Assessment of the Environmental Status of the Mangrove Ecosystem in the United Arab Emirates
Abstract
1. Introduction
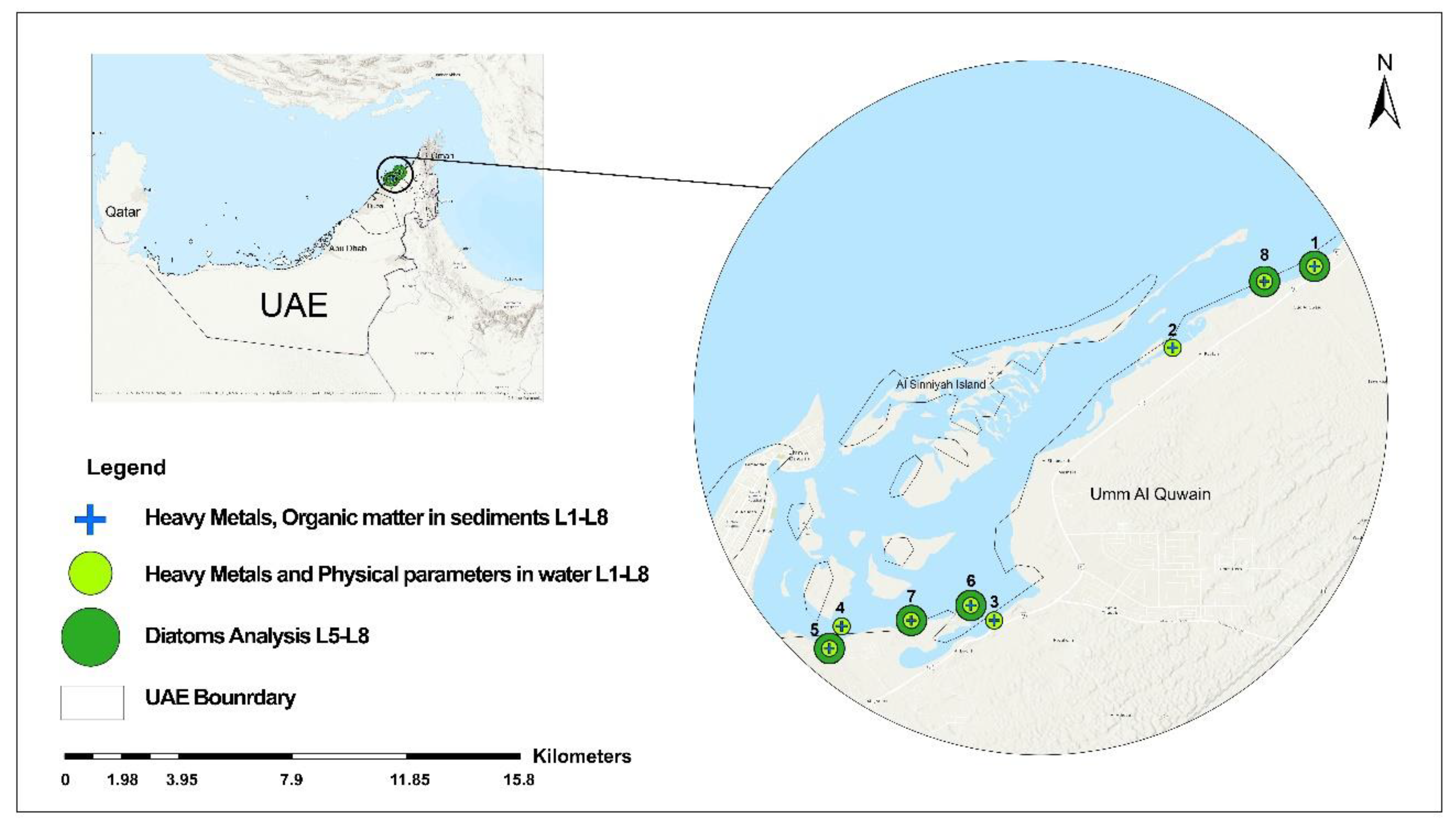
2. Materials and Methods
2.1. Sampling and Storage
2.2. Elemental Analysis of the Water and Sediment Samples
2.3. Organic Carbon and Organic Matter Analysis
2.4. Diatom Analysis
2.5. Assessment of Hazard
2.6. Multivariate Statistical Analysis
3. Results
3.1. Physical Water Quality Analysis and Total Organic Carbon in Water
3.2. Elemental Analysis
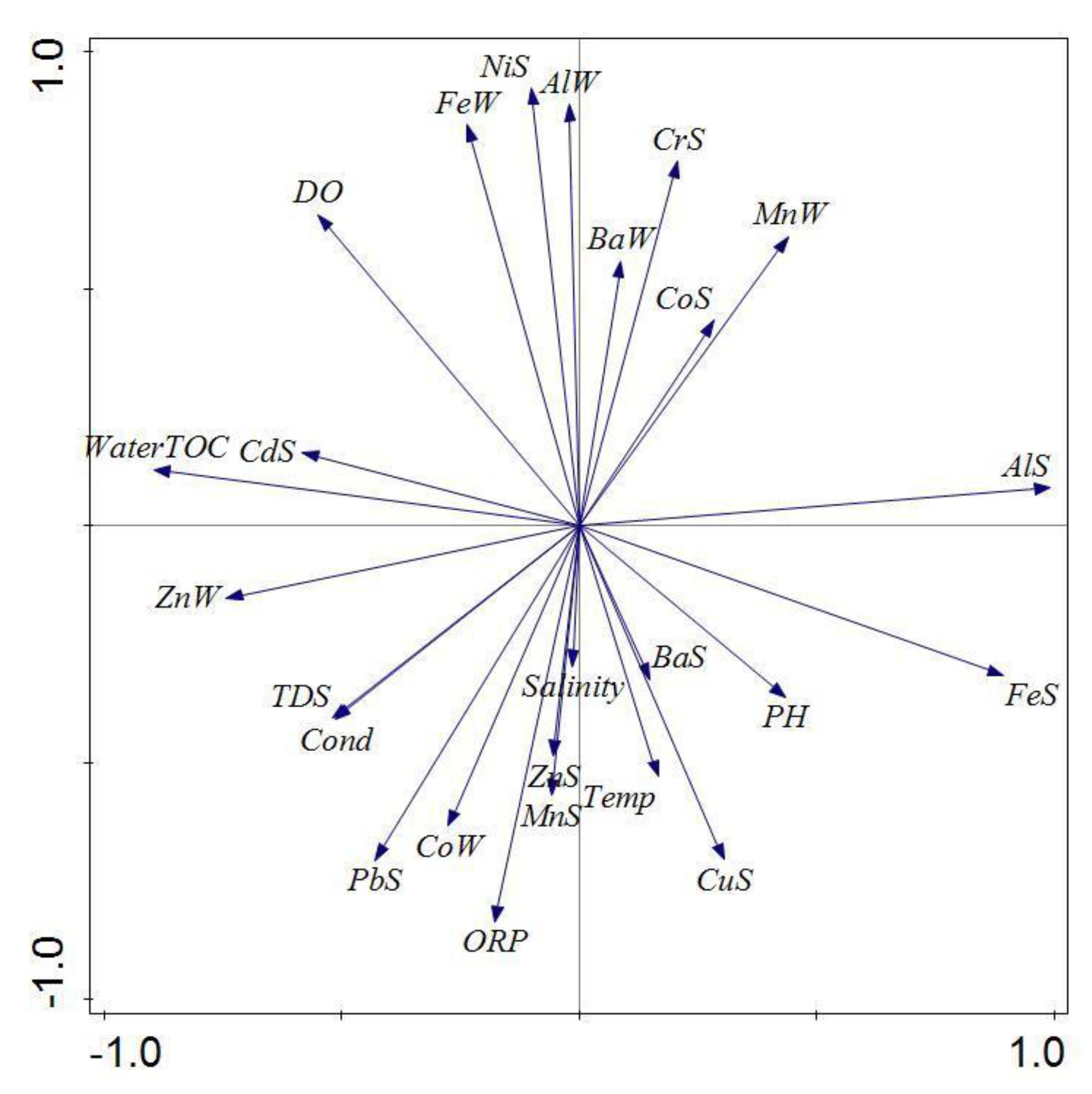
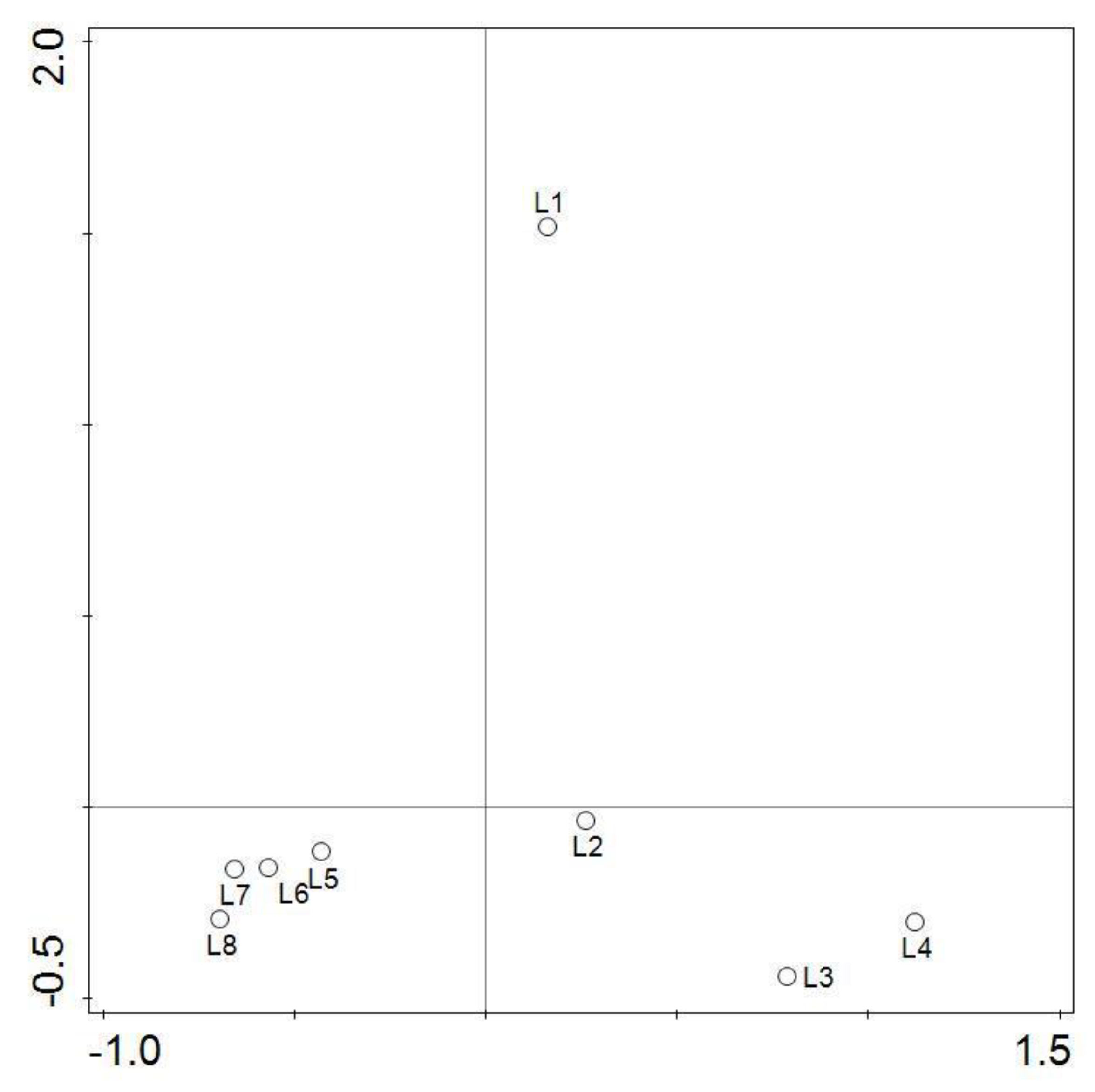
3.3. Principal Component Analysis
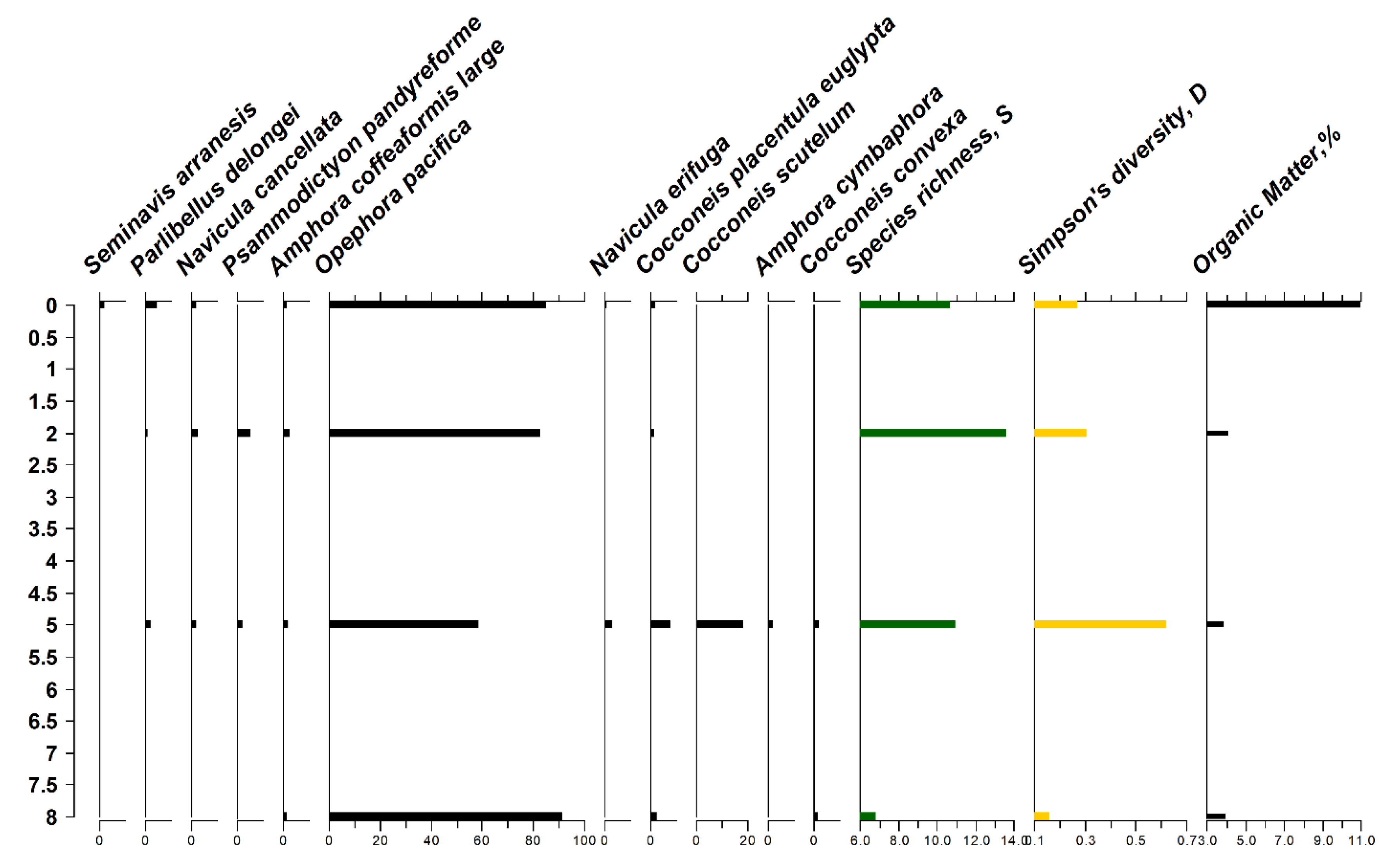
3.4. Diatom Analysis and Sediment Organic Matter (OM)%
4. Discussion
4.1. Physical Water Quality Analysis and Total Organic Carbon in Water
4.2. Elemental Analysis
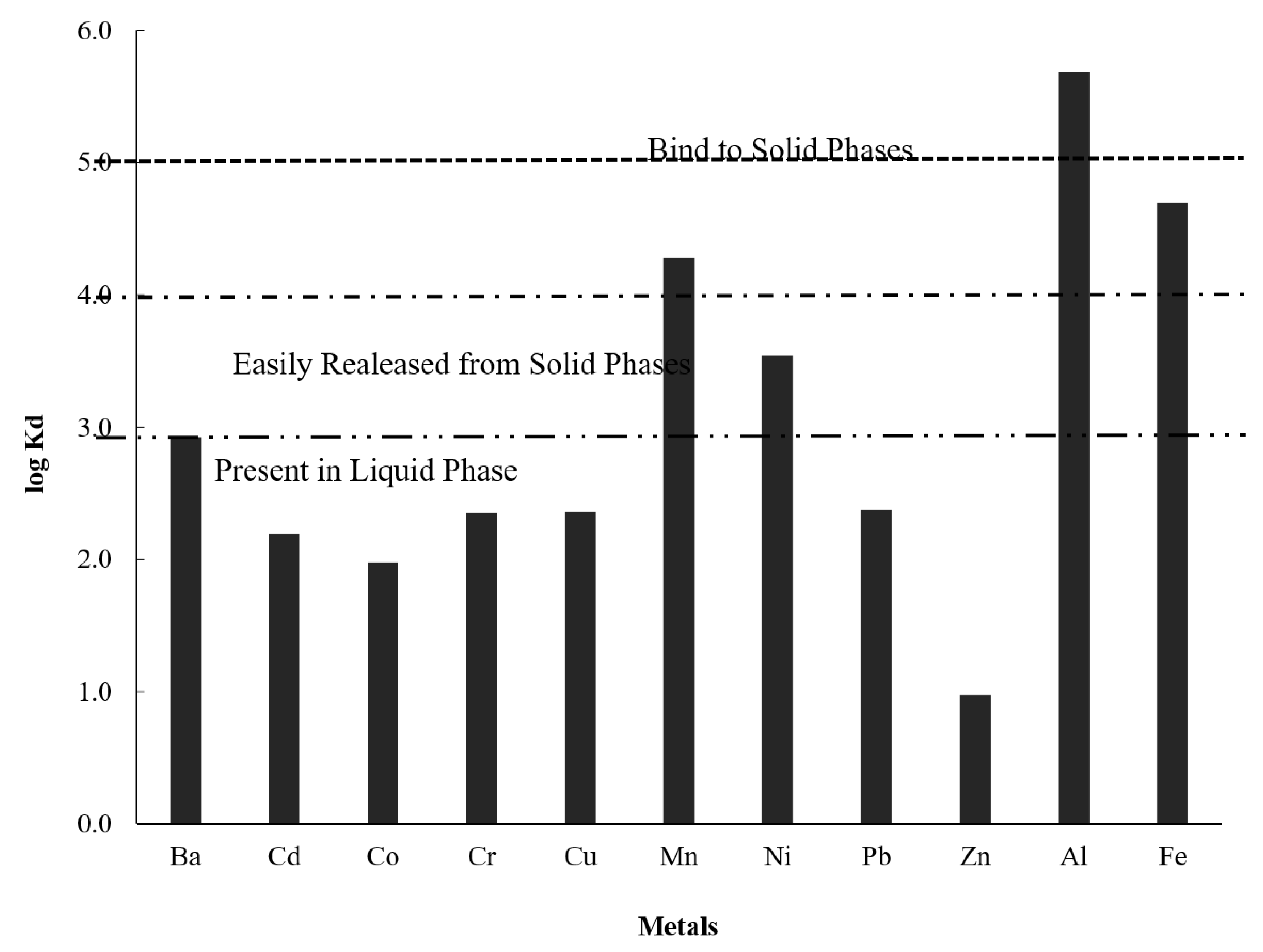
4.3. Distribution of Water and Surface Sediment Chemistry Parameters
4.4. Assessment of Hazard
4.5. Diatom Analysis and Organic Matter
- Ubiquitous cosmopolitan epipsammic Achnanthidium minutissimum prevailed in the algal mat in location L6 together with epiphytic Mastogloia macdonaldii and Fragilaria fasciculata. Mastogloia citrus also occurred predominantly in the algal mat in location L6. It was observed as attached to the filaments of Enteromorpha and Rhizoclonium. Achnanthidium minutissimum is a widespread freshwater taxon and its presence at the algal mat may indicate occasional influx of freshwater into the mangrove, although there is no permanent source of freshwater in Khor al Beida. Potentially, it may also occur in the algal mat through air transport from a freshwater source. Nitzschia sp. (type 5) also occurred at high abundance in this location.
- Surface diatom assemblage in location L5 (both algal mat and surface sediment sample) shows similarities with the assemblages in location 7AM (right hand side bottom part of diagram in Figure 7). This assemblage was dominated by epiphytic Cocconeis placentula var. euglipta and epipelic Amphora coffeaeformis large form. Amphora coffeaeformis commonly occurs in marine and brackish intertidal sediments, it was found in large quantities in Kuwaiti mud flats [19] and in the Shatt Al Arab estuary in Iraq [21].
- Another large diatom assemblage (Assemblage 3 in Figure 7) comprises mainly epipelic and epipsammic diatoms from locations L6, L7, and L8 (these exclude algal mats). Several epiphytic Cocconies taxa (C. scutellum and C. convexa), which are quite common marine taxa, with C. convexa also found on Kuwaiti macrophytes [19], in the sediments of Shatt Al Arab estuary in Iraq [21] and in the northern coast of Jeddah [55]. Epipelic taxa (e.g., A. coffeaeformis small, A. cymbaphora, Navicula erifuga, N. besarensis and N. digitoradiata) and epiphytic Achnanthes brevipes dominated this diatom assemblage. These species commonly occur in Kuwaiti coastal sediments [19]. Epiphytic Achanthes brevipes was also abundant in Huwaiza marsh in Iraq [56].
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Full Diatom Name | Abbreviation in Figure 7 |
|---|---|
| Achnanthidium minutissimum agg | AchnMint |
| Achnanthes brevipes | AchnBrev |
| Amphora coffeaeformis large form | AmpCoffL |
| Amphora coffeaeformis small form | AmpCoffSm |
| Amphora cymbaphora | AmphCymb |
| Cocconeis placentula var. euglipta | CoccPlac |
| Cocconeisscutellum | CoccScut |
| Cocconeis convexa | CoccConv |
| Craspedostauros amphoroides | CrasAmph |
| Fragilaria fasciculata | FraFasIc |
| Mastogloia citrus | MastMacd |
| Mastogloia macdonaldii | MastCitr |
| Navicula cancellata | NavCanc |
| Navicula erifuga | NavcErif |
| Navicula besarensis | NavcBesr |
| Navicula cancellata | NavCanc |
| Navicula digitoradiata | NavcDigt |
| Navicula erifuga | NavcErif |
| Opephora pacifica | OpePac |
| Opephora mutabilis | OpepMut |
| Parlibellus delongei | ParlDeln |
| Seminavisarranensis | SemnArrn |
| Nitzschia cf. perminuta’ | NitPerm |
| Nitzschia sp. type 5 | NitSp5 |
References
- Carugati, L.; Gatto, B.; Rastelli, E.; Lo Martire, M.; Coral, C.; Greco, S.; Danovaro, R. Impact of Mangrove Forests Degradation on Biodiversity and Ecosystem Functioning. Sci. Rep. 2018, 8, 13298. [Google Scholar] [CrossRef] [PubMed]
- Holguin, G.; Vazquez, P.; Bashan, Y. The Role of Sediment Microorganisms in the Productivity, Conservation, and Rehabilitation of Mangrove Ecosystems: An Overview. Biol. Fertil. Soils 2001, 33, 265–278. [Google Scholar] [CrossRef]
- United Arab Emirates Ministry of Climate Change and Environment. Available online: https://www.moccae.gov.ae/assets/download/98f3baf3/UAEMangroves-Mooreetal.2013.pdf.aspx?view=true (accessed on 2 October 2019).
- Polidoro, B.A.; Carpenter, K.E.; Collins, L.; Duke, N.C.; Ellison, A.M.; Ellison, J.C.; Farnsworth, E.J.; Fernando, E.S.; Kathiresan, K.; Koedam, N.E.; et al. The Loss of Species: Mangrove Extinction Risk and Geographic Areas of Global Concern. PLoS ONE 2010, 5, e10095. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.; Losada, I.J.; Hendriks, I.; Mazarrasa, I.; Marba, N. The Role of Coastal Plant Communities for Climate Change Mitigation and Adaptation. Nat. Clim. Chang. 2013, 3, 961–968. [Google Scholar] [CrossRef]
- Kandasamy, K.; Rajendran, N. Mangrove Ecosystems of the Indian Ocean Region. Indian J. Mar. Sci. 2005, 34, 104–113. [Google Scholar]
- Spalding, M.; Kainuma, M.; Collins, L. World Atlas of Mangroves, 1st ed.; Earthscan: London, UK, 2010. [Google Scholar]
- Moore, G. Distribution, Pore-Water Chemistry, and Stand Characteristics of the Mangroves of the United Arab Emirates. J. Coast. Res. 2015, 31, 957–963. [Google Scholar] [CrossRef]
- Loughland, R.A.; Saenger, P.; Luker, G.; Siddiqui, K.; Saji, B.; Belt, M.; Crawford, K. Changes in the Coastal Zone of Abu Dhabi Determined Using Satellite Imagery (1972–2003). Aquat. Ecosyst. Health Manag. 2007, 10, 301–308. [Google Scholar] [CrossRef]
- Ward, R.D.; Friess, D.A.; Day, R.H.; MacKenzie, R.A. Impacts of Climate Change on Mangrove Ecosystems: A Region by Region Overview. Ecosyst. Health Sustain. 2016, 2. [Google Scholar] [CrossRef]
- Wabnitz, C.C.C.; Lam, V.W.Y.; Reygondeau, G.; Teh, L.C.L.; Al-Abdulrazzak, D.; Khalfallah, M.; Pauly, D.; Palomares, M.L.D.; Zeller, D.; Cheung, W.W.L. Climate Change Impacts on Marine Biodiversity, Fisheries and Society in the Arabian Gulf. PLoS ONE 2018, 13, e0194537. [Google Scholar] [CrossRef]
- Dodd, R.S.; Blasco, F.; Rafii, Z.A.; Torquebiau, E. Mangroves of the United Arab Emirates: Ecotypic Diversity in Cuticular Waxes at the Bioclimatic Extreme. Aquat. Bot. 1999, 63, 291–304. [Google Scholar] [CrossRef]
- Al Habshi, A.; Youssef, T.; Aizpuru, M.; Blasco, F. New Mangrove Ecosystem Data along the UAE Coast Using Remote Sensing. Aquat. Ecosyst. Health Manag. 2007, 10, 309–319. [Google Scholar] [CrossRef]
- Boer, B.; Lieth, H. Halophytes for Seawater Irrigation in the Arabian Peninsula-a Review. In Halophyte Crop Development for Different Climates, Ecological and Eco-Physiological Contributions; Lieth, H., Hamdy, A., Koyro, H.W., Moschenko, M., Eds.; University of Paderborn: Paderborn, Germany, 1999. [Google Scholar]
- Almahasheer, H. High Levels of Heavy Metals in Western Arabian Gulf Mangrove Soils. Mol. Biol. Rep. 2019, 46. [Google Scholar] [CrossRef] [PubMed]
- Shriadah, M.A. Metals Pollution in Marine Sediments of the United Arab Emirates Creeks along the Arabian Gulf Shoreline. Bull. Environ. Contam. Toxicol. 1998, 60, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Muzaffar, S.B.; Whelan, R.; Clarke, C.; Gubiani, R.; Benjamin, S. Breeding Population Biology in Socotra Cormorants (Phalacrocorax Nigrogularis) in the United Arab Emirates. Waterbirds 2017, 40, 1–10. [Google Scholar] [CrossRef]
- Battarbee, R.W.; Jones, V.J.; Flower, R.J.; Cameron, N.G.; Bennion, H.; Carvalho, L. Tracking Environmental Change Using Lake Sediments. In Tracking Environmental Change Using Lake Sediments: Volume 3: Terrestrial, Algal and Siliceous Indicators; Smol, J.P., Birks, H.J., Last, W.M., Eds.; Springer: Berlin, Germany, 2001; pp. 155–202. [Google Scholar] [CrossRef]
- Al-Yamani, F.; Saburova, M. Illustrated Guide on the Benthic Diatoms of Kuwait’s Marine Environment; Kuwait Institute for Scientific Research: Chestnut Hill, MA, USA, 2011. [Google Scholar]
- Desrosiers, C.; Leflaive, J.; Eulin, A.; Ten-Hage, L. Bioindicators in Marine Waters: Benthic Diatoms as a Tool to Assess Water Quality from Eutrophic to Oligotrophic Coastal Ecosystems. Ecol. Indic. 2013, 32, 25–34. [Google Scholar] [CrossRef]
- Al-Handal, A.Y. Littoral Diatoms from the Shatt Al-Arab Estuary, North West Arabian Gulf. Cryptogam. Algol. 2009, 30, 153–183. [Google Scholar]
- Nabelkova, J.; Komínková, D. Trace Metals in the Bed Sediment of Small Urban Streams. Open Environ. Biol. Monit. J. 2012, 5, 48–55. [Google Scholar] [CrossRef]
- Al Obaidy, A.H.; Al-Janabi, Z.; Al-Mashhady, A. Distribution of some heavy metals in sediments and water in Tigris River. J. Glob. Ecol. Environ. 2016, 4, 140–146. [Google Scholar]
- Clements, W.; Carlisle, D.; Lazorchak, J.; Johnson, P. Heavy Metals Structure Benthic Communities in Colorado Mountain Streams. Ecol. Appl. 2000, 10. [Google Scholar] [CrossRef]
- Barnthouse, L.W.; DeAngelis, D.L.; Gardner, R.H.; Ońeill, R.V.; Suter, G.V.; Vaughan, D.S. Methodology for Environmental Risk Analysis; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1986.
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using CANOCO 5, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar] [CrossRef]
- Hill, M. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Birks, H.J.B.; Line, J.M. The Use of Rarefaction Analysis for Estimating Palynological Richness from Quaternary Pollen-Analytical Data. Holocene 1992, 2, 1–10. [Google Scholar] [CrossRef]
- Juggins, S. Software for Ecological and Palaeoecological Data Analysis and Visualisation; Newcastle University: Newcastle upon Tyne, UK, 2005. [Google Scholar]
- Samara, F.; Ali, T.; Haverila, J.; Knuteson, S. Investigation of Fecal Contamination of Groundwater and Surface Water at Al Wasit Nature Reserve, Sharjah, United Arab Emirates. Asian J. Microbiol. Biotechnol. Environ. Sci. 2016, 18, 35–45. [Google Scholar]
- Shriadah, M.M.A. Heavy Metals in Mangrove Sediments of the United ARAB Emirates Shoreline (Arabian Gulf). Water Air Soil Pollut. 1999, 116, 523–534. [Google Scholar] [CrossRef]
- Jitthaisong, O.; Dhanmanonda, P.; Chunkao, K.; Teejuntuk, S. Water Quality from Mangrove Forest: The King’s Royally Initiated Laem Phak Bia Environmental Research and Development Project, Phetchaburi Province, Thailand. Mod. Appl. Sci. 2012, 6. [Google Scholar] [CrossRef]
- Radojevic, M.; Bashkin, V. Practical Environmental Analysis; The Royal Society of Chemistry: London, UK, 2006. [Google Scholar] [CrossRef]
- Samara, F.; Elsayed, Y.; Soghomonian, B.; Knuteson, S. Chemical and Biological Assessment of Sediments and Water of Khalid Khor, Sharjah, United Arab Emirates. Mar. Pollut. Bull. 2016, 111. [Google Scholar] [CrossRef]
- Hossain, M.D.; Nuruddin, A.A. Soil and Mangrove: A Review. J. Environ. Sci. Technol. 2016, 9, 198–207. [Google Scholar] [CrossRef]
- Ish-Shalom-Gordon, N.; Dubinsky, Z. Diurnal Pattern of Salt Secretion in Leaves of the Black Mangrove, Avicennia Marina, on the Sinai Coast of the Red Sea. Pac. Sci. 1993, 47, 51–58. [Google Scholar]
- Emara, H.I. Total Organic Carbon Content in the Waters of the Arabian Gulf. Environ. Int. 1998, 24, 97–103. [Google Scholar] [CrossRef]
- Al-Said, T.; Naqvi, S.W.A.; Al-Yamani, F.; Goncharov, A.; Fernandes, L. High Total Organic Carbon in Surface Waters of the Northern Arabian Gulf: Implications for the Oxygen Minimum Zone of the Arabian Sea. Mar. Pollut. Bull. 2018, 129, 35–42. [Google Scholar] [CrossRef]
- Emara, H.I. Nutrient Salts, Inorganic and Organic Carbon Contents in the Waters of the Persian Gulf and the Gulf of Oman. J. Persian Gulf 2010, 1, 33–44. [Google Scholar]
- Sippo, J.Z.; Maher, D.T.; Tait, D.R.; Ruiz-Halpern, S.; Sanders, C.J.; Santos, I.R. Mangrove Outwelling Is a Significant Source of Oceanic Exchangeable Organic Carbon. Limnol. Oceanogr. Lett. 2017, 2, 1–8. [Google Scholar] [CrossRef]
- Zeri, C.; Kontoyiannis, H.; Giannakourou, A. Distribution, Fluxes and Bacterial Consumption of Total Organic Carbon in a Populated Mediterranean Gulf. Cont. Shelf Res. 2009, 29, 886–895. [Google Scholar] [CrossRef]
- Rajagopal, K. Analysis of Heavy Metals Accumulation in Mangroves and Associated Mangroves Species of Ennore Mangrove Ecosystem, East Coast India. Indian J. Sci. Technol. 2016, 9, 1–11. [Google Scholar] [CrossRef]
- Trakhees. Available online: https://www.trakhees.ae/en/ehs/env/Documents/Regulations/RegulationEN-5.0,WaterEnvironment.pdf (accessed on 20 May 2019).
- Chapman, D.V.; World Health Organization; United Nations Environment Programme. Water Quality Assessments: A Guide to the Use of Biota, Sediments and Water in Environmental Monitoring/Edited by Deborah Chapman, 2nd ed.; E & FN Spon: London, UK, 1996. [Google Scholar]
- U.S. Environmental Protection Agency (US EPA). National Recommended Water Quality Criteria. US EPA 40 CFR Part 1; U.S. Environmental Protection Agency (US EPA): Washington, DC, USA, 2004.
- Sharma, R.; Agrawal, M. Biological Effects of Heavy Metals: An Overview. J. Environ. Biol. 2005, 26, 301–313. [Google Scholar] [PubMed]
- U.S. Environmental Protection Agency (US EPA). Guidelines for the Pollution Classification of Great Lakes Harbor Sediments; U.S. Environmental Protection Agency (US EPA): Washington, DC, USA, 1977.
- Eggleton, J.; Thomas, K.V. A Review of Factors Affecting the Release and Bioavailability of Contaminants during Sediment Disturbance Events. Environ. Int. 2004, 30, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.; Batley, G. Disturbances to Metal Partitioning during Toxicity Testing Fe(II)-Rich Estuarine Porewaters and Whole-Sediments. Environ. Toxicol. Chem. 2003, 22, 424–432. [Google Scholar] [CrossRef]
- Masoud, M.; Elewa, A.A.; Ali, A.E.; Mohamed, E. Distribution of Some Metal Concentration in Water and Sediments of Lake Edku, Egypt. Bull. Chem. Technol. Maced. 2005, 24, 21–34. [Google Scholar]
- Gonneea, M.E.; Paytan, A.; Herrera-Silveira, J.A. Tracing Organic Matter Sources and Carbon Burial in Mangrove Sediments over the Past 160 Years. Estuar. Coast. Shelf Sci. 2004, 61, 211–227. [Google Scholar] [CrossRef]
- Hinokidani, K.; Nakanishi, Y. Dissolved Iron Elution from Mangrove Ecosystem Associated with Polyphenols and a Herbivorous Snail. Ecol. Evol. 2019, 9, 6772–6784. [Google Scholar] [CrossRef]
- Al-Yamani, F.Y.; Bishop, J.; Ramandhan, E.; Al-Husaini, M.; Al-Ghadban, A.N. Oceonagraphic Atlas of Kuwait’s Waters; Kuwait Institute for Scientific Research: Kuwait City, Kuwait, 2004. [Google Scholar]
- Al-Harbi, S. Epiphytic Microalgal Species Composition and Dynamics on Host Green Seaweeds (Ulvaphyceae) on the Northern Coast of Jeddah, Saudi Arabia. Environ. Ecol. Res. 2017, 5, 212–219. [Google Scholar] [CrossRef]
- Al-Handal, A.Y.; Dawood, S.A.; Wulff, A.; Abdulwahab, M.T. Epiphytic Diatoms of the Mesopotamian Wetland: Huwaiza Marsh, South Iraq. Diatom 2014, 30, 164–178. [Google Scholar] [CrossRef]
- Bouillon, S.; Dahdouh-Guebas, F.; Rao, A.V.V.S.; Koedam, N.; Dehairs, F. Sources of Organic Carbon in Mangrove Sediments: Variability and Possible Ecological Implications. Hydrobiologia 2003, 495, 33–39. [Google Scholar] [CrossRef]
- Sebastian, R.; Chacko, J. Distribution of Organic Carbon in Tropical Mangrove Sediments (Cochin, India). Int. J. Environ. Stud. 2006, 63, 303–311. [Google Scholar] [CrossRef]
- Sanders, C.J.; Smoak, J.M.; Waters, M.N.; Sanders, L.M.; Brandini, N.; Patchineelam, S.R. Organic Matter Content and Particle Size Modifications in Mangrove Sediments as Responses to Sea Level Rise. Mar. Environ. Res. 2012, 77, 150–155. [Google Scholar] [CrossRef]


| Location | Temperature (°C) | pH | DO (mg/L) | Conductivity (mS/cm) | TDS (ppt) | ORP | Salinity (ppt) | Water TOC (mg C/L) |
|---|---|---|---|---|---|---|---|---|
| L1 | 28.25 | 7.72 | 24.27 | 55.08 | 27.58 | 26.90 | 36.44 | 33.19 |
| L2 | 29.85 | 8.00 | 18.20 | 54.57 | 27.39 | 53.70 | 36.10 | 36.71 |
| L3 | 34.18 | 8.08 | 15.25 | 60.46 | 30.24 | 61.10 | 40.17 | 20.73 |
| L4 | 29.75 | 8.33 | 15.71 | 55.36 | 27.66 | 53.30 | 36.50 | 19.59 |
| L5 | 28.74 | 7.34 | 22.85 | 60.76 | 30.38 | 81.80 | 40.66 | 36.26 |
| L6 | 29.02 | 8.15 | 21.73 | 58.47 | 29.24 | 66.00 | 38.91 | 33.64 |
| L7 | 32.75 | 7.97 | 17.27 | 65.31 | 32.65 | 48.60 | 34.96 | 42.92 |
| L8 | 30.36 | 7.94 | 19.33 | 58.57 | 29.31 | 55.91 | 37.68 | 52.01 |
| Max | 34.18 | 8.33 | 24.27 | 65.31 | 32.65 | 81.80 | 40.66 | 52.01 |
| Min | 28.25 | 7.34 | 15.25 | 54.57 | 27.39 | 26.90 | 34.96 | 19.59 |
| Average | 30.36 | 7.94 | 19.33 | 58.57 | 29.31 | 55.91 | 37.68 | 34.38 |
| Range | 5.93 | 0.99 | 9.02 | 10.74 | 5.26 | 54.90 | 5.70 | 32.42 |
| St Dev | 2.06 | 0.30 | 3.33 | 3.63 | 1.80 | 15.61 | 2.05 | 10.69 |
| Water Metal Concentrations (mg/L) | |||||||||||
| Sites | Ba | Cd | Co | Cr | Cu | Mn | Ni | Pb | Zn | Al | Fe |
| L1 | 0.024 | ND | 0.000 | 0.077 | 0.001 | 0.011 | 0.014 | 0.011 | 2.729 | 0.080 | 0.054 |
| L2 | 0.021 | 0.001 | 0.007 | ND * | 0.024 | 0.004 | 0.005 | 0.013 | 2.688 | 0.015 | 0.024 |
| L3 | 0.016 | ND | 0.003 | ND | ND | 0.009 | 0.001 | ND | 3.003 | 0.025 | 0.022 |
| L4 | 0.016 | ND | 0.012 | ND | ND | 0.004 | 0.008 | 0.003 | 2.187 | 0.008 | 0.015 |
| L5 | 0.008 | ND | 0.007 | ND | ND | 0.005 | 0.019 | ND | 2.905 | 0.037 | 0.031 |
| L6 | 0.016 | 0.001 | 0.011 | ND | 0.001 | 0.003 | 0.009 | ND | 2.887 | 0.010 | 0.015 |
| L7 | 0.020 | 0.000 | 0.012 | ND | ND | 0.005 | ND | 0.006 | 3.091 | 0.015 | 0.031 |
| L8 | 0.018 | 0.000 | 0.009 | ND | ND | 0.001 | 0.016 | 0.004 | 3.495 | 0.023 | 0.031 |
| Max | 0.024 | 0.001 | 0.012 | 0.077 | 0.024 | 0.011 | 0.019 | 0.013 | 3.495 | 0.080 | 0.054 |
| Min | 0.008 | 0.000 | 0.000 | 0.001 | 0.001 | 0.001 | 0.003 | 2.187 | 0.008 | 0.015 | |
| Average | 0.017 | 0.001 | 0.008 | 0.009 | 0.005 | 0.010 | 0.007 | 2.873 | 0.027 | 0.028 | |
| Range | 0.016 | 0.001 | 0.012 | 0.023 | 0.010 | 0.018 | 0.010 | 1.308 | 0.072 | 0.039 | |
| St. Dev | 0.005 | 0.001 | 0.004 | 0.013 | 0.003 | 0.006 | 0.004 | 0.374 | 0.024 | 0.012 | |
| * ND-non-detectable | |||||||||||
| Sediment Metal Concentrations (mg/kg) | |||||||||||
| Sites | Ba | Cd | Co | Cr | Cu | Mn | Ni | Pb | Zn | Al | Fe |
| L1 | 11.72 | 0.08 | 1.25 | 44.59 | 0.42 | 33.89 | 177.42 | 0.40 | 17.21 | 11693.33 | 872.10 |
| L2 | 9.64 | 0.06 | 0.34 | 7.20 | 1.18 | 24.36 | 8.36 | 0.77 | 12.10 | 13393.51 | 955.43 |
| L3 | 13.32 | 0.02 | 0.75 | 12.41 | 3.71 | 94.95 | 9.30 | 1.90 | 27.66 | 24970.37 | 2223.76 |
| L4 | 20.54 | 0.02 | 0.86 | 20.40 | 2.69 | 171.38 | 13.77 | 1.43 | 37.26 | 40923.43 | 3527.65 |
| L5 | 20.61 | 0.16 | 1.09 | 17.56 | 3.11 | 169.82 | 29.60 | 2.36 | 36.56 | 3236.11 | 1018.99 |
| L6 | 13.50 | 0.11 | 0.75 | 13.00 | 2.50 | 110.17 | 17.71 | 1.31 | 27.79 | 2460.24 | 785.63 |
| L7 | 12.72 | 0.17 | 0.08 | 12.05 | 1.25 | 112.01 | 17.66 | 2.40 | 30.62 | 2483.39 | 738.79 |
| L8 | 13.88 | 0.00 | 0.63 | 11.23 | 1.03 | 82.49 | 11.16 | 3.53 | 26.02 | 2303.78 | 798.72 |
| Max | 20.61 | 0.17 | 1.25 | 44.59 | 3.71 | 171.38 | 177.42 | 3.53 | 37.26 | 40923.43 | 3527.65 |
| Min | 9.64 | 0.00 | 0.08 | 7.20 | 0.42 | 24.36 | 8.36 | 0.40 | 12.10 | 2303.78 | 738.79 |
| Average | 14.49 | 0.08 | 0.72 | 17.31 | 1.99 | 99.88 | 35.62 | 1.76 | 26.90 | 12683.02 | 1365.13 |
| Range | 10.97 | 0.16 | 1.17 | 37.39 | 3.29 | 147.02 | 169.06 | 3.14 | 25.16 | 38619.65 | 2788.86 |
| St. Dev | 3.98 | 0.10 | 0.40 | 11.70 | 1.20 | 54.30 | 57.69 | 1.00 | 8.70 | 13915.50 | 999.53 |
| Water (mg/L) | Ba | Cd | Co | Cr | Cu | Mn | Ni | Pb | Zn | Al | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dubai marine water [44] | - | 0.003 | - | 0.010 | 0.005 | - | - | 0.010 | 0.020 | 0.200 | - |
| Canada fisheries and aquatic life [45] | - | 0.0002–0.0018 | - | 0.002–0.02 | 0.002–0.004 | - | - | 0.001–0.007 | 0.030 | 0.005–0.1 | 0.300 |
| US EPA salt water [46] | - | 0.04–0.009 | - | 0.05–1.1 | 0.003–0.005 | - | 0.008–0.007 | 0.008–0.2 | 0.08–0.09 | - | - |
| India [43] | - | 6.82 | - | 10.24 | 2.94 | - | - | 18.12 | 2.00 | - | - |
| Average this study | 0.017 | 0.001 | 0.008 | 0.077 | 0.009 | 0.005 | 0.010 | 0.007 | 2.873 | 0.027 | 0.028 |
| Hazard Quotient (HQ) | 0.33 | 7.70 | 1.80 | 1.43 | 0.70 | 143.65 | 0.14 | 0.09 | |||
| Sediments (mg/kg) | Ba | Cd | Co | Cr | Cu | Mn | Ni | Pb | Zn | Al | Fe |
| Dubai Land [44] | - | 5.00 | - | 250.00 | 100.00 | 700.00 | - | 200.00 | 500.00 | - | - |
| US EPA Harbor sediments [48] | - | 6.00 | - | 25–75 | 25–50 | 300–500 | 20–50 | 40–60 | 90–200 | - | 1.7–2.5 |
| UAE mangroves [32] | - | 4.49 | 10.50 | 11.70 | 6.31 | 95.20 | 20.41 | 26.10 | 10.10 | - | - |
| Average this study | 14.49 | 0.08 | 0.72 | 17.31 | 1.99 | 99.88 | 35.62 | 1.76 | 26.90 | 12,683.02 | 1365.13 |
| Hazard Quotient (HQ) | 0.02 | 0.07 | 0.02 | 0.14 | 0.71 | 0.01 | 0.05 | 546.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samara, F.; Solovieva, N.; Ghalayini, T.; Nasrallah, Z.A.; Saburova, M. Assessment of the Environmental Status of the Mangrove Ecosystem in the United Arab Emirates. Water 2020, 12, 1623. https://doi.org/10.3390/w12061623
Samara F, Solovieva N, Ghalayini T, Nasrallah ZA, Saburova M. Assessment of the Environmental Status of the Mangrove Ecosystem in the United Arab Emirates. Water. 2020; 12(6):1623. https://doi.org/10.3390/w12061623
Chicago/Turabian StyleSamara, Fatin, Nadia Solovieva, Thouraya Ghalayini, Zaina Anwar Nasrallah, and Maria Saburova. 2020. "Assessment of the Environmental Status of the Mangrove Ecosystem in the United Arab Emirates" Water 12, no. 6: 1623. https://doi.org/10.3390/w12061623
APA StyleSamara, F., Solovieva, N., Ghalayini, T., Nasrallah, Z. A., & Saburova, M. (2020). Assessment of the Environmental Status of the Mangrove Ecosystem in the United Arab Emirates. Water, 12(6), 1623. https://doi.org/10.3390/w12061623





