Driving Forces Analysis of Non-structural Carbohydrates for Phragmites australis in Different Habitats of Inland River Wetland
Abstract
1. Introduction
2. Materials and Methods
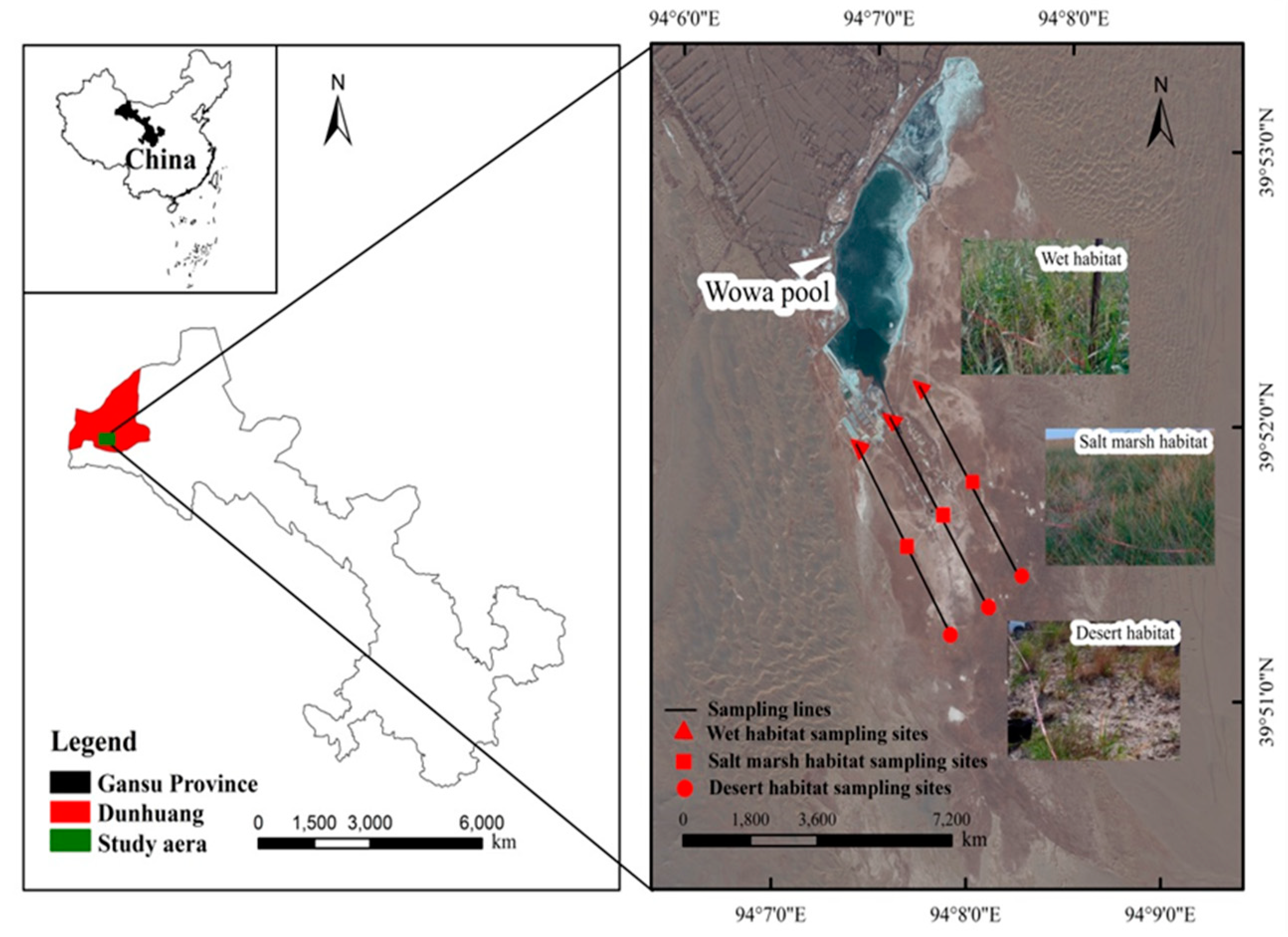
2.1. Study Area
2.2. Research Methods
2.2.1. Sample Collection
2.2.2. Sample Analysis Method
2.2.3. Data Processing
3. Results
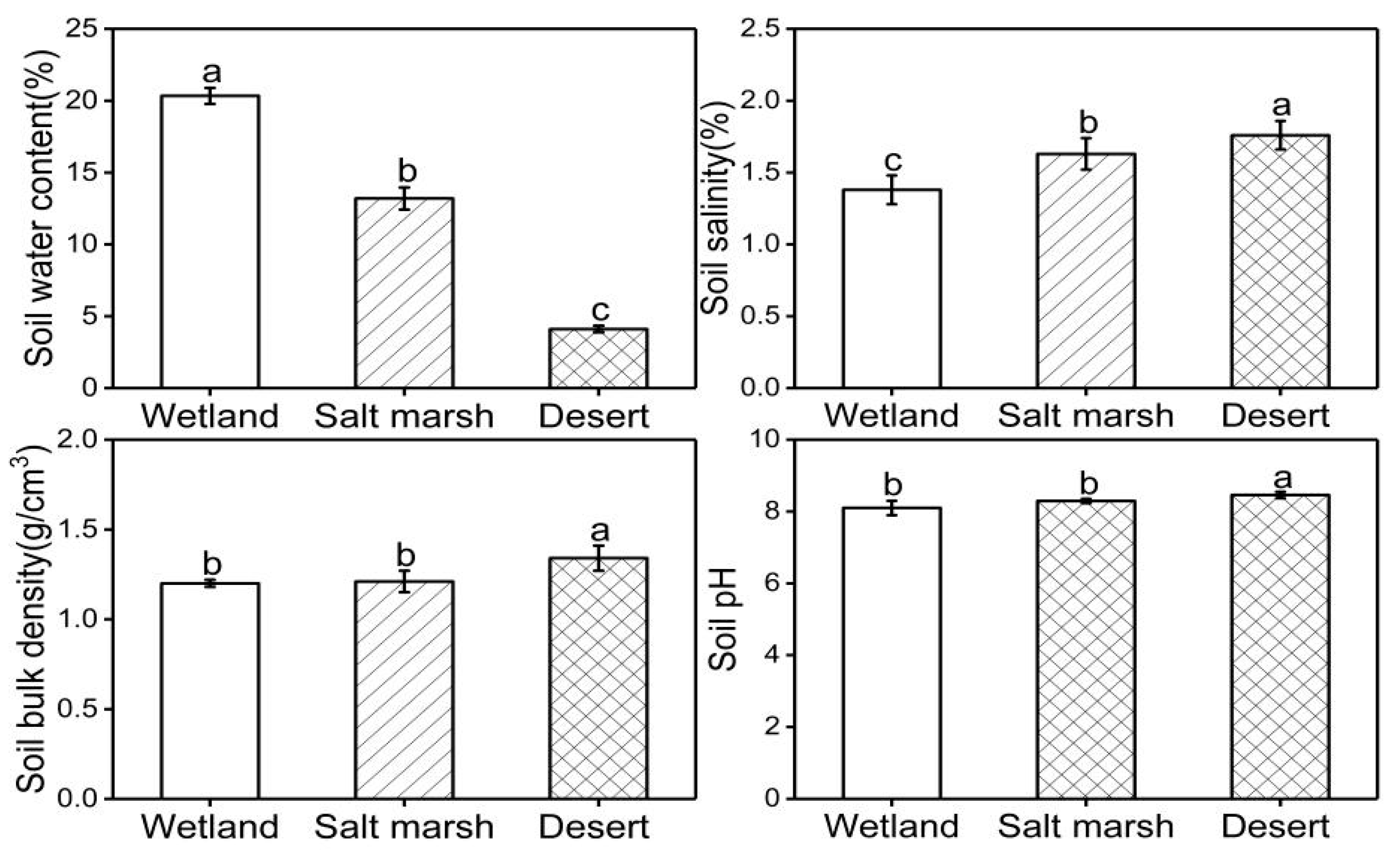
3.1. Characteristics of the Soil Environmental Factors in Different Habitats
3.2. Spatial Differences of Reed NSC and Its Components
3.3. Spatial Differences in the Ratio of Soluble Sugar and Starch in Reeds
3.4. Relative Importance of Soil Environmental Factors to Reed NSC and Its Components in Different Habitats
4. Discussion
4.1. Distribution Trade-Offs of NSC and Components in Phragmites australis in Different Habitats
4.2. Distribution Driving Forces of NSC and Components for Phragmites australis in Different Habitats
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural Carbon in Woody Plants. Annu. Rev. Plant Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Martinezvilalta, J.; Sala, A.; Asensio, D.; Galiano, L.; Hoch, G.; Palacio, S. Dynamics of Non-Structural Carbohydrates in Terrestrial Plants: A Global Synthesis. Ecol. Monogr. 2016, 86, 495–516. [Google Scholar] [CrossRef]
- Wurth, M.K.; Pelaezriedl, S.; Wright, S.J.; Korner, C. Non-Structural Carbohydrate Pools in a Tropical Forest. Oecologia 2005, 143, 11–24. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S.E. Understanding the Roles of Nonstructural Carbohydrates in Forest Trees–from What We Can Measure to What We Want to Know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef] [PubMed]
- Latt, C.R.; Nair, P.K.; Kang, B.T. Reserve Carbohydrate Levels in the Boles and Structural Roots of Five Multipurpose Tree Species in a Seasonally Dry Tropical Climate. For. Ecol. Manag. 2001, 146, 145–158. [Google Scholar] [CrossRef]
- Trifilò, P.; Casolo, V.; Raimondo, F.; Petrussa, E.; Boscutti, F.; Gullo, M.A.L.; Nardini, A. Effects of Prolonged Drought on Stem Non-Structural Carbohydrates Content and Post-Drought Hydraulic Recovery in Laurus nobilis L.: The Possible Link Between Carbon Starvation and Hydraulic failUre. Plant Physiol. Biochem. 2017, 120, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, Y.V.; Kartashov, A.V.; Zlobin, I.E.; Sarvin, B.; Stavrianidi, A.N.; Kuznetsov, V.V. Water Deficit-Dependent Changes in Non-Structural Carbohydrate Profiles, Growth and Mortality of Pine and Spruce Seedlings in Hydroculture. Environ. Exp. Bot. 2019, 157, 151–160. [Google Scholar] [CrossRef]
- Shi, L.X.; Guo, J.X. Changes in Photosynthetic and Growth Characteristics of Leymus chinensis Community along the Retrogression on the Songnen Grassland in Northeastern China. Photosynthetica 2006, 44, 542–547. [Google Scholar] [CrossRef]
- Michelot, A.; Simard, S.; Rathgeber, C.B.; Dufrene, E.; Damesin, C. Comparing the Intra-Annual Wood Formation of Three European Species (Fagus sylvatica, Quercus petraea and Pinus sylvestris) as Related to Leaf Phenology and Non-Structural Carbohydrate Dynamics. Tree Physiol. 2012, 32, 1033–1045. [Google Scholar] [CrossRef]
- Li, M.H.; Xiao, W.F.; Wang, S.G.; Cheng, G.W.; Cherubini, P.; Cai, X.H.; Liu, X.L.; Wang, X.D.; Zhu, W.Z. Mobile Carbohydrates in Himalayan Treeline Trees I. Evidence for Carbon Gain Limitation but not for Growth Limitation. Tree Physiol. 2008, 28, 1287–1296. [Google Scholar] [CrossRef]
- Dong, M.; Yu, F.; Alpert, P. Ecological Consequences of Plant Clonality. Ann. Bot. 2014, 114, 367–367. [Google Scholar] [CrossRef] [PubMed]
- Lopp, J.; Sammul, M. Benefits of Clonal Propagation: Impact of Imported Assimilates from Connected Ramets. Plant Ecol. 2016, 217, 315–329. [Google Scholar] [CrossRef]
- Law, R.; Mclellan, A.; Mahdi, A.S. Spatio-Temporal Processes in a Calcareous Grassland. Plant Species Biol. 1993, 8, 175–193. [Google Scholar] [CrossRef]
- Hara, T.; Herben, T. Shoot Growth Dynamics and Size-dependent Shoot Fate of a Clonal Plant, Festuca rubra, in a Mountain Grassland. Res. Popul. Ecol. 1997, 39, 83–93. [Google Scholar] [CrossRef]
- Liang, K.; Fan, Y.; Feng, H.J. Concentration and Distribution Pattern of Non-Structural Carbohydrate of Phyllostachys glauca in Different Limestone Habitats. Sci. Silvae Sin. 2019, 55, 22–27. [Google Scholar] [CrossRef]
- Zhu, B.J.; Yin, B.F.; Zhang, Y.M. Seasonal Dynamics of Non-structural Carbohydrates Contents of Syntrichia caninervis among Different Microhabitats. J. Desert Res. 2017, 37, 268–275. [Google Scholar] [CrossRef]
- Newell, E.; Mulkey, S.S.; Wright, J.S. Seasonal Patterns of Carbohydrate Storage in Four Tropical Tree Species. Oecologia 2002, 131, 333–342. [Google Scholar] [CrossRef]
- Shi, C.; Silva, L.C.; Zhang, H.; Zheng, Q.; Xiao, B.; Wu, N.; Sun, G. Climate Warming Alters Nitrogen Dynamics and Total Non-Structural Carbohydrate Accumulations of Perennial Herbs of Distinctive Functional Groups during the Plant Senescence in Autumn in an Alpine Meadow of the Tibetan Plateau. Agric. For. Meteorol. 2015, 200, 21–29. [Google Scholar] [CrossRef]
- Shen, C.; Ji, R.Y.; Yu, X. Changes of Non-Structural Carbohydrates in Caryopteris mongolica Seedlings during the Process of Drought-Induced Mortality. J. Appl. Ecol. 2019, 30, 2541–2548. [Google Scholar] [CrossRef]
- Ohare, M.T.; Aguiar, F.C.; Asaeda, T.; Bakker, E.S.; Chambers, P.A.; Clayton, J.S.; Wood, K.A. Plants in Aquatic Ecosystems: Current Trends and Future Directions. Hydrobiologia 2018, 812, 1–11. [Google Scholar] [CrossRef]
- Hamilton, S.K. Prairie Wetland Ecology. The Contribution of the Marsh Ecology Research Program. Limnol. Oceanogr. 2001, 46, 747–748. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, T.; Liu, Q. Physiological Responses of Phragmites australis to the Combined Effects of Water and Salinity Stress. Ecohydrology 2014, 7, 420–426. [Google Scholar] [CrossRef]
- Lezberg, A.L.; Halpern, C.B.; Antos, J.A. Clonal development of Maianthemum dilatatum in Forests of Differing Age and Structure. Can. J. Bot. 2001, 79, 1028–1038. [Google Scholar] [CrossRef]
- Van Drunen, W.E.; Van Kleunen, M.; Dorken, M.E. Consequences of Clonality for Sexual Fitness: Clonal Expansion Enhances Fitness under Spatially Restricted Dispersal. Proc. Natl. Acad. Sci. USA 2015, 112, 8929–8936. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chen, J.; Wang, Q. Trade-offs between Sexual and Asexual Reproduction in a Monoecious Species Sagittaria pygmaea (Alismataceae): The Effect of Different Nutrient Levels. Plant Syst. Evol. 2009, 277, 61–65. [Google Scholar] [CrossRef]
- Srivastava, J.; Kalra, S.J.; Naraian, R. Environmental Perspectives of Phragmites australis (Cav.) Trin. Ex. Steudel. Appl. Water Sci. 2014, 4, 193–202. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.P.; Xie, J.P.; Zhao, T.W.; Chao, J.J. Distribution and Influencing Factors of Soil Organic Carbon in Dunhuang Yangguan Wetland. Chin J Eco. 2017, 36, 2455–2464. [Google Scholar] [CrossRef]
- Harper, J.L. Population Biology of Plant; Academic Press: London, UK; New York, NY, USA, 1977; pp. 56–62. [Google Scholar]
- Dong, M.; Yu, F.H.; Chen, Y.F.; Song, M.H.; Liu, J.; Chen, J.S.; Li, J.M.; Liu, F.H. Clonal Plant Ecology; Beijing Science press: Beijing, China, 2011; pp. 23–28. [Google Scholar]
- Quentin, A.G.; Pinkard, E.A.; Ryan, M.G.; Tissue, D.T.; Baggett, L.S.; Adams, H.D.; Gibon, Y. Non-Structural Carbohydrates in Woody Plants Compared among Laboratories. Tree Physiol. 2015, 35, 1146–1165. [Google Scholar] [CrossRef]
- Landhäusser, S.M.; Chow, P.S.; Dickman, L.T.; Furze, M.E.; Kuhlman, I.; Schmid, S.; Hoch, G. Standardized Protocols and Procedures can Precisely and Accurately Quantify Non-Structural Carbohydrates. Tree Physiol. 2018, 38, 1764–1778. [Google Scholar] [CrossRef]
- Sørensen, S.T.; Campbell, M.L.; Duke, E.; Manley-Harris, M. A Standard, Analytical Protocol for the Quantitation of Non-Structural Carbohydrates in Seagrasses that Permits Inter-Laboratory Comparison. Aquat. Bot. 2018, 151, 71–79. [Google Scholar] [CrossRef]
- Israeli, O. A Shapley-Based Decomposition of the R-Square of a Linear Regression. J. Econ. Inequal. 2007, 5, 199–212. [Google Scholar] [CrossRef]
- An, Y.Y.; Liang, Z.S.; Hao, W.F. Growth and Physiological Responses of Periploca sepium Seedlings to Drought Stress of Different Intensity. Acta Ecol. Sinica. 2011, 31, 716–725. [Google Scholar]
- Lv, Y.Y.; Li, D.Z.; Xu, J.; Xu, L.L.; Gao, J.J.; Pan, Y.; Zhao, M.X.; Cheng, L.L.; Wang, H.; He, Y.Y. Spatial and Temporal Characteristics of Non-structural Carbohydrates Contents of Phragmites australis and Spartina alterniflora at Chongming Dongtan Wetland. J. Northeast For. Univ. 2013, 41, 85–89. [Google Scholar] [CrossRef]
- Valentino, C.; Enrico, B.; Elisa, P. Relationships between Population Traits, Nonstructural Carbohydrates, and Elevation in Alpine Stands of Vaccinium myrtillus. Am. J. Bot. 2020, 107, 639–649. [Google Scholar] [CrossRef]
- Ikegami, M.; Whigham, D.F.; Werger, M.J. Ramet Phenology and Clonal Architectures of the Clonal Sedge Schoenoplectus americanus (Pers.) Volk. ex Schinz & R. Keller. Plant Ecol. 2009, 200, 287–301. [Google Scholar] [CrossRef]
- Sperry, J.S.; Nichols, K.L.; Sullivan, J.E.; Eastlack, S.E. Xylem Embolism in Ring-Porous, Diffuse-Porous, and Coniferous Trees of Northern Utah and Interior Alaska. Ecology 1994, 75, 1736–1752. [Google Scholar] [CrossRef]
- Sala, A.; Woodruff, D.R.; Meinzer, F.C. Carbon Dynamics in Trees: Feast or Famine? Tree Physiol. 2012, 32, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.N.; Ferreirasilva, S.L.; Viegas, R.A.; Silveira, J.A. The Role of Organic and Inorganic Solutes in the Osmotic Adjustment of Drought-Stressed Jatropha curcas Plants. Environ. Exp. Bot. 2010, 69, 279–285. [Google Scholar] [CrossRef]
- Scartazza, A.; Moscatello, S.; Matteucci, G.; Battistelli, A.; Brugnoli, E. Seasonal and Inter-Annual Dynamics of Growth, Non-Structural Carbohydrates and C Stable Isotopes in a Mediterranean beech Forest. Tree Physiol. 2013, 33, 730–742. [Google Scholar] [CrossRef]
- Zhang, T.; Cao, Y.; Chen, Y.; Liu, G. Non-Structural Carbohydrate Dynamics in Robinia pseudoacacia Saplings under Three Levels of Continuous Drought Stress. Trees 2015, 29, 1837–1849. [Google Scholar] [CrossRef]
- Schadel, C.; Blochl, A.; Richter, A.; Hoch, G. Short-Term Dynamics of Nonstructural Carbohydrates and Hemicelluloses in Young Branches of Temperate Forest Trees during Bud Break. Tree Physiol. 2009, 29, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Kobe, R.K.; Iyer, M.; Walters, M.B. Optimal Partitioning Theory Revisited: Nonstructural Carbohydrates Dominate Root Mass Responses to Nitrogen. Ecology 2010, 91, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Trumbore, S.E. Persistence of Soil Organic Matter as an Ecosystem Property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, M.; Su, Y.; Yang, R. Stoichiometry of Leaf Carbon, Nitrogen, and Phosphorus along a Geographic, Climatic, and Soil Gradients in Temperate Desert of Hexi Corridor, Northwest China. J. Plant Ecol. 2019, 13, 114–121. [Google Scholar] [CrossRef]
- Logsdon, S.D.; Karlen, D.L. Bulk Density as a Soil Quality Indicator during Conversion to Notillage. Soil Tillage Res. 2004, 78, 143–149. [Google Scholar] [CrossRef]
- Passioura, J.B. Soil Structure and Plant Growth. Soil Res. 1991, 29, 717–728. [Google Scholar] [CrossRef]
- Kuss, F.R. A Review of Major Factors Influencing Plant Responses to Recreation Impacts. Environ. Manag. 1986, 10, 637–650. [Google Scholar] [CrossRef]
- Parida, A.; Das, A.B. Salt Tolerance and Salinity Effects on Plants: A Review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Richardson, A.D.; Carbone, M.S.; Keenan, T.F.; Czimczik, C.I.; Hollinger, D.Y.; Murakami, P.; Xu, X. Seasonal Dynamics and Age of Stemwood Nonstructural Carbohydrates in Temperate Forest Trees. New Phytol. 2013, 197, 850–861. [Google Scholar] [CrossRef]
- Petrussa, E.; Boscutti, F.; Vianello, A.; Casolo, V. ‘Last In-First Out’: Seasonal Variations of Non-Structural Carbohydrates, Glucose-6-Phospate and ATP in Tubers of Two Arum Species. Plant Biol. 2018, 20, 346–356. [Google Scholar] [CrossRef]
- Purdy, S.J.; Maddison, A.L.; Cunniff, J.; Donnison, I.; Clifton-Brown, J. Non-Structural Carbohydrate Profiles and Ratios between Soluble Sugars and Starch Serve as Indicators of Productivity for a Bioenergy grass. AoB Plants 2015, 7, 032–032. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Liu, G.B.; Li, P.; Xue, S. Responses of Photosynthesis and Non-Structural Carbohydrates of Bothriochloa ischaemum to Doubled CO2 Concentration and Drought Stress. J. Plant Nutr. Fertil. 2017, 23, 389–397. [Google Scholar] [CrossRef]
- Lei, H.; Wang, K.; Tian, H. Responses of Non-Structural Carbohydrates Accumulation and Distribution of Caragana mi-crophylla Seedlings to Drought Stress. Chin. J. Ecol. 2017, 36, 3168–3175. [Google Scholar] [CrossRef]
- Guicherd, P.; Peltier, J.; Gout, E.; Bligny, R.; Marigo, G. Osmotic Adjustment in Fraxinus excelsior L.: Malate and Mannitol Accumulation in Leaves under Drought Conditions. Trees 1977, 11, 155–161. [Google Scholar] [CrossRef]
- Peltier, J.P.; Marigo, D.; Marigo, G. Involvement of Malate and Mannitol in the Diurnal Regulation of the Water Status in Members of Oleaceae. Trees 1997, 12, 27–34. [Google Scholar] [CrossRef]



| Habitat | Leafs | Stems | Rhizomes | Roots |
|---|---|---|---|---|
| Wetland | 20.50 ± 0.47 a | 19.11 ± 0.66 a | 18.82 ± 0.70 a | 14.59 ± 1.46 a |
| Salt marsh | 15.79 ± 0.29 b | 18.44 ± 0.53 a | 19.84 ± 0.38 a | 13.26 ± 0.50 a |
| Desert | 12.79 ± 0.36 c | 17.78 ± 0.48 a | 19.31 ± 0.45 a | 12.44 ± 0.95 a |
| Habitat | Leafs | Stems | Rhizomes | Roots |
|---|---|---|---|---|
| Wetland | 8.22 ± 0.22 a | 5.04 ± 0.19 b | 4.34 ± 0.31 c | 6.14 ± 0.40 a |
| Salt marsh | 6.83 ± 0.63 b | 6.48 ± 0.29 b | 8.22 ± 0.25 b | 4.64 ± 0.31 b |
| Desert | 6.09 ± 0.17 b | 8.45 ± 0.31 a | 10.19 ± 0.31 a | 3.62 ± 0.32 c |
| Habitat | Leafs | Stems | Rhizomes | Roots |
|---|---|---|---|---|
| Wetland | 12.28 ± 0.57 a | 14.07 ± 0.92 a | 14.48 ± 0.74 a | 8.44 ± 0.13 a |
| Salt marsh | 8.96 ± 0.46 b | 11.96 ± 0.47 b | 11.62 ± 0.27 b | 8.62 ± 0.13 a |
| Desert | 6.70 ± 0.63 c | 9.34 ± 0.36 c | 9.13 ± 0.32 c | 8.82 ± 0.86 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, L.; Zhou, Y.; Liu, X.; Wang, S.; Li, F. Driving Forces Analysis of Non-structural Carbohydrates for Phragmites australis in Different Habitats of Inland River Wetland. Water 2020, 12, 1700. https://doi.org/10.3390/w12061700
Jiao L, Zhou Y, Liu X, Wang S, Li F. Driving Forces Analysis of Non-structural Carbohydrates for Phragmites australis in Different Habitats of Inland River Wetland. Water. 2020; 12(6):1700. https://doi.org/10.3390/w12061700
Chicago/Turabian StyleJiao, Liang, Yi Zhou, Xuerui Liu, Shengjie Wang, and Fang Li. 2020. "Driving Forces Analysis of Non-structural Carbohydrates for Phragmites australis in Different Habitats of Inland River Wetland" Water 12, no. 6: 1700. https://doi.org/10.3390/w12061700
APA StyleJiao, L., Zhou, Y., Liu, X., Wang, S., & Li, F. (2020). Driving Forces Analysis of Non-structural Carbohydrates for Phragmites australis in Different Habitats of Inland River Wetland. Water, 12(6), 1700. https://doi.org/10.3390/w12061700





