Feeding the Building Plumbing Microbiome: The Importance of Synthetic Polymeric Materials for Biofilm Formation and Management
Abstract
1. Drinking Water Microbiology from Source to Tap
1.1. Bacteria Are Omnipresent in Drinking Water Treatment and Distribution Systems
1.2. The Microbiology of DWDS Is Studied, Monitored, and Regulated
1.3. The Microbiology of DWDS Is Prone to (Environmental) Temporal and Spatial Changes
1.4. Changes in Microbiological Quality Are Problematic
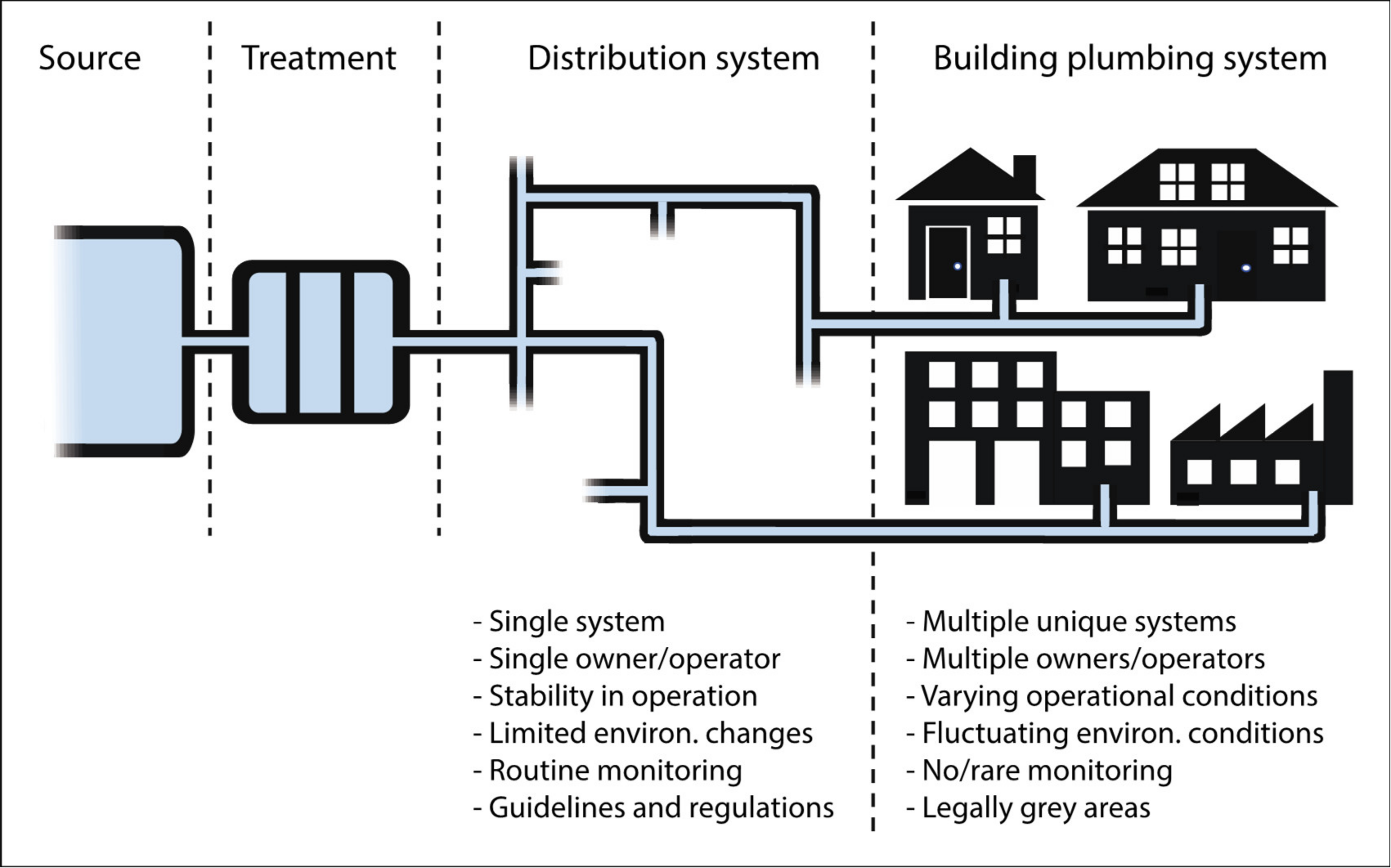
2. Building Plumbing Systems Change the Microbiology
2.1. The Microbiological Black Box Between the Water Meter and the Tap
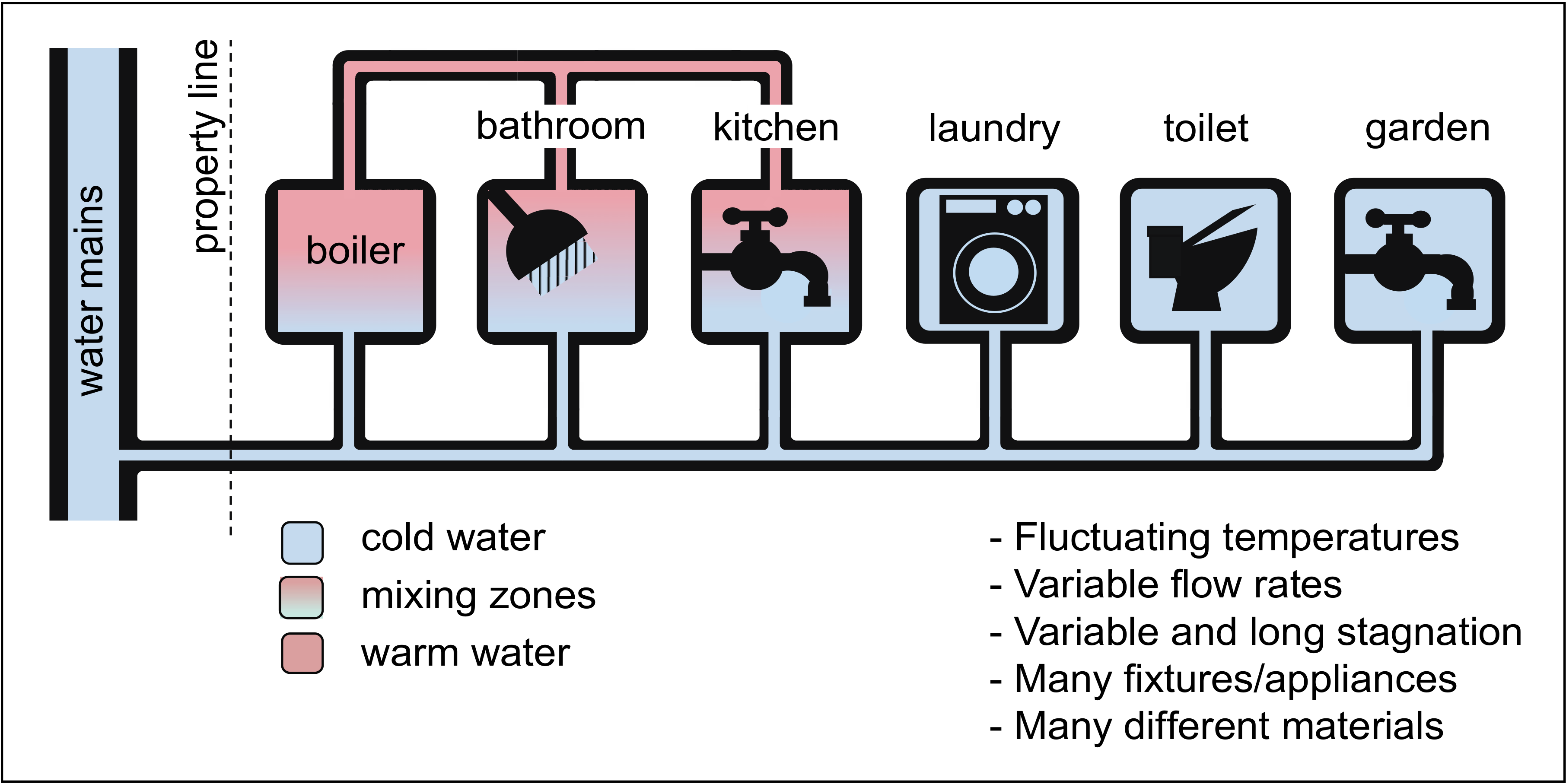
2.2. Specific Building Plumbing System Conditions Alter Microbiological Water Quality
3. Synthetic Polymeric Materials in Building Plumbing Systems
3.1. The Variety of Materials Used in Building Plumbing Systems Creates Numerous Ecological Niches
3.2. Carbon Migrates from Synthetic Polymeric Materials
3.3. Migrating Organic Carbon Compounds Drive Biofilm Formation and Selection
4. Quantifying Initial Biofilm Formation on Flexible Synthetic Polymeric Materials
4.1. Dispersal and Selection as Main Parameters for Initial Biofilm Formation
- Inorganic nutrients from the water: The tap water in this example is typical for Zurich (CH), meaning non-chlorinated, biologically stable (i.e., assimilable organic carbon (AOC) < 10 µg/L, [81]), oligotrophic water with approximately 1 mg/L dissolved organic carbon (DOC), 3 mg/L total nitrogen (TN), and 5 µg/L total phosphorous (TP) [82]. This converts to 0.1 mg-TN/hose and 0.5 µg-TP/hose.
- Water-to-surface dispersal: Zurich tap water comprises ~5 × 104 cells/mL (i.e., 4.5 × 106 cells/hose) and >5000 different bacterial taxa [50]. Water-to-surface dispersal rates for initial colonization remain poorly characterized for drinking water systems, but it is known that bacterial attachment starts within seconds to minutes of the first exposure [83,84]. Here, we assume an attachment of 1% of the total cell concentration (TCC) from the water phase during 1 h of stagnation, which means ~1.1 × 106 cells/hose/day in the absence of any growth.
- Bacterial growth: Based on a conversion factor of 107 cells/µg-AOC [85,86] and following the rule-of-thumb for growth requirements of bacteria (i.e., a C:N:P ratio of 100:10:1 [85]), bacterial growth in the water would be carbon-limited (allowing for the growth of ~9 × 106 cells/hose/day). However, this is reversed due to the excessive AOC that migrates continuously from the material, rendering the shower-hose environment phosphorous-limited.Ultimately, the maximum growth potential of the combined system (i.e., water and hose) is ~1 × 108 cells/hose/day, assuming that 100% of the phosphorous is biologically available.
- Nutrient-based selection: The composition and biodiversity of biofilm communities is influenced by the type of material they grow on [87]. Based on the selection observed in previous studies, we assume for our example that only 10% of the bacteria present in the water phase and of those dispersing to the material’s surface can actually utilize the migrated organic carbon and grow (i.e., 4.5 × 105 cells/hose/day).
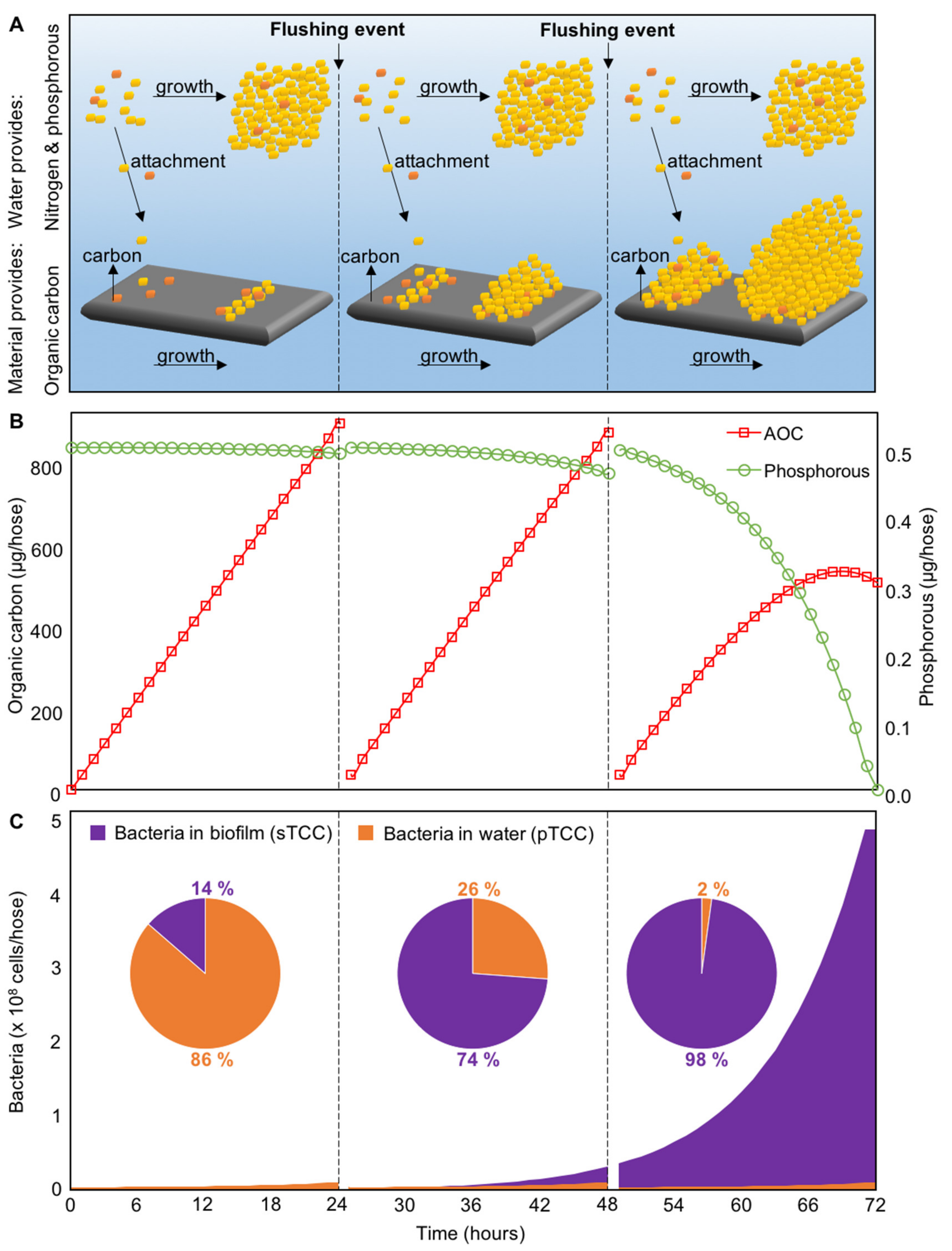
4.2. Initial Colonization, Growth, and Biofilm Formation
- During the first stagnation period, i.e., within the first 24 h of stagnation, planktonic growth dominates the shower hose system, with 1.2 × 107 planktonic (86%) and 1.8 × 106 attached (14%) cells/hose. However, in the subsequent day(s) (with daily shower/flushing events), the water phase is exchanged every 24 h, meaning a replacement of the grown planktonic cells by the source water community, and a replenishment of inorganic nutrients in the otherwise carbon-rich environment. Sessile cells remain in the biofilm and therefore continue growing at the concentration of sTCC24 after the first flushing event, subsequently rendering the system biofilm dominated; with 1 × 107 pTCC/hose (26%) versus 3 × 107 sTCC/hose (74%) after 48 h.
- Assuming continuous growth in the biofilm, the shower hose system will reach phosphorous-limitation after approximately 70 h (Figure 4B), limiting further growth until a replenishment of inorganic nutrients.
- During the initial stagnation period, the biofilm community is dominated by the initial water-to-surface dispersal-driven colonization. However, the continuous growth of adapted cells in the biofilm results in a highly selective growth and biofilm development. More precisely, the original drinking water community in our example comprised around 5000 different species. Due to the ability of (initially) only 10% to grow (i.e., 500 species), species heterogeneity in the biofilm inevitably declines. As a result, we can state that nutrient-based selection is important for the subsequent development of the biofilm and its microbial community composition.
5. The Relevance and Management of Building Plumbing Biofilms
5.1. Why Should We Care?
5.2. What Can We Do?
- A quality label for good materials: Important for the widespread use of high-quality materials are sensible and standardized quality control procedures. Irrespective of legal guidelines, assays for the assessment of carbon migration and growth potential exist (Section 3 [69]) and can be used by both material producers and policy makers. The result would be a material-grading system that is ideally freely available to all stakeholders, including plumbers, planners, and architects. This material grading system can, for example, be in form of a quality label, which enables easy identification of high-quality materials for both professional and private costumers. Here, the incentive for producers would be the competitive advantage gained over lower quality products from competitors.
- Information sharing: To address microbiological challenges requires extensive information sharing between the diverse stakeholders in building plumbing systems. Here, scientists have an opportunity to contribute knowledge on how the basic principles in microbial ecology relates to different plumbing materials and ultimately water quality. One example can be to incorporate microbiology courses in basic training and teaching modules for plumbers and architects. On a different level, opportunities exist to collaborate with producers of plumbing materials and fixtures on applied research projects focusing on the evaluation of (existing) material properties, their interaction with microorganisms, and their dependency on environmental conditions in buildings. Finally, it is important to engage the public as the end-users who are operating the building plumbing systems and therefore create the conditions that influence material behavior and microbiological growth potential. Elucidating the impact of low-quality materials on drinking water microbial quality will incentivize users to invest in high-quality materials, for example, when purchasing a new shower hose or fixture.
- Further research: Considerable knowledge gaps exist in our understanding of the microbial ecology of building plumbing systems. There is a clear need for additional pilot- and full-scale experiments dealing with the interplay between existing materials, the developing microbial community, and water quality. More precisely, a better understanding is needed of a materials’ behavior in the context of complex building plumbing systems (e.g., fluctuating water temperatures, stagnation, disinfectant residuals, different materials in concert, etc.). Additionally, it is still completely unclear whether material-specific microbial communities establish when similar plumbing materials are used in different locations, or what exactly the impact of source water differences (e.g., community composition) are on the microbiome development. In a similar vein, research is needed on whether specific materials (additives) favor the establishment of specific opportunistic pathogens. Finally, with respect to building plumbing, there are clear research opportunities in the field of new material design/development. On the one hand, there is interest in developing anti-microbial strategies focusing on surface-coatings (e.g., copper or silver [99]). Similarly, there is ongoing research on materials with anti-adhesive properties to combat fouling [100]. On the other hand, we propose that material design might also move towards exploring the management of a “good,” stable microbial community composition. In this regard, Wang et al. [65] proposed a probiotic approach in which they would introduce specific bacteria into the building plumbing system, potentially coupled with a prebiotic approach of creating favorable conditions for such organisms in building plumbing systems. With respect to the latter, one option would be to tailor the leaching properties of a specific material (nutrient type, rate) to selected probiotic microorganisms in order to sustain their presence/dominance in a plumbing system.
6. Conclusions
- Conditions within building plumbing systems impact and change the microbial community composition of the water, potentially resulting in quality deterioration.
- Flexible synthetic materials leach organic carbon, which not only increases the potential for bacterial growth but also drives selection within the establishing biofilm community.
- Ecological principles can be used to understand and quantify microbial growth dynamics and their dependency on engineered components of plumbing systems.
- Gaining and sharing knowledge on the interaction between material properties and microbiology provides stakeholders with the possibility to actively manage building plumbing microbiology through material design, material selection, and operation.
- The exclusive use of high-quality materials in new building plumbing systems poses a straightforward strategy towards managing the building plumbing microbiome.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Proctor, C.R.; Hammes, F. Drinking Water Microbiology—From Measurement to Management. Curr. Opin. Biotechnol. 2015, 33, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Hammes, F.; Berney, M.; Wang, Y.; Vital, M.; Koster, O.; Egli, T. Flow-Cytometric Total Bacterial Cell Counts as a Descriptive Microbiological Parameter for Drinking Water Treatment Processes. Water Res. 2008, 42, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Rinta-Kanto, J.M.; Lehtola, M.J.; Vartiainen, T.; Martikainen, P.J. Rapid Enumeration of Virus-like Particles in Drinking Water Samples Using SYBR Green I-Staining. Water Res. 2004, 38, 2614–2618. [Google Scholar] [CrossRef] [PubMed]
- Vital, M.; Dignum, M.; Magic-Knezev, A.; Ross, P.; Rietveld, L.; Hammes, F. Flow Cytometry and Adenosine Tri-Phosphate Analysis: Alternative Possibilities to Evaluate Major Bacteriological Changes in Drinking Water Treatment and Distribution Systems. Water Res. 2012, 46, 4665–4676. [Google Scholar] [CrossRef]
- Besmer, M.D.; Hammes, F. Short-Term Microbial Dynamics in a Drinking Water Plant Treating Groundwater with Occasional High Microbial Loads. Water Res. 2016, 107, 11–18. [Google Scholar] [CrossRef]
- Stadler, H.; Klock, E.; Skritek, P.; Mach, R.L.; Zerobin, W.; Farnleitner, A.H. The Spectral Absorption Coefficient at 254nm as a Real-Time Early Warning Proxy for Detecting Faecal Pollution Events at Alpine Karst Water Resources. Water Sci. Technol. 2010, 62, 1898–1906. [Google Scholar] [CrossRef]
- Jung, A.-V.; Le Cann, P.; Roig, B.; Thomas, O.; Baurè, E.; Thomas, M.-F. Microbial Contamination Detection in Water Resources: Interest of Current Optical Methods, Trends and Needs in the Context of Climate Change. Int. J. Environ. Res. Public Health 2014, 11, 4292–4310. [Google Scholar] [CrossRef]
- Ma, X.; Vikram, A.; Casson, L.; Bibby, K. Centralized Drinking Water Treatment Operations Shape Bacterial and Fungal Community Structure. Environ. Sci. Technol. 2017, 51, 7648–7657. [Google Scholar] [CrossRef]
- Bruno, A.; Sandionigi, A.; Bernasconi, M.; Panio, A.; Labra, M.; Casiraghi, M. Changes in the Drinking Water Microbiome: Effects of Water Treatments Along the Flow of Two Drinking Water Treatment Plants in a Urbanized Area, Milan (Italy). Front. Microbiol. 2018, 9, 2557. [Google Scholar] [CrossRef]
- Pinto, A.J.; Xi, C.; Raskin, L. Bacterial Community Structure in the Drinking Water Microbiome Is Governed by Filtration Processes. Environ. Sci. Technol. 2012, 46, 8851–8859. [Google Scholar] [CrossRef]
- Niquette, P.; Servais, P.; Savoir, R. Bacterial Dynamics in the Drinking Water Distribution System of Brussels. Water Res. 2001, 35, 675–682. [Google Scholar] [CrossRef]
- Henne, K.; Kahlisch, L.; Brettar, I.; Höfle, M.G. Analysis of Structure and Composition of Bacterial Core Communities in Mature Drinking Water Biofilms and Bulk Water of a Citywide Network in Germany. Appl. Environ. Microbiol. 2012, 78, 3530–3538. [Google Scholar] [CrossRef] [PubMed]
- Lührig, K.; Canback, B.; Paul, C.J.; Johansson, T.; Persson, K.M.; Radström, P. Bacterial Community Analysis of Drinking Water Biofilms in Southern Sweden. Microbes Environ. 2015, 30, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ling, F.; Whitaker, R.; LeChevallier, M.W.; Liu, W.-T. Drinking Water Microbiome Assembly Induced by Water Stagnation. ISME J. 2018, 12, 1520–1531. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Rhoads, W.J.; Edwards, M.A.; Pruden, A. Impact of Water Heater Temperature Setting and Water Use Frequency on the Building Plumbing Microbiome. ISME J. 2017, 11, 1318–1330. [Google Scholar] [CrossRef]
- Inkinen, J.; Kaunisto, T.; Pursiainen, A.; Miettinen, I.T.; Kusnetsov, J.; Riihinen, K.; Keina, M.M. Drinking Water Quality and Formation of Biofilms in an Office Building during Its First Year of Operation, a Full Scale Study. Water Res. 2014, 9, 83–91. [Google Scholar] [CrossRef]
- Liu, G.; Bakker, G.L.; Li, S.; Vreeburg, J.H.; Verberk, J.Q.; Medema, G.J.; Liu, W.T.; Van Dijk, J.C. Pyrosequencing Reveals Bacterial Communities in Unchlorinated Drinking Water Distribution System: An Integral Study of Bulk Water, Suspended Solids, Loose Deposits, and Pipe Wall Biofilm. Environ. Sci. Technol. 2014, 48, 5467–5476. [Google Scholar] [CrossRef]
- Wingender, J.; Flemming, H.-C. Contamination Potential of Drinking Water Distribution Network Biofilms. Water Sci. 2004, 49, 277–286. [Google Scholar] [CrossRef]
- Lehtola, M.J.; Miettinen, I.T.; Keina, M.M.; Kekki, T.K.; Laine, O.; Hirvonen, A.; Vartiainen, T.; Martikainen, P.J. Microbiology, Chemistry and Biofilm Development in a Pilot Drinking Water Distribution System with Copper and Plastic Pipes. Water Res. 2004, 38, 3769–3779. [Google Scholar] [CrossRef]
- Departement of Industrial Plants. Drinking water distribution, City of Zurich. Available online: https://www.stadt-zuerich.ch/dib/de/index/wasserversorgung/trinkwasser.html (accessed on 31 August 2019).
- Liu, J.; Ren, H.; Ye, X.; Wang, W.; Liu, Y.; Lou, L.; Cheng, D.; He, X.; Zhou, X.; Qiu, S.; et al. Bacterial Community Radial-Spatial Distribution in Biofilms along Pipe Wall in Chlorinated Drinking Water Distribution System of East China. Appl. Microbiol. Biotechnol. 2017, 101, 749–759. [Google Scholar] [CrossRef]
- EU Comission, D.W.D. DWD 98/83/EC. Off. J. Eur. Communities 1998, 41, 54. [Google Scholar]
- EPA. SAFE DRINKING WATER ACT (SDWA); EPA: Washington, DC, USA, 2018; pp. 1–144. [Google Scholar]
- Prest, E.I.; Hammes, F.; van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Biological Stability of Drinking Water: Controlling Factors, Methods, and Challenges. Front. Microbiol. 2016, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Nescerecka, A.; Rubulis, J.; Vital, M.; Juhna, T.; Hammes, F. Biological Instability in a Chlorinated Drinking Water Distribution Network. PLoS ONE 2014, 9, e96354. [Google Scholar] [CrossRef] [PubMed]
- Prest, E.I.; Weissbrodt, D.G.; Hammes, F.; Van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Long-Term Bacterial Dynamics in a Full-Scale Drinking Water Distribution System. PLoS ONE 2016, e0164445. [Google Scholar] [CrossRef] [PubMed]
- McCoy, S.T.; Vanbriesen, J.M.; Asce, M. Temporal Variability of Bacterial Diversity in a Chlorinated Drinking Water Distribution System. J. Environ. Eng. 2012, 138, 786–795. [Google Scholar] [CrossRef]
- Stanish, L.F.; Hull, N.M.; Robertson, C.E.; Harris, J.K.; Stevens, M.J.; Spear, J.R.; Pace, N.R. Factors Influencing Bacterial Diversity and Community Composition in Municipal Drinking Waters in the Ohio River Basin, USA. PLoS ONE 2016, 11, e0157966. [Google Scholar] [CrossRef]
- Potgieter, S.; Pinto, A.; Sigudu, M.; du Preez, H.; Ncube, E.; Venter, S. Long-Term Spatial and Temporal Microbial Community Dynamics in a Large-Scale Drinking Water Distribution System with Multiple Disinfectant Regimes. Water Res. 2018, 139, 406–419. [Google Scholar] [CrossRef]
- Haig, S.; Kotlarz, N.; Lipuma, J.J.; Raskin, L. A High-Throughput Approach for Identification of Nontuberculous Mycobacteria in Drinking Water Reveals Relationship between Water Age and Mycobacterium avium. MBio 2018, 9, e02354-17. [Google Scholar] [CrossRef]
- LeChevallier, M.W.; Welch, N.J.; Smith, D.B. Full-Scale Studies of Factors Related to Coliform Regrowth in Drinking Water. Appl. Environ. Microbiol. 1996, 62, 2201–2211. [Google Scholar] [CrossRef]
- Buse, H.Y.; Lu, J.; Lu, X.; Mou, X.; Ashbolt, N.J. Microbial Diversities (16S and 18S rRNA Gene Pyrosequencing) and Environmental Pathogens within Drinking Water Biofilms Grown on the Common Premise Plumbing Materials Unplasticized Polyvinylchloride and Copper. FEMS Microbiol. Ecol. 2014, 88, 280–295. [Google Scholar] [CrossRef]
- Williams, M.M.; Armbruster, C.R.; Arduino, M.J. Plumbing of Hospital Premises Is a Reservoir for Opportunistically Pathogenic Microorganisms: A Review. Biofouling 2013, 29, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Moritz, M.M.; Flemming, H.C.; Wingender, J. Integration of Pseudomonas Aeruginosa and Legionella Pneumophila in Drinking Water Biofilms Grown on Domestic Plumbing Materials. Int. J. Hyg. Environ. Health 2010, 213, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Zlatanovic, L.; Van Der Hoek, J.P.; Vreeburg, J.H.G. An Experimental Study on the in Fl Uence of Water Stagnation and Temperature Change on Water Quality in a Full-Scale Domestic Drinking Water System. Water Res. 2017, 123, 761–772. [Google Scholar] [CrossRef]
- Bédard, E.; Laferrière, C.; Déziel, E.; Prévost, M. Impact of Stagnation and Sampling Volume on Water Microbial Quality Monitoring in Large Buildings. PLoS ONE 2018, e0199429. [Google Scholar] [CrossRef]
- Trinkwasserverordnung und Legionellen. Available online: https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/T/Trinkwasserverordnung/Stammtext_TrinkwV_und_Legionellen_250418.pdf (accessed on 31 August 2019).
- Bundesministerium für Wirtschaft. Verordnung Über Allgemeine Bedingungen Für Die Versorgung Mit Wasser (AVBWasserV). 2014, 1980. Available online: https://www.gesetze-im-internet.de/avbwasserv/BJNR007500980.html (accessed on 31 August 2019).
- Open Data Zürich. Gebäude und Wohnungen nach Gebäudeart und Stadtquartier seit 2008. Available online: https://data.stadt-zuerich.ch/dataset/bau_best_geb_whg_bev_gebaeudeart_quartier_seit2008 (accessed on 31 August 2019).
- Liu, G.; Van der Mark, E.J.; Verberk, J.Q.; Van Dijk, J.C. Flow Cytometry Total Cell Counts: A Field Study Assessing Microbiological Water Quality and Growth in Unchlorinated Drinking Water Distribution Systems. Biomed. Res. Int. 2013, 2013, 595872. [Google Scholar] [CrossRef]
- Lin, S.; Wang, X.; Chao, Y.; He, Y.; Liu, M. Predicting Biofilm Thickness and Biofilm Viability Based on the Concentration of Carbon-Nitrogen-Phosphorus by Support Vector Regression. Environ. Sci. Pollut. Res. 2016, 23, 418–425. [Google Scholar] [CrossRef]
- Prévost, M.; Rompré, A.; Coallier, J.; Servais, P.; Laurent, P.; Clément, B.; Lafrance, P. Suspended Bacterial Biomass and Activity in Full-Scale Drining Water Distribution Systems: Impact of Water Treatment. Water Res. 1998, 32, 1393–1406. [Google Scholar] [CrossRef]
- Liu, R.; Zhu, J.; Yu, Z.; Joshi, D.; Zhang, H.; Lin, W.; Yang, M. Molecular Analysis of Long-Term Biofilm Formation on PVC and Cast Iron Surfaces in Drinking Water Distribution System. J. Environ. Sci. 2014, 26, 865–874. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Han, S.; Lee, J.; Oh, J.; Chung, S.; Park, H.-D. Hydrodynamic Effects on Bacterial Biofilm Development in a Microfluidic Environment. RSC Publ. 2013, 13, 1846–1849. [Google Scholar] [CrossRef]
- Fang, H.; Chen, Y.; Huang, L.; He, G. Analysis of Biofilm Bacterial Communities under Different Shear Stresses Using Size-Fractionated Sediment. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Douterelo, I.; Sharpe, R.L.; Boxall, J.B. Influence of Hydraulic Regimes on Bacterial Community Structure and Composition in an Experimental Drinking Water Distribution System. Water Res. 2013, 47, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Fish, K.; Osborn, A.M.; Boxall, J.B. Biofilm Structures (EPS and Bacterial Communities) in Drinking Water Distribution Systems Are Conditioned by Hydraulics and Influence Discolouration. Sci. Total Environ. 2017, 593–594, 571–580. [Google Scholar] [CrossRef]
- Henne, K.; Kahlisch, L.; Ho, M.G.; Brettar, I. Seasonal Dynamics of Bacterial Community Structure and Composition in Cold and Hot Drinking Water Derived from Surface Water Reservoirs. Water Res. 2013, 47, 5614–5630. [Google Scholar] [CrossRef] [PubMed]
- Lautenschlager, K.; Boon, N.; Wang, Y.; Egli, T.; Hammes, F. Overnight Stagnation of Drinking Water in Household Taps Induces Microbial Growth and Changes in Community Composition. Water Res. 2010, 44, 4868–4877. [Google Scholar] [CrossRef]
- Proctor, C.R.; Reimann, M.; Vriens, B.; Hammes, F. Biofilms in Shower Hoses. Water Res. 2018, 131, 274–286. [Google Scholar] [CrossRef]
- Moerman, A.; Blokker, M.; Vreeburg, J.; Van Der Hoek, J.P. Drinking Water Temperature Modelling in Domestic Systems. Procedia Eng. 2014, 89, 143–150. [Google Scholar] [CrossRef]
- Nguyen, C.; Elfland, C.; Edwards, M. Impact of Advanced Water Conservation Features and New Copper Pipe on Rapid Chloramine Decay and Microbial Regrowth. Water Res. 2011, 46, 611–621. [Google Scholar] [CrossRef]
- Rhoads, W.J.; Pruden, A.; Edwards, M.A. Survey of Green Building Water Systems Reveals Elevated Water Age and Water Quality Concerns. Environ. Sci. Water Res. Technol. 2016, 2, 164–173. [Google Scholar] [CrossRef]
- Hogt, A.H.; Dankert, J.; Feijen, J. Adhesion of Staphylococcus Epidermidis and Staphylococcus Saprophyticus to a Hydrophobic Biomaterial. J. Gen. Microbiol. 1985, 131, 2485–2491. [Google Scholar] [CrossRef]
- Connell, M.; Stenson, A.; Weinrich, L.; Lechevallier, M.; Boyd, S.L.; Ghosal, R.R.; Dey, R.; Whelton, A.J. PEX and PP Water Pipes: Assimilable Carbon, Chemicals, and Odors. J. Am. Water Works Assoc. 2016, 108, E192–E204. [Google Scholar] [CrossRef]
- Bucheli-Witschel, M.; Koetzsch, S.; Darr, S.; Widler, R.; Egli, T. A New Method to Assess the Influence of Migration from Polymeric Materials on the Biostability of Drinking Water. Water Res. 2012, 46, 4246–4260. [Google Scholar] [CrossRef] [PubMed]
- Krzeminski, P.; Vogelsang, C.; Meyn, T.; Köhler, S.J.; Poutanen, H.; de Wit, H.A.; Uhl, W. Natural Organic Matter Fractions and Their Removal in Full-Scale Drinking Water Treatment under Cold Climate Conditions in Nordic Capitals. J. Environ. Manag. 2019, 241, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Lee, S.H.; Choi, S.C.; Kim, K. Characteristics of Biofilm Community Formed in the Chlorinated Biodegradable Organic Matter-Limited Tap Water. Environ. Technol. 2006, 27, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Schiller, M. PVC Additives; Carl Hanser Verlag: München, Germany, 2015. [Google Scholar]
- Skjevrak, I.; Due, A.; Gjerstad, K.O.; Herikstad, H. Volatile Organic Components Migrating from Plastic Pipes (HDPE, PEX and PVC) into Drinking Water. Water Res. 2003, 37, 1912–1920. [Google Scholar] [CrossRef]
- BASF. Irgafos® 168. 2010. Available online: http://www.mohe021.com/uploads/soft/180202/TDS_Irgafos-168_e.pdf (accessed on 31 August 2019).
- Löschner, D.; Rapp, T.; Schlosser, F.U.; Schuster, R.; Stottmeister, E.; Zander, S. Experience with the Application of the Draft European Standard prEN 15768 to the Identification of Leachable Organic Substances from Materials in Contact with Drinking Water by GC-MS. Anal. Methods 2011, 3, 2547–2556. [Google Scholar] [CrossRef]
- Graham, P.R. Phthalate Ester Plasticizers-Why and How They Are Used. Environ. Health Perspect. 1973, 3, 3–12. [Google Scholar]
- Erythropel, H.C.; Maric, M.; Nicell, J.A.; Leask, R.L.; Yargeau, V. Leaching of the Plasticizer di(2-Ethylhexyl)phthalate (DEHP) from Plastic Containers and the Question of Human Exposure. Appl. Microbiol. Biotechnol. 2014, 98, 9967–9981. [Google Scholar] [CrossRef]
- Wen, G.; Koetzsch, S.; Vital, M.; Egli, T.; Ma, J. BioMig—A Method to Evaluate the Potential Release of Compounds from and the Formation of Biofilms on Polymeric Materials in Contact with Drinking Water. Environ. Sci. Technol. 2015, 49, 11659–11669. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, S. Investigation of Organic Compounds Migration from Polymeric Pipes into Drinking Water under Long Retention Times. Procedia Eng. 2014, 70, 1753–1761. [Google Scholar] [CrossRef]
- BSI. Influence of Materials on Water for Human Consumption Enhancement of Microbial Growth (EMG); BS EN 16421:2014; BSI: London, UK, 2014. [Google Scholar]
- Umweltbundesamt. Leitlinie Zur Hygienischen Beurteilung von Organischen Materialien Im Kontakt Mit Trinkwasser; Umweltbundesamt: Dessau-Roßlau, Germany, 2016. [Google Scholar]
- Koetzsch, S.; Egli, T. Kunststoffe in Kontakt Mit Trinkwasser. Aqua Gas 2016, 3, 44–52. [Google Scholar]
- Mao, G.; Wang, Y.; Hammes, F. Short-Term Organic Carbon Migration from Polymeric Materials in Contact with Chlorinated Drinking Water. Sci. Total Environ. 2018, 613–614, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Lund, V.; Anderson-Glenna, M.; Skjevrak, I.; Steffensen, I.L. Long-Term Study of Migration of Volatile Organic Compounds from Cross-Linked Polyethylene (PEX) Pipes and Effects on Drinking Water Quality. J. Water Health 2011, 9, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; He, C.; He, Q. Fate of Free Chlorine in Drinking Water during Distribution in Premise Plumbing. Ecotoxicology 2015, 24, 2151–2155. [Google Scholar] [CrossRef] [PubMed]
- Proctor, C.R.; Gächter, M.; Kötzsch, S.; Rölli, F.; Sigrist, R.; Walser, J.-C.; Hammes, F. Biofilms in Shower Hoses—Choice of Pipe Material Influences Bacterial Growth and Communities. Environ. Sci. Water Res. Technol. 2016, 2, 670–682. [Google Scholar] [CrossRef]
- Van der Kooij, D.; van der Wielen, P.W. Microbial Growth in Drinking-Water Supplies; Iwa Publishing: London, UK, 2014. [Google Scholar]
- Eilers, K.G.; Lauber, C.L.; Knight, R.; Fierer, N. Shifts in Bacterial Community Structure Associated with Inputs of Low Molecular Weight Carbon Compounds to Soil. Soil Biol. Biochem. 2010, 42, 896–903. [Google Scholar] [CrossRef]
- Reintjes, G.; Arnosti, C.; Fuchs, B.; Amann, R. Selfish, Sharing and Scavenging Bacteria in the Atlantic Ocean: A Biogeographical Study of Bacterial Substrate Utilisation. ISME J. 2019, 13, 1119–1132. [Google Scholar] [CrossRef]
- Freilich, S.; Zarecki, R.; Eilam, O.; Segal, E.S.; Henry, C.S.; Kupiec, M.; Gophna, U.; Sharan, R.; Ruppin, E. Competitive and Cooperative Metabolic Interactions in Bacterial Communities. Nat. Commun. 2011, 2. [Google Scholar] [CrossRef]
- Benölken, J.; Dorsch, T.; Wichmann, K.; Bendinger, B. Praxisnahe Untersuchungen Zur Kontamination von Trinkwasser in Halbtechnischen Trinkwasser-Installationen. In IWW Schriftenreihe—Vermeidung und Sanierung von Trinkwasserkontaminationen Durch Hygienisch Relevante Mikroorganismen aus Biofilmen der Hausinstallation; IWW Schriftenreihe: Mülheim, Germany, 2010; pp. 101–180. [Google Scholar]
- Nemergut, D.R.; Schmidt, S.K.; Fukami, T.; Neill, S.P.O.; Bilinski, T.M.; Stanish, L.F.; Knelman, J.E.; Darcy, J.L.; Lynch, R.C.; Wickey, P. Patterns and Processes of Microbial Community Assembly. Microbiol. Mol. Biol. Rev. 2013, 77, 342–356. [Google Scholar] [CrossRef]
- Vellend, M. Conceptual Synthesis in Community Ecology. Q. Rev. Biol. 2010, 85, 183–206. [Google Scholar] [CrossRef]
- Van der Kooij, D. Assimilable Organic Carbon as an Indicator of Bacterial Regrowth. J. Am. Water Work. Assoc. 1992, 84, 57–65. [Google Scholar] [CrossRef]
- Stadt Zurich, W. The Quality of Zurich’s Drinking Water. 1–2. Available online: https://www.stadt-zuerich.ch/dib/de/index/wasserversorgung/Qualitaetsueberwachung/qualitaetswerte.html (accessed on 31 October 2019).
- Boks, N.P.; Busscher, H.J.; van der Mei, H.C.; Norde, W. Bond-Strengthening in Staphylococcal Adhesion to Hydrophilic and Hydrophobic Surfaces Using Atomic Force Microscopy. Langmuir 2008, 24, 12990–12994. [Google Scholar] [CrossRef] [PubMed]
- Schwab, U.; Hu, Y.; Wiedmann, M.; Boor, K.J. Alternative Sigma Factor σB Is Not Essential for Listeria Monocytogenes Surface Attachment. J. Food Prot. 2005, 68, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Van Der Kooij, D.; Visser, A.; Hijnen, W.A.M. Determing the Concentration of Easily Assimilable Organic Carbon in Drinking. Am. Water Work. Assoc. 1982. [Google Scholar] [CrossRef]
- Hammes, F.A.; Egli, T. New Method for Assimilable Organic Carbon Determination Using Flow-Cytometric Enumeration and a Natural Microbial Consortium as Inoculum. Environ. Sci. Technol. 2005, 39, 3289–3294. [Google Scholar] [CrossRef] [PubMed]
- Waines, P.L.; Moate, R.; Moody, A.J.; Allen, M.; Bradley, G. The Effect of Material Choice on Biofilm Formation in a Model Warm Water Distribution System. Biofouling 2011, 27, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Whelton, A.J.; Cooney, M.F. We Need Our Customers to Complain. Opflow 2004, 30, 1–7. [Google Scholar] [CrossRef]
- Shah, P.; Barskey, A.; Binder, A.; Edens, C.; Lee, S.; Smith, J.; Schrag, S.; Whitney, C.; Cooley, L. Legionnaires’ Disease Surveillance Summary Report, United States. Available online: https://www.cdc.gov/legionella/health-depts/surv-reporting/2016-17-surv-report-508.pdf (accessed on 31 October 2019).
- Bundesamt für Gesundheit BAG. Zahlen Zu Infektionskrankheiten: Legionellose; Bundesamt für Gesundheit BAG: Bern, Switzerland, 2019. [Google Scholar]
- Richards, A.M.; Von Dwingelo, J.E.; Price, C.T.; Kwaik, Y.A. Cellular Microbiology and Molecular Ecology of Legionella-Amoeba Interaction. Virulence 2013, 4, 307–314. [Google Scholar] [CrossRef]
- Falkinham, J.; Pruden, A.; Edwards, M. Opportunistic Premise Plumbing Pathogens: Increasingly Important Pathogens in Drinking Water. Pathogens 2015, 4, 373–386. [Google Scholar] [CrossRef]
- Völker, S.; Schreiber, C.; Kistemann, T. Drinking Water Quality in Household Supply Infrastructure—A Survey of the Current Situation in Germany. Int. J. Hyg. Environ. Health 2010, 213, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Bédard, E.; Prévost, M.; Déziel, E. Pseudomonas Aeruginosa in Premise Plumbing of Large Buildings. Microbiologyopen 2016, 5, 937–956. [Google Scholar] [CrossRef] [PubMed]
- Feazel, L.M.; Baumgartner, L.K.; Peterson, K.L.; Frank, D.N.; Harris, J.K.; Pace, N.R. Opportunistic Pathogens Enriched in Showerhead Biofilms. PNAS 2009, 106, 16393–16399. [Google Scholar] [CrossRef] [PubMed]
- Falkinham, J.O. Reducing Human Exposure to Mycobacterium avium. Ann. Am. Thorac. Soc. 2013, 10, 378–382. [Google Scholar] [CrossRef]
- Gebert, M.J.; Delgado-Baquerizo, M.; Oliverio, A.M.; Webster, T.M.; Nichols, L.M.; Honda, J.R.; Chan, E.D.; Adjemian, J.; Dunn, R.R.; Fierer, N. Ecological Analyses of Mycobacteria in Showerhead Biofilms and Their Relevance to Human Health. MBio 2018, 9, e01614-18. [Google Scholar] [CrossRef]
- Vital, M.; Stucki, D.; Egli, T.; Hammes, F. Evaluating the Growth Potential of Pathogenic Bacteria in Water. Appl. Environ. Microbiol. 2010, 76, 6477–6484. [Google Scholar] [CrossRef]
- Stüken, A.; Haverkamp, T.H.A.; Dirven, H.A.A.M.; Gilfillan, G.D.; Leithaug, M.; Lund, V. Microbial Community Composition of Tap Water and Biofilms Treated with or without Copper-Silver Ionization. Environ. Sci. Technol. 2018, 52, 3354–3364. [Google Scholar] [CrossRef]
- Golberg, K.; Emuna, N.; Vinod, T.P.; Van Moppes, D.; Marks, R.S.; Arad, S.M.; Kushmaro, A. Novel Anti-Adhesive Biomaterial Patches: Preventing Biofi Lm with Metal Complex Films (MCF) Derived from a Microalgal Polysaccharide. Adv. Mater. Interfaces 2016, 1500486. [Google Scholar] [CrossRef]
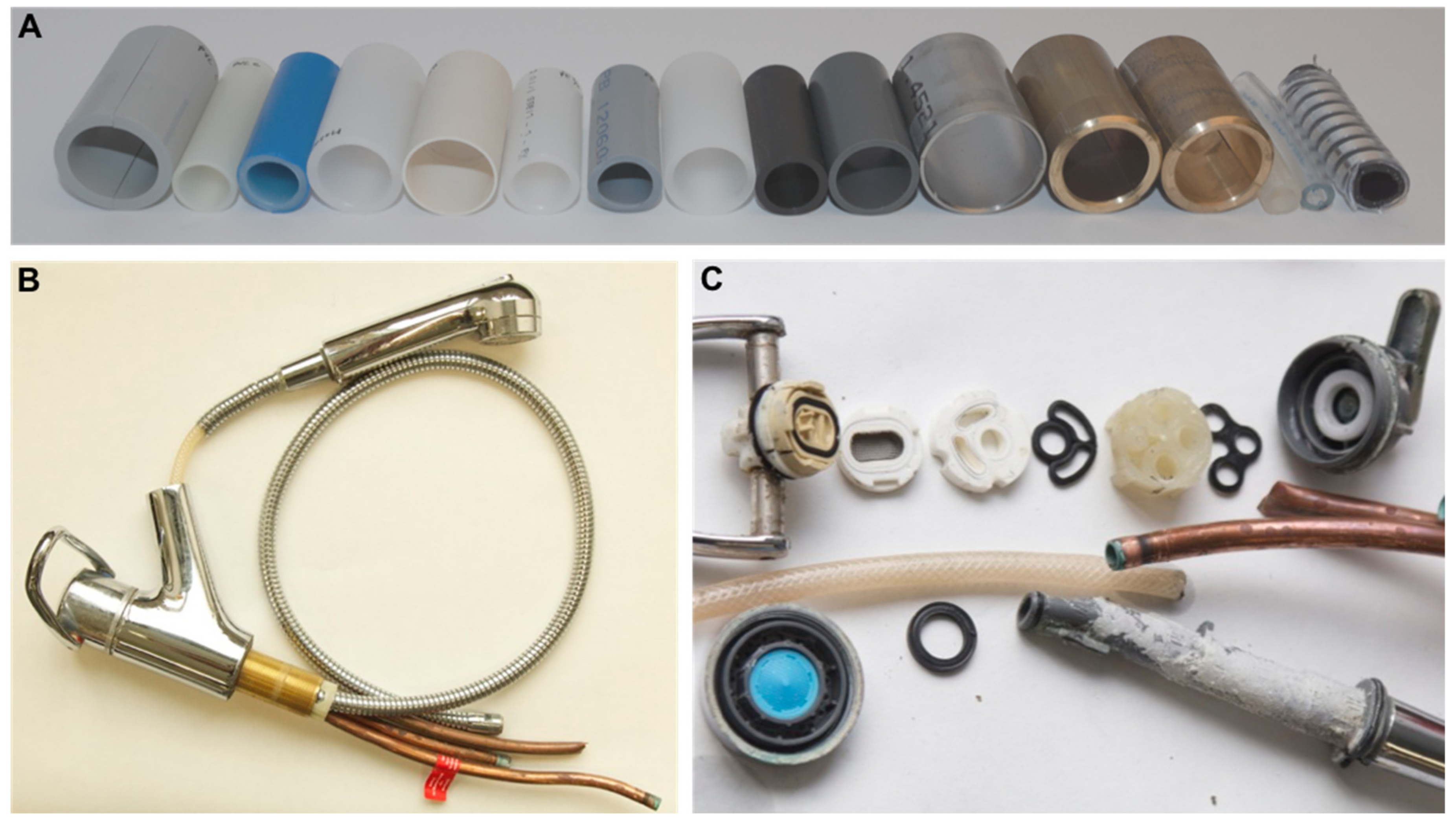
| Material | Application |
|---|---|
| Metals | |
| Copper and copper alloys | Pipes and fittings |
| Brass (copper alloy) | Taps, valves, pipes, and fittings |
| Galvanized steel (GI) | Pipes and taps |
| Stainless steel | Fittings |
| Ductile iron | Pipes and fittings |
| Malleable iron | Nipples |
| Galvanized iron | Pipes and fittings |
| Synthetic materials | |
| Ethylene propylene diene monomer (EPDM) | O-rings, seals |
| Polyvinyl chloride (PVC) | |
| PVC-U (unplasticized) | Pipes and fittings |
| PVC-C (chlorinated) | Pipes and fittings |
| PVC-P (plasticized) | (Shower) hoses |
| Polyethylene (PE) | |
| PE-X (crosslinked; a, b, c) | Pipes (hot water pipes) |
| Multilayer pipes | |
| PE-RT (raised temperature resistant) | Pipes (hot water pipes) |
| Multilayer pipes | |
| Polybutylene (PB) | Pipes and fittings |
| Polypropylene (PP) | |
| PP-R (random Co-polymer) | Tubes and fittings |
| PP-C (Copolymer) | Tubes and fittings |
| PP-H (Holopolymer) | Tubes and fittings |
| Polyphenylsulfone (PPSU) | Fittings |
| Polyoxymethylen (POM) | Valve elements |
| Polyvinylidene fluoride (PVDF) | Fittings |
| Polytetrafluorethylen (PTFE; Teflon) | Valve elements, seals |
| Silicone rubber | Seals |
| Epoxy resin | Inline coating |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neu, L.; Hammes, F. Feeding the Building Plumbing Microbiome: The Importance of Synthetic Polymeric Materials for Biofilm Formation and Management. Water 2020, 12, 1774. https://doi.org/10.3390/w12061774
Neu L, Hammes F. Feeding the Building Plumbing Microbiome: The Importance of Synthetic Polymeric Materials for Biofilm Formation and Management. Water. 2020; 12(6):1774. https://doi.org/10.3390/w12061774
Chicago/Turabian StyleNeu, Lisa, and Frederik Hammes. 2020. "Feeding the Building Plumbing Microbiome: The Importance of Synthetic Polymeric Materials for Biofilm Formation and Management" Water 12, no. 6: 1774. https://doi.org/10.3390/w12061774
APA StyleNeu, L., & Hammes, F. (2020). Feeding the Building Plumbing Microbiome: The Importance of Synthetic Polymeric Materials for Biofilm Formation and Management. Water, 12(6), 1774. https://doi.org/10.3390/w12061774




