Leaf Traits of Drought Tolerance for 37 Shrub Species Originating from a Moisture Gradient
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Climate Variables
2.3. ΨTLP and Pressure–Volume Traits
2.4. Morphological Measurements
2.5. Statistical Analyses
3. Results
3.1. Mean Climatic Parameters for Each Species
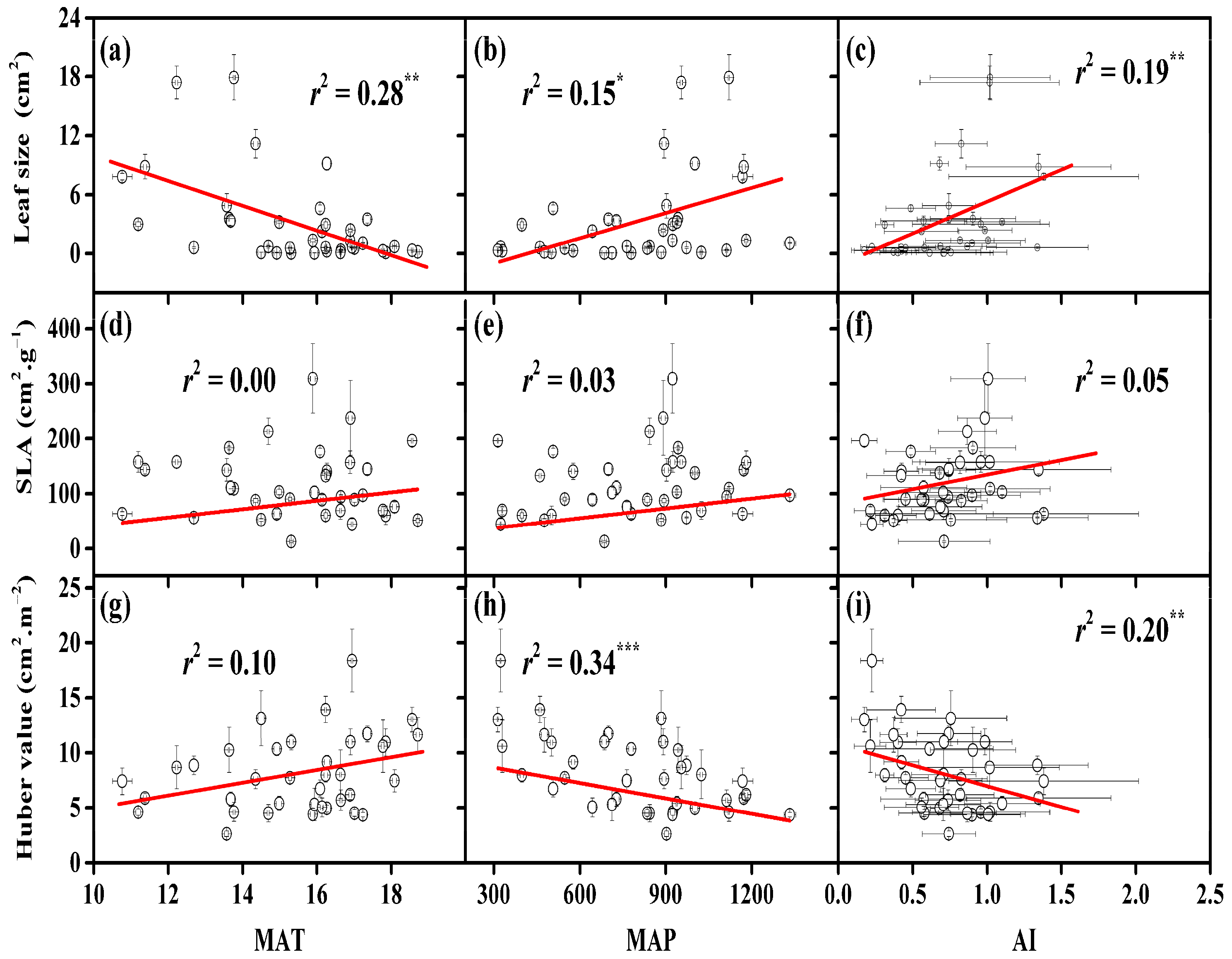
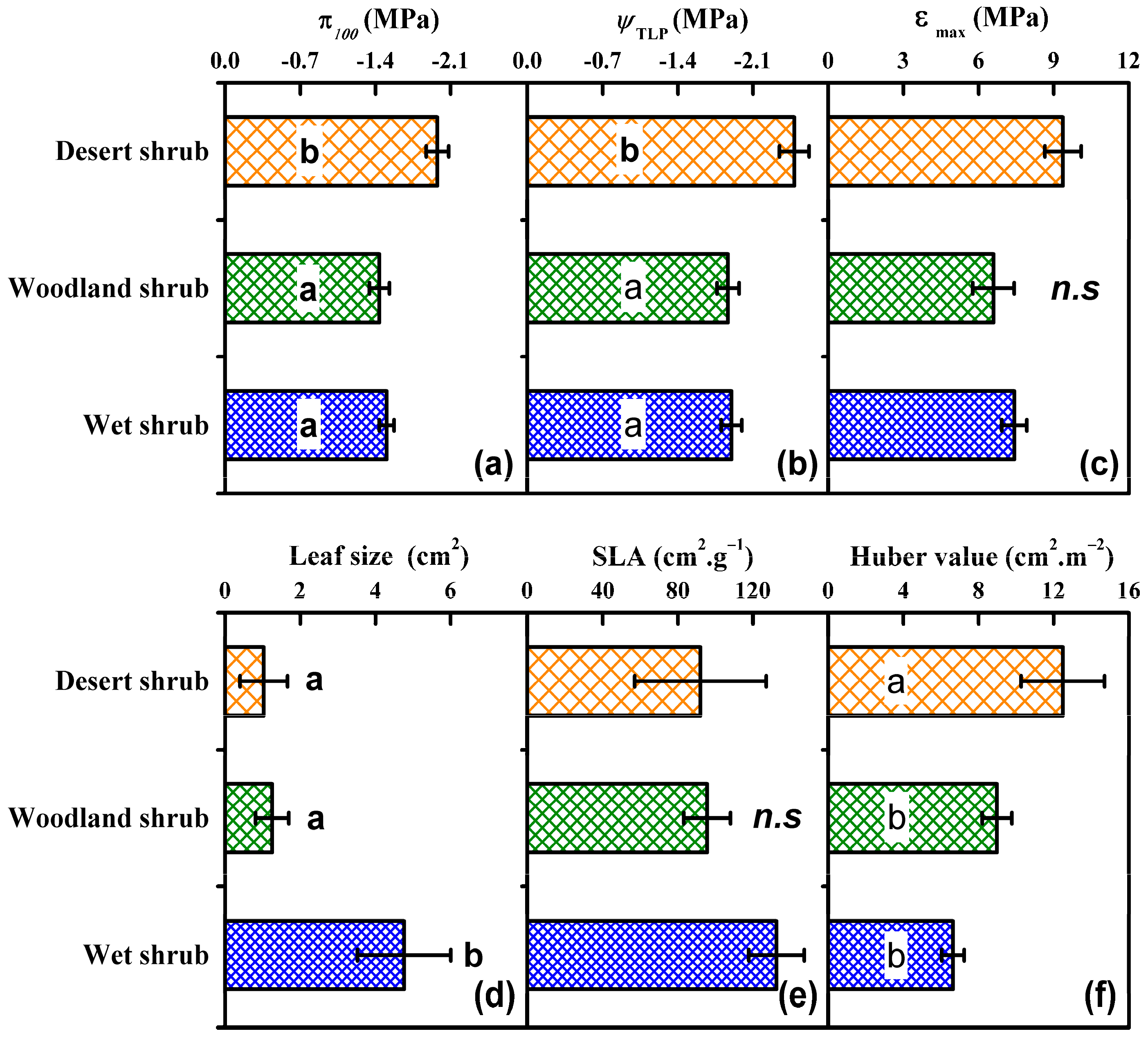
3.2. Dependencies of Leaf Size, SLA, and Huber Value on Provenance Climate
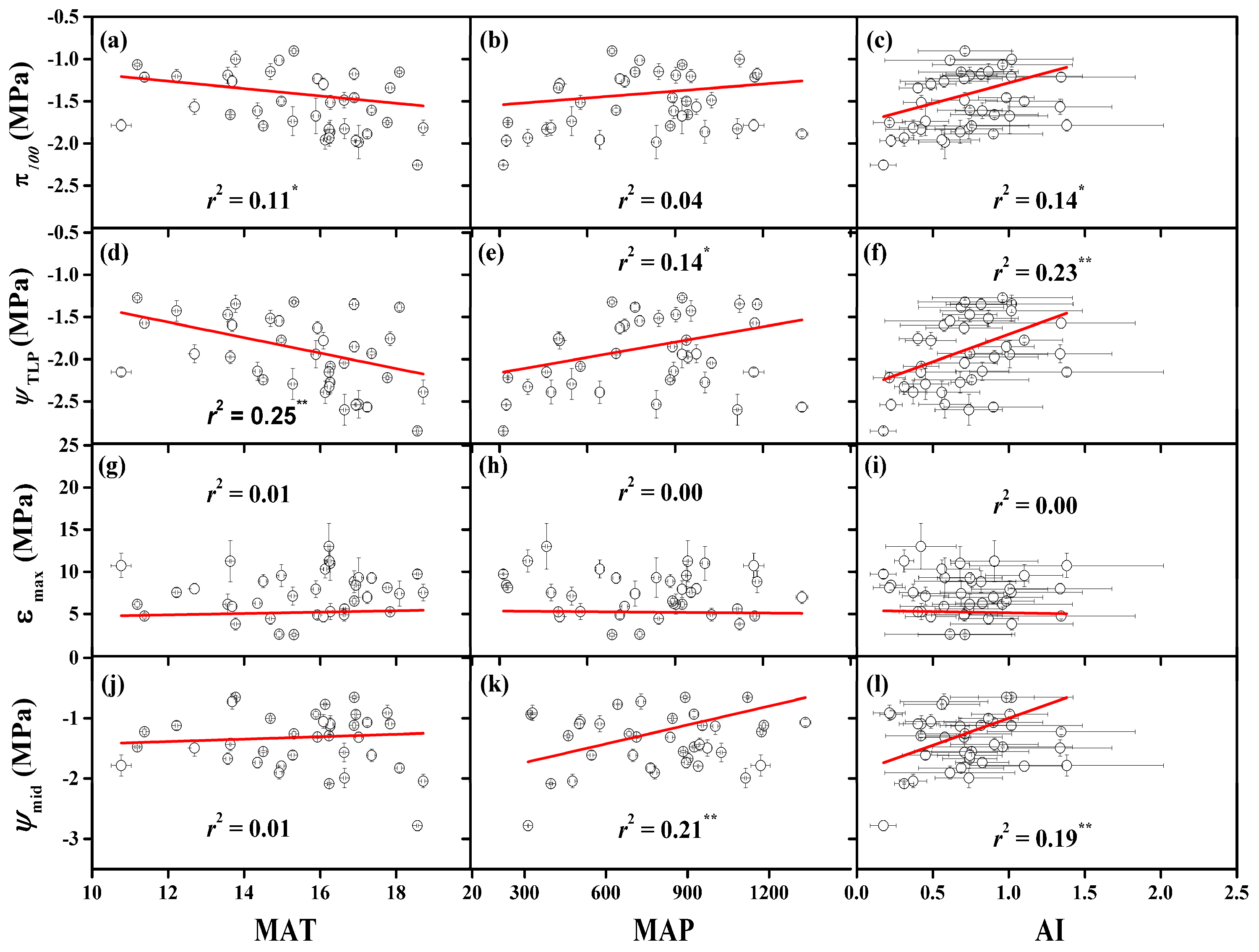
3.3. Dependence of Turgor Loss Traits on the Native Climate
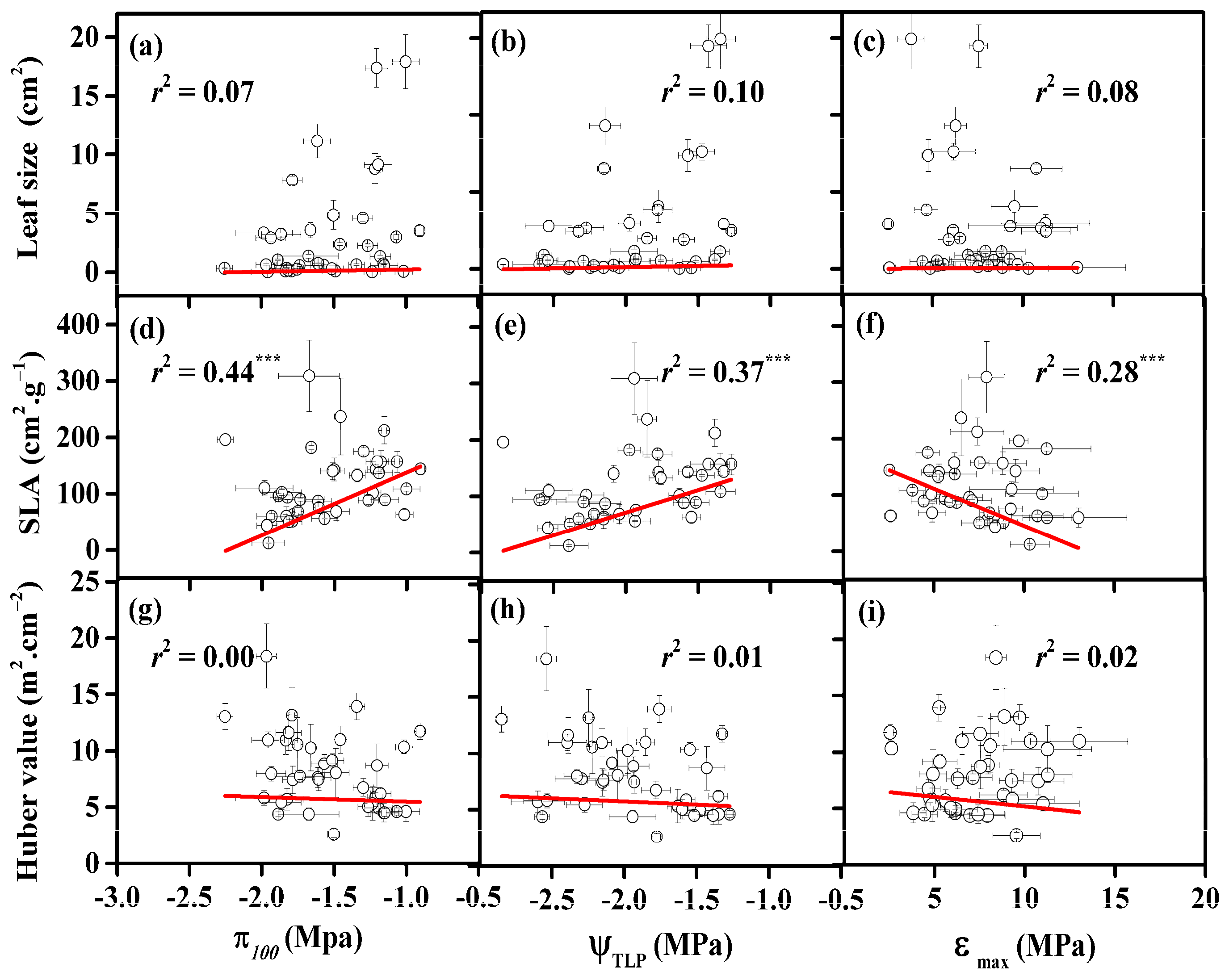
3.4. Correlations between Turgor Loss and Other Leaf Traits
3.5. Leaf Water-Related Traits Correlated with Native Climates across Biomes
4. Discussion
4.1. The Relevance of Native Climate to Morphological Traits
4.2. The Importance of Physiological Traits to Ecological Drought Tolerance
4.3. Coordination among Morphological and Physiological Traits and Their Correlations with Native Climates
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sheffield, J.; Wood, E.F. Global trends and variability in soil moisture and drought characteristics, 1950–2000, from observation-driven Simulations of the terrestrial hydrologic cycle. J. Clim. 2008, 21, 432–458. [Google Scholar] [CrossRef]
- Adams, H.D.; Guardiola-Claramonte, M.; Barron-Gafford, G.A.; Villegas, J.C.; Breshears, D.D.; Zou, C.B.; Troch, P.A.; Huxman, T.E. Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought. Proc. Natl. Acad. Sci. USA 2009, 106, 7063–7066. [Google Scholar] [CrossRef] [PubMed]
- van Mantgem, P.J.; Stephenson, N.L.; Byrne, J.C.; Daniels, L.D.; Franklin, J.F.; Fule, P.Z.; Harmon, M.E.; Larson, A.J.; Smith, J.M.; Taylor, A.H.; et al. Widespread Increase of Tree Mortality Rates in the Western United States. Science 2009, 323, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Breshears, D.D.; Lopez-Hoffman, L.; Graumlich, L.J. When Ecosystem Services Crash: Preparing for Big, Fast, Patchy Climate Change. AMBIO 2011, 40, 256–263. [Google Scholar] [CrossRef]
- Mitchell, P.J.; O’Grady, A.P. Adaptation of Leaf Water Relations to Climatic and Habitat Water Availability. Forests 2015, 6, 2281–2295. [Google Scholar] [CrossRef]
- Delzon, S. New insight into leaf drought tolerance. Funct. Ecol. 2015, 29, 1247–1249. [Google Scholar] [CrossRef]
- Hacke, U.G.; Sperry, J.S.; Pockman, W.T.; Davis, S.D.; McCulloh, K.A. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 2001, 126, 457–461. [Google Scholar] [CrossRef]
- Sack, L.; Cowan, P.D.; Jaikumar, N.; Holbrook, N.M. The ‘hydrology’ of leaves: Co-ordination of structure and function in temperate woody species. Plant Cell Environ. 2003, 26, 1343–1356. [Google Scholar] [CrossRef]
- Bucci, S.J.; Scholz, F.G.; Goldstein, G.; Meinzer, F.C.; Sternberg, L.D.L. Dynamic changes in hydraulic conductivity in petioles of two savanna tree species: Factors and mechanisms contributing to the refilling of embolized vessels. Plant Cell Environ. 2003, 26, 1633–1645. [Google Scholar] [CrossRef]
- Lamont, B.B.; Groom, P.K.; Cowling, R.M. High leaf mass per area of related species assemblages may reflect low rainfall and carbon isotope discrimination rather than low phosphorus and nitrogen concentrations. Funct. Ecol. 2002, 16, 403–412. [Google Scholar] [CrossRef]
- Warren, C.R.; Tausz, M.; Adams, M.A. Does rainfall explain variation in leaf morphology and physiology among populations of red ironbark (Eucalyptus sideroxylon subsp tricarpa) grown in a common garden? Tree Physiol. 2005, 25, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, M.K.; Scoffoni, C.; Sack, L. The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: A global meta-analysis. Ecol. Lett. 2012, 15, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Zanne, A.E.; Westoby, M.; Falster, D.S.; Ackerly, D.D.; Loarie, S.R.; Arnold, S.E.J.; Coomes, D.A. Angiosperm wood structure: Global patterns in vessel anatomy and their relation to wood density and potential conductivity. Am. J. Bot. 2010, 97, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Fortunel, C.; Ruelle, J.; Beauchene, J.; Fine, P.V.A.; Baraloto, C. Wood specific gravity and anatomy of branches and roots in 113 Amazonian rainforest tree species across environmental gradients. New Phytol. 2014, 202, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Maréchaux, I.; Bartlett, M.K.; Sack, L.; Baraloto, C.; Engel, J.; Joetzjer, E.; Chave, J. Drought tolerance as predicted by leaf water potential at turgor loss point varies strongly across species within an Amazonian forest. Funct. Ecol. 2015, 29, 1268–1277. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Zhang, Y.; Kreidler, N.; Sun, S.; Ardy, R.; Cao, K.; Sack, L. Global analysis of plasticity in turgor loss point, a key drought tolerance trait. Ecol. Lett. 2014, 17, 1580–1590. [Google Scholar] [CrossRef]
- Mitchell, P.J.; Veneklaas, E.J.; Lambers, H.; Burgess, S.S.O. Leaf water relations during summer water deficit: Differential responses in turgor maintenance and variation in leaf structure among different plant communities in south-western Australia. Plant Cell Environ. 2008, 31, 1791–1802. [Google Scholar] [CrossRef]
- Ramírez-Valiente, J.A.; Sánchez-Gómez, D.; Aranda, I.; Valladares, F. Phenotypic plasticity and local adaptation in leaf ecophysiological traits of 13 contrasting cork oak populations under different water availabilities. Tree Physiol. 2010, 30, 618–627. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Sancho-Knapik, D.; Barrón, E.; Camarero, J.J.; Vilagrosa, A.; Gil-Pelegrín, E. Morphological and physiological divergences within Quercus ilex support the existence of different ecotypes depending on climatic dryness. Ann. Bot. 2014, 114, 301–313. [Google Scholar] [CrossRef]
- Niinemets, U. Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology 2001, 82, 453–469. [Google Scholar] [CrossRef]
- Nardini, A.; Pedà, G.; Rocca, N.L. Trade-offs between leaf hydraulic capacity and drought vulnerability: Morpho-anatomical bases, carbon costs and ecological consequences. New Phytol. 2012, 196, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Costa-Saura, J.M.; Martínez-Vilalta, J.; Trabucco, A.; Spano, D.; Mereu, S. Specific leaf area and hydraulic traits explain niche segregation along an aridity gradient in Mediterranean woody species. Perspect. Plant Ecol. Evol. Syst. 2016, 21, 23–30. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; Holbrook, N.M. Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiol. 2003, 132, 2166–2173. [Google Scholar] [CrossRef]
- Choat, B.; Sack, L.; Holbrook, N.M. Diversity of hydraulic traits in nine Cordia species growing in tropical forests with contrasting precipitation. New Phytol. 2007, 175, 686–698. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Scoffoni, C.; Ardy, R.; Zhang, Y.; Sun, S.; Cao, K.; Sack, L. Rapid determination of comparative drought tolerance traits: Using an osmometer to predict turgor loss point. Methods Ecol. Evol. 2012, 3, 880–888. [Google Scholar] [CrossRef]
- Higgins, S.I.; O’Hara, R.B.; Roemermann, C. A niche for biology in species distribution models. J. Biogeogr. 2012, 39, 2091–2095. [Google Scholar] [CrossRef]
- Arndt, S.K.; Irawan, A.; Sanders, G.J. Apoplastic water fraction and rehydration techniques introduce significant errors in measurements of relative water content and osmotic potential in plant leaves. Physiol. Plant. 2015, 155, 355–368. [Google Scholar] [CrossRef]
- Abrams, M.D.; Kubiske, M.E.; Steiner, K.C. Drought adaptations and responses in 5 genotypes of Fraxinus pennsylvanica Marsh: Photosynthesis, water relations and leaf morphology. Tree Physiol. 1990, 6, 305–315. [Google Scholar] [CrossRef]
- Engelbrecht, B.M.J.; Comita, L.S.; Condit, R.; Kursar, T.A.; Tyree, M.T.; Turner, B.L.; Hubbell, S.P. Drought sensitivity shapes species distribution patterns in tropical forests. Nature 2007, 447, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Baltzer, J.L.; Davies, S.J.; Bunyavejchewin, S.; Noor, N.S.M. The role of desiccation tolerance in determining tree species distributions along the Malay–Thai Peninsula. Funct. Ecol. 2008, 22, 221–231. [Google Scholar] [CrossRef]
- Lenz, T.I.; Wright, I.J.; Westoby, M. Interrelations among pressure–volume curve traits across species and water availability gradients. Physiol. Plant. 2006, 127, 423–433. [Google Scholar] [CrossRef]
- Blackman, C.J.; Gleason, S.M.; Chang, Y.; Cook, A.M.; Laws, C.; Westoby, M. Leaf hydraulic vulnerability to drought is linked to site water availability across a broad range of species and climates. Ann. Bot. 2014, 114, 435–440. [Google Scholar] [CrossRef]
- Revell, D.K.; Norman, H.C.; Vercoe, P.E.; Phillips, N.; Toovey, A.; Bickell, S.; Hulm, E.; Hughes, S.; Emms, J. Australian perennial shrub species add value to the feed base of grazing livestock in low- to medium-rainfall zones. Anim. Prod. Sci. 2013, 53, 1221–1230. [Google Scholar] [CrossRef]
- Du, P.; Arndt, S.K.; Farrell, C. Relationships between plant drought response, traits, and climate of origin for green roof plant selection. Ecol. Appl. 2018, 28, 1752–1761. [Google Scholar] [CrossRef]
- Guerin, G.R.; Wen, H.; Lowe, A.J. Leaf morphology shift linked to climate change. Biol. Lett. 2012, 8, 882–886. [Google Scholar] [CrossRef]
- Mitchell, P.J.; O’Grady, A.P.; Hayes, K.R.; Pinkard, E.A. Exposure of trees to drought- induced die- off is defined by a common climatic threshold across different vegetation types. Ecol. Evol. 2014, 4, 1088–1101. [Google Scholar] [CrossRef]
- Tyree, M.T.; Hammel, H.T. The Measurement of the Turgor Pressure and the Water Relations of Plants by the Pressure-bomb Technique. J. Exp. Bot. 1972, 23, 267–282. [Google Scholar] [CrossRef]
- Schulte, P.J.; Hinckley, T.M. A comparison of pressure-volume curve data-analysis techniques. J. Exp. Bot. 1985, 36, 1590–1602. [Google Scholar] [CrossRef]
- Knutzen, F.; Meier, I.C.; Leuschner, C. Does reduced precipitation trigger physiological and morphological drought adaptations in European beech (Fagus sylvatica L.)? Comparing provenances across a precipitation gradient. Tree Physiol. 2015, 35, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.-D.; Chen, Y.-J.; Ye, Q.; He, P.-C.; Liu, H.; Li, R.-H.; Fu, P.-L.; Jiang, G.-F.; Cao, K.-F. Leaf turgor loss point is correlated with drought tolerance and leaf carbon economics traits. Tree Physiol. 2018, 38, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Bourne, A.E.; Creek, D.; Peters, J.M.R.; Ellsworth, D.S.; Choat, B. Species climate range influences hydraulic and stomatal traits in Eucalyptus species. Ann. Bot. 2017, 120, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Givnish, T.J. Leaf and Canopy Adaptations in Tropical Forests. In Physiological Ecology of Plants of the Wet Tropics: Proceedings of an International Symposium Held in Oxatepec and Los Tuxtlas, Mexico, June 29 to July 6, 1983; Medina, E., Mooney, H.A., Vázquez-Yánes, C., Eds.; Springer: Dordrecht, The Netherlands, 1984; pp. 51–84. [Google Scholar] [CrossRef]
- Fonseca, C.R.; Overton, J.M.; Collins, B.; Westoby, M. Shifts in trait-combinations along rainfall and phosphorus gradients. J. Ecol. 2000, 88, 964–977. [Google Scholar] [CrossRef]
- Cunningham, S.A.; Summerhayes, B.; Westoby, M. Evolutionary divergences in leaf structure and chemistry, comparing rainfall and soil nutrient gradients. Ecol. Monogr. 1999, 69, 569–588. [Google Scholar] [CrossRef]
- Ackerly, D.; Knight, C.; Weiss, S.; Barton, K.; Starmer, K. Leaf size, specific leaf area and microhabitat distribution of chaparral woody plants: Contrasting patterns in species level and community level analyses. Oecologia 2002, 130, 449–457. [Google Scholar] [CrossRef]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Díaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Law, E.A.; et al. Global climatic drivers of leaf size. Science 2017, 357, 917–921. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M. Strategy shifts in leaf physiology, structure and nutrient content between species of high- and low-rainfall and high- and low-nutrient habitats. Funct. Ecol. 2001, 15, 423–434. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, U.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Walter, H.; Breckle, S.-W. The Arid Regions of Australia. In Ecological Systems of the Geobiosphere: 2 Tropical and Subtropical Zonobiomes; Springer: Berlin/Heidelberg, Germany, 1986; pp. 330–365. [Google Scholar] [CrossRef]
- Addington, R.N.; Donovan, L.A.; Mitchell, R.J.; Vose, J.M.; Pecot, S.D.; Jack, S.B.; Hacke, U.G.; Sperry, J.S.; Oren, R. Adjustments in hydraulic architecture of Pinus palustris maintain similar stomatal conductance in xeric and mesic habitats. Plant Cell Environ. 2006, 29, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Mencuccini, M.; Grace, J. Climate influences the leaf area/sapwood area ration in Scots pine. Tree Physiol. 1995, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Magnani, F.; Grace, J.; Borghetti, M. Adjustment of tree structure in response to the environment under hydraulic constraints. Funct. Ecol. 2002, 16, 385–393. [Google Scholar] [CrossRef]
- Edwards, E.J. Correlated evolution of stem and leaf hydraulic traits in Pereskia (Cactaceae). New Phytol. 2006, 172, 479–489. [Google Scholar] [CrossRef]
- Vander Willigen, C.; Pammenter, N.W. Relationship between growth and xylem hydraulic characteristics of clones of Eucalyptus spp. at contrasting sites. Tree Physiol. 1998, 18, 595–600. [Google Scholar] [CrossRef]
- Eamus, D.; O’Grady, A.P.; Hutley, L. Dry season conditions determine wet season water use in the wet–tropical savannas of northern Australia. Tree Physiol. 2000, 20, 1219–1226. [Google Scholar] [CrossRef]
- Macinnis-Ng, C.; McClenahan, K.; Eamus, D. Convergence in hydraulic architecture, water relations and primary productivity amongst habitats and across seasons in Sydney. Funct. Plant Biol. 2004, 31, 429–439. [Google Scholar] [CrossRef]
- O’Grady, A.P.; Cook, P.G.; Eamus, D.; Duguid, A.; Wischusen, J.D.H.; Fass, T.; Worldege, D. Convergence of tree water use within an arid-zone woodland. Oecologia 2009, 160, 643–655. [Google Scholar] [CrossRef]
- Blackman, C.J.; Brodribb, T.J.; Jordan, G.J. Leaf hydraulic vulnerability is related to conduit dimensions and drought resistance across a diverse range of woody angiosperms. New Phytol. 2010, 188, 1113–1123. [Google Scholar] [CrossRef]
- Blackman, C.J. Leaf turgor loss as a predictor of plant drought response strategies. Tree Physiol. 2018, 38, 655–657. [Google Scholar] [CrossRef]
- White, D.A.; Turner, N.C.; Galbraith, J.H. Leaf water relations and stomatal behavior of four allopatric Eucalyptus species planted in Mediterranean southwestern Australia. Tree Physiol. 2000, 20, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Ngugi, M.R.; Doley, D.; Hunt, M.A.; Dart, P.; Ryan, P. Leaf water relations of Eucalyptus cloeziana and Eucalyptus argophloia in response to water deficit. Tree Physiol. 2003, 23, 335–343. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clifford, S.C.; Arndt, S.K.; Corlett, J.E.; Joshi, S.; Sankhla, N.; Popp, M.; Jones, H.G. The role of solute accumulation, osmotic adjustment and changes in cell wall elasticity in drought tolerance in Ziziphus mauritiana (Lamk). J. Exp. Bot. 1998, 49, 967–977. [Google Scholar] [CrossRef]
- Joly, R.J.; Zaerr, J.B. Alteration of cell-wall water content and elasticity in Douglas-fir during periods of water deficit. Plant Physiol. 1987, 83, 418–422. [Google Scholar] [CrossRef]
- Salleo, S.; Gullo, M.A.L. Sclerophylly and plant water relations in 3 Mediterranean Quercus species. Ann. Bot. 1990, 65, 259–270. [Google Scholar] [CrossRef]
- Corcuera, L.; Camarero, J.J.; Gil-Pelegrín, E. Functional groups in Quercus species derived from the analysis of pressure–volume curves. Trees 2002, 16, 465–472. [Google Scholar] [CrossRef]
- Villagra, M.; Campanello, P.I.; Bucci, S.J.; Goldstein, G. Functional relationships between leaf hydraulics and leaf economic traits in response to nutrient addition in subtropical tree species. Tree Physiol. 2013, 33, 1308–1318. [Google Scholar] [CrossRef]
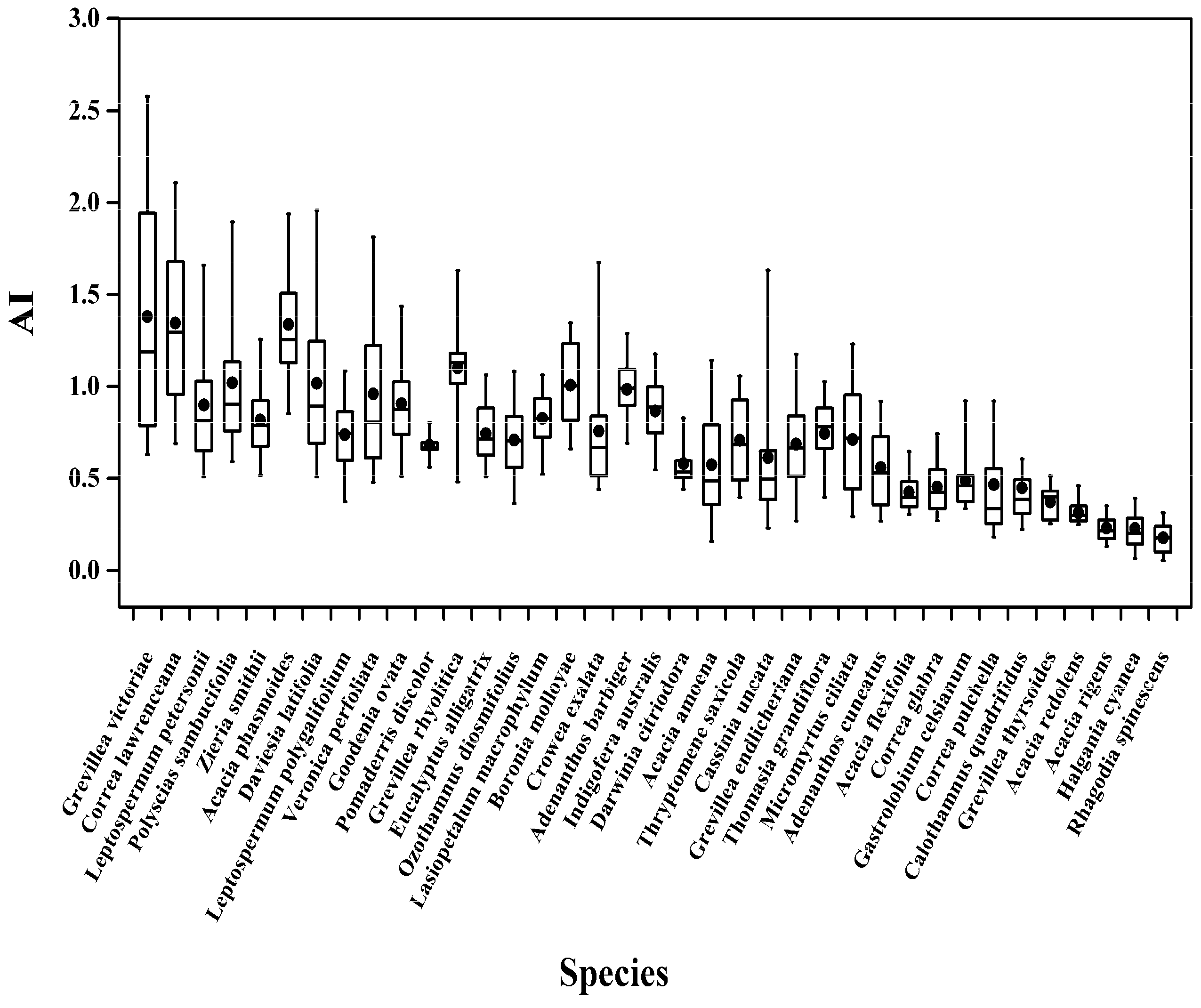

| No. | Family | Genus | Species | AI | MAP (mm) | MAT (°C) | Median AI |
|---|---|---|---|---|---|---|---|
| 1 | Proteaceae | Grevillea | Grevillea victoriae | 1.381 | 1169 (631, 2004) | 10.77 | 1.189 |
| 2 | Rutaceae | Correa | Correa lawrenceana | 1.345 | 1172 (546, 2683) | 11.38 | 1.296 |
| 3 | Myrtaceae | Leptospermum | Leptospermum petersonii | 0.899 | 1334 (614, 2360) | 17.24 | 0.814 |
| 4 | Araliaceae | Polyscias | Polyscias sambucifolia | 1.019 | 1120 (444, 2604) | 13.77 | 0.902 |
| 5 | Rutaceae | Zieria | Zieria smithii | 0.817 | 1181 (278, 3742) | 16.89 | 0.788 |
| 6 | Fabaceae | Acacia | Acacia phasmoides | 1.337 | 973 (614, 1200) | 12.69 | 1.254 |
| 7 | Fabaceae | Daviesia | Daviesia latifolia | 1.017 | 954 (379, 2524) | 12.22 | 0.893 |
| 8 | Myrtaceae | Leptospermum | Leptospermum polygalifolium | 0.737 | 1113 (199, 3404) | 16.64 | 0.744 |
| 9 | Plantaginaceae | Veronica | Veronica perfoliata | 0.959 | 924 (409, 2368) | 11.19 | 0.806 |
| 10 | Goodeniaceae | Goodenia | Goodenia ovata | 0.905 | 944 (196, 2524) | 13.64 | 0.876 |
| 11 | Rhamnaceae | Pomaderris | Pomaderris discolor | 0.680 | 1002 (392, 1609) | 16.26 | 0.664 |
| 12 | Proteaceae | Grevillea | Grevillea rhyolitica | 1.101 | 938 (864, 1016) | 14.98 | 1.127 |
| 13 | Myrtaceae | Eucalyptus | Eucalyptus alligatrix | 0.743 | 902 (459, 1312) | 13.57 | 0.714 |
| 14 | Asteraceae | Ozothamnus | Ozothamnus diosmifolius | 0.709 | 1024 (247, 2360) | 16.63 | 0.702 |
| 15 | Malvaceae | Lasiopetalum | Lasiopetalum macrophyllum | 0.826 | 895 (317, 1531) | 14.35 | 0.826 |
| 16 | Rutaceae | Boronia | Boronia molloyae | 1.006 | 923 (308, 1245) | 15.89 | 1.002 |
| 17 | Rutaceae | Crowea | Crowea exalata | 0.757 | 884 (408, 2073) | 14.50 | 0.669 |
| 18 | Proteaceae | Adenanthos | Adenanthos barbiger | 0.984 | 891 (256, 1152) | 16.90 | 0.990 |
| 19 | Fabaceae | Indigofera | Indigofera australis | 0.867 | 844 (158, 2359) | 14.69 | 0.887 |
| 20 | Myrtaceae | Darwinia | Darwinia citriodora | 0.579 | 836 (335, 1124) | 17.01 | 0.535 |
| 21 | Fabaceae | Acacia | Acacia amoena | 0.574 | 728 (524, 1380) | 13.68 | 0.485 |
| 22 | Myrtaceae | Thryptomene | Thryptomene saxicola | 0.706 | 712 (362, 1181) | 15.93 | 0.683 |
| 23 | Asteraceae | Cassinia | Cassinia uncata | 0.613 | 779 (222, 2287) | 14.92 | 0.496 |
| 24 | Proteaceae | Grevillea | Grevillea endlicheriana | 0.687 | 763 (350, 1087) | 18.09 | 0.666 |
| 25 | Malvaceae | Thomasia | Thomasia grandiflora | 0.744 | 699 (345, 1099) | 17.35 | 0.781 |
| 26 | Myrtaceae | Micromyrtus | Micromyrtus ciliata | 0.711 | 685 (280, 2004) | 15.31 | 0.718 |
| 27 | Proteaceae | Adenanthos | Adenanthos cuneatus | 0.560 | 643 (282, 1219) | 16.13 | 0.529 |
| 28 | Fabaceae | Acacia | Acacia flexifolia | 0.424 | 577 (255, 1155) | 16.27 | 0.396 |
| 29 | Rutaceae | Correa | Correa glabra | 0.453 | 547 (229, 1233) | 15.28 | 0.424 |
| 30 | Fabaceae | Gastrolobium | Gastrolobium celsianum | 0.487 | 506 (312, 1039) | 16.08 | 0.459 |
| 31 | Rutaceae | Correa | Correa pulchella | 0.422 | 461 (275, 835) | 16.23 | 0.334 |
| 32 | Myrtaceae | Melaleuca | Calothamnus quadrifidus | 0.402 | 501 (195, 1252) | 17.84 | 0.384 |
| 33 | Proteaceae | Grevillea | Grevillea thyrsoides | 0.371 | 476 (353, 653) | 18.71 | 0.398 |
| 34 | Fabaceae | Acacia | Acacia redolens | 0.312 | 397 (329, 675) | 16.24 | 0.298 |
| 35 | Fabaceae | Acacia | Acacia rigens | 0.226 | 323 (175, 818) | 16.94 | 0.214 |
| 36 | Ehretiaceae | Halgania | Halgania cyanea | 0.214 | 329 (151, 1252) | 17.77 | 0.202 |
| 37 | Amaranthaceae | Chenopodium | Rhagodia spinescens | 0.175 | 313 (142, 1189) | 18.56 | 0.176 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.-Q.; Arndt, S.K.; Farrell, C. Leaf Traits of Drought Tolerance for 37 Shrub Species Originating from a Moisture Gradient. Water 2020, 12, 1626. https://doi.org/10.3390/w12061626
Xu G-Q, Arndt SK, Farrell C. Leaf Traits of Drought Tolerance for 37 Shrub Species Originating from a Moisture Gradient. Water. 2020; 12(6):1626. https://doi.org/10.3390/w12061626
Chicago/Turabian StyleXu, Gui-Qing, Stefan K. Arndt, and Claire Farrell. 2020. "Leaf Traits of Drought Tolerance for 37 Shrub Species Originating from a Moisture Gradient" Water 12, no. 6: 1626. https://doi.org/10.3390/w12061626
APA StyleXu, G.-Q., Arndt, S. K., & Farrell, C. (2020). Leaf Traits of Drought Tolerance for 37 Shrub Species Originating from a Moisture Gradient. Water, 12(6), 1626. https://doi.org/10.3390/w12061626






