Effects of Harmful Blooms of Large-Sized and Colonial Cyanobacteria on Aquatic Food Webs
Abstract
1. Introduction
2. The Cyanobacteria–Zooplankton Interface
2.1. Cyanobacterial Anti-Grazing Strategies
2.1.1. Size
2.1.2. Chemical Defense
2.1.3. Induction of Defenses
2.2. Cyanobacterial Anti-Grazing Strategies
2.2.1. Grazing Inhibition
2.2.2. Vital Rates of Zooplankton
2.2.3. Zooplankton Defense
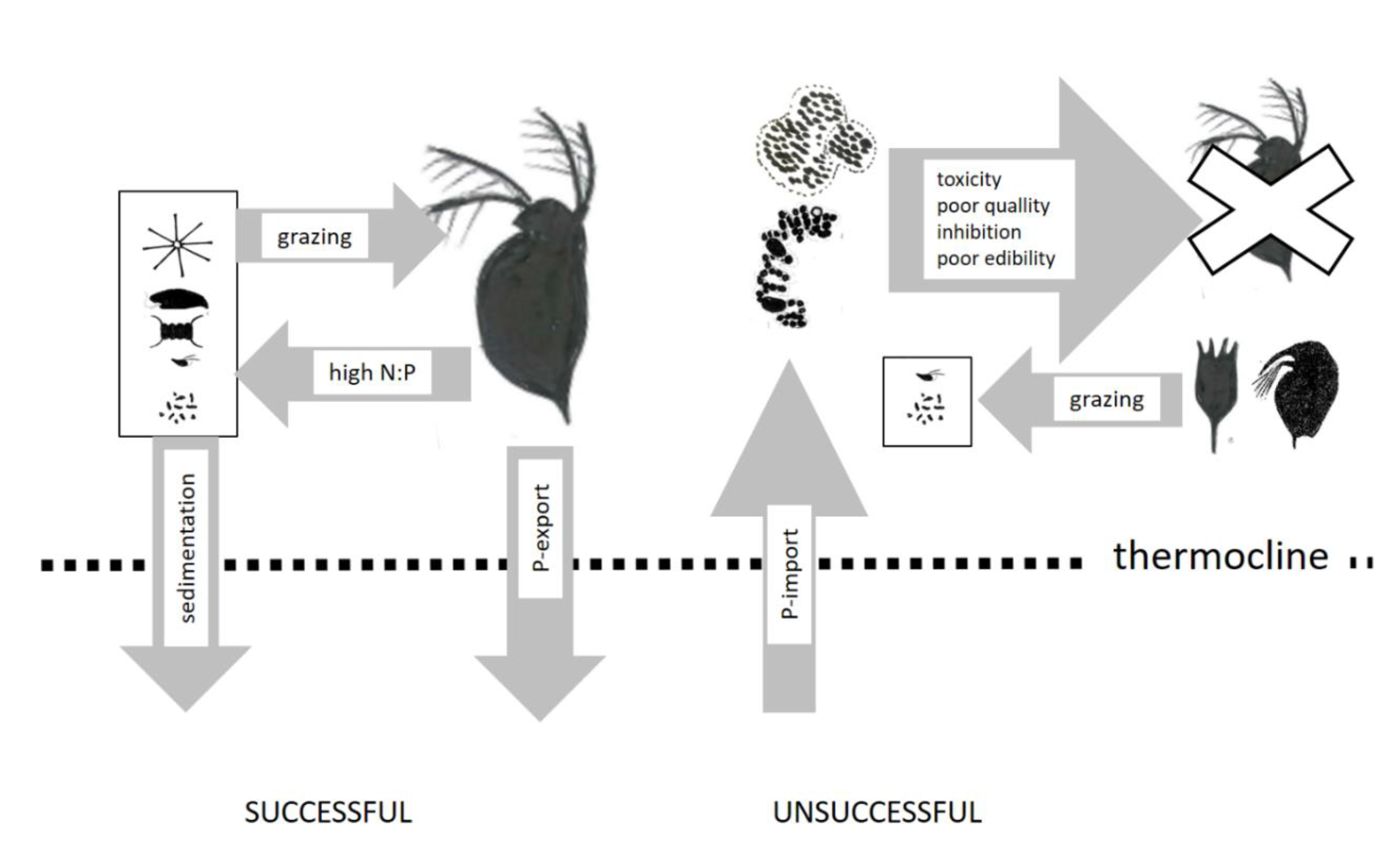
2.2.4. Shifts towards Smaller Zooplankton Species
3. The Cyanobacteria–Fish Interface
3.1. Fish Kills
3.2. Cascade Effects of Fish on Phytoplankton
3.3. Fish Feeding on Cyanobacteria
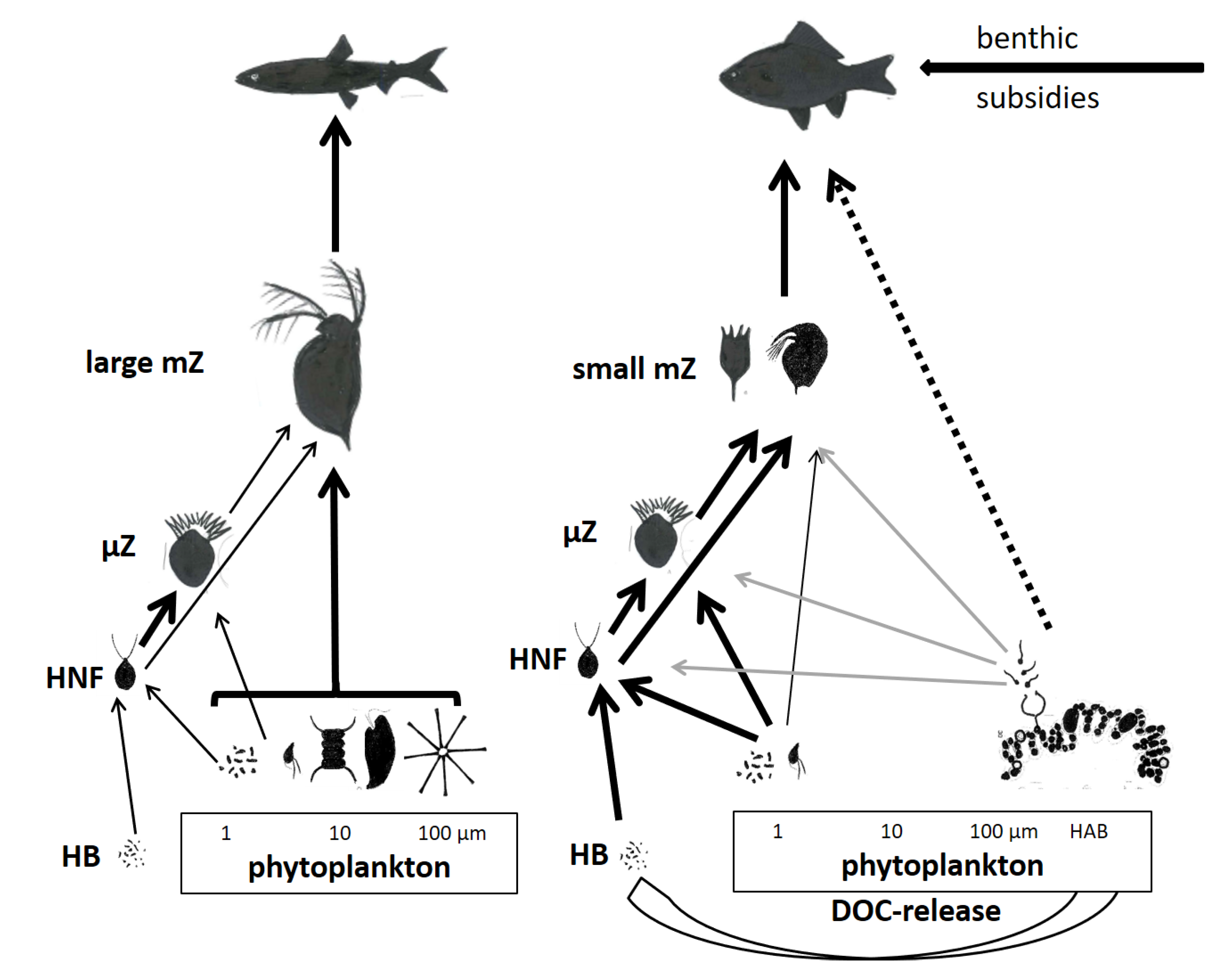
4. Food Web Structure and Production of Animal Trophic Levels
4.1. Food Web Structure
4.2. N-Fertilization of Food Webs
4.3. Impacts on an Animal Biomass and Production
4.3.1. Studies within Restricted Regions
4.3.2. Large Scale Surveys
5. Conclusions
- Filamentous and large colonial Cyanobacteria resist zooplankton grazing by size, toxicity and low nutritional values;
- Grazing resistance is not complete. Thus, cyanobacteria are not a complete dead end in the food web;
- There is an evolutionary arms race between cyanobacterial grazing resistance and zooplankton ability, to overcome grazing resistance;
- Cyanobacteria drive zooplankton composition towards species of smaller body size, thus reducing the potential for top-down control on phytoplankton;
- Top-down control of cyanobacterial blooms by large zooplankton is more effective in the preventing the onset of blooms than in controlling already-developed blooms;
- Daphnia. can reduce the competitive advantage for cyanobacteria by fecal sedimentation and increase the competitive advantage of nitrogen-fixing cyanobacteria by a reduction in N:P supply rates;
- Fish can be harmed by cyanobacterial toxins, harmful effects sometimes even leading to fish kills;
- However, coexistence of fish, often cyprinids, with cyanobacterial blooms is often observed;
- Cyanobacterial dominance in phytoplankton reduces the efficiency of matter and energy transfer from primary production via zooplankton to fish;
- Chytrid parasites might transfer part of primary production from cyanobacteria to mesozooplankton through zoospores ingested by zooplankton;
- Obligate and facultative filter-feeding fish have been found to ingest cyanobacteria;
- Some fish species (silver carp and tilapia) can exert top-down control on cyanobacteria;
- Some fish species can grow on a cyanobacterial diet;
- Further research is needed, to see whether this applies also to widespread bentho-pelagic fish like bream and roach;
- The probability of fish kills increases with increasing eutrophication and with increasing cyanobacterial and other harmful algal blooms;
- To date, there is no firm evidence that the ratio of fish production: to primary production declines with eutrophication or increased cyanobacterial biomass, as long as there are no fish kills. Multi-lake surveys with simultaneous measurements of primary production, phytoplankton and cyanobacterial biomass, zooplankton and fish production are needed.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moustaka-Gouni, M.; Sommer, U.; Katsiapi, M.; Vardaka, E. Monitoring of cyanobacteria for water quality: Doing the necessary right or wrong? Mar. Freshw. Res. 2019. [Google Scholar] [CrossRef]
- Moustaka-Gouni, M.; Sommer, U.; Economou-Amilli, A.; Arhonditsis, G.B.; Katsiapi, M.; Papastergiadou, E.; Kormas, K.A.; Vardaka, E.; Karayanni, H.; Papadimitriou, T. Implementation of the Water Framework Directive: Lessons learned and future perspectives for an ecologically meaningful classification based on phytoplankton of the status of Greek lakes, Mediterranean region. Environ. Manag. 2019, 64, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Ger, K.A.; Urrutia-Cordero, P.; Frost, P.C.; Hansson, L.A.; Sarnelle, O.; Wilson, A.E.; Lurling, M. The interaction between cyanobacteria and zooplankton in a more eutrophic world. Harmful Algae 2016, 54, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Fulton, R.S., III; Paerl, H.W. Effects of colonial morphology on zooplankton utilization of algal resource during blue-green algal (Microcystes aeruginosa) blooms. Limnol. Oceanogr. 1987, 32, 634–644. [Google Scholar] [CrossRef]
- Lehman, J.T. Selective herbivory and it role in the evolution of phytoplankton growth strategies. In Growth and Reproductive Strategies of Freshwater Phytoplankton; Sandgren, C.D., Ed.; Cambridge University Press: Cambridge, UK, 1988; pp. 369–387. [Google Scholar]
- Epp, G.T. Grazing on filamentous cyanobacteria by Daphnia pulicaria. Limnol. Oceanogr. 1996, 41, 560–567. [Google Scholar] [CrossRef]
- Burns, C.W. The relationship between body size of filter feeding Cladocera and the maximal size of particles ingested. Limnol. Oceanogr. 1968, 13, 675–678. [Google Scholar] [CrossRef]
- Gliwicz, Z.M. Food size selection and seasonal succession of filter feeding zooplankton in an eutrophic lake. Ekologia Polska 1977, 25, 179–225. [Google Scholar]
- Sommer, U.; Charalampous, E.; Genitsaris, S.; Moustaka-Gouni, M. Costs, benefits and taxonomic distribution of phytoplankton body size. J. Plankton Res. 2017, 39, 494–508. [Google Scholar] [CrossRef]
- Sommer, F.; Hansen, T.; Sommer, U. Transfer of diazotrophic nitrogen to mesozooplankton in Kiel Fjord, Baltic Sea: A mesocosm study. Mar. Ecol. Progr. Ser. 2006, 324, 105–112. [Google Scholar] [CrossRef]
- Woodland, R.J.; Holland, D.P.; Beardall, J.; Smith, J.; Scicluna, T.; Cook, P.L.M. Assimilation of diazotrophic nitrogen into pelagic food webs. PLoS ONE 2013, 6, e67588. [Google Scholar] [CrossRef]
- Engstöm, K.; Koski, M.; Viitasalo, M.; Reinikainen, M.; Pepka, S.; Sivonen, K. Feeding interactions of the copepods Eurytemora affinis and Acartia bifilosa with the cyanobacteria Nodularia sp. J. Plankton Res. 2000, 22, 1403–1409. [Google Scholar] [CrossRef]
- Motwani, N.H.; Duberg, J.; Sveden, J.B.; Gorokhova, E. Grazing on cyanobacteria and transfer of diazotrophic nitrogen to zooplankton in the Baltic Sea. Limnol. Oceanogr. 2018, 63, 672–686. [Google Scholar] [CrossRef]
- Canter, H.M.; Heaney, S.I.; Lund, J.W. The ecological significance of grazing on planktonic populations of cyanobacteria by the ciliate Nassula. New Phytol. 1990, 114, 247–263. [Google Scholar] [CrossRef]
- Lampert, W. Toxicity of the blue-green Microcystis aeruginosa: Effective defence mechanism against grazing pressure by Daphnia. Verh. Int. Verein. Limnol. 1981, 21, 1436–1440. [Google Scholar] [CrossRef]
- Rantala, A.; Fewer, D.P.; Hisbergues, M.; Rouhiainen, L.; Vaitomaa, J.; Borner, T.; Sivonen, K. Phylogenetic evidence for the early evolution of microcystin synthesis. Proc. Natl. Acad. Sci. USA 2004, 101, 568–573. [Google Scholar] [CrossRef] [PubMed]
- DeMott, W.R. Discrimination between algae and artificial particles by freshwater and marine copepods. Limnol. Oceanogr. 1988, 33, 397–408. [Google Scholar] [CrossRef]
- DeMott, W.R.; Moxter, F. Foraging on cyanobacteria by copepods: Responses to chemical defenses and resource abundance. Ecology 1991, 72, 1820–1834. [Google Scholar] [CrossRef]
- Haney, J.F.; Forsyth, D.J.; James, M.R. Inhibition of zooplankton filtering rates by dissolved inhibitors produced aby naturally occurring cyanobacteria. Arch. Hydrobiol. 1994, 132, 1–13. [Google Scholar]
- Rohrlack, T.; Dittman, G.; Henning, M.; Börner, T.; Kohl, J.G. Role of microcystins in poisoning and food ingestion inhibition of Daphnia galeata caused by the cyanobacterium Microcystis aeruginosa. Appl. Environ. Microbiol. 1999, 65, 737–739. [Google Scholar] [CrossRef] [PubMed]
- Lürling, M.; van der Grinten, E. Life-history characteristics of Daphnia exposed to dissolved microcystin-LR and to the cyanobacterium Microcytis aeruginosa with and without microcystins. Environ. Toxicol. Chem. 2003, 22, 1281–1287. [Google Scholar] [CrossRef]
- Ferrao-Filho, A.d.S.; Kozlowsky-Suzuki, B. Cyanotoxins: Bioaccumulation and Effects on Aquatic Animals. Mar. Drugs 2011, 9, 2729–2772. [Google Scholar] [CrossRef] [PubMed]
- Martin-Creuzburg, D.; von Elert, E.; Hoffmann, K. Nutritional constraints at the cyanobacteria-Daphnia interface: The role of sterols. Limnol. Oceanogr. 2008, 53, 456–468. [Google Scholar] [CrossRef]
- Müller-Navarra, D.C.; Brett, M.T.; Liston, A.M.; Goldman, C.R. A highly unsaturated fatty acid predicts carbon transfer between primary producers and consumers. Nature 2000, 403, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Von Elert, E.; Martin-Creuzburg, D.; Le Coz, J.R. Absence of sterols constrains carbon transfer between cyanobacteria and a freshwater herbivore (Daphnia galeata). Proc. R. Soc. B 2003, 270, 1209–1214. [Google Scholar] [CrossRef]
- Tollrian, R.; Harvell, C.D. The evolution of inducible defenses: Current ideas. In The Ecology and Evolution of Inducible Defenses; Tollrian, R., Harvell, C.D., Eds.; Princeton University Press: Princeton, NJ, USA, 1999; pp. 306–321. [Google Scholar]
- Lynch, M. Aphanizomenon blooms: Alternate control and cultivation by Daphnia pulex. Am. Soc. Limnol. Oceanog. Spec. Symp. 1980, 299–304. [Google Scholar]
- Wejnerowski, L.; Cerbin, S.; Wojciechowicz, M.; Jurczak, T.; Glama, M.; Meriluoto, J.; Dziuba, M. Effects of Daphnia exudates and sodium octyl sulphates on filament morphology and cell wall thickness of Aphanizomenon gracile (Nostocales), Cylindrospermopsis raciborskii (Nostocales) and Planktothrix agardhii (Oscillatoriales). Eur. J. Phycol. 2018, 53, 280–289. [Google Scholar] [CrossRef]
- Van Gremberghe, I.; Vanormelingen, P.; Vanelslander, B.; Van der Gucht, K.; D’Hont, S.; De Meester, L.; Vyverman, W. Genotype-depenndent interactions among sympatric Microcystis strains mediated by Daphnia grazing. Oikos 2009, 118, 1647–1658. [Google Scholar] [CrossRef]
- Yang, Z.; Kong, F.X. Formation of large colonies: A defense mechanism of Microcystis aeruginosa under continuous grazing pressure by flagellate Ochromonas sp. J. Limnol. 2012, 71, 61–66. [Google Scholar] [CrossRef]
- Lundgren, V.; Graneli, E.; Pflugmacher, S. Influence of Acartia cf. bifilosa (Copepoda) on morphology and toxicity of Nodularia spumigena (Cyanophyceae). Harmful Algae 2012, 18, 35–46. [Google Scholar] [CrossRef]
- Jang, M.H.; Ha, K.; Joo, G.J.; Takamura, N. Toxin production of cyanobacteria is increased by exposure to zooplankton. Freshw. Biol. 2003, 48, 1540–1550. [Google Scholar] [CrossRef]
- Gorokhova, E.; Engström-Öst, J. Toxin concentration in Nodularia spumigena is modulated by mesozooplankton grazers. J. Plankton Res. 2009, 31, 1235–1247. [Google Scholar] [CrossRef][Green Version]
- Engström-Öst, J.; Hogfors, H.; El-Shehawy, R.; De Stasio, B.; Vehmaa, A.; Gorokhova, E. Toxin-producing cyanobacterium Nodulariaspumigena, potential competitors and grazers: Testing mechanisms of reciprocal interactions. Aquat. Microb. Ecol. 2011, 62, 39–48. [Google Scholar] [CrossRef]
- Sadler, T.; von Elert, E. Dietary exposure of Daphnia to microcystins: No in vivo relevance of biotransformation. Aquat. Toxicol. 2014, 150, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Sadler, T.; von Elert, E. Physiological interaction of Daphnia and Microcystis with regard to cyanobacteria secondary metabolites. Aquat. Toxicol. 2014, 156, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Harke, M.J.; Jankowiak, J.G.; Morrell, B.K.; Gobler, C.J. Transcriptomic responses in the bloom-forming cyanobacterium Microcystis induced during exposure to zooplankton. Appl. Environ. Microbiol. 2017, 83, e02832. [Google Scholar] [CrossRef] [PubMed]
- Gliwicz, Z.M.; Siedlar, E. Food size limitation and algae interfering with food collection in Daphnia. Arch. Hydrobiol. 1980, 88, 155–177. [Google Scholar]
- Gilbert, J.J.; Durand, M.W. Effect of Anabaena flos-aquae on the abilities of Daphnia and Keratella to feed and reproduce on unicellular algae. Freshw. Biol. 1990, 24, 577–596. [Google Scholar] [CrossRef]
- Davis, T.W.; Koch, F.; Marcoval, M.A.; Wilhelm, S.W.; Gobler, C.J. Mesozooplankton and microzooplankton grazing during cyanobacterial blooms in the western basin of Lake Erie. Harmful Algae 2012, 15, 26–35. [Google Scholar] [CrossRef]
- Sellner, K.G.; Olson, M.M.; Kononen, K. Copepod grazing in a summer cyanobacteria bloom in the Gulf of Finland. Hydrobiologia 1994, 292/293, 249–254. [Google Scholar] [CrossRef]
- Gilbert, J.J. Different effects of Anabaena affinis on cladocerans and rotifers: Mechanisms and implication. Ecology 1990, 71, 1727–1740. [Google Scholar] [CrossRef]
- Gliwicz, Z.M. Daphnia growth at different concentration of blue-green filament. Arch. Hydrobiol. 1990, 120, 51–65. [Google Scholar]
- Gliwicz, Z.M. Food thresholds and body size in cladocerans. Nature 1990, 343, 638–640. [Google Scholar] [CrossRef]
- Gliwicz, Z.M.; Lampert, W. Food thresholds in Daphnia species in the absence and presence of blue-green filaments. Ecology 1990, 71, 691–702. [Google Scholar] [CrossRef]
- DeMott, W.R.; Zhang, Q.-X.; Carmichael, W. Effects of toxic cyanobacteria and purified toxins on the survival and feeding of a copepod and three species of Daphnia. Limnol. Oceanogr. 1991, 36, 1346–1357. [Google Scholar] [CrossRef]
- Rohrlack, T.; Christoffersen, K.; Dittman, E.; Nogueira, I.; Vasconcelos, V.; Borner, T. Ingestiion of microcystins by Daphnia: Intestinal uptake and toxic effects. Limnol. Oceanogr. 2005, 50, 440–448. [Google Scholar] [CrossRef]
- Wilson, A.E.; Hay, M.E. A direct test of cyanobacterial defense: Variable effects of microcystin-treated food on two Daphnia pulicaria clones. Limnol. Oceanogr. 2007, 52, 1467–1479. [Google Scholar] [CrossRef]
- Šulčius, S.; Montvydienė, D.; Mazur-Marzec, M.; Kasperovičienė, J.; Rulevičius, R.; Cibulskaitė, Z. The profound effect of harmful cyanobacterial blooms: From food-weband management perspectives. Sci. Total Environ. 2017, 609, 1443–1450. [Google Scholar] [CrossRef]
- Benndorf, J.; Henning, M. Daphnia and toxic blooms of Microcystis aeruginosa in Bautzen Reservoir (GDR). Int. Rev. Hydrobiol. 1989, 74, 233–248. [Google Scholar] [CrossRef]
- Ger, K.A.; Teh, S.J.; Goldman, C.R. Microcystin-LR toxicity on dominant copepods Eurytemora affinis and Pseudodiaptomus forbesi of the upper San Francisco Estuary. Sci. Total Environ. 2009, 407, 4852–4857. [Google Scholar] [CrossRef]
- Gustafsson, S.; Hansson, L.A. Development of tolerance against toxic cyanobacteria in Daphnia. Aquat. Ecol. 2004, 38, 37–44. [Google Scholar] [CrossRef]
- Gustafsson, S.; Rengefors, K.; Hansson, L.A. Increased consumer fitness following transfer of toxin tolerance to offspring via maternal effects. Ecology 2005, 86, 2561–2567. [Google Scholar] [CrossRef]
- Hairston, N.G.; Holtmeier, C.L.; Lampert, W.; Weider, L.J.; Post, D.M.; Fischer, J.M.; Caceres, C.E.; Fox, J.A.; Gaedke, U. Natural selection for grazer resistance to toxic cyanobacteria: Evolution of phenotypic plasticity? Evolution 2001, 55, 2203–2214. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S.; Du, J.J.; Lin, H.; Kong, X.S.; Sun, S.C.; Tian, X.J. Understanding interactive inducible defenses of Daphnia and its phytoplankton prey. Harmful Algae 2017, 66, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Hairston, N.G.; Lampert, W.; Caceres, C.E.; Holtmeier, C.L.; Weider, L.J.; Gaedke, U.; Fischer, J.M.; Fox, J.A.; Post, D.M. Lake ecosystems—Rapid evolution revealed by dormant eggs. Nature 1991, 401, 446. [Google Scholar] [CrossRef]
- Medina, M.H.; Correa, J.A.; Barata, C. Micro-evolution due to pollution: Possible consequences for ecosystem responses to toxic stress. Chemosphere 2007, 67, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Haney, J.F.; Lampert, W. Spatial avoidance of Microcystis aeruginosa by Daphnia: Fitness consequences and evolutionary implications. Limnol. Oceanogr. 2013, 58, 2122–2132. [Google Scholar] [CrossRef]
- Chislock, M.F.; Sarnelle, O.; Olsen, B.K.; Doster, E.; Wilson, A.E. Large effects of consumer offense on ecosystem structure and function. Ecology 2013, 94, 2375–2380. [Google Scholar] [CrossRef]
- Sommer, U.; Gliwicz, Z.M.; Lampert, W.; Duncan, A. The PEG model of seasonal succession of planktonic events in fresh waters. Arch. Hydrobiol. 1986, 106, 433–471. [Google Scholar]
- DeMott, W.R.; Gulati, R.D.; Van Donk, E. Daphnia food limitation in three hypereutrophic Dutch lakes: Evidence for exclusion of large-bodied species by interfering filaments of cyanobacteria. Limnol. Oceanogr. 2001, 46, 2054–2060. [Google Scholar] [CrossRef]
- Hansson, L.A.; Gustafsson, S.; Rengefors, K.; Bomark, L. Cyanobacterial chemical warfare affects zooplankton community composition. Freshw. Biol. 2007, 52, 1290–1301. [Google Scholar] [CrossRef]
- Stamou, G.; Katsiapi, M.; Moustaka-Gouni, M.; Michaloudi, E. Grazing potential—A functional plankton food web metric for ecological water quality assessment in Mediterranean lakes. Water 2019, 11, 1274. [Google Scholar] [CrossRef]
- Moustaka-Gouni, M.; Vardaka, E.; Michaloudi, E.; Kormas, K.A.; Tryfon, E.; Mihalatou, H.; Gkelis, S.; Lanaras, T. Plankton food web structure in a eutrophic polymictic lake with a history of toxic cyanobacterial blooms. Limnol. Oceanog. 2006, 51, 715–727. [Google Scholar] [CrossRef]
- Leonard, J.A.; Paerl, H.W. Zooplankton community structure, micro-zooplankton grazing impact, and seston energy content in the St. Johns river system, Florida as influenced by the toxic cyanobacterium Cylindrospermopsis raciborskii. Hydrobiologia 2005, 537, 89–97. [Google Scholar] [CrossRef]
- Christoffersen, K.; Riemann, B.; Hansen, L.R.; Klysner, A.; Sørensen, H.B. Quantitative importance of the microbial loop in a eutrophic lake during a bloom of cyanobacteria. Microb. Ecol. 1990, 20, 253–272. [Google Scholar] [CrossRef] [PubMed]
- Hrbáček, J. Species composition and the amount of zooplankton in relation to fish stock. ČSAV 1962, 72, 1–116. [Google Scholar]
- Brooks, J.L.; Dodson, S.I. Predation, body size and composition of plankton. Science 1965, 150, 28–35. [Google Scholar] [CrossRef]
- Chorus, I. Effects of Microcystis spp. and Selected Cyanotoxins on Freshwater Organisms. In Cyanotoxins; Chorus, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 239–280. [Google Scholar]
- Ernst, B.; Hoeger, S.J.; O’Brien, E.; Dietrich, D.R. Oral toxicity of the microcystin-containing cyanobacterium Planktothrix rubescens in European whitefish (Coregonus lavaretus). Aquat. Toxicol. 2006, 79, 31–40. [Google Scholar] [CrossRef]
- Malbrouck, C.; Kestemont, P. Effects of microcystins on fish. Environ. Toxicol. Chem. 2006, 26, 957–966. [Google Scholar] [CrossRef]
- Svirčev, Z.; Lalić, D.; Savić, G.B.; Tokodi, N.; Backović, D.D.; Chen, L.; Meriluoto, J.; Cood, J.A. Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Arch. Toxicol. 2019, 93, 2429–2481. [Google Scholar] [CrossRef]
- Rodger, H.D.; Turnbull, T.; Edwards, C.; Codd., G.A. Cyanobacterial (blue-green-algal) bloom associated pathology in brown trout, Salmo trutta L., in Loch Leven, Scotland. J. Fish Dis. 1994, 17, 177–181. [Google Scholar] [CrossRef]
- Kankaanpää, H.T.; Sipia, V.O.; Kuparinen, J.S.; Ott, J.L.; Carmichael, W.W. Nodularin analyses and toxicity of a Nodulariaspumigena (Nostocales, Cyanobacteria) water-bloom in the western Gulf of Finland, Baltic Sea, in August 1999. Phyciologia 2001, 40, 268–274. [Google Scholar] [CrossRef]
- Zimba, P.V.; Khoo, L.; Gaunt, P.S.; Brittain, S.; Carmichael, W.W. Confirmation of catfish, Ictalurus punctatus (Rafinesque), mortality from Microcystis toxins. J. Fish. Dis. 2001, 24, 41–47. [Google Scholar] [CrossRef]
- Starling, F.; Lazzaro, X.; Cavalcanti, C.; Moreira, R. Contribution of omnivorous tilapia to eutrophication of a shallow tropical reservoir: Evidence from a fish kill. Freshw. Biol. 2002, 47, 2443–2452. [Google Scholar] [CrossRef]
- Bürgi, H.; Stadelmann, P. Change of phytoplankton composition and biodiversity in Lake Sempach before and during restoration. Hydrobiologia 2002, 496, 33–48. [Google Scholar] [CrossRef]
- Moustaka-Gouni, M.; Hiskia, A.; Genitsaris, S.; Katsiapi, M.; Manolidi, K.; Zervou, S.K.; Christophoridis, C.; Triantis, T.M.; Kaloudis, T.; Orfanidis, S. First report of Aphanizomenon favaloroi occurrence in Europe associated with saxitoxins and a massive fish kill in Lake Vistonis, Greece. Mar. Freshw. Res. 2017, 68, 793–800. [Google Scholar] [CrossRef]
- Nõges, T.; Laugaste, R.; Loigu, E.; Nedogarko, I.; Skakalski, B.; Nõges, P. Is the destabilisation of Lake Peipsi ecosystem caused by increased phosphorus loading or decreased nitrogen loading? Water Sci. Technol. 2005, 51, 267–274. [Google Scholar] [CrossRef]
- Lindholm, T.; Ohman, P.; Kurki-Helasmo, K.; Kincaid, B.; Meriluoto, J. Toxic algae and fish mortality in a brackish-water lake in angstrom land, SW Finland. Hydrobiologia 1999, 397, 109–120. [Google Scholar] [CrossRef]
- Oikonomou, A.; Κatsiapi, M.; Karayanni, H.; Moustaka-Gouni, M.; Kormas, K. Plankton microorganisms coinciding with two consecutive mass fish kills in a newly reconstructed lake. Sci. World J. 2012, 2012, 504135. [Google Scholar] [CrossRef]
- Michaloudi, E.; Moustaka-Gouni, M.; Gkelis, S.; Pantelidakis, K. Plankton community structure during an ecosystem disruptive algal bloom of Prymnesium parvum. J. Plankton Res. 2009, 31, 301–309. [Google Scholar] [CrossRef]
- Papadimitriou, T.; Katsiapi, M.; Vlachopoulos, K.; Christopoulos, A.; Laspidou, C.; Moustaka-Gouni, M.; Kormas, K. Cyanotoxins as the “common suspects” for the Dalmatian pelican (Pelecanuscrispus) deaths in a Mediterranean reconstructed reservoir. Environ. Pollut. 2018, 234, 779–787. [Google Scholar] [CrossRef]
- McInnes, A.S.; Quigg, A. Near-Annual Fish Kills in Small Embayments: Casual vs. Causal Factors. J. Coast. Res. 2010, 26, 957–966. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Kitchell, J.F.; Hodgson, J.R. Cascading trophic interactions and lake productivity. Bioscience 1985, 35, 634–639. [Google Scholar] [CrossRef]
- Shapiro, J.; Wright, D.J. Lake restoration by biomanipulation: Rund Lake, Minnesota, the first two years. Freshw. Biol. 1984, 14, 371–382. [Google Scholar]
- Ekvall, M.K.; Urrutia-Cordero, P.; Hansson, L.A. Linking cascading effects of fish predation and zooplankton grazing to reduced cyanobacterial biomass and toxin levels following biomanipulation. PLoS ONE 2014, 9, e112956. [Google Scholar] [CrossRef] [PubMed]
- Urrutia-Cordero, P.; Ekvall, M.K.; Hansson, L.A. Responses of cyanobacteria to herbivorous zooplankton across predator regimes: Who mows the bloom? Freshw. Biol. 2015, 60, 960–972. [Google Scholar] [CrossRef]
- Rose, V.; Rollwagen-Bollens, G.; Bollens, S.M. Interactive effects of phosphorus and zooplankton grazing on cyanobacterial blooms in a shallow temperate lake. Hydrobiologia 2017, 788, 345–359. [Google Scholar] [CrossRef]
- Rollwagen-Bollens, G.; Lee, T.; Rose, V.; Bollens, S.M. Beyond eutrophication: Vancouver Lake, WA, USA as a model system for assessing multiple, interacting biotic and abiotic drivers of harmful cyanobacterial blooms. Water 2018, 10, 757. [Google Scholar] [CrossRef]
- Brett, M.T.; Goldman, C.R. A meta-analysis of the freshwater trophic cascade. Proc. Nat. Acad. Sci. USA 1996, 93, 7723–7726. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Christensen, D.L.; Cole, J.J.; Cottingham, K.L.; He, X.; Hodgsoan, J.R.; Kitchell, J.F.; Knight, S.E.; Pace, M.L.; Post, D.M.; et al. Biological control of eutrophication in lakes. Environ. Sci. Technol. 1995, 29, 784–786. [Google Scholar] [CrossRef]
- Ghadouani, A.; Pinel-Alloul, B.; Prepas, E.E. Effects of experimentally induced cyanobacterial blooms on crustacean zooplankton communities. Freshw. Biol. 2003, 48, 363–381. [Google Scholar] [CrossRef]
- Triest, L.; Stiers, I.; Van Onsem, S. Biomanipulation as a nature-based solution to reduce cyanobacterial blooms. Aquat. Ecol. 2016, 50, 461–483. [Google Scholar] [CrossRef]
- Benndorf, J.; Boing, W.; Koop, J.; Neubauer, I. Top-down control of phytoplankton: The role of time scale, lake depth and trophic state. Freshw. Biol. 2002, 47, 2282–2295. [Google Scholar] [CrossRef]
- Sommer, U.; Adrian, R.; De SenerpontDomis, L.; Elser, J.J.; Gaedke, U.; Ibelings, B.; Jeppesen, E.; Lürling, M.; Molinero, J.C.; Mooij, W.M.; et al. Beyond the Plankton Ecology Group (PEG) model: Mechanisms driving plankton succession. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 429–448. [Google Scholar] [CrossRef]
- Scheffer, M.; Hosper, S.H.; Meijer, M.-L.; Moss, B.; Jeppesen, E. Alternative equlibria in shallow lakes. Trends. Ecol. Evol. 1993, 8, 275–279. [Google Scholar] [CrossRef]
- Moustaka-Gouni, M.; Michaloudi, E.; Sommer, U. Modifying the PEG model for the Mediterranean—No biological winter and strong fish predation. Freshw. Biol. 2014, 59, 1136–1144. [Google Scholar] [CrossRef]
- Gliwicz, Z.M. Why do cladocerans fail to control algal blooms? Hydrobiologia 1990, 200, 83–97. [Google Scholar] [CrossRef]
- Elser, J.J. The pathway to noxious cyanobacteria blooms in lakes: The food web as the final turn. Freshw. Biol. 1999, 42, 537–543. [Google Scholar] [CrossRef]
- Sommer, U. The scientific basis of eutrophication management. Reconciling basic physiology and empirical biomass models. Memorie dell’Istituto Italiano di Idrobiologia 1993, 52, 89–111. [Google Scholar]
- Walsby, A.E.; Reynolds, C.S. Sinking and floating. In The Physiological Ecology of Phytoplankton; Morris, I., Ed.; Blackwell: Oxford, UK, 1980; pp. 371–412. [Google Scholar]
- Sommer, U. P-limited Daphnia: Intraspecific facilitation instead of competition. Limnol. Oceanogr. 1992, 37, 966–973. [Google Scholar] [CrossRef]
- Yoshioka, T.; Wada, E.; Hayashi, H. A stable isotope study on seasonal food web dynamics in a eutrophic lake. Ecology 1984, 20, 129–138. [Google Scholar] [CrossRef]
- Lewin, W.C.; Kamjunke, N.; Mehner, T. Phosphorus uptake by Microcystis during passage through fish guts. Limnol. Oceanogr. 2003, 48, 2392–2396. [Google Scholar] [CrossRef]
- Starling, F.; Beveridge, M.; Lazzaro, X.; Baird, D. Silver carp biomass effects on the plankton community in Paranoa reservoir (Brazil) and an assessment of its potential for improving water quality in lacustrine environments. Int. Rev. Hydrobiol. 1998, 83, 499–507. [Google Scholar]
- Ke, Z.X.; Xie, P.; Guo, L.G. Impacts of two biomanipulation fishes stocked in a large pen on the plankton abundance and water quality during a period of phytoplankton seasonal succession. Ecol. Eng. 2009, 35, 1610–1618. [Google Scholar] [CrossRef]
- Torres, G.S.; Silva, L.H.S.; Rangel, L.M.; Attayde, J.L.; Huszar, V.L.M. Cyanobacteria are controlled by omnivorous filter-feeding fish (Nile tilapia) in a tropical eutrophic reservoir. Hydrobiologia 2016, 765, 115–129. [Google Scholar] [CrossRef]
- Wang, Y.P.; Gu, X.H.; Zeng, Q.F.; Mao, Z.G.; Wang, W.K. Contrasting response of a plankton community to two filter feeding fish and their feces: An in-situ enclosure experiment. Aquaculture 2016, 465, 330–340. [Google Scholar] [CrossRef]
- Yi, C.; Guo, L.; Ni, L.; Luo, C. Silver carp exhibited an enhanced ability of biomanipulation to control cyanobacteria bloom compared to bighead carp in hypereutrophic Lake Taihu mesocosms. Ecol. Eng. 2016, 89, 7–13. [Google Scholar] [CrossRef]
- Radke, R.J.; Kahl, U. Effects of a filter-feeding fish [silver carp, Hypophthalmichthys molitrix (Val.)] on phyto- and zooplankton in a mesotrophic reservoir: Results from an enclosure experiment. Freshw. Biol. 2002, 47, 2337–2344. [Google Scholar] [CrossRef]
- Volta, P.; Jeppesen, E.; Leoni, B.; Campi, B.; Sala, P.; Garibaldi, L.; Lauridsen, T.L.; Winfield, I.J. Recent invasion by a non-native cyprinid (common bream Abramisbrama) is followed by major changes in the ecological quality of a shallow lake in southern Europe. Biol. Invasions 2013, 15, 2065–2079. [Google Scholar] [CrossRef]
- Gulati, R.D.; van Donk, E. Lakes in the Netherlands, their origin, eutrophication and restoration: State-of-the-art review. Hydrobiologia 2002, 478, 73–106. [Google Scholar] [CrossRef]
- Kamjunke, N.; Schmidt, K.; Pflugmacher, S.; Mehner, T. Consumption of cyanobacteria by roach (Rutilus rutilus): Useful or harmful to the fish? Freshw. Biol. 2002, 47, 243–250. [Google Scholar] [CrossRef]
- Fujibayashi, M.; Okano, K.; Takada, Y.; Mizutani, H.; Uchida, N.; Nishimura, O.; Miyata, N. Transfer of cyanobacterial carbon to a higher trophic-level fish community in a eutrophic lake food web: Fatty acid and stable isotope analyses. Oecologia 2018, 188, 901–912. [Google Scholar] [CrossRef]
- Thingstad, T.F.; Havskum, H.; Garde, K.; Riemann, B. On the strategy of ’’eating your competitor’’: A mathematical analysis of algal mixotrophy. Ecology 1996, 77, 2108–2118. [Google Scholar] [CrossRef]
- Work, K.; Havens, K.; Sharfstein, B.; East, T. How important is bacterial carbon to planktonic grazers in a turbid, subtropical lake? J. Plankton Res. 2005, 27, 357–372. [Google Scholar] [CrossRef]
- Agha, R.; Saebelfeld, M.; Manthey, C.; Rohrlack, T.; Wolinska, J. Chytrid parasitism facilitates trophic transfer between bloom-forming cyanobacteria and zooplankton (Daphnia). Sci. Rep. 2016, 6, 35039. [Google Scholar] [CrossRef]
- Haraldsson, M.; Gerphagnon, M.; Bazin, P.; Colombet, J.; Tecchio, S.; Sime-Ngando, T.; Niquil, N. Microbial parasites make cyanobacteria blooms less of a trophic dead end than commonly assumed. ISME J. 2018, 12, 1008–1021. [Google Scholar] [CrossRef]
- Sommer, U.; Stibor, H.; Katechakis, A.; Sommer, F.; Hansen, T. Pelagic food web configurations at different levels of nutrient richness and their implications for the ratio fish production:primary production. Hydrobiologia 2002, 484, 11–20. [Google Scholar] [CrossRef]
- Patoine, A.; Graham, M.D.; Leavitt, P.R. Spatial variation of nitrogen fixation in lakes of the northern Great Plains. Limnol. Oceanogr. 2006, 51, 1665–1677. [Google Scholar] [CrossRef]
- Loick-Wilde, N.; Dutz, J.; Miltner, A.; Gehre, M.; Montoya, J.P.; Voss, M. Incorporation of nitrogen from N2 fixation into amino acids of zooplankton. Limnol. Oceanogr. 2012, 57, 199–210. [Google Scholar] [CrossRef]
- Loick-Wilde, N.; Fernandez-Urruzola, I.; Eglite, E.; Liskow, I.; Nausch, M.; Schulz-Bull, D.; Wodarg, D.; Wasmund, N.; Mohrholz, V. Stratification, nitrogen fixation, and cyanobacterial bloom stage regulate the planktonic food web structure-Glob. Chang. Biol. 2019, 25, 794–810. [Google Scholar] [CrossRef]
- Wannicke, N.; Korth, F.; Liskow, I.; Voss, M. Incorporation of diazotrophic fixed N-2 by mesozooplankton—Case studies in the southern Baltic Sea. J. Mar. Syst. 2013, 117, 1–13. [Google Scholar] [CrossRef]
- Smith, V. Predictive models for the biomass of blue-green algae in lakes. Water Resourc. Bull. 1985, 21, 433–439. [Google Scholar] [CrossRef]
- Gerdeaux, D.; Anneville, O.; Hefti, D. Fishery changes during re-oligotrophication in 11 peri-alpine Swiss and French lakes over the past 30 years. Acta Oecol. 2006, 30, 161–167. [Google Scholar] [CrossRef]
- Persson, L.; Diehl, S.; Johansson, L.; Andersson, G.; Harmin, S.F. Shifts in fish communities along the productivity gradient of temperate lakes-patterns and the importance of size-structured interactions. J. Fish. Biol. 1991, 38, 281–293. [Google Scholar] [CrossRef]
- Jeppesen, E.; Jensen, J.P.; Sondergaard, M.; Lauridsen, T.; Landkildehus, F. Trophic structure, species richness and biodiversity in Danish lakes: Changes along a phosphorus gradient. Freshw. Biol. 2000, 45, 201–218. [Google Scholar] [CrossRef]
- Mülller, R. Trophic state and its implications for natural reproduction of salmonid fish. Hydrobiologia 1999, 243, 261–268. [Google Scholar]
- Dillon, P.J.; Rigler, F.H. The phosphorus-chlorophyll relationship in lakes. Limnol. Oceanogr. 1974, 19, 767–773. [Google Scholar] [CrossRef]
- Vollenweider, R.; Kerekes, J. Eutrophication of Waters, Monitoring, Assessment and Control; OECD: Paris, France, 1982. [Google Scholar]
- Watson, S.; Kalff, J. Relationships between nanoplankton and lake trophic status. Can. J. Fish. Aquat. Sci. 1981, 38, 960–967. [Google Scholar] [CrossRef]
- Hanson, J.M.; Peters, R.H. Empirical prediction of zooplankton and profundal macrobenthos biomass in lakes. Can. J. Fish. Aquat. Sci. 1984, 41, 439–445. [Google Scholar] [CrossRef]
- Pace, M.L. An empirical analysis of zooplankton community size structure across lake trophic gradient. Limnol. Oceanogr. 1986, 31, 45–55. [Google Scholar] [CrossRef]
- Hanson, J.M.; Leggett, W.C. Empirical prediction of fish biomass and yield. Can. J. Fish. Aquat. Sci. 1982, 39, 257–263. [Google Scholar] [CrossRef]
- Håkanson, L.; Boulion, V.V. Regularities in primary production, Secchi depth and fish yield and a new system to define trophic and humic state indices for lake ecosystems. Int. Rev. Hydrobiol. 2001, 86, 23–62. [Google Scholar] [CrossRef]

| Dependent Variable | Independent Variable | a | b | Source |
|---|---|---|---|---|
| Phytoplankton | ||||
| Chlorophyll, summer (µg L−1) | TP, spring (µg L−1) | 0.073 | 1.44 | [130] |
| Chlorophyll, annual (µg L−1) | TP, annual (µg L−1) | 0.28 | 0.96 | [131] |
| Chlorophyll, annual maximum (µg L−1) | TP, annual (µg L−1) | 0.64 | 1.05 | [131] |
| Wetweight, growing season (µg L−1) | TP, growing season (µg L−1) | 30 | 1.4 | [132] |
| Cyanobacteria, wwt, growing season (µg L−1) | TP, growing season (µg L−1) | 43 | 0.98 | [125] |
| Primary production, annual (g C m−2 year−1) | TP, annual (µg L−1) | 31.1 | 0.54 | [131] |
| Primary production, growing season (g C m−2day−1) | Chlorophyll, growing season (µg L−1) | 30.6 | 0.927 | [136] |
| Zooplankton | ||||
| Dry weight, ice free season (µg L−1) | TP, ice free season (µg L−1) | 38 | 0.64 | [134] |
| Crustacean dwt, ice free season (µg L−1) | TP, ice free season (µg L−1) | 5.7 | 0.91 | [133] |
| Crustacean dwt, seasonal coverage not uniform between study lakes (µg L−1) | Chlorophyll, ice free season (µg L−1) | 27.5 | 0.53 | [133] |
| Fish | ||||
| Wet weight (mg m−2) | TP, annual (µg L−1) | 590 | 0.71 | [135] |
| Yield (mg wwt m−2 year−1) | TP, annual (µg L−1) | 7.1 | 1.02 | [135] |
| Yield(mg wwt m−2 year−1) | Primary production(mg wwt m−2 year−1) | 0.0023 | 0.90 | [136] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moustaka-Gouni, M.; Sommer, U. Effects of Harmful Blooms of Large-Sized and Colonial Cyanobacteria on Aquatic Food Webs. Water 2020, 12, 1587. https://doi.org/10.3390/w12061587
Moustaka-Gouni M, Sommer U. Effects of Harmful Blooms of Large-Sized and Colonial Cyanobacteria on Aquatic Food Webs. Water. 2020; 12(6):1587. https://doi.org/10.3390/w12061587
Chicago/Turabian StyleMoustaka-Gouni, Maria, and Ulrich Sommer. 2020. "Effects of Harmful Blooms of Large-Sized and Colonial Cyanobacteria on Aquatic Food Webs" Water 12, no. 6: 1587. https://doi.org/10.3390/w12061587
APA StyleMoustaka-Gouni, M., & Sommer, U. (2020). Effects of Harmful Blooms of Large-Sized and Colonial Cyanobacteria on Aquatic Food Webs. Water, 12(6), 1587. https://doi.org/10.3390/w12061587





