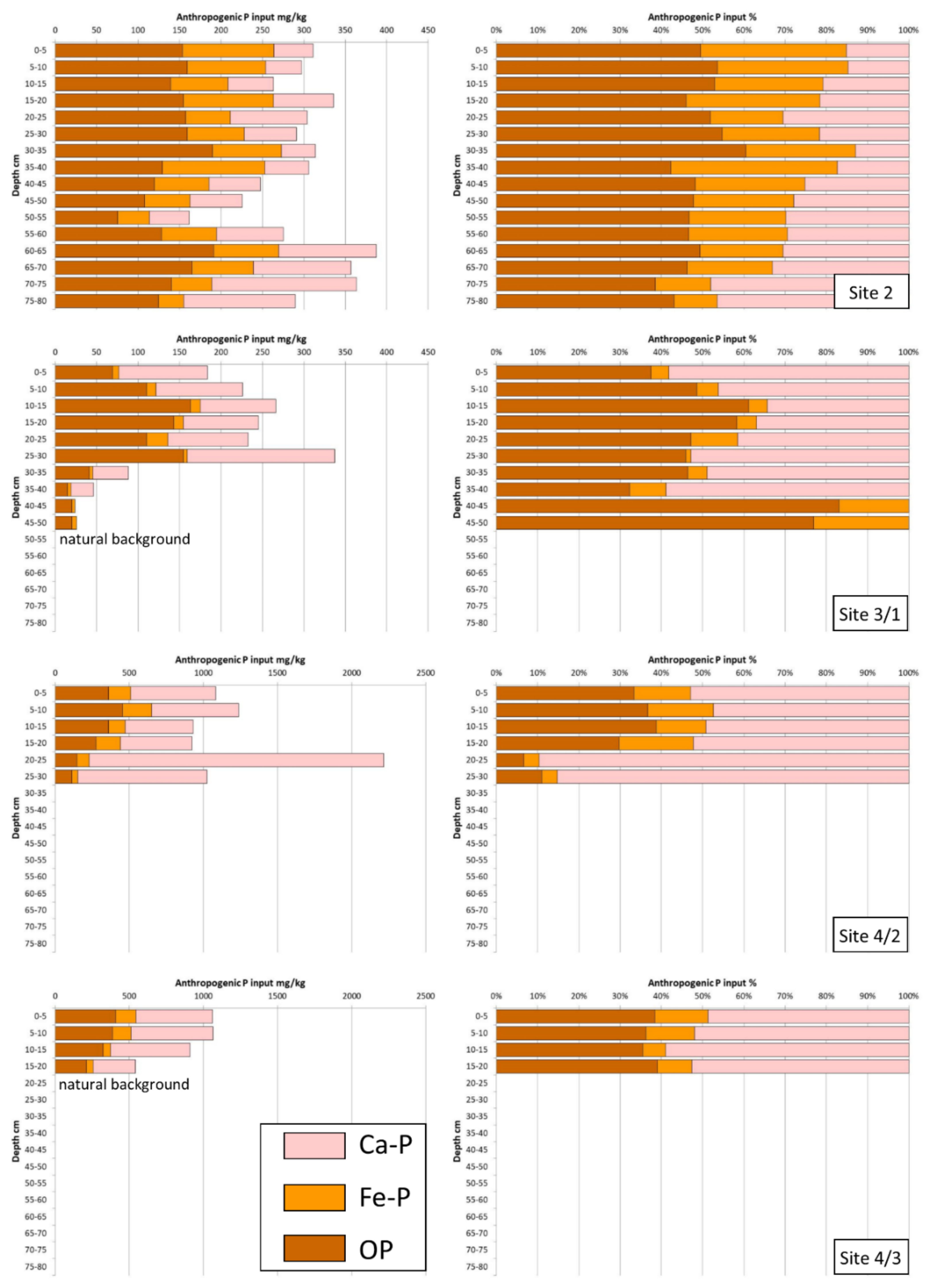
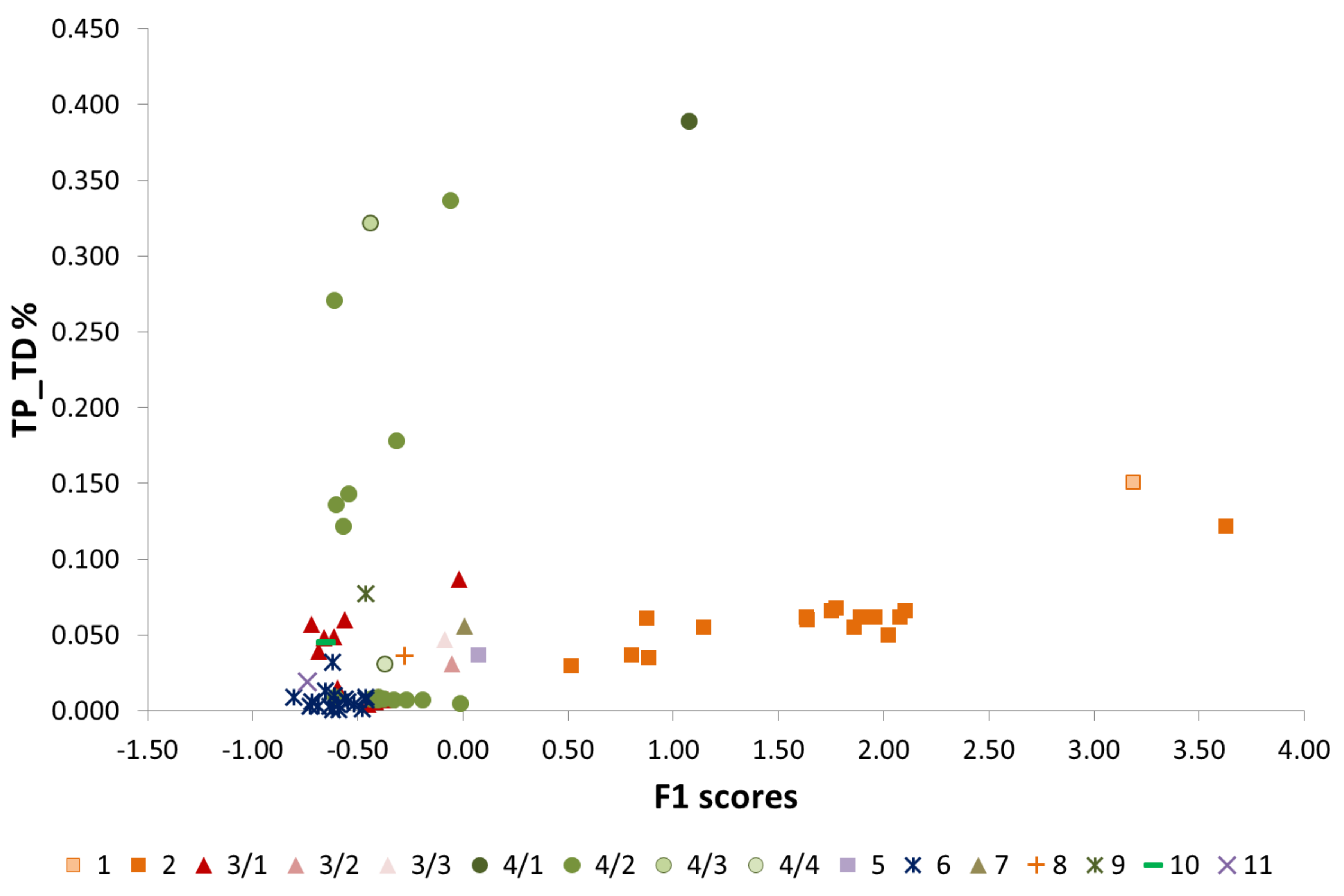
| Site | Longitude (UTM) | Latitude (UTM) | Environmental Setting | Potential Nearby Pollution Sources | Samples Collected |
|---|---|---|---|---|---|
| 1 | 47Q 0283427 | 2284690 | Nanlit stream—inflow | Naung Shwe Township and motorboat circulation | G |
| 2 | 47Q 0283122 | 2280515 | Nanlit stream—lake entrance | Motorboat circulation | G, C |
| 3/1 | 47Q 0282707 | 2276910 | Inle lake—wide area—Centre | Detrital input form the Nanlit stream | G, C |
| 3/2 | 47Q 0281508 | 2277202 | Inle lake—wide area—West | None | G |
| 3/3 | 47Q 0283305 | 2276640 | Inle lake—wide area—East | None | G |
| 4/1 | 47Q 0281104 | 2270131 | Floating gardens area—Northern entrance | Floating gardens | G |
| 4/2 | 47Q 0281190 | 2269152 | Floating gardens area—Centre | Floating gardens and Kaylar village | G, C |
| 4/3 | 47Q 0282356 | 2268878 | Floating gardens area—Eastern entrance | Floating gardens | G, C |
| 4/4 | 47Q 0283103 | 2268840 | Inle lake—narrow area | Floating gardens | G |
| 5 | 47Q 0279397 | 2259847 | Lower Belu stream—outflow | Helon village | G |
| 6 | 47Q 0282396 | 2265350 | Inle lake—narrow area | None | G |
| 7 | 47Q 0279908 | 2276921 | Inle lake—Western bank | Floating gardens | G |
| 8 | 47Q 0280995 | 2276119 | Inle lake—Western bank | Floating gardens | G |
| 9 | 47Q 0281155 | 2273786 | Inle lake—Western bank | Floating gardens | G |
| 10 | 47Q 0284373 | 2273034 | Inle lake—Eastern bank | None | G |
| 11 | 47Q 0284409 | 2275275 | Inle lake—Eastern bank | Tourist resorts | G |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thin, M.M.; Sacchi, E.; Setti, M.; Re, V. A Dual Source of Phosphorus to Lake Sediments Indicated by Distribution, Content, and Speciation: Inle Lake (Southern Shan State, Myanmar). Water 2020, 12, 1993. https://doi.org/10.3390/w12071993
Thin MM, Sacchi E, Setti M, Re V. A Dual Source of Phosphorus to Lake Sediments Indicated by Distribution, Content, and Speciation: Inle Lake (Southern Shan State, Myanmar). Water. 2020; 12(7):1993. https://doi.org/10.3390/w12071993
Chicago/Turabian StyleThin, Myat Mon, Elisa Sacchi, Massimo Setti, and Viviana Re. 2020. "A Dual Source of Phosphorus to Lake Sediments Indicated by Distribution, Content, and Speciation: Inle Lake (Southern Shan State, Myanmar)" Water 12, no. 7: 1993. https://doi.org/10.3390/w12071993
APA StyleThin, M. M., Sacchi, E., Setti, M., & Re, V. (2020). A Dual Source of Phosphorus to Lake Sediments Indicated by Distribution, Content, and Speciation: Inle Lake (Southern Shan State, Myanmar). Water, 12(7), 1993. https://doi.org/10.3390/w12071993






