An LC-MS/MS Method for a Comprehensive Determination of Metabolites of BTEX Anaerobic Degradation in Bacterial Cultures and Groundwater
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Procedures
2.2. Samples
2.3. Instrumentation
2.4. Procedures
3. Results and Discussion
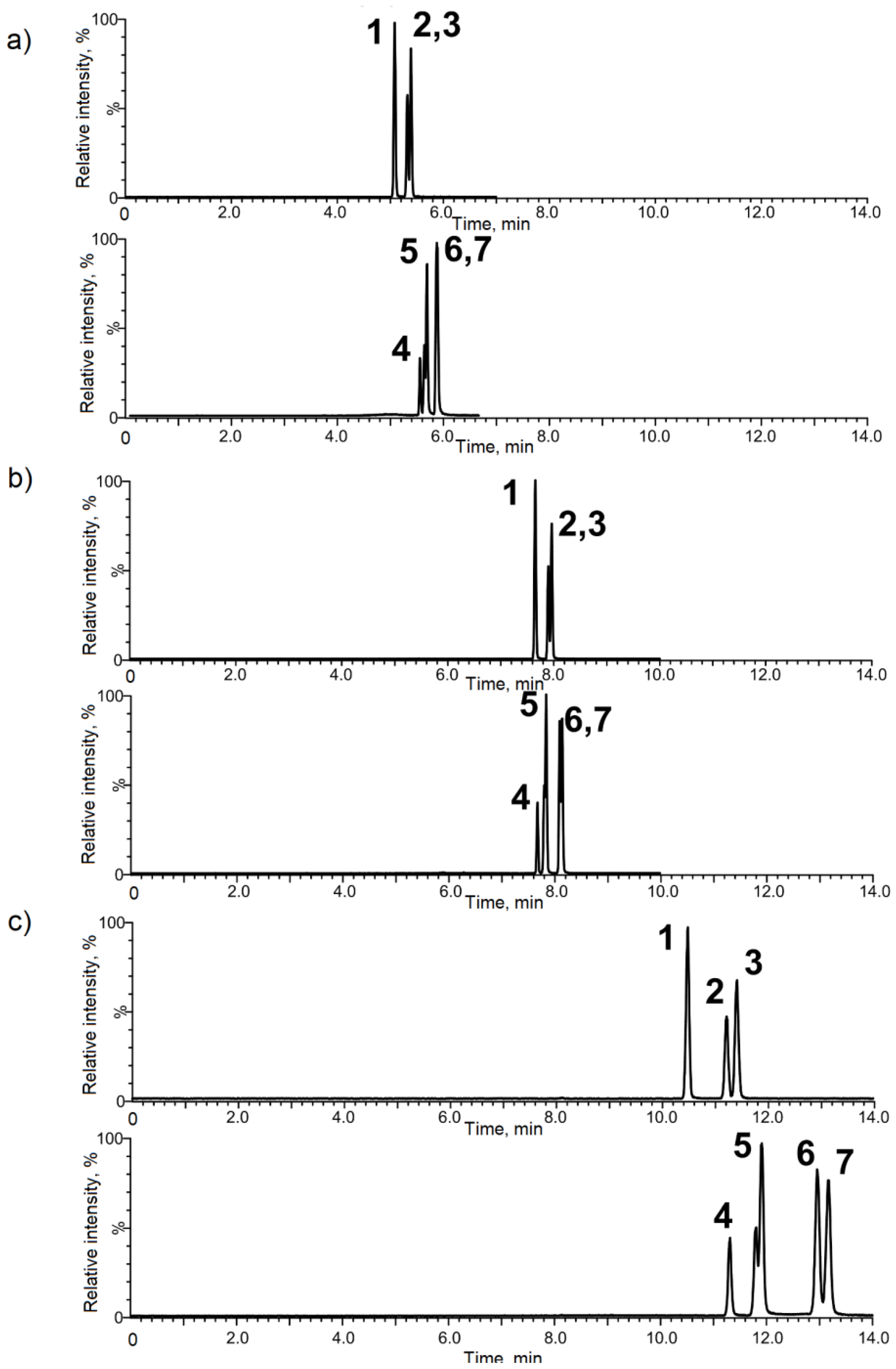
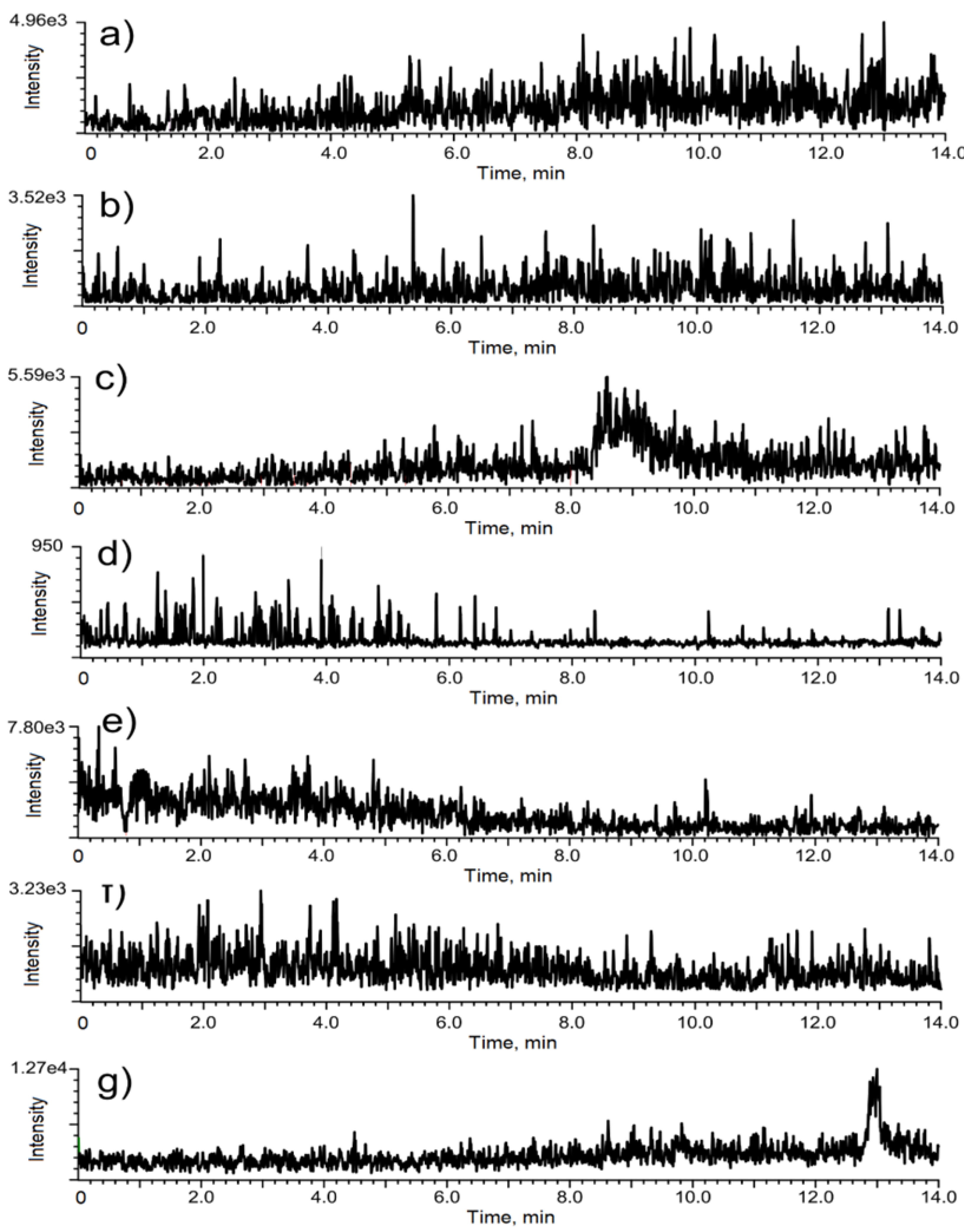
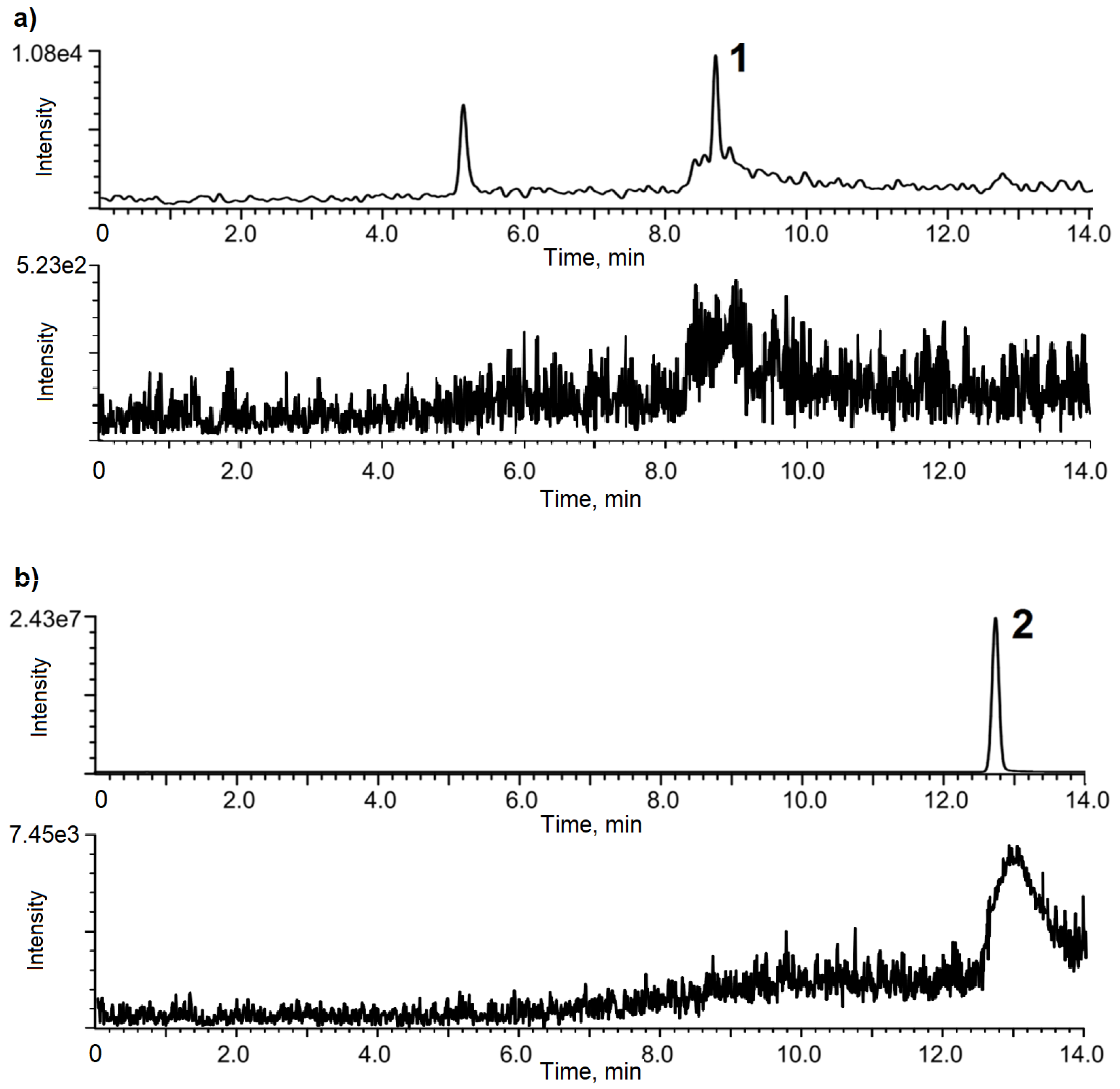
3.1. Chromatographic Optimization
3.2. Validation of the Method
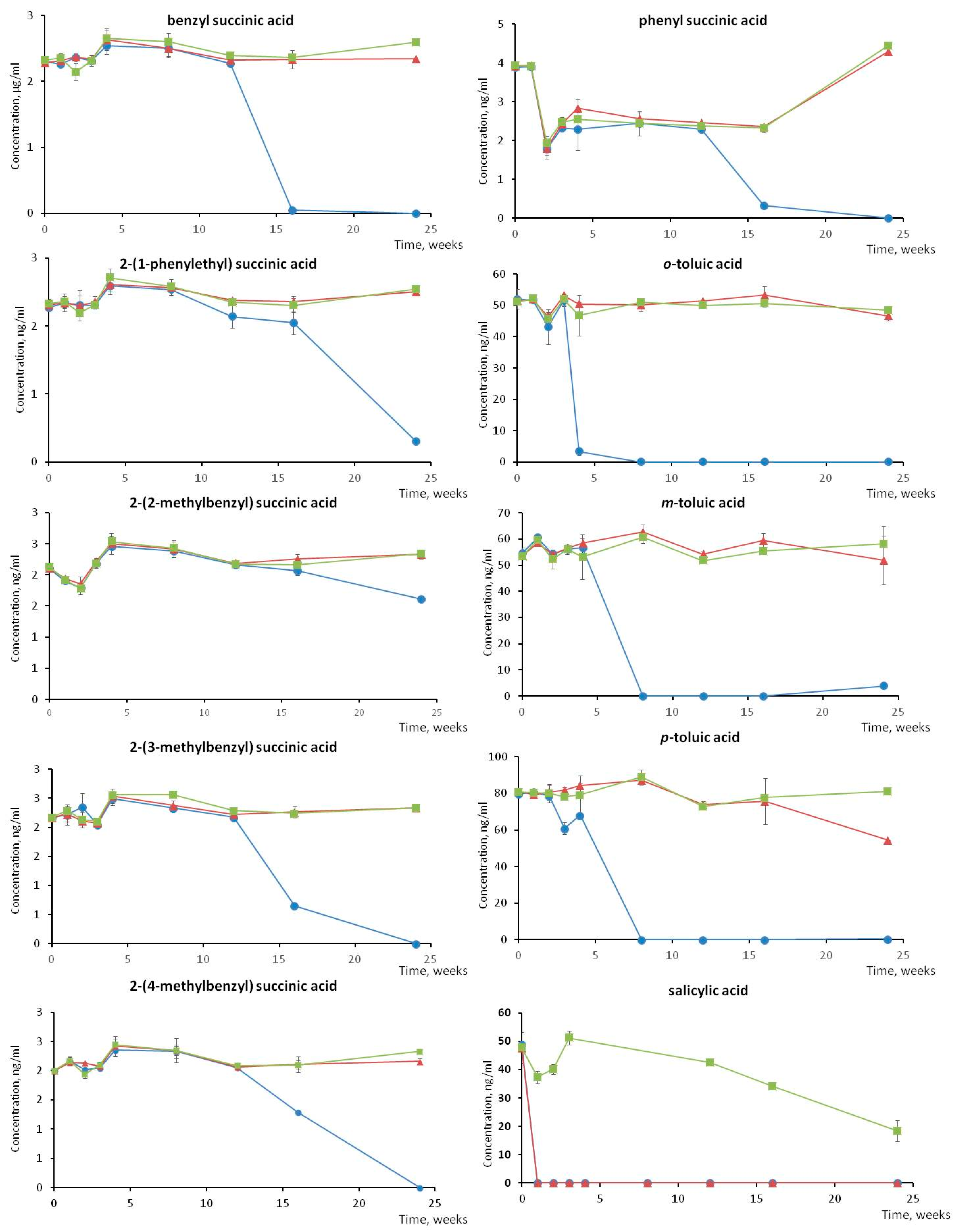
3.3. Stability Study of BTEX Metabolites during Sample Storage
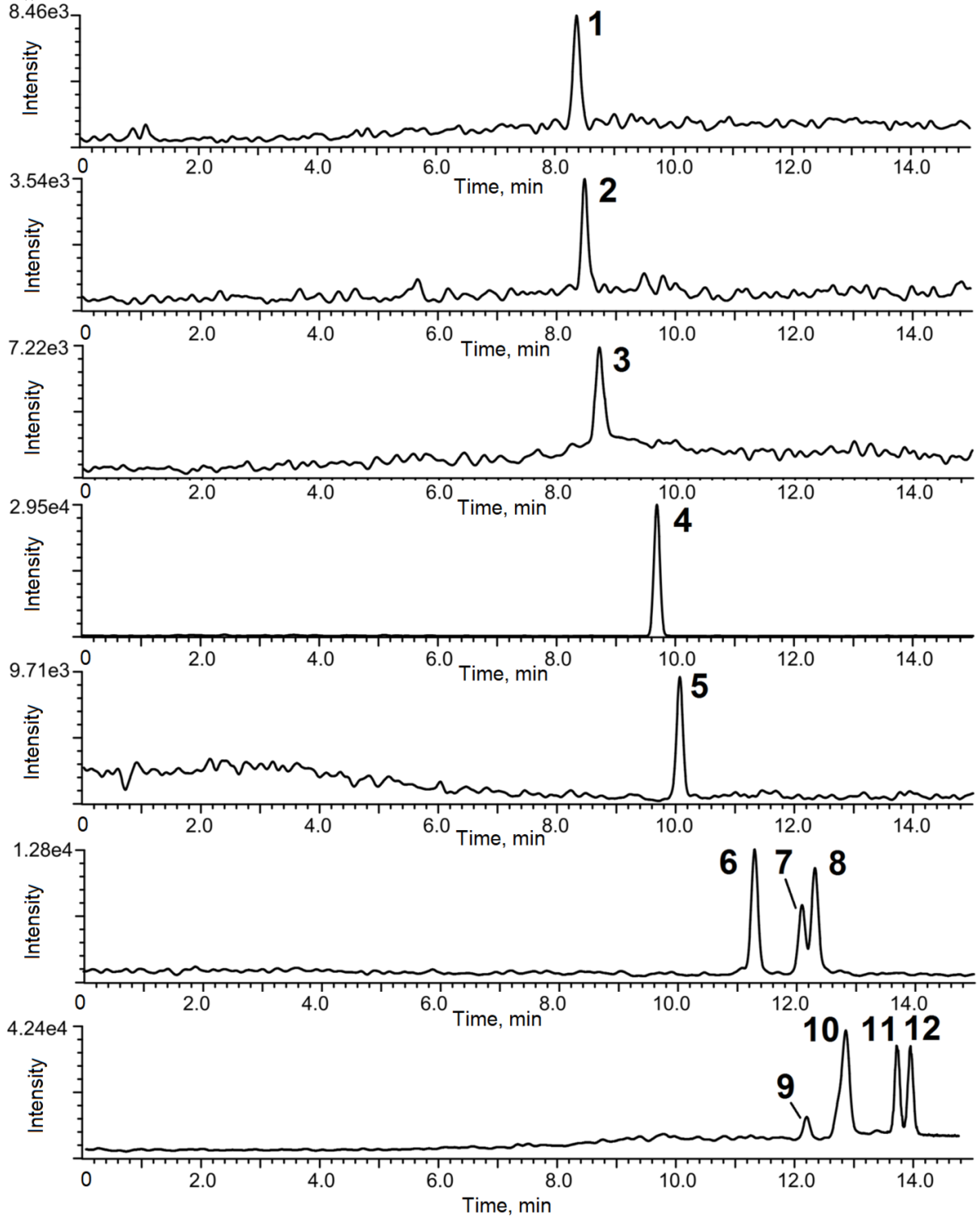
3.4. Analysis of Ground Water
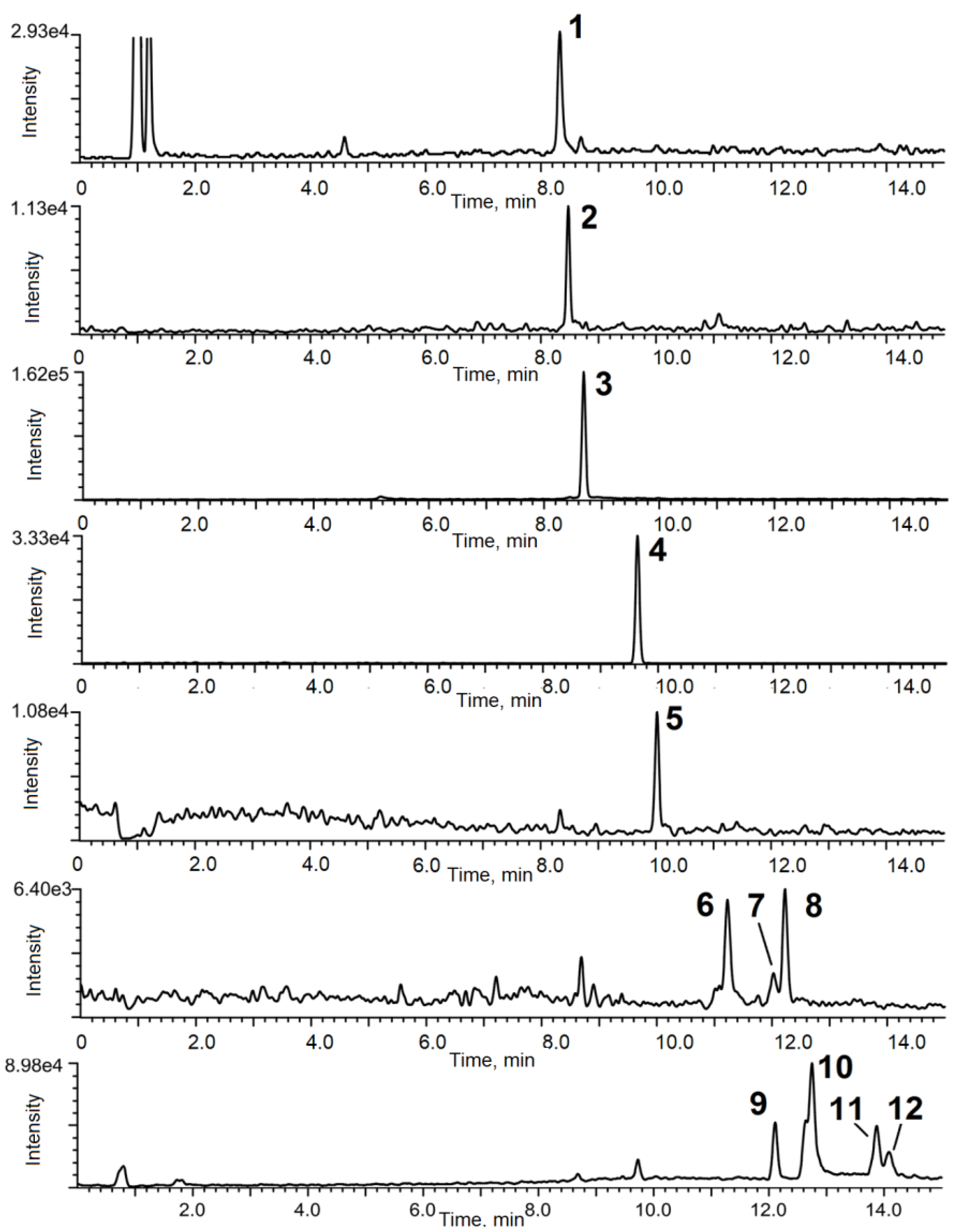
3.5. Analysis of Bacterial Cultures
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Van Agteren, M.H.; Keuning, S.; Janssen, D. Handbook on Biodegradation and Biological Treatment of Hazardous Organic Compounds; Kluwer Academic Publishers: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Winderl, C.; Schaefer, S.; Lueders, T. Detection of anaerobic toluene and hydrocarbon degraders in contaminated aquifers using benzylsuccinate synthase (bssA) genes as a functional marker. Environ. Microbiol. 2007, 9, 1035–1046. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Public Health Service, Interaction profile for: Benzene, toluene, ethylbenzene, and xylenes (BTEX). Agency Toxic Subst. Dis. Regist. 2004. Available online: https://www.atsdr.cdc.gov/interactionprofiles/ip05.html (accessed on 28 May 2020).
- Bombach, P.; Richnow, H.H.; Kästner, M.; Fischer, A. Current approaches for the assessment of in situ biodegradation. Appl. Microbiol. Biotechnol. 2010, 86, 839–852. [Google Scholar] [CrossRef]
- Agrawal, A.; Gieg, L.M. In situ detection of anaerobic alkane metabolites in subsurface environments. Front. Microbiol. 2013, 4, 140. [Google Scholar] [CrossRef]
- Gibson, D.T. Initial stages in microbial degradation of aromatic hydrocarbons. J. Am. Oil Chem. Soc. 1970, 47, 337. [Google Scholar]
- Hopper, D.J. Incorporation of O-18 water in formation of para-hydroxybenzyl alcohol by para-cresol methylhydroxylase from Pseudomonas-putida. Biochem. J. 1978, 175, 345–347. [Google Scholar] [CrossRef]
- Kuhn, E.P.; Colberg, P.J.; Schnoor, J.L.; Wanner, O.; Zehnder, A.J.P.; Schwarzenbach, R.P. Microbial transformations of substituted benzenes during infiltration of river water to groundwater—Laboratory column studies. Environ. Sci. Technol. 1985, 19, 961–968. [Google Scholar] [CrossRef]
- Wilson, B.H.; Smith, G.B.; Rees, J.F. Biotransformations of selected alkylbenzenes and halogenated aliphatic-hydrocarbons in methanogenic aquifer material—A microcosm study. Environ. Sci. Technol. 1986, 20, 997–1002. [Google Scholar] [CrossRef]
- Muller, S.; Vogt, C.; Laube, M.; Harms, H.; Kleinsteuber, S. Community dynamics within a bacterial consortium during growth on toluene under sulfate-reducing conditions. FEMS Microbiol. Ecol. 2009, 70, 586–596. [Google Scholar] [CrossRef]
- Van der Zaan, B.M.; Saia, F.T.; Stams, A.J.M.; Plugge, C.M.; de Vos, W.M.; Smidt, H.; Langenhoff, A.A.M.; Gerritse, J. Anaerobic benzene degradation under denitrifying conditions: Peptococcaceae as dominant benzene degraders and evidence for a syntrophic process. Environ. Microbiol. 2012, 14, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.T.; Rooney-Varga, J.N.; Gaw, C.V.; Lovley, D.R. Anaerobic benzene oxidation in the fe (III) reduction zone of petroleum-contaminated aquifers. Environ. Sci. Technol. 1998, 32, 1222–1229. [Google Scholar] [CrossRef]
- Masumoto, H.; Kurisu, F.; Kasuga, I.; Tourlousse, D.M.; Furumai, H. Complete mineralization of benzene by a methanogenic enrichment culture and effect of putative metabolites on the degradation. Chemosphere 2012, 86, 822–828. [Google Scholar] [CrossRef]
- Cervantes, F.J.; Mancilla, A.R.; Toro, E.E.R.; Alpuche-Solís, A.G.; Lorenzana, L.M. Anaerobic degradation of benzene by enriched consortia with humic acids as terminal electron acceptors. J. Hazard. Mater. 2011, 195, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Grbic-Galic, D. O-demethylation, dehydroxylation, ring-reduction and cleavage of aromatic substrates by enterobacteriaceae under anaerobic conditions. J. Appl. Bacteriol. 1986, 61, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Grbic-Galic, D.; Vogel, T.M. Transformation of toluene and benzene by mixed methanogenic cultures. Appl. Environ. Microbiol. 1987, 53, 254–260. [Google Scholar] [CrossRef]
- Weiner, J.M.; Lovley, D.R. Rapid benzene degradation in methanogenic sediments from a petroleum-contaminated aquifer. Appl. Environ. Microbiol. 1998, 64, 1937–1939. [Google Scholar] [CrossRef]
- Coates, J.D.; Chakraborty, R.; McInerney, M.J. Anaerobic benzene biodegradation—A new era. Res. Microbiol. 2002, 153, 621–628. [Google Scholar] [CrossRef]
- Ulrich, A.C.; Beller, H.R.; Edwards, E.A. Metabolites detected during biodegradation of 13C6-benzene in nitrate-reducing and methanogenic enrichment cultures. Environ. Sci. Technol. 2005, 39, 6681–6691. [Google Scholar] [CrossRef]
- Boll, M. Dearomatizing benzene ring reductases. J. Mol. Microbiol. Biotechnol. 2005, 10, 132–142. [Google Scholar] [CrossRef]
- Holmes, D.E.; Risso, C.; Smith, J.A.; Lovley, D.R. Anaerobic oxidation of benzene by the hyperthermophilic archaeon ferroglobus placidus. Appl. Environ. Microbiol. 2011, 77, 5926–5933. [Google Scholar] [CrossRef]
- Abu Laban, N.; Selesi, D.; Jobelius, C.; Meckenstock, R.U. Anaerobic benzene degradation by gram-positive sulfate-reducing bacteria. FEMS Microbiol. Ecol. 2009, 68, 300–311. [Google Scholar] [CrossRef]
- Kunapuli, U.; Griebler, C.; Beller, H.R.; Meckenstock, R.U. Identification of intermediates formed during anaerobic benzene degradation by an iron-reducing enrichment culture. Environ. Microbiol. 2008, 10, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Harwood, C.S.; Burchhardt, G.; Herrmann, H.; Fuchs, G. Anaerobic metabolism of aromatic compounds via the benzoyl-CoA pathway. FEMS Microbiol. Rev. 1999, 22, 439–458. [Google Scholar] [CrossRef]
- Frazer, A.C.; Coschigano, P.W.; Young, L.Y. Toluene metabolism under anaerobic conditions: A review. Anaerobe 1995, 1, 293–303. [Google Scholar] [CrossRef]
- Langenhoff, A.A.M.; Brouwers-Ceiler, D.L.; Engelberting, J.H.L.; Quist, J.J.; Wolkenfelt, J.G.P.N.; Zehnder, A.J.B.; Schraa, G. Microbial reduction of manganese coupled to toluene oxidation. FEMS Microbiol. Ecol. 1995, 22, 119–127. [Google Scholar] [CrossRef]
- Langenhoff, A.A.M.; Nijenhuis, I.; Tan, N.C.G.; Briglia, M.; Zehnder, A.J.B.; Schraa, G. Characterisation of a manganese-reducing, toluene-degrading enrichment culture. FEMS Microbiol. Ecol. 1997, 24, 113–125. [Google Scholar] [CrossRef]
- Beller, H.R.; Spormann, A.M. Benzylsuccinate formation as a means of anaerobic toluene activation by sulfate-reducing strain prtol1. Appl. Environ. Microbiol. 1997, 63, 3729–3731. [Google Scholar] [CrossRef]
- Biegert, T.; Fuchs, G.; Heider, J. Evidence that anaerobic oxidation of toluene in the denitrifying bacterium Thauera aromatica is initiated by formation of benzylsuccinate from toluene and fumarate. Eur. J. Biochem. FEBS 1996, 238, 661–668. [Google Scholar] [CrossRef]
- Leuthner, B.; Heider, J. Anaerobic toluene catabolism of Thauera aromatica: The bbs operon codes for enzymes of β oxidation of the intermediate benzylsuccinate. J. Bacteriol. 2000, 182, 272–277. [Google Scholar] [CrossRef]
- Philipp, B.; Schink, B. Different strategies in anaerobic biodegradation of aromatic compounds: Nitrate reducers versus strict anaerobes. Environ. Microbiol. Rep. 2012, 4, 469–478. [Google Scholar] [CrossRef]
- Krieger, C.J.; Beller, H.R.; Reinhard, M.; Spormann, A.M. Initial reactions in anaerobic oxidation of m-xylene by the denitrifying bacterium Azoarcus sp strain T. J. Bacteriol. 1999, 181, 6403–6410. [Google Scholar] [CrossRef]
- Morasch, B.; Meckenstock, R.U. Anaerobic degradation of p-xylene by a sulfate-reducing enrichment culture. Curr. Microbiol. 2005, 51, 127–130. [Google Scholar] [CrossRef] [PubMed]
- 34 Morasch, B.; Schink, B.; Tebbe, C.C.; Meckenstock, R.U. CDegradation of o-xylene and m-xylene by a novel sulfate-reducer belonging to the genus Desulfotomaculum. Arch. Microbiol. 2004, 181, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Raymond, R.L.; Jamison, V.W.; Hudson, J.O. Microbial Hydrocarbon Co-oxidation I. Oxidation of mono- and dicyclic hydrocarbons by soil isolates of the genus Nocardia. Appl. Microbiol. 1967, 15, 357–865. [Google Scholar]
- Beller, H.R. Analysis of benzylsuccinates in groundwater by liquid chromatography tandem mass spectrometry and its use for monitoring in situ BTEX biodegradetion. Environ. Sci. Technol. 2002, 36, 2724–2728. [Google Scholar] [CrossRef] [PubMed]
- Lotte Ask Reitzel, A.L.; Bjerg, P.L. Quantitative determination of toluene, ethylbenzene and xylene degradation products in contaminated groundwater by solid-phase extraction and in-vial derivatization. Int. J. Environ. Anal. Chem. 2005, 85, 1075–1087. [Google Scholar] [CrossRef]
- Alumbaugh, R.E.; Gieg, L.M.; Field, J.A. Determination of alkylbenzene metabolites in groundwater by solid-phase extraction and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2004, 1042, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Basso, O.; Lascourrèges, J.F.; Jarry, M.; Magot, M. The effect of cleaning and disinfecting the sampling well on the microbial communities of deep subsurface water samples. Environ. Microbiol. 2005, 7, 13–21. [Google Scholar] [CrossRef]
- Berlendis, S.; Lascourreges, J.F.; Schraauwers, B.; Sivadon, P.; Magoy, M. Anaerobic biodegradation of BTEX by original bacterial communities from an underground gas storage aquifer. Environ. Sci. Technol. 2010, 44, 3621–3628. [Google Scholar] [CrossRef]
- Pfennig, N.; Widdel, F.; Trüper, H.G. The dissimilatory sulfate-reducing bacteria. Prokaryotes 1981, 1, 926–940. [Google Scholar]
- Beller, H.R. Metabolic indicators for detecting in situ anaerobic alkylbenzene degradation. Biodegradation 2000, 11, 125–139. [Google Scholar] [CrossRef]
- Heitzer, A.; Malachowsky, K.; Thonnard, J.E.; Bienkowski, P.R.; White, D.C.; Sayler, G.S. Optical biosensor for environmental on-line monitoring of naphthalene and salicylate bioavailability with an immobilized bioluminescent catabolic reporter bacterium. Appl. Environ. Microbiol. 1994, 60, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Eaton, R.W.; Chapman, P.J. Bacterial metabolism of naphthalene: construction and use of recombinant bacteria to study ring cleavage of 1, 2-dihydroxynaphthalene and subsequent reactions. J. Bacteriol. 1992, 174, 7542–7554. [Google Scholar] [CrossRef]
- Lyu, Y.; Zheng, W.; Zheng, T.; Tian, Y. Biodegradation of polycyclic aromatic hydrocarbons by Novosphingobium pentaromativorans US6-1. PLoS ONE 2014, 9, e101438. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Gallardo, L.; Gómez-Álvarez, H.; Santero, E.; Floriano, B. Combination of degradation pathways for naphthalene utilization in R hodococcus sp. strain TFB. Microb. Biotechnol. 2013, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Bakker, P.A.H.M.; Ran, L.X.; Mercado-Blanco, J. Rhizobacterial salicylate production provokes headaches! Plant. Soil 2014, 382, 1. [Google Scholar] [CrossRef]
- Von Netzer, F.; Pilloni, G.; Kleindienst, S.; Krüger, M.; Knittel, K.; Gründger, F.; Lueders, T. Enhanced gene detection assays for fumarate-adding enzymes allow uncovering of anaerobic hydrocarbon degraders in terrestrial and marine systems. Appl. Environ. Microbiol. 2013, 79, 543–552. [Google Scholar] [CrossRef]
- Acosta-González, A.; Rossellï, R.; Marquï, S. Diversity of benzylsuccinate synthase-like (bssA) genes in hydrocarbon-polluted marine sediments suggests substrate-dependent clustering. Appl. Environ. Microbiol. 2013, 79, 3667–3676. [Google Scholar] [CrossRef]
- Funk, M.A.; Judd, E.T.; Marsh, E.N.G.; Elliott, S.J.; Drennan, C.L. Structures of benzylsuccinate synthase elucidate roles of accessory subunits in glycyl radical enzyme activation and activity. PNAS 2014, 111, 10161–10166. [Google Scholar] [CrossRef]
- Lueders, T.; von Netzer, F. Primers: Functional Genes for Anaerobic Hydrocarbon Degrading Microbes; Springer Protocols Handbooks: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Carmona, M.; Zamarro, M.T.; Blázquez, B.; Durante-Rodríguez, G.; Juárez, J.F.; Valderrama, J.A.; Barragán, M.J.L.; García, J.L.; Díaz, E. Anaerobic catabolism of aromatic compounds: a genetic and genomic view. Microbiol. Mol. Biol. Rev. 2009, 73, 71. [Google Scholar] [CrossRef]
| Name | Formula | Mass | Ion Transition | Cone (V) | Collision (V) |
|---|---|---|---|---|---|
| o-toluic acid | 136.15 | 135.0 → 91.0 | 24 | 10 | |
| m-toluic acid | C8H8O2 | 22 | 12 | ||
| p-toluic acid | 26 | 10 | |||
| benzoate | C7H6O2 | 122.12 | 120.9 → 77.0 | 22 | 10 |
| salicylic acid | C7H6O3 | 138.12 | 135.9 → 93 | 25 | 16 |
| 2-(2-methylbenzyl)-succinic acid | 221.1 → 177.1 | 24 | 14 | ||
| 2-(3-methylbenzyl)-succinic acid | C12H14O4 | 222.23 | 25 | 14 | |
| 2-(4-methylbenzyl)-succinic acid | 22 | 14 | |||
| 2-(1-phenylethyl)-succinic acid | C12H14O4 | 222.23 | 221.1 → 177.1 | 28 | 15 |
| Phenyl-succinic acid | C10H10O4 | 194.18 | 193.0 →149.0 | 14 | 10 |
| Benzyl-succinic acid | C11H12O4 | 208.21 | 207.0 → 163.1 | 24 | 14 |
| iso-4FBA (IS) | C6D413CO2HF | 145.13 | 144.0 → 99.1 | 20 | 14 |
| Compound | Added, (ng/mL) | Found ± SD, (ng/mL) | % of the Expected Value |
|---|---|---|---|
| benzoic acid | 2 | 2.18 ± 0.07 | 109 |
| 5 | 5.25 ± 0.28 | 105 | |
| 25 | 23.50 ± 0.55 | 94 | |
| o-toluic acid | 2 | 2.18 ± 0.14 | 109 |
| 5 | 4.97 ± 0.11 | 99 | |
| 25 | 23.14 ± 1.06 | 93 | |
| m-toluic acid | 2 | 1.86 ± 0.09 | 93 |
| 5 | 4.90 ± 0.63 | 98 | |
| 25 | 23.97 ± 0.89 | 96 | |
| p-toluic acid | 2 | 2.16 ± 0.08 | 108 |
| 5 | 4.80 ± 0.20 | 96 | |
| 25 | 26.02 ± 0.95 | 104 | |
| Salicylic acid | 2 | 1.99 ± 0.07 | 99 |
| 5 | 5.21 ± 0.09 | 104 | |
| 25 | 26.18 ± 0.70 | 105 | |
| phenylsuccinic acid | 2 | 1.80 ± 0.02 | 90 |
| 5 | 4.69 ± 0.16 | 94 | |
| 25 | 23.59 ± 1.03 | 94 | |
| benzylsuccinic acid | 2 | 1.91 ± 0.09 | 96 |
| 5 | 5.51 ± 0.19 | 110 | |
| 25 | 24.28 ± 0.97 | 97 | |
| 2-(1-phenylethyl) succinic acid | 2 | 2.10 ± 0.03 | 105 |
| 5 | 5.02 ± 0.08 | 100 | |
| 25 | 25.61 ± 1.42 | 102 | |
| 2-(2-methylbenzyl) succinic acid | 2 | 1.72 ± 0.24 | 86 |
| 5 | 4.84 ± 0.06 | 97 | |
| 25 | 25.04 ± 1.65 | 100 | |
| 2-(3-methylbenzyl) succinic acid | 2 | 1.81 ± 0.19 | 90 |
| 5 | 4.69 ± 0.23 | 94 | |
| 25 | 23.60 ± 1.13 | 94 | |
| 2-(4-methylbenzyl) succinic acid | 2 | 1.82 ± 0.11 | 91 |
| 5 | 4.35 ± 0.25 | 87 | |
| 25 | 23.66 ± 1.15 | 95 |
| Compound | ng/mL |
|---|---|
| benzoate | 85.3 ± 3.8 |
| o-toluic acid | 0.8 ± 0.1 |
| m-toluic acid | 0.3 ± 0.1 |
| p-toluic acid | 0.8 ± 0.1 |
| salicylic acid | 3.9 ± 0.3 |
| phenyl succinic acid | 3.8 ± 0.4 |
| benzyl succinic acid | 1.7 ± 0.3 |
| 2-(1-phenyl-ethyl) succinic acid | 0.5 ± 0.1 |
| 2-(2-methyl-benzyl) succinic acid | 0.5 ± 0.1 |
| 2-(3- methyl-benzyl) succinic acid | 0.2 + 0.04 |
| 2-(4- methyl-benzyl) succinic acid | 1.0 ± 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godin, S.; Kubica, P.; Ranchou-Peyruse, A.; Le Hecho, I.; Patriarche, D.; Caumette, G.; Szpunar, J.; Lobinski, R. An LC-MS/MS Method for a Comprehensive Determination of Metabolites of BTEX Anaerobic Degradation in Bacterial Cultures and Groundwater. Water 2020, 12, 1869. https://doi.org/10.3390/w12071869
Godin S, Kubica P, Ranchou-Peyruse A, Le Hecho I, Patriarche D, Caumette G, Szpunar J, Lobinski R. An LC-MS/MS Method for a Comprehensive Determination of Metabolites of BTEX Anaerobic Degradation in Bacterial Cultures and Groundwater. Water. 2020; 12(7):1869. https://doi.org/10.3390/w12071869
Chicago/Turabian StyleGodin, Simon, Pawel Kubica, Anthony Ranchou-Peyruse, Isabelle Le Hecho, Delphine Patriarche, Guilhem Caumette, Joanna Szpunar, and Ryszard Lobinski. 2020. "An LC-MS/MS Method for a Comprehensive Determination of Metabolites of BTEX Anaerobic Degradation in Bacterial Cultures and Groundwater" Water 12, no. 7: 1869. https://doi.org/10.3390/w12071869
APA StyleGodin, S., Kubica, P., Ranchou-Peyruse, A., Le Hecho, I., Patriarche, D., Caumette, G., Szpunar, J., & Lobinski, R. (2020). An LC-MS/MS Method for a Comprehensive Determination of Metabolites of BTEX Anaerobic Degradation in Bacterial Cultures and Groundwater. Water, 12(7), 1869. https://doi.org/10.3390/w12071869






