Factors Controlling Hypoxia Occurrence in Estuaries, Chester River, Chesapeake Bay
Abstract
1. Introduction
2. Materials and Methods
2.1. Water Quality Model
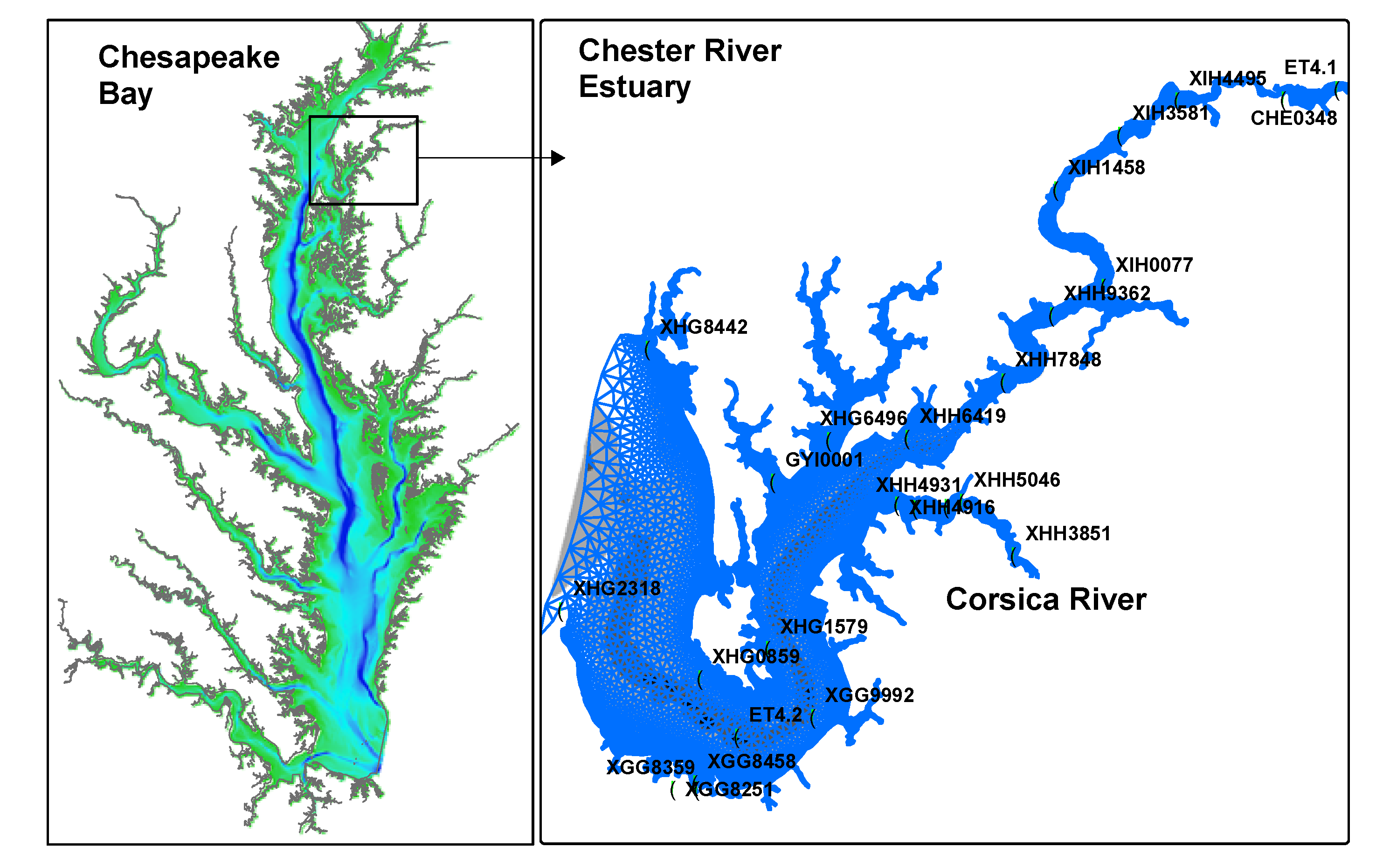
2.2. Forcing Data
2.3. Observation Data and Statistical Analysis
3. Results
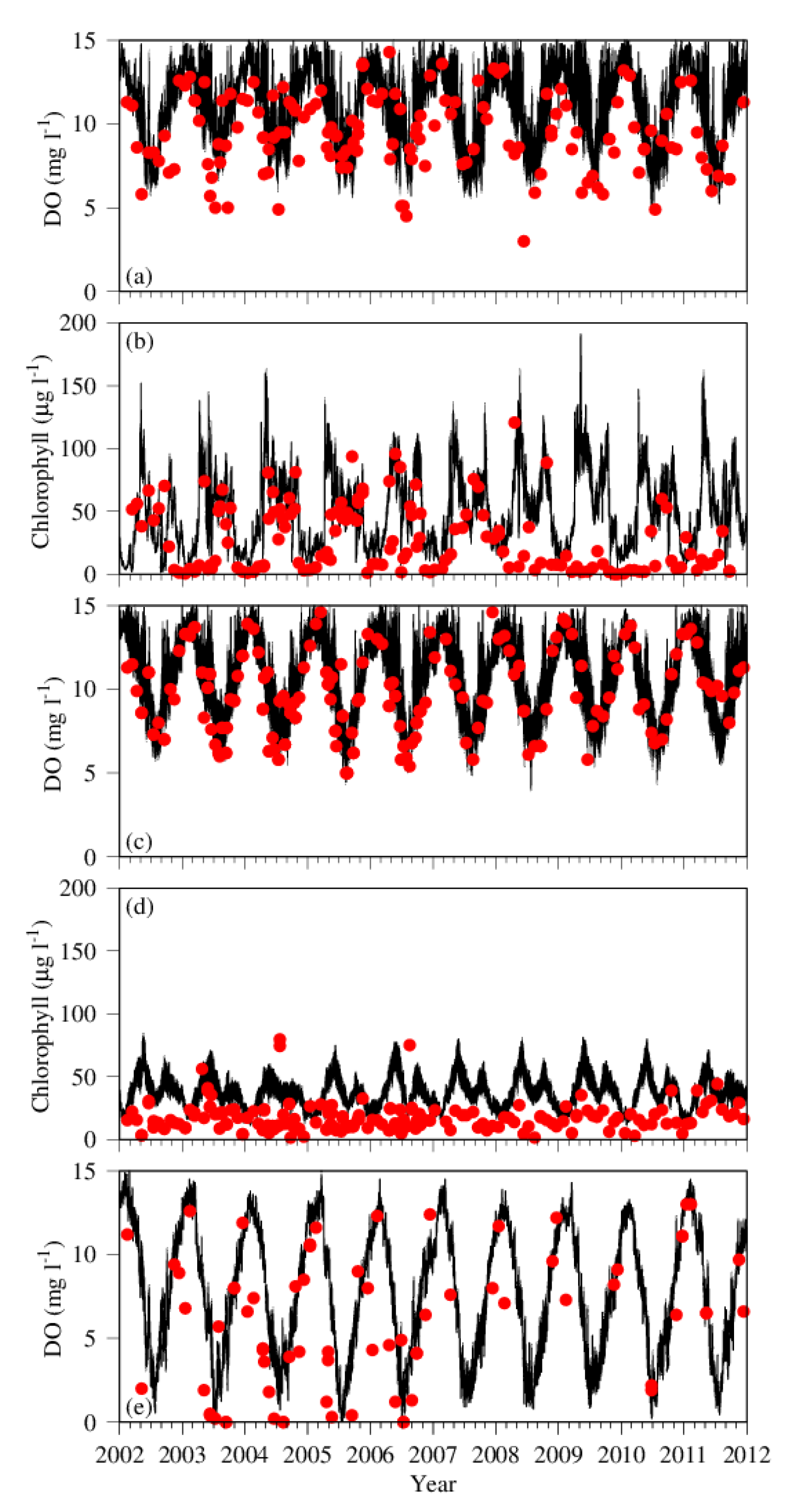
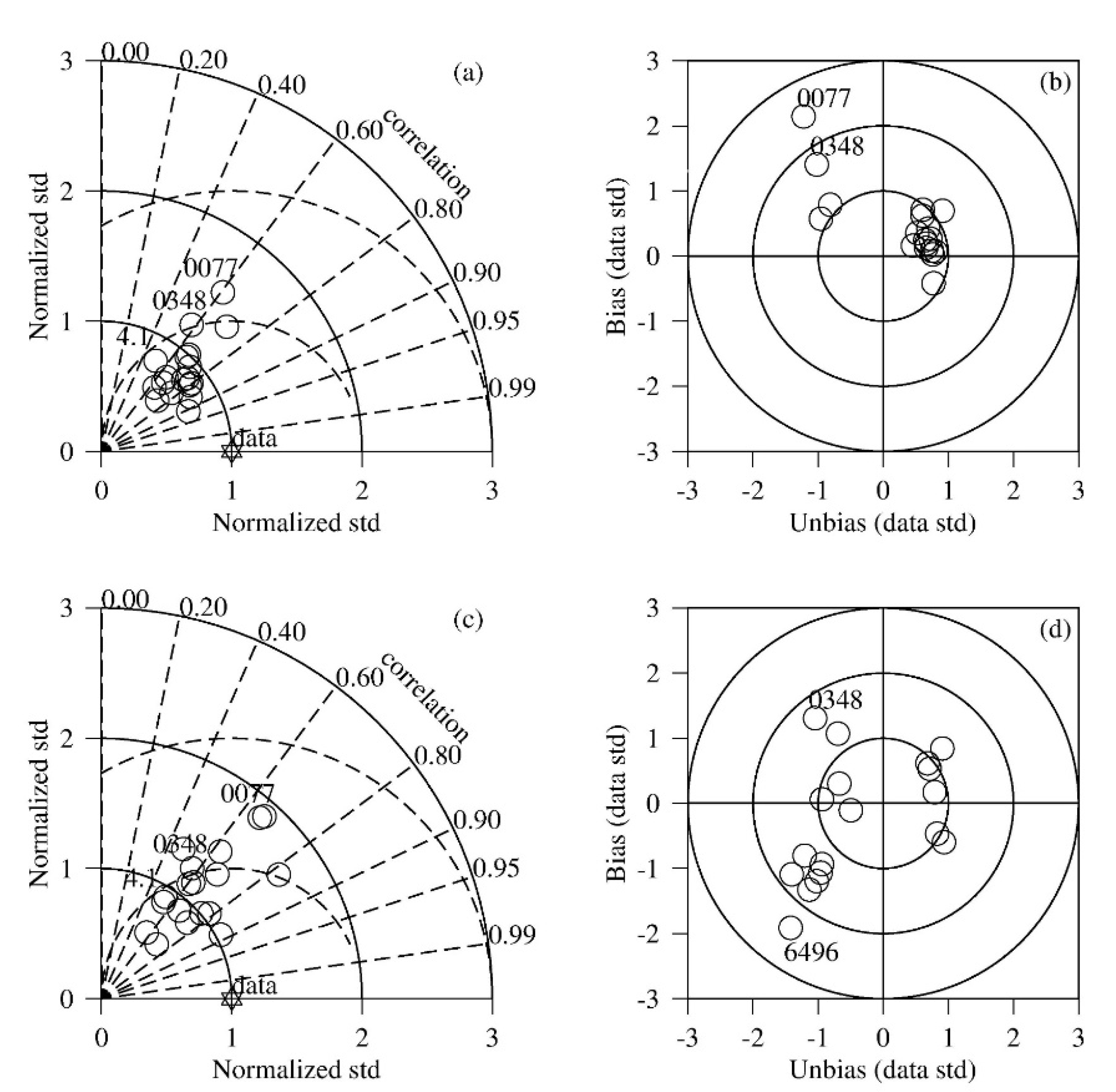
3.1. Comparison between Simulation and Observation
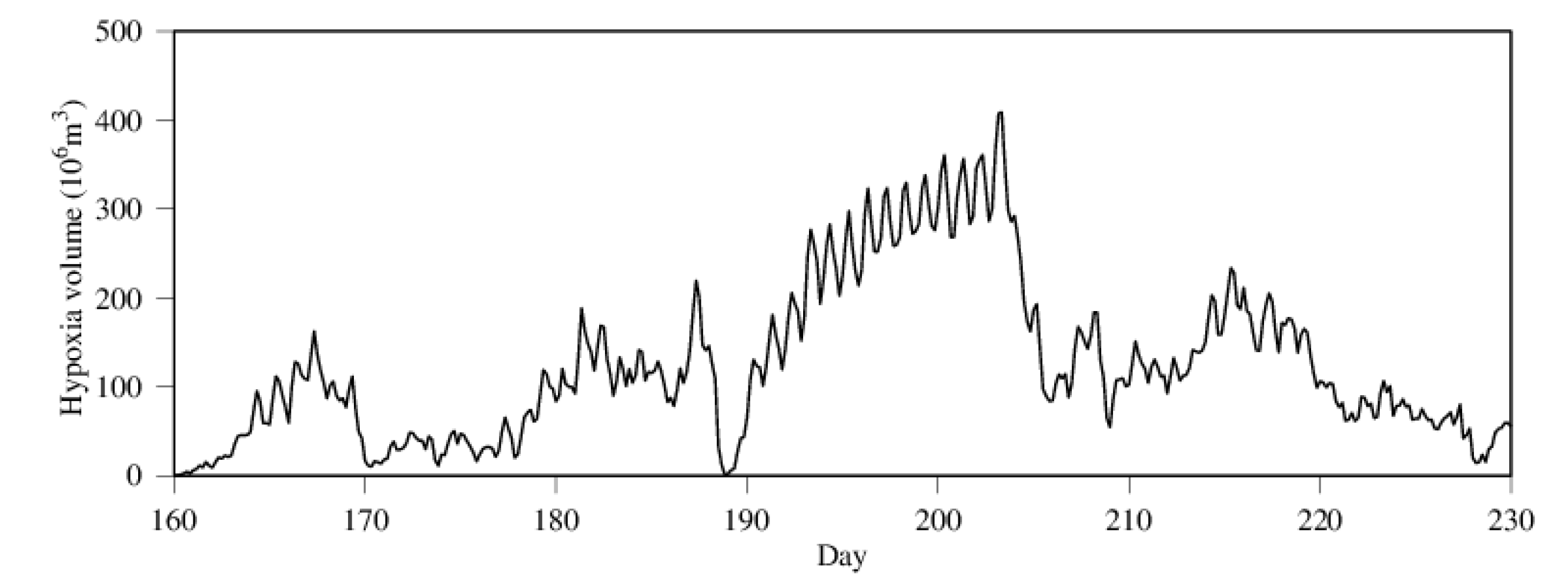
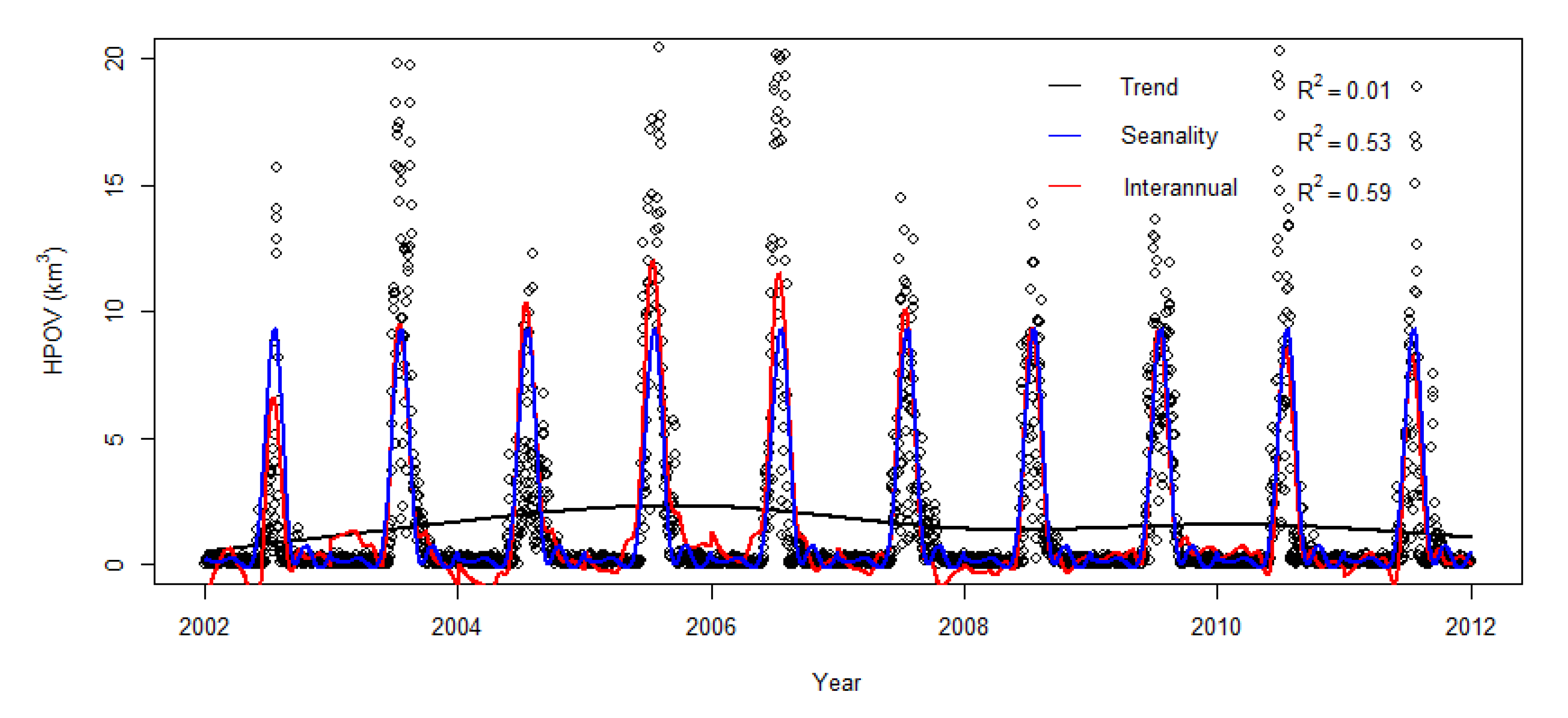
3.2. Seasonality and Interannual Variations in Hypoxia Volume
3.3. GAM Decomposition of Time-Series Hypoxia Volume and PCA Causality Analysis
4. Discussion
4.1. Time-Series Pattern
4.2. Major Drivers of Hypoxia Occurrence
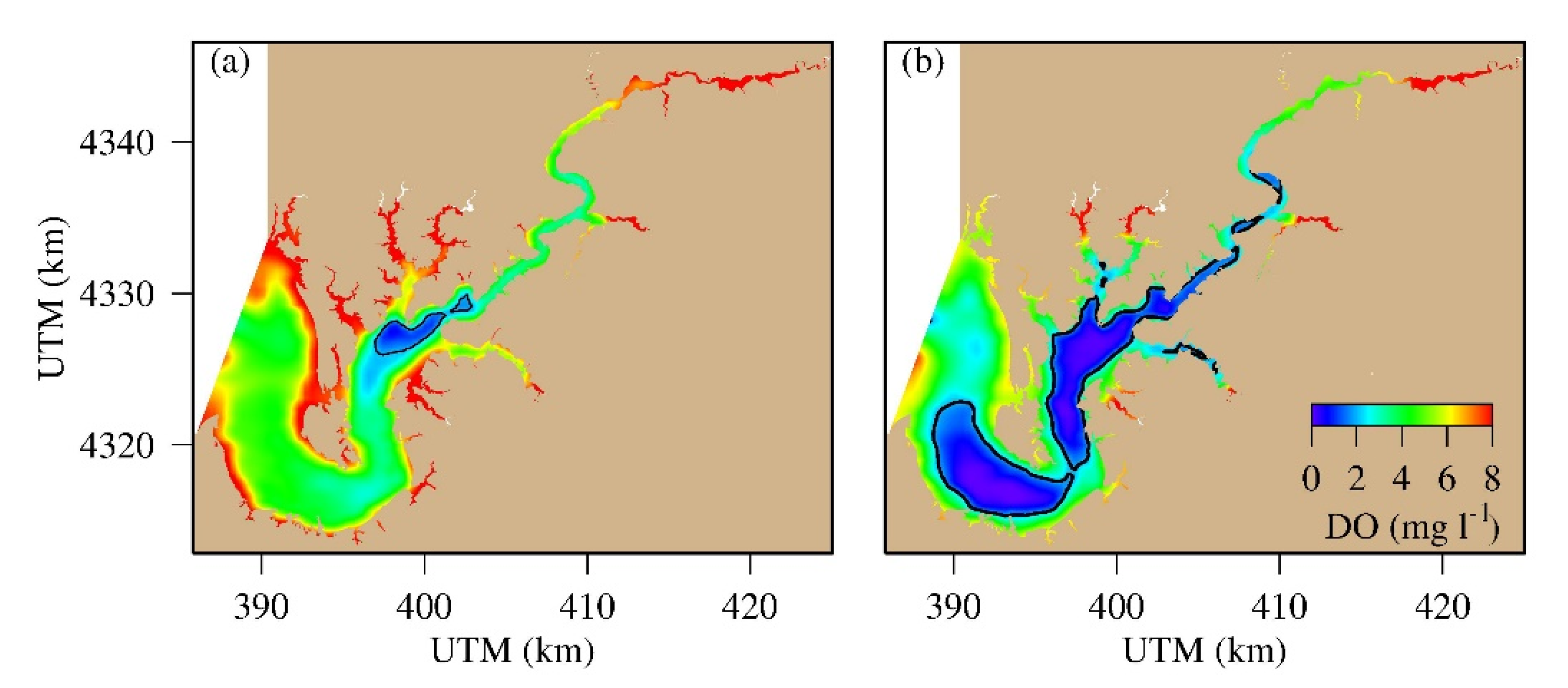
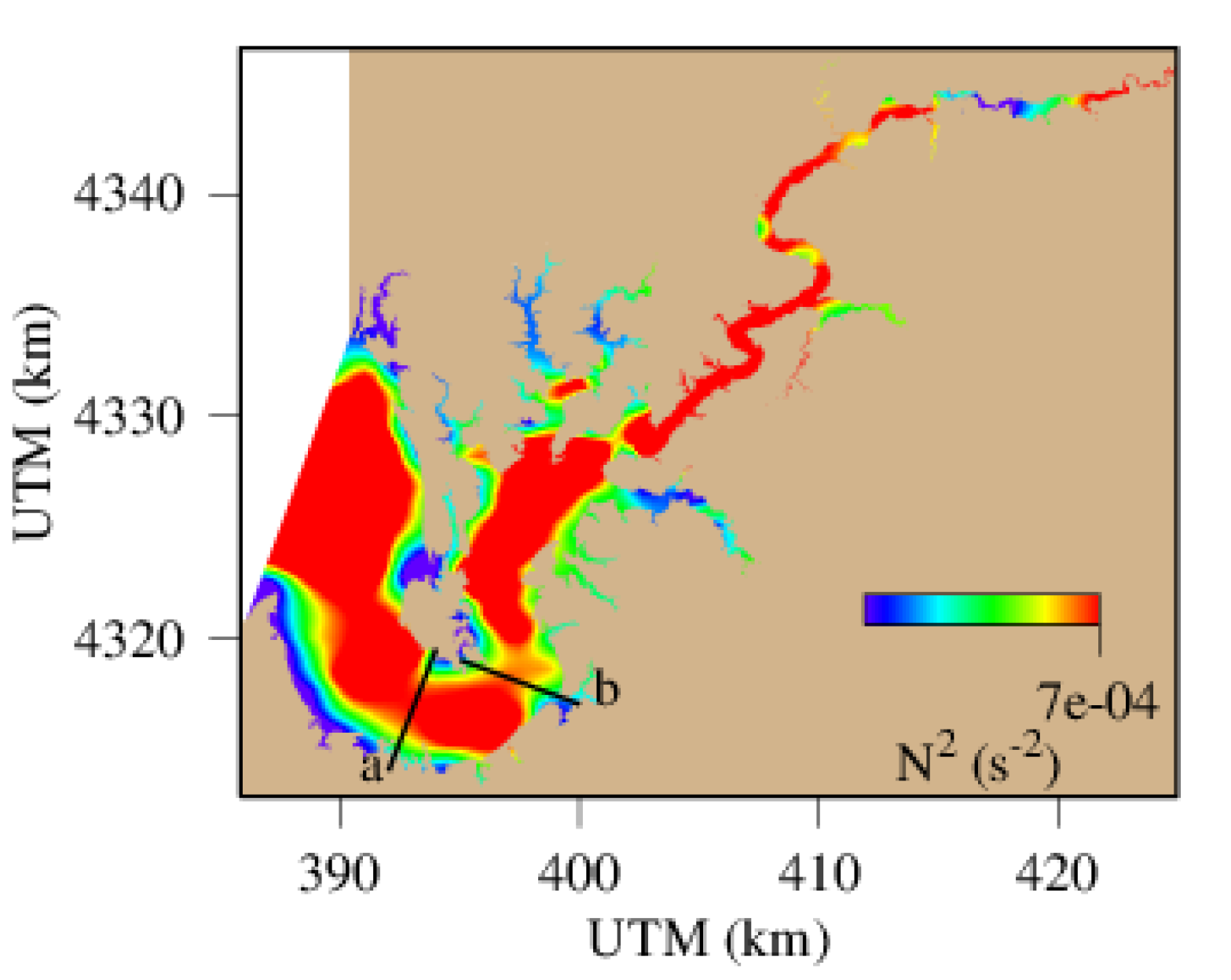
4.3. Spatial Distribution of Hypoxic Water
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Renaud, M.L. Hypoxia in Louisiana coastal waters during 1983: Implications for fisheries. Fish. Bull. 1986, 84, 19–26. [Google Scholar]
- Diaz, R.J. Overview of hypoxia around the world. J. Environ. Qual. 2001, 30, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Rabalais, N.N.; Turne, R.E.; Wiseman, W.J. Gulf of Mexico hypoxia, aka “The dead zone”. Ann. Rev. Ecol. Syst. 2002, 33, 235–263. [Google Scholar] [CrossRef]
- Diaz, R.J.; Rosenberg, R. Spreading dead zones and consequences for marine ecosystems. Science 2008, 321, 926–929. [Google Scholar] [CrossRef]
- Howarth, R.F.; Chan, D.J.; Conley, J.; Garnier, S.C.; Doney, R.M.; Billen, G. Coupled biogeochemical cycles: Eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems. Front. Ecol. Environ. 2011, 9, 18–26. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Cai, W.J.; Carstensen, J.; Conley, D.J.; Fry, B.; Hu, X.; Quiñones-Rivera, Z.; Rosenberg, R.; Slomp, C.P.; Turner, R.E.; et al. Eutrophication-driven deoxygenation in the coastal ocean. Oceanography 2014, 27, 172–183. [Google Scholar] [CrossRef]
- Hale, S.S.; Cicchetti, G.; Deacutis, C.F. Eutrophication and hypoxia diminish ecosystem functions of benthic communities in a New England estuary. Front. Mar. Sci. 2016, 3. [Google Scholar] [CrossRef]
- Raballais, N.N.; Turner, R.E.; Wiseman, W.J., Jr. Hypoxia in the Gulf of Mexico. J. Environ. Qual. 2001, 30, 320–329. [Google Scholar] [CrossRef]
- Zhang, J.; Gilbert, D.; Gooday, A.J.; Levin, L.; Naqvi, S.W.A. Natural and human-induced hypoxia and consequences for coastal areas: Synthesis and future development. Biogeosciences 2010, 7, 1443–1467. [Google Scholar] [CrossRef]
- Manuel, J. Nutrient pollution: A persistent threat to waterways. Environ. Health Perspect. 2014, 122, 304–309. [Google Scholar] [CrossRef]
- Larsen, C.E. The Chesapeake Bay: Geologic product of rising sea level. USGS Fact Sheet 1998, 1–12, 102–198. [Google Scholar]
- Boynton, W.R. Chesapeake Bay eutrophication Current status, historical trends, nutrient limitation and management actions. In Proceedings of the Coastal Nutrients Workshop, Australian Water & Wastewater Association Incorporated, Artamon, Australia, 30–31 October 1997; pp. 6–13. [Google Scholar]
- Murphy, R.R.; Kemp, W.M.; Ball, W.P. Long-term trends in Chesapeake Bay seasonal hypoxia, stratification, and nutrient loading. Estuar. Coasts 2011, 34, 1293–1309. [Google Scholar] [CrossRef]
- Sale, J.W.; Skinner, W.W. The vertical distribution of dissolved oxygen and the precipitation by saltwater in certain tidal areas. J. Franklin I. 1917, 184, 837–848. [Google Scholar] [CrossRef]
- Newcombe, C.L.; Horne, W.A. Oxygen-poor waters of the Chesapeake Bay. Science 1938, 88, 80–81. [Google Scholar] [CrossRef] [PubMed]
- Hagy, J.D.; Boynton, W.R.; Keefe, C.W.; Wood, K.V. Hypoxia in Chesapeake Bay, 1950-2001: Long-term change in relation to nutrient loading and river flow. Estuaries 2004, 27, 634–658. [Google Scholar] [CrossRef]
- Rothschild, B.J.; Ault, J.S.; Goulletquer, P.; Heral, M. Decline of the Chesapeake Bay oyster population: A century of habitat destruction and overfishing. Mar. Ecol. Prog. Ser. 1994, 111, 29–39. [Google Scholar] [CrossRef]
- Breitburg, D.L.; Hondorp, D.W.; Davias, L.A.; Diaz, R.J. Hypoxia, nitrogen, and fisheries: Integrating effects across local and global landscapes. Annu. Rev. Mar. Sci. 2009, 1, 329–349. [Google Scholar] [CrossRef]
- Schubel, J.R.; Pritchard, D.W. Responses of upper Chesapeake Bay to variations in discharge of the Susquehanna River. Estuaries 1986, 9, 236–249. [Google Scholar] [CrossRef]
- Kemp, W.M.; Boyton, W.R.; Adolf, J.E.; Boesch, D.F.; Boicourt, W.C.; Brush, G.; Cornwell, J.C.; Fisher, T.R.; Gilbert, P.M.; Hagy, J.D.; et al. Eutrophication of Chesapeake Bay: Historical trends and ecological interactions. Mar. Ecol. Prog. Ser. 2005, 303, 1–29. [Google Scholar] [CrossRef]
- Kemp, W.M.; Testa, J.M.; Conley, D.J.; Gilbert, D.; Hagy, J.D. Temporal responses of coastal hypoxia to nutrient loading and physical controls. Biogeosciences 2009, 6, 2985–3008. [Google Scholar] [CrossRef]
- Li, M.; Lee, Y.J.; Testa, J.M.; Li, Y.; Ni, W.; Kemp, W.M.; Di Toro, D.M. What drives interannual variability of hypoxia in Chesapeake Bay: Climate forcing versus nutrient loading? Geophys. Res. Lett. 2016, 43, 2127–2134. [Google Scholar] [CrossRef]
- Scully, M.E. Wind modulation of dissolved oxygen in Chesapeake Bay. Estuar. Coasts 2010, 33, 1164–1175. [Google Scholar] [CrossRef]
- Testa, J.M.; Li, Y.; Lee, Y.J.; Li, M.; Brady, D.C.; Di Toro, D.M.; Kemp, W.M.; Fitzpatrick, J.J. Quantifying the effects of nutrient loading on dissolved O2 cycling and hypoxia in Chesapeake Bay using a coupled hydrodynamic–biogeochemical model. J. Mar. Syst. 2014, 139, 139–158. [Google Scholar] [CrossRef]
- Irby, I.D.; Friedrichs, M.A.M.; Friedrichs, C.T.; Bever, A.J.; Hood, R.; Lanerolle, L.W.J.; Li, M.; Linker, L.; Scully, S.E.; Sellner, K.; et al. Challenges associate with modeling low-oxygen waters in Chesapeake Bay: A multiple model comparison. Biogeosciences 2016, 13, 2011–2028. [Google Scholar] [CrossRef]
- Xia, N.; Jiang, L. Application of an unstructured grid-based water quality model to Chesapeake Bay and its adjacent coastal ocean. J. Mar. Sci. Eng. 2016, 4, 52. [Google Scholar] [CrossRef]
- Jiang, L.; Xia, M. Wind effects on the spring phytoplankton dynamics in the middle reach of the Chesapeake Bay. Ecol. Model. 2017, 363, 68–80. [Google Scholar] [CrossRef]
- Kuo, A.Y.; Moustafa, M. Hypoxia in the Lower Rappahannock Estuary. In Special Report in Applied Marine Science and Ocean Engineering No. 302; W&M Publish, William and Mary College: Williamsburg, WV, USA, 1989; pp. 1–133. [Google Scholar]
- Sturdivant, S.K.; Brush, M.J.; Diaz, R.J. Modeling the effect of hypoxia on macrobenthos production in the Lower Rappahannock River, Chesapeake Bay, USA. PLoS ONE 2013, 8, e84140. [Google Scholar] [CrossRef]
- Breitburg, D.L.; Admack, A.; Rose, K.A.; Kolesar, S.E.; Decker, M.B.; Purcell, J.E.; Keister, J.E.; Cowan, J.H., Jr. The pattern and influence of low dissolved oxygen in the Patuxent River, a seasonally hypoxic estuary. Estuaries 2003, 26, 280–297. [Google Scholar] [CrossRef]
- Testa, J.M.; Kemp, W.M.; Boynton, W.R.; Hagy, J.D., III. Long-term changes in water quality and productivity in the Patuxent River estuary: 1985 to 2003. Estuar. Coasts 2008, 31, 1021–1037. [Google Scholar] [CrossRef]
- Kuo, A.Y.; Neilson, B.J. Hypoxia and salinity in Virginia estuaries. Estuaries 1987, 10, 277–283. [Google Scholar] [CrossRef]
- MDE. Watershed Report for Biological Impairment of Middle Chester River Watershed in Kent and Queen Anne’s Counties, Maryland; EPA Report, BSID Analysis; Middle Chester River: Annapolis, MD, USA, 2013; 33p. [Google Scholar]
- DNR. Maryland Chesapeake Bay Oyster Management Plan; DNR 17-012319-117; Maryland Department of Natural Resource: Annapolis, MD, USA, 2019.
- Cooney, J.J.; Martin, F.D.; Roosenberg, W.H.; Freeman, D.H.; Bostater, C.R. An Evaluation of Chester River Oyster Mortality; MDDNR: Annapolis, MD, USA, 1990; 129p. [Google Scholar]
- Boynton, W.R.; Testa, J.M.; Kemp, W.M. An Ecological Assessment of the Corsica River Estuary and Watershed: Scientific Advice for Future Water Quality Management; CBL 09-117; UMCES: Cambridge, MD, USA, 2009. [Google Scholar]
- Leight, A.; Jacobs, J.; Gonsalves, L.; Messick, G.; McLaughpin, S.; Lewis, J.; Brush, J.; Daniels, E.; Rhodes, M.; Collier, L.; et al. Coastal Ecosystem Assessment of Chesapeake Bay Watershed: A Story of Three Rivers–The Corsica, Magothy and Rhode; NOAA Technical Memorandum NOS NCCOS 189; NOAA: Silver Spring, MD, USA, 2014; 93p. [Google Scholar]
- Tian, R.C. Factors controlling saltwater intrusion across multi-time scales in estuaries, Chester River, Chesapeake Bay. Estuar. Coast. Shelf Sci. 2019, 223, 61–73. [Google Scholar] [CrossRef]
- Cerco, C.F.; Noel, M.R. The 2002 Chesapeake Bay Eutrophication Model; US EPA report 903-R-04-004; US Army Corps of Engineers, Waterways Experiment Station: Washington, DC, USA, 2004. [Google Scholar]
- Sanderson, B.G.; Redden, A.M.; Evens, K. Grazing constants are not constant: Microzooplankton grazing is a function of phytoplankton production in an Australian lagoon. Estuar. Coasts 2012, 35, 1270–1284. [Google Scholar] [CrossRef]
- Jassby, A.; Platt, T. Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol. Oceanogr. 1976, 21, 540–547. [Google Scholar] [CrossRef]
- Garcia, H.W.; Gordon, L.I. Oxygen solubility in seawater: Better fitting equations. Limnol. Oceanogr. 1992, 37, 1307–1312. [Google Scholar] [CrossRef]
- Morel, F. Principles of Aquatic Chemistry; John Wiley and Sons: New York, NY, USA, 1983; 150p. [Google Scholar]
- DiToro, D.; Fitzpatrick, J. Chesapeake Bay Sediment Flux Model; Contract Report EL-93-2; US Army Engineer Experiment Station: Vicksburg, MS, USA, 1993; 200p. [Google Scholar]
- Westrich, J.; Berner, R. The role of sedimentary organic matter in bacterial sulfate reduction: The G model tested. Limnol. Oceanogr. 1984, 29, 236–249. [Google Scholar] [CrossRef]
- Kirk, J.T.O. Light and Photosynthesis in Aquatic Environment; Cambridge University Press: Cambridge, UK, 1983; 401p. [Google Scholar]
- Taylor, K.E. Summarizing multiple aspects of model performance in a single diagram. J. Geophys. Res. Oceans 2001, 106, 7183–7192. [Google Scholar] [CrossRef]
- Tian, R.; Chen, C.; Qi, J.; Ji, R.; Beardsley, R.C.; Davis, C. Modeling study of nutrient and phytoplankton dynamics in the Gulf of Maine: Patterns and drivers for seasonal and interannual variability. ICES J. Mar. Sci. 2014, 72, 388–402. [Google Scholar] [CrossRef][Green Version]
- Jolliff, J.K.; Kindle, J.C.; Shulman, I.; Penta, B.; Friedrichs, M.A.M.; Helber, R.; Arnone, R.A. Summary diagrams for coupled hydrodynamic-ecosystem model skill assessment. J. Mar. Syst. 2009, 76, 64–82. [Google Scholar] [CrossRef]
- Harding, L.W., Jr.; Adolf, J.E.; Mallonee, M.E.; Miller, W.D.; Gallegos, C.L.; Perry, E.S.; Johnson, J.M.; Sellner, K.G.; Paerl, H.W. Climate effects on phytoplankton floral composition in Chesapeake Bay. Estuar. Coast. Shelf Sci. 2015, 162, 53–68. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Ellison, A.M. A Primer of Ecological Statistics; Sinauer: Sunderland, MA, USA, 2004; 510p. [Google Scholar]
- Roman, M.R.; Brandt, S.B.; House, E.D.; Pierson, J.J. Interactive effects of hypoxia and temperature on coastal pelagic zooplankton and fish. Front. Mar. Sci. 2019. [Google Scholar] [CrossRef]
- Fisher, T.R.; Peele, E.R.; Ammerman, J.W.; Harding, L.W., Jr. Nutrient limitation of phytoplankton in Chesapeake Bay. Mar. Ecol. Prog. Ser. 1992, 82, 51–63. [Google Scholar] [CrossRef]
- Fisher, T.R.; Gustafson, A.B.; Sellner, K.; Lacouture, R.; Haas, L.W.; Wetzel, R.L.; Magnien, R.; Everitt, D.; Michaels, B.; Karrh, R. Spatial and temporal variation of resource limitation in Chesapeake Bay. Mar. Biol. 1999, 133, 763–778. [Google Scholar] [CrossRef]
- Lacy, J.R.; Monismith, S.G. Secondary currents in a curved, stratified, estuarine channel. J. Geophys. Res. Oceans 2001, 106, 31283–31302. [Google Scholar] [CrossRef]
- Chant, R.J. Secondary circulation in a region of flow curvature: Relationship with tidal forcing and river discharge. J. Geophys. Res. Oceans 2002, 107, 3131. [Google Scholar] [CrossRef]
- Huijts, K.M.H.; Schuttelaars, B.H.M.; De Swart, H.E.; Friedrichs, C.T. Analytical study of the transverse distribution of along-channel and transverse residual flows in tidal estuaries. Cont. Shelf Res. 2009, 9, 89–100. [Google Scholar] [CrossRef]
- Basdurak, N.B.; Valle-Levinson, A. Tidal variability of lateral advection in a coastal plain estuary. Cont. Shelf Res. 2013, 61, 85–97. [Google Scholar] [CrossRef]





| Symbol | Definition | Value | Unit |
|---|---|---|---|
| B | Phytoplankton biomass | variable | g C m−3 |
| VW | Wind speed | variable | m s−1 |
| aDOC | DOC fraction from metabolism and grazing | 0.2 | dimensionless |
| aNC | Fraction of metabolism leading to NH4 | 0.5 | dimensionless |
| aOC | O:C ratio in metabolism and remineralization | 2.67 | g O2 g−1 C |
| aON | O:N ratio in nitrification | 4.33 | g O2 g−1 N |
| αair | Reaeration rate | ∝VW | s−1 |
| αCOD | COD oxidation rate | 5 | day−1 |
| αDOC | DOC remineralization rate | 0.1 | day−1 |
| αDON | DON remineralization rate | 0.15 | day−1 |
| αLPOC | Dissolution coefficient for labile POC | 0.15 | day−1 |
| αNT | Maximum nitrification rate of NH4 | 0.4 | day−1 |
| αRPOC | Dissolution coefficient for refractory POC | 0.005 | day−1 |
| αm | Metabolism coefficient | 0.02 | day−1 |
| αp | Grazing coefficient | 0.06 | day−1 |
| αDOC | Denitrification rate | 0.05 | day−1 |
| KCOD | Half-saturation constant for COD oxidation | 0.1 | g O m−3 |
| KDN | Half-saturation constant of NO3 for denitrification | 0.1 | g N m−3 |
| KI | Light constant for phytoplankton growth | 50 | Watt |
| KN | Half-saturation constant for nitrogen uptake | 0.5 | g N m−3 |
| KNNT | Half-saturation constant of NH4 for nitrification | 0.5 | g N m−3 |
| KOC | Half-saturation constant of DO for DOC remineralization | 0.5 | g DO m−3 |
| KONT | Half-saturation constant of DO for nitrification | 1.0 | g DO m−3 |
| KP | Half-saturation constant for phosphorus uptake | 0.0025 | g P m−3 |
| KT | Temperature coefficient for phytoplankton growth | 0.02 | °C −1 |
| To | Optimal reference temperature for phytoplankton growth | 22,8,15 | °C |
| μmax | Phytoplankton maximum growth rate | 200,300,300 | g C g−1 Chl day−1 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, R. Factors Controlling Hypoxia Occurrence in Estuaries, Chester River, Chesapeake Bay. Water 2020, 12, 1961. https://doi.org/10.3390/w12071961
Tian R. Factors Controlling Hypoxia Occurrence in Estuaries, Chester River, Chesapeake Bay. Water. 2020; 12(7):1961. https://doi.org/10.3390/w12071961
Chicago/Turabian StyleTian, Richard. 2020. "Factors Controlling Hypoxia Occurrence in Estuaries, Chester River, Chesapeake Bay" Water 12, no. 7: 1961. https://doi.org/10.3390/w12071961
APA StyleTian, R. (2020). Factors Controlling Hypoxia Occurrence in Estuaries, Chester River, Chesapeake Bay. Water, 12(7), 1961. https://doi.org/10.3390/w12071961




