Evaluation of Groundwater and Grey Water Contamination with Heavy Metals and Their Adsorptive Remediation Using Renewable Carbon from a Mixed-Waste Source
Abstract
1. Introduction
2. Experimental
2.1. Water Samples from Different Regions of Riyadh City and Surrounding Cities
2.2. Determining Heavy Metals and Optimizing the Adsorptive Remediation Process
3. Results and Discussion
3.1. Evaluation of the Adsorptive Remediation Process for Separation of Pb(II), Zn(II), Cu(II), and Fe(II) from an Aqueous Medium Using the Model Solutions
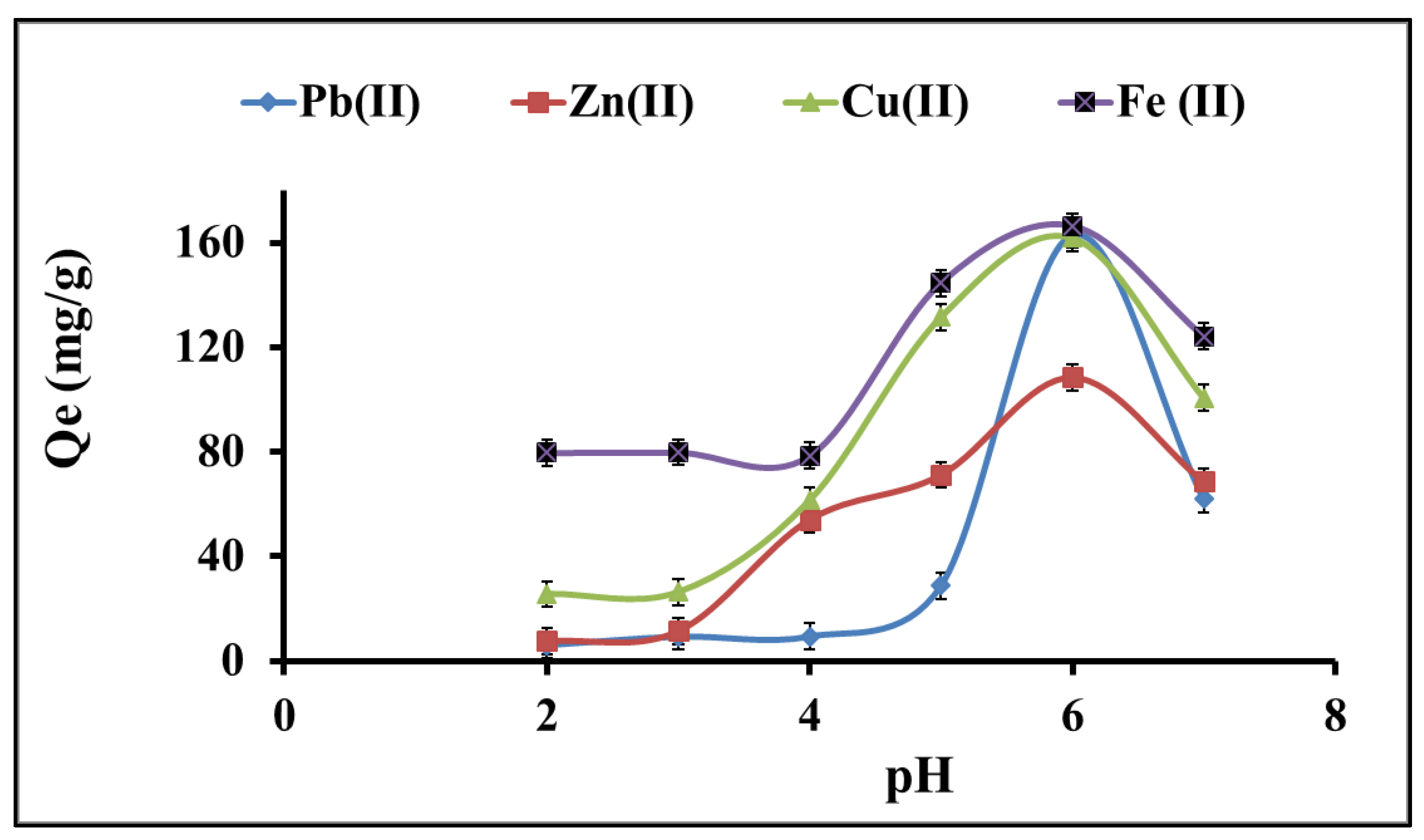
3.1.1. Effect of pH on the Metal Ion Solution Medium
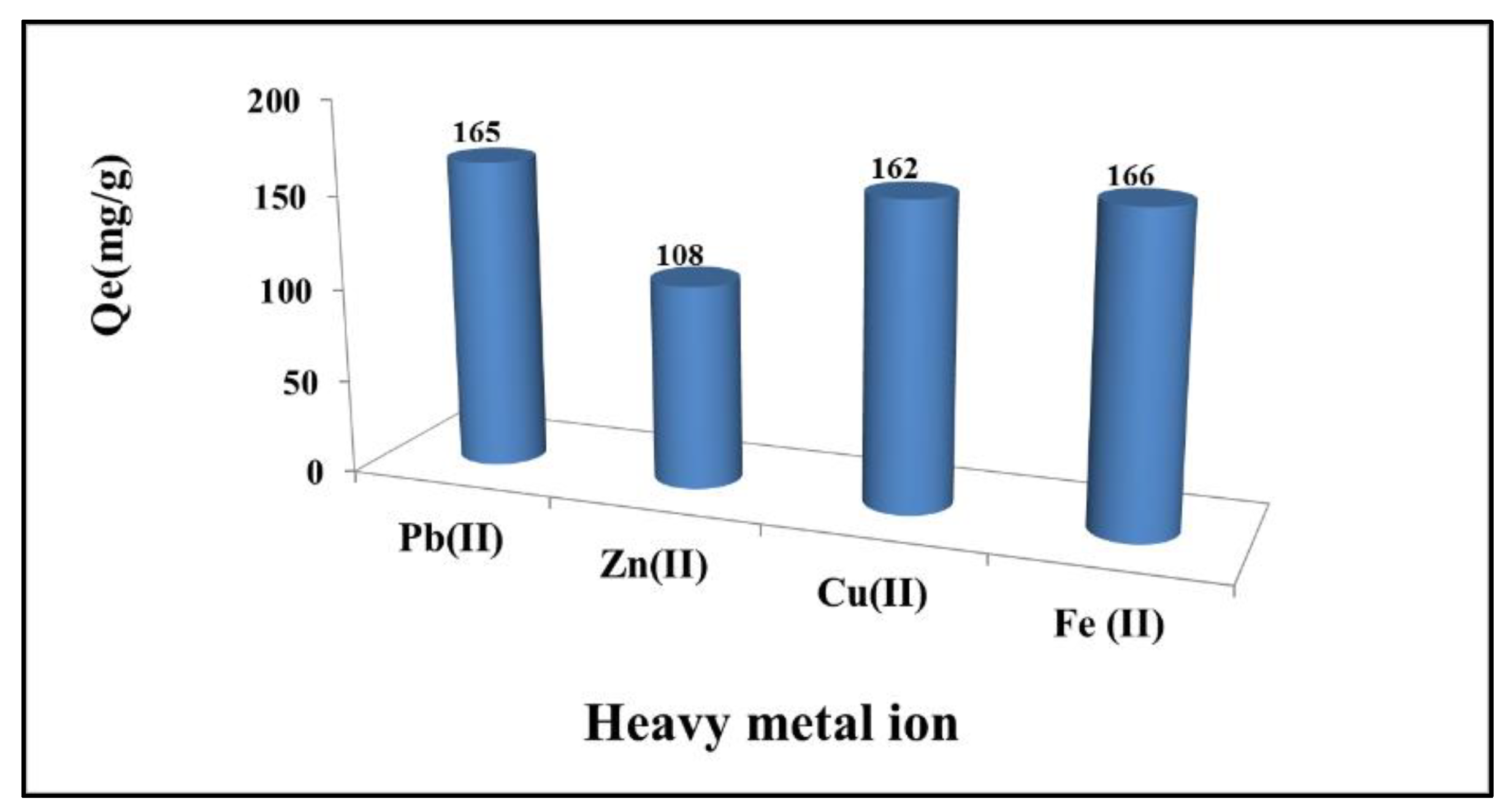
3.1.2. Competition for the Adsorption of Pb(II), Zn(II), Cu(II), and Fe(II) Onto RC-MWS
3.1.3. Kinetic Studies on the Adsorption of Pb(II), Zn(II), Cu(II), and Fe(II) Onto RC-MWS
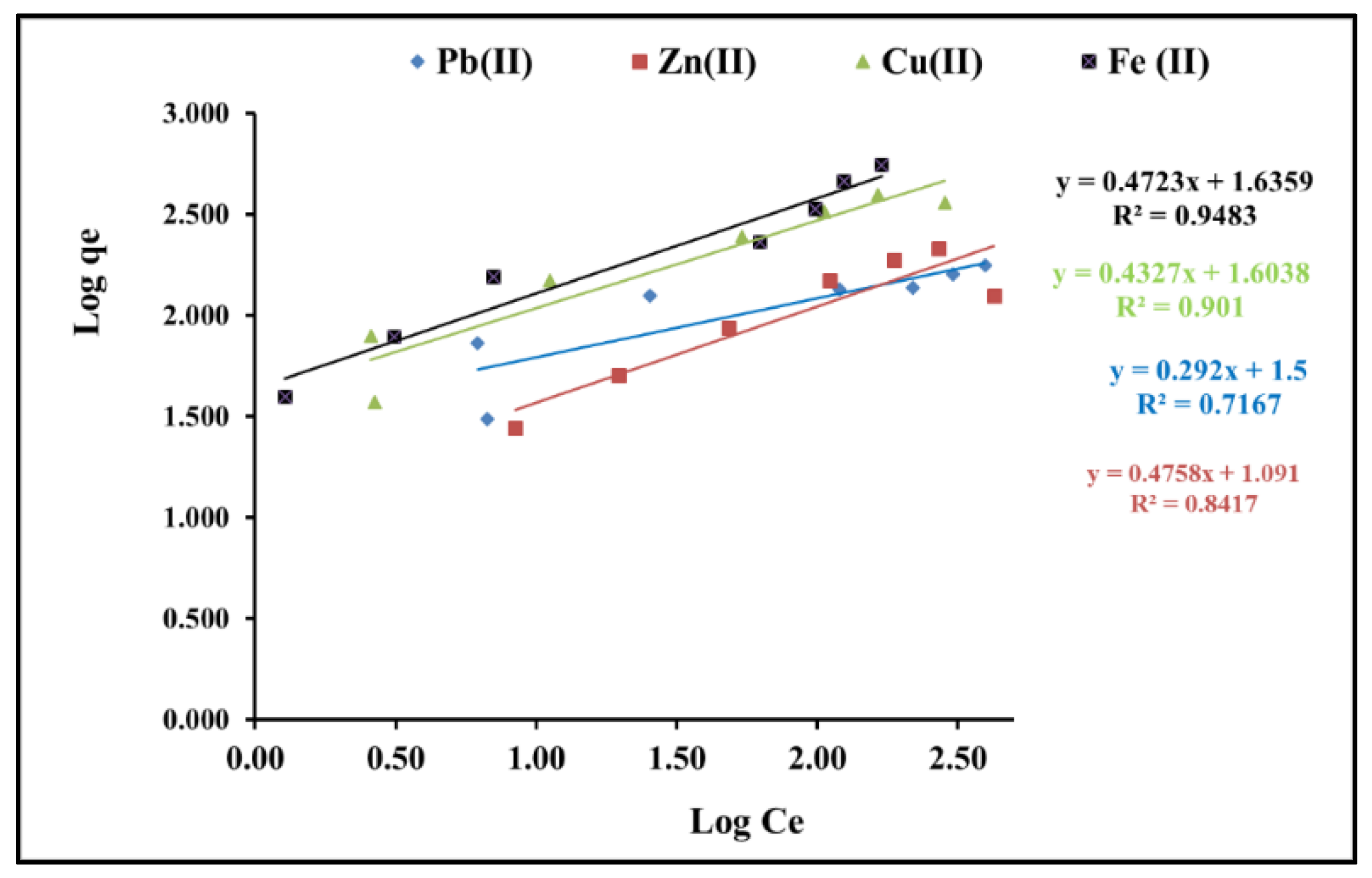
3.1.4. Isotherm Studies
The Application of the Adsorption Process to Actual Water Samples
4. Conclusions and Recommendations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alharbi, S.K.; Shafiquzzaman, M.; Haider, H.; AlSaleem, S.S.; Ghumman, A.R. Treatment of Ablution Greywater for Recycling by Alum Coagulation and Activated Carbon Adsorption. Arab. J. Sci. Eng. 2019, 44, 8389–8399. [Google Scholar] [CrossRef]
- Pradhan, S.; Al-Ghamdi, S.G.; Mackey, H.R. Greywater recycling in buildings using living walls and green roofs: A review of the applicability and challenges. Sci. Total Environ. 2019, 652, 330–344. [Google Scholar] [CrossRef]
- Al-Jasser, A.O. Greywater reuse in Saudi Arabia: Current situation and future potential. WIT Trans. Ecol. Environ. 2011, 153, 159–169. [Google Scholar]
- Abdula’aly, A.I.; Chammem, A.A. Groundwater treatment in the Central Region of Saudi Arabia. Desalination 1994, 96, 203–214. [Google Scholar] [CrossRef]
- Villamar, C.A.; Vera-Puerto, I.; Rivera, D.; De la Hoz, F.D. Reuse and recycling of livestock and municipal wastewater in Chilean agriculture: A preliminary assessment. Water (Switzerland) 2018, 10, 817. [Google Scholar] [CrossRef]
- Amin, S.; Farjoud, M.R.; Shabani, A. Groundwater Contamination by Heavy Metals in Water Resources of Shiraz Area. Iran Agric. Res. 2011, 30, 21–32. [Google Scholar]
- Vetrimurugan, E.; Brindha, K.; Elango, L.; Ndwandwe, O.M. Human exposure risk to heavy metals through groundwater used for drinking in an intensively irrigated river delta. Appl. Water Sci. 2017, 7, 3267–3280. [Google Scholar] [CrossRef]
- Ouda, O.K.M. Towards Assessment of Saudi Arabia Public Awareness of Water Shortage Problem. Resour. Environ. 2013, 3, 10–13. [Google Scholar]
- DeNicola, E.; Aburizaiza, O.S.; Siddique, A.; Khwaja, H.; Carpenter, D.O. Climate change and water scarcity: The case of Saudi Arabia. Ann. Glob. Health 2015, 81, 342–353. [Google Scholar] [CrossRef]
- Drewes, J.E.; Garduño, C.P.R.; Amy, G.L. Water reuse in the kingdom of Saudi Arabia—Status, prospects and research needs. Water Sci. Technol. Water Supply 2012, 12, 926–936. [Google Scholar] [CrossRef]
- Abdel-Satar, A.M.; Al-Khabbas, M.H.; Alahmad, W.R.; Yousef, W.M.; Alsomadi, R.H.; Iqbal, T. Quality assessment of groundwater and agricultural soil in Hail region, Saudi Arabia. Egypt. J. Aquat. Res. 2017, 43, 55–64. [Google Scholar] [CrossRef]
- Balkhair, K.S.; Ashraf, M.A. Field accumulation risks of heavy metals in soil and vegetable crop irrigated with sewage water in western region of Saudi Arabia. Saudi J. Biol. Sci. 2016, 23, S32–S44. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmadi, M.E. Groundwater quality assessment in Wadi Fayd, Western Saudi Arabia. Arab. J. Geosci. 2013, 6, 247–258. [Google Scholar] [CrossRef]
- Alhababy, A.; Al-Rajab, A. Groundwater Quality Assessment in Jazan Region, Saudi Arabia. Curr. World Environ. 2015, 10, 22–28. [Google Scholar] [CrossRef][Green Version]
- Mohamaden, M.I.I.; Khalil, M.K.; Draz, S.E.O.; Hamoda, A.Z.M. Ecological risk assessment and spatial distribution of some heavy metals in surface sediments of New Valley, Western Desert, Egypt. Egypt. J. Aquat. Res. 2017, 43, 31–43. [Google Scholar] [CrossRef]
- Gupta, V.K.; Gupta, M.; Sharma, S. Process development for the removal of lead and chromium from aqueous solutions using red mud—An aluminium industry waste. Water Res. 2001, 35, 1125–1134. [Google Scholar] [CrossRef]
- Iqbal, M.; Edyvean, R.G.J. Loofa sponge immobilized fungal biosorbent: A robust system for cadmium and other dissolved metal removal from aqueous solution. Chemosphere 2005, 61, 510–518. [Google Scholar] [CrossRef]
- Dubey, A.; Shiwani, S. Adsorption of lead using a new green material obtained from Portulaca plant. Int. J. Environ. Sci. Technol. 2012, 9, 15–20. [Google Scholar] [CrossRef]
- Tyler, G.; Påhlsson, A.M.B.; Bengtsson, G.; Bååth, E.; Tranvik, L. Heavy-metal ecology of terrestrial plants, microorganisms and invertebrates—A review. Water Air Soil Pollut. 1989, 47, 189–215. [Google Scholar] [CrossRef]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Shukia, S.; Mostaghimi, S.; Shanholtz, V.O.; Collins, M.C. A gis-based modeling approach for evaluating groundwater vulnerability to pesticides. J. Am. Water Resour. Assoc. 1998, 34, 1275–1293. [Google Scholar] [CrossRef]
- Al-Jassim, N.; Ansari, M.I.; Harb, M.; Hong, P.-Y. Removal of bacterial contaminants and antibiotic resistance genes by conventional wastewater treatment processes in Saudi Arabia: Is the treated wastewater safe to reuse for agricultural irrigation? Water Res. 2015, 73, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, A.I.; Matis, K.A.; Lanara, B.G.; Loos-Neskovic, C. Removal of Cadmium from Dilute Solutions by Hydroxyapatite. II. Flotation Studies. Sep. Sci. Technol. 1997, 32, 1755–1767. [Google Scholar] [CrossRef]
- Esalah, J.O.; Weber, M.E.; Vera, J.H. Removal of lead, cadmium and zinc from aqueous solutions by precipitation with sodium Di-(n-octyl) phosphinate. Can. J. Chem. Eng. 2000, 78, 948–954. [Google Scholar] [CrossRef]
- Canet, L.; Ilpide, M.; Seta, P. Efficient facilitated transport of lead, cadmium, zinc, and silver across a flat-sheet-supported liquid membrane mediated by lasalocid A. Sep. Sci. Technol. 2002, 37, 1851–1860. [Google Scholar] [CrossRef]
- Senthilkumar, R.; Vijayaraghavan, K.; Thilakavathi, M.; Iyer, P.V.R.; Velan, M. Application of seaweeds for the removal of lead from aqueous solution. Biochem. Eng. J. 2007, 33, 211–216. [Google Scholar] [CrossRef]
- El-Toni, A.M.; Habila, M.A.; Ibrahim, M.A.; Labis, J.P.; ALOthman, Z.A. Simple and facile synthesis of amino functionalized hollow core-mesoporous shell silica spheres using anionic surfactant for Pb(II), Cd(II), and Zn(II) adsorption and recovery. Chem. Eng. J. 2014, 251, 441–451. [Google Scholar] [CrossRef]
- Symposium on Waste Water—Treatment and Reuse | British Council Saudi Arabia. Available online: https://www.britishcouncil.sa/en/events/symposium-waste-water-treatment-and-reuse (accessed on 11 October 2019).
- Polat, H.; Erdogan, D. Heavy metal removal from waste waters by ion flotation. J. Hazard. Mater. 2007, 148, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wen, S.; Han, G.; Xu, L.; Feng, Q. Activation mechanism of lead ions in the flotation of sphalerite depressed with zinc sulfate. Miner. Eng. 2020, 146, 106132. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Azizi, A.; Kouachi, S.; Karimi, M.; Celik, M.S. Estimation of flotation rate constant and particle-bubble interactions considering key hydrodynamic parameters and their interrelations. Miner. Eng. 2019, 141, 105836. [Google Scholar] [CrossRef]
- Feng, Q.; Wen, S.; Bai, X.; Chang, W.; Cui, C.; Zhao, W. Surface modification of smithsonite with ammonia to enhance the formation of sulfidization products and its response to flotation. Miner. Eng. 2019, 137, 1–9. [Google Scholar] [CrossRef]
- Kyzas, G.; Matis, K. Flotation in Water and Wastewater Treatment. Processes 2018, 6, 116. [Google Scholar] [CrossRef]
- Kitchener, J.A.; Gochin, R.J. The mechanism of dissolved air flotation for potable water: Basic analysis and a proposal. Water Res. 1981, 15, 585–590. [Google Scholar] [CrossRef]
- Özer, A. Removal of Pb (II) ions from aqueous solutions by sulphuric acid-treated wheat bran. J. Hazard. Mater. 2007, 141, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Badmus, M.O.A.; Audu, T.O.K.; Anyata, B. Removal of copper from industrial wastewaters by activated carbon prepared from periwinkle shells. Korean J. Chem. Eng. 2007, 24, 246–252. [Google Scholar] [CrossRef]
- Amarasinghe, B.M.W.P.K.; Williams, R.A. Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater. Chem. Eng. J. 2007, 132, 299–309. [Google Scholar] [CrossRef]
- Qaiser, S.; Saleemi, A.R.; Umar, M. Biosorption of lead from aqueous solution by Ficus religiosa leaves: Batch and column study. J. Hazard. Mater. 2009, 166, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Othman, Z.A.A.; Hashem, A.; Habila, M.A. Kinetic, equilibrium and thermodynamic studies of cadmium (II) adsorption by modified agricultural wastes. Molecules 2011, 16, 10443–10456. [Google Scholar] [CrossRef] [PubMed]
- (PDF) Adsorption of Chromium Ion from Industrial Effluent Using Activated Carbon Derived from Plantain. Available online: https://www.researchgate.net/publication/322677407_Adsorption_of_Chromium_Ion_from_Industrial_Effluent_Using_Activated_Carbon_Derived_from_Plantain (accessed on 11 October 2019).
- Bulut, Y.; Tez, Z. Adsorption studies on ground shells of hazelnut and almond. J. Hazard. Mater. 2007, 149, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Amuda, O.S.; Giwa, A.A.; Bello, I.A. Removal of heavy metal from industrial wastewater using modified activated coconut shell carbon. Biochem. Eng. J. 2007, 36, 174–181. [Google Scholar] [CrossRef]
- Gong, J.; Liu, J.; Chen, X.; Jiang, Z.; Wen, X.; Mijowska, E.; Tang, T. Converting real-world mixed waste plastics into porous carbon nanosheets with excellent performance in the adsorption of an organic dye from wastewater. J. Mater. Chem. A 2015, 3, 341–351. [Google Scholar] [CrossRef]
- Habila, M.A.; ALOthman, Z.A.; Al-Tamrah, S.A.; Ghafar, A.A.; Soylak, M. Activated carbon from waste as an efficient adsorbent for malathion for detection and removal purposes. J. Ind. Eng. Chem. 2015, 32, 336–344. [Google Scholar] [CrossRef]
- Saleem, J.; Shahid, U.B.; Hijab, M.; Mackey, H.; McKay, G. Production and applications of activated carbons as adsorbents from olive stones. Biomass Convers. Biorefin. 2019, 9, 775–802. [Google Scholar] [CrossRef]
- Balcerek, M.; Pielech-Przybylska, K.; Patelski, P.; Dziekońska-Kubczak, U.; Jusel, T. Treatment with activated carbon and other adsorbents as an effective method for the removal of volatile compounds in agricultural distillates. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2017, 34, 714–727. [Google Scholar] [CrossRef]
- Ghaedi, M.; Nasab, A.G.; Khodadoust, S.; Rajabi, M.; Azizian, S. Application of activated carbon as adsorbents for efficient removal of methylene blue: Kinetics and equilibrium study. J. Ind. Eng. Chem. 2014, 20, 2317–2324. [Google Scholar] [CrossRef]
- Dias, J.M.; Alvim-Ferraz, M.C.M.; Almeida, M.F.; Rivera-Utrilla, J.; Sánchez-Polo, M. Waste materials for activated carbon preparation and its use in aqueous-phase treatment: A review. J. Environ. Manag. 2007, 85, 833–846. [Google Scholar] [CrossRef]
- Wong, S.; Ngadi, N.; Inuwa, I.M.; Hassan, O. Recent advances in applications of activated carbon from biowaste for wastewater treatment: A short review. J. Clean. Prod. 2018, 175, 361–375. [Google Scholar] [CrossRef]
- Wang, A.; Zheng, Z.; Li, R.; Hu, D.; Lu, Y.; Luo, H.; Yan, K. Biomass-derived porous carbon highly efficient for removal of Pb (II) and Cd (II). Green Energy Environ. 2019, 4, 414–423. [Google Scholar] [CrossRef]
- Deng, J.; Li, M.; Wang, Y. Biomass-derived carbon: Synthesis and applications in energy storage and conversion. Green Chem. 2016, 18, 4824–4854. [Google Scholar] [CrossRef]
- Yang, S.; Fang, X.; Duan, L.; Yang, S.; Lei, Z.; Wen, X. Comparison of ultrasound-assisted cloud point extraction and ultrasound-assisted dispersive liquid liquid microextraction for copper coupled with spectrophotometric determination. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 148, 72–77. [Google Scholar] [CrossRef]
- Alothman, Z.A.; Habila, M.A.; Yilmaz, E.; Soylak, M.; Alfadul, S.M. Ultrasonic supramolecular microextration of nickel (II) as N,N′-Dihydroxy-1,2-cyclohexanediimine chelates from water, tobacco and fertilizer samples before FAAS determination. J. Mol. Liq. 2016, 221, 773–777. [Google Scholar] [CrossRef]
- Lagergren, S. About the Theory of So-Called Adsorption of Soluble Substances. Sven. Vetensk. 1898, 24, 1–39. Available online: https://www.oalib.com/references/5821801 (accessed on 12 June 2020).
- McKay, G.; Ho, Y.S.; Ng, J.C.Y. Biosorption of copper from waste waters: A review. Sep. Purif. Methods 1999, 28, 87–125. [Google Scholar] [CrossRef]
- Blanchard, G.; Maunaye, M.; Martin, G. Removal of heavy metals from waters by means of natural zeolites. Water Res. 1984, 18, 1501–1507. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the Adsorption in Solution. J. Phys. Chem. A 1906, 57, 385–470. [Google Scholar]
- Mohanty, K.; Jha, M.; Meikap, B.C.; Biswas, M.N. Removal of chromium (VI) from dilute aqueous solutions by activated carbon developed from Terminalia arjuna nuts activated with zinc chloride. Chem. Eng. Sci. 2005, 60, 3049–3059. [Google Scholar] [CrossRef]
- Arshadi, M.; Amiri, M.J.; Mousavi, S. Kinetic, equilibrium and thermodynamic investigations of Ni(II), Cd(II), Cu(II) and Co(II) adsorption on barley straw ash. Water Resour. Ind. 2014, 6, 1–17. [Google Scholar] [CrossRef]
- Abdel Salam, O.E.; Reiad, N.A.; ElShafei, M.M. A study of the removal characteristics of heavy metals from wastewater by low-cost adsorbents. J. Adv. Res. 2011, 2, 297–303. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Zhao, R.; Li, Y.; Li, C.; Zhang, C. Adsorption of Pb(II) on activated carbon prepared from Polygonum orientale Linn.: Kinetics, isotherms, pH, and ionic strength studies. Bioresour. Technol. 2010, 101, 5808–5814. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef] [PubMed]
- Albratty, M.; Arbab, I.; Alhazmi, H.; Attafi, I.; Al-Rajab, A. Icp-Ms Determination of Trace Metals in Drinking Water Sources in Jazan Area, Saudi Arabia. Curr. World Environ. 2017, 12, 06–17. [Google Scholar] [CrossRef][Green Version]
- Kausar, R.; Ahmad, Z. Determination of toxic inorganic elements pollution in ground waters of Kahuta Industrial Triangle Islamabad, Pakistan using inductively coupled plasma mass spectrometry. Environ. Monit. Assess. 2009, 157, 347–354. [Google Scholar] [CrossRef] [PubMed]

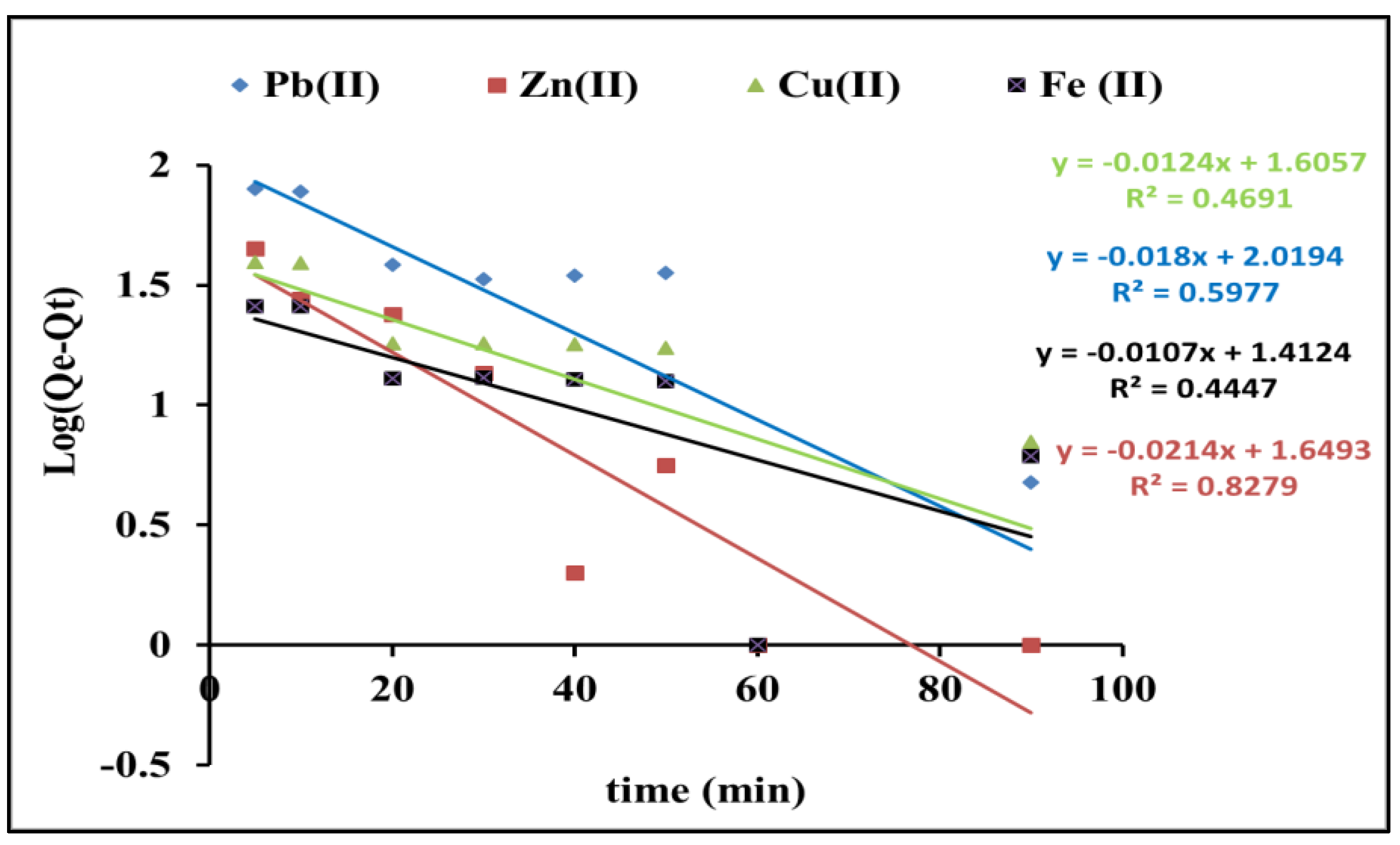
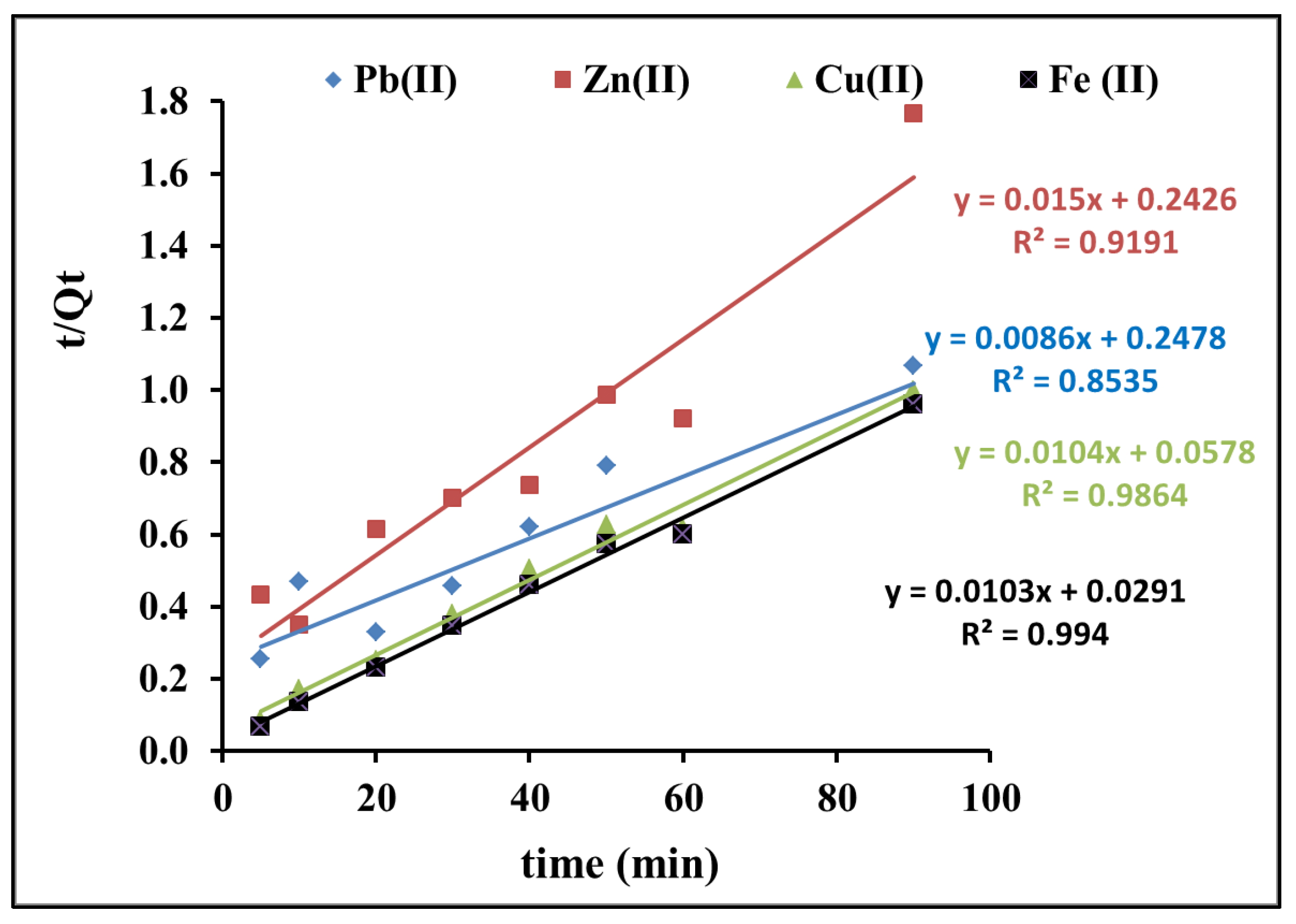

| Pseudo First Order | R2 | Pseudo Second Order | R2 | ||||
|---|---|---|---|---|---|---|---|
| qe, exp (mg/g) | k1 (min−1) | qe, cal (mg/g) | k2 (g/mg·min) | qe, cal (mg/g) | |||
| Pb(II) | 99 | 0.041 | 104.5 | 0.59 | 9.2 × 10−4 | 66.6 | 0.92 |
| Zn(II) | 65 | 0.049 | 44.5 | 0.82 | 3.6 × 10−3 | 97 | 0.99 |
| Cu(II) | 97 | 0.028 | 40.3 | 0.46 | 2.9 × 10−4 | 116.2 | 0.85 |
| Fe(II) | 100 | 0.024 | 25.8 | 0.44 | 1.8 × 10−3 | 96.1 | 0.98 |
| Langmuir Constants | Correlation Coefficients R2 | Freundlich Constants | Correlation Coefficients R2 | |||
|---|---|---|---|---|---|---|
| KL (L/mg) | (mg/g) | Kf (mg/g)/(mg/L)n | n | |||
| Pb(II) | 0.05 | 161.2 | 0.98 | 31.6 | 0.29 | 0.71 |
| Zn(II) | 0.01 | 285.7 | 0.98 | 12.3 | 0.47 | 0.84 |
| Cu(II) | 0.039 | 434.7 | 0.97 | 40.1 | 0.43 | 0.90 |
| Fe(II) | 0.049 | 434.7 | 0.86 | 43.2 | 0.47 | 0.94 |
| Pb(II) Investigations | Zn(II) Investigations | Cu(II) Investigations | Fe(II) Investigations | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water Sample | Initial Concentration of Pb(II) (ppm) | Final Concentration of Pb(II) (ppm) | Efficiency % | Initial Concentration of Zn(II) (ppm) | Final Concentration of Zn(II) (ppm) | Efficiency % | Initial Concentration of Cu(II) (ppm) | Final Concentration of Cu(II) (ppm) | Efficiency % | Initial Concentration of Fe(II) (ppm) | Final Concentration of Fe(II) (ppm) | Efficiency % |
| Deria-1 | 0.2151 | 0 | 100 | 1.3 | 0 | 100 | 1.26 | 0.00 | 100 | 3.54 | 1.17 | 97 |
| Deria-2 | 0.1357 | 0 | 100 | 1.2 | 0.091 | 92 | 0.33 | 0.00 | 100 | 0.70 | 3.31 | 53 |
| Deria-3 | 0.2313 | 0 | 100 | 2.1 | 0.064 | 97 | 0.11 | 0.00 | 100 | 2.42 | 8.71 | 64 |
| Deria-4 | 0.0436 | 0 | 100 | 1.4 | 0.075 | 95 | 0.25 | 0.00 | 100 | 0.28 | 0.18 | 94 |
| Deria-5 | 0.1305 | 0 | 100 | 1.6 | 0 | 100 | 0.16 | 0.00 | 100 | 0.22 | 0.66 | 70 |
| Deria-6 | 0 | 0 | - | 1.8 | 0.019 | 99 | 0.46 | 0.00 | 100 | 0.36 | 0.05 | 99 |
| Deria-7 | 0 | 0 | - | 1.4 | 0.063 | 96 | 0.00 | 0.00 | - | 1.23 | 1.90 | 85 |
| Deria-8 | 0 | 0 | - | 1.4 | 0 | 100 | 1.10 | 0.00 | 100 | 5.42 | 2.89 | 95 |
| Deria-9 | 0 | 0 | - | 1.2 | 0 | 100 | 0.22 | 0.00 | 100 | 4.47 | 2.23 | 95 |
| Deria-10 | 0 | 0 | - | 1.1 | 0.08 | 93 | 0.24 | 0.29 | 88 | 1.94 | 2.31 | 88 |
| Deria-11 | 0.0405 | 0 | 100 | 0.8 | 0.01 | 99 | 0.25 | 0.00 | 100 | 14.60 | 1.48 | 99 |
| Mozahemia-1 | 0.0833 | 0 | 100 | 2.1 | 0.056 | 97 | 0.25 | 0.00 | 100 | 0.31 | 0.14 | 96 |
| Mozahemia-2 | 0 | 0 | - | 1.2 | 0.038 | 97 | 0.00 | 0.00 | - | 0.17 | 0.00 | 100 |
| Mozahemia-3 | 0 | 0 | - | 1.0 | 0.033 | 97 | 0.06 | 0.00 | 100 | 11.19 | 2.22 | 98 |
| Pb(II) Investigations | Zn(II) Investigations | Cu(II) Investigations | Fe(II) Investigations | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water Samples | Initial Concentration of Pb(II) (ppm) | Final Concentration of Pb(II) ppm) | Efficiency % | Initial Concentration of Zn(II) (ppm) | Final Concentration of Zn(II) (ppm) | Efficiency % | Initial Concentration of Cu(II) (ppm) | Final Concentration of Cu(II) (ppm) | Efficiency % | Initial Concentration of Fe(II) (ppm) | Final Concentration of Fe(II) (ppm) | Efficiency % |
| clothes-1 | 0 | 0 | - | 905.21 | 12.59 | 99 | 11.18 | 1.92 | 98 | 5.31 | 3.87 | 93 |
| clothes-2 | 0 | 0 | - | 997.08 | 1.69 | 100 | 0.16 | 0.00 | 100 | 2.20 | 1.09 | 95 |
| clothes-3 | 6.1151 | 0.215386 | 96.5 | 862.14 | 0.85 | 100 | 1.84 | 0.87 | 95 | 1.32 | 3.17 | 76 |
| clothes-4 | 0.4854 | 0.003688 | 99.2 | 4600.01 | 1.09 | 100 | 1.86 | 1.16 | 94 | 4.21 | 9.25 | 78 |
| clothes-5 | 5.039 | 0.025297 | 99.5 | 1019.94 | 0.87 | 100 | 0.39 | 0.00 | 100 | 2.15 | 3.42 | 84 |
| clothes-6 | 0 | 0 | - | 638.83 | 0.35 | 100 | 0.53 | 0.00 | 100 | 4.48 | 3.09 | 93 |
| clothes-7 | 0.0024 | 0 | 100 | 977.57 | 0.00 | 100 | 0.24 | 0.00 | 100 | 9.42 | 3.08 | 97 |
| clothes-8 | 0.2102 | 0 | 100 | 904.20 | 1.56 | 100 | 0.71 | 0.62 | 91 | 4.56 | 5.68 | 88 |
| cars-1 | 0 | 0 | - | 923.95 | 3.78 | 100 | 15.09 | 28.41 | 81 | 47.54 | 0.59 | 100 |
| cars-2 | 0 | 0 | - | 1217.16 | 7.21 | 99 | 21.11 | 7.31 | 97 | 1.95 | 0.18 | 99 |
| cars-3 | 1.787 | 0 | 100 | 842.75 | 14.36 | 98 | 39.94 | 73.41 | 82 | 15.87 | 6.13 | 96 |
| cars-4 | 0 | 0 | - | 865.13 | 8.00 | 99 | 92.67 | 270.09 | 71 | 373.65 | 3.71 | 100 |
| cars-5 | 0 | 0 | - | 1056.29 | 12.91 | 99 | 50.99 | 79.33 | 84 | 7.86 | 1.00 | 99 |
| cars-6 | 0 | 0 | - | 1228.33 | 0.00 | 100 | 0.30 | 0.00 | 100 | 41.20 | 19.86 | 95 |
| cars-7 | 0 | 0 | - | 956.51 | 0.25 | 100 | 0.11 | 0.00 | 100 | 9.18 | 0.36 | 100 |
| cars-8 | 0 | 0 | - | 1174.11 | 10.11 | 99 | 30.34 | 27.44 | 91 | 81.47 | 2.91 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alomar, T.S.; Habila, M.A.; Alothman, Z.A.; AlMasoud, N.; Alqahtany, S.S. Evaluation of Groundwater and Grey Water Contamination with Heavy Metals and Their Adsorptive Remediation Using Renewable Carbon from a Mixed-Waste Source. Water 2020, 12, 1802. https://doi.org/10.3390/w12061802
Alomar TS, Habila MA, Alothman ZA, AlMasoud N, Alqahtany SS. Evaluation of Groundwater and Grey Water Contamination with Heavy Metals and Their Adsorptive Remediation Using Renewable Carbon from a Mixed-Waste Source. Water. 2020; 12(6):1802. https://doi.org/10.3390/w12061802
Chicago/Turabian StyleAlomar, Taghrid S., Mohamed A. Habila, Zeid A. Alothman, Najla AlMasoud, and Saad Saeed Alqahtany. 2020. "Evaluation of Groundwater and Grey Water Contamination with Heavy Metals and Their Adsorptive Remediation Using Renewable Carbon from a Mixed-Waste Source" Water 12, no. 6: 1802. https://doi.org/10.3390/w12061802
APA StyleAlomar, T. S., Habila, M. A., Alothman, Z. A., AlMasoud, N., & Alqahtany, S. S. (2020). Evaluation of Groundwater and Grey Water Contamination with Heavy Metals and Their Adsorptive Remediation Using Renewable Carbon from a Mixed-Waste Source. Water, 12(6), 1802. https://doi.org/10.3390/w12061802






