Control of Nuisance Cyanobacteria in Drinking Water Resources Using Alternative Algae-Blocking Mats
Abstract
1. Introduction
2. Materials and Methods
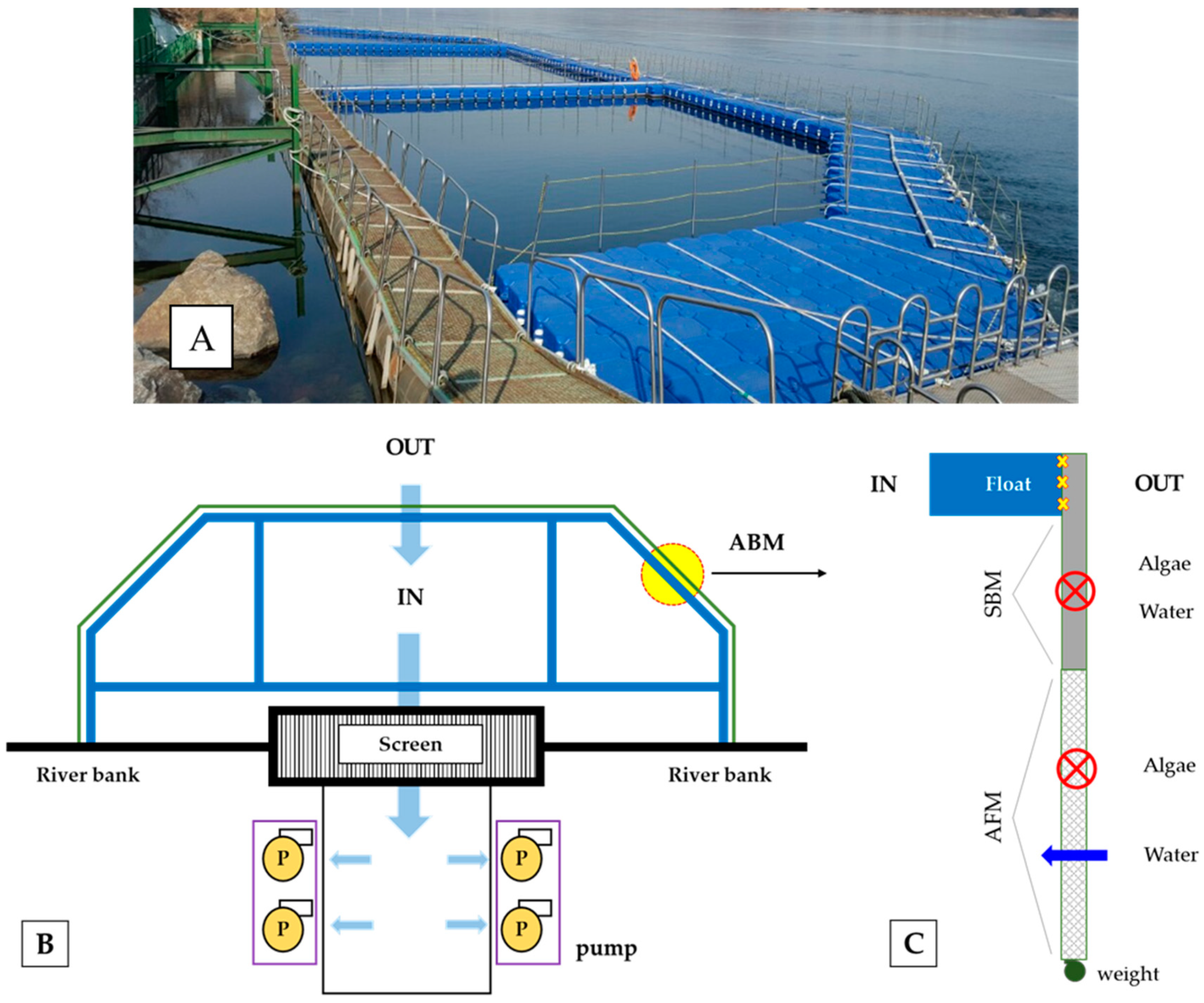
2.1. Description of the Test Site
2.2. Experimental Design
2.3. Sample Analysis
2.3.1. Phytoplankton Analysis
2.3.2. Algae-Related Odor Analysis
2.3.3. Photometry
3. Results and Discussion
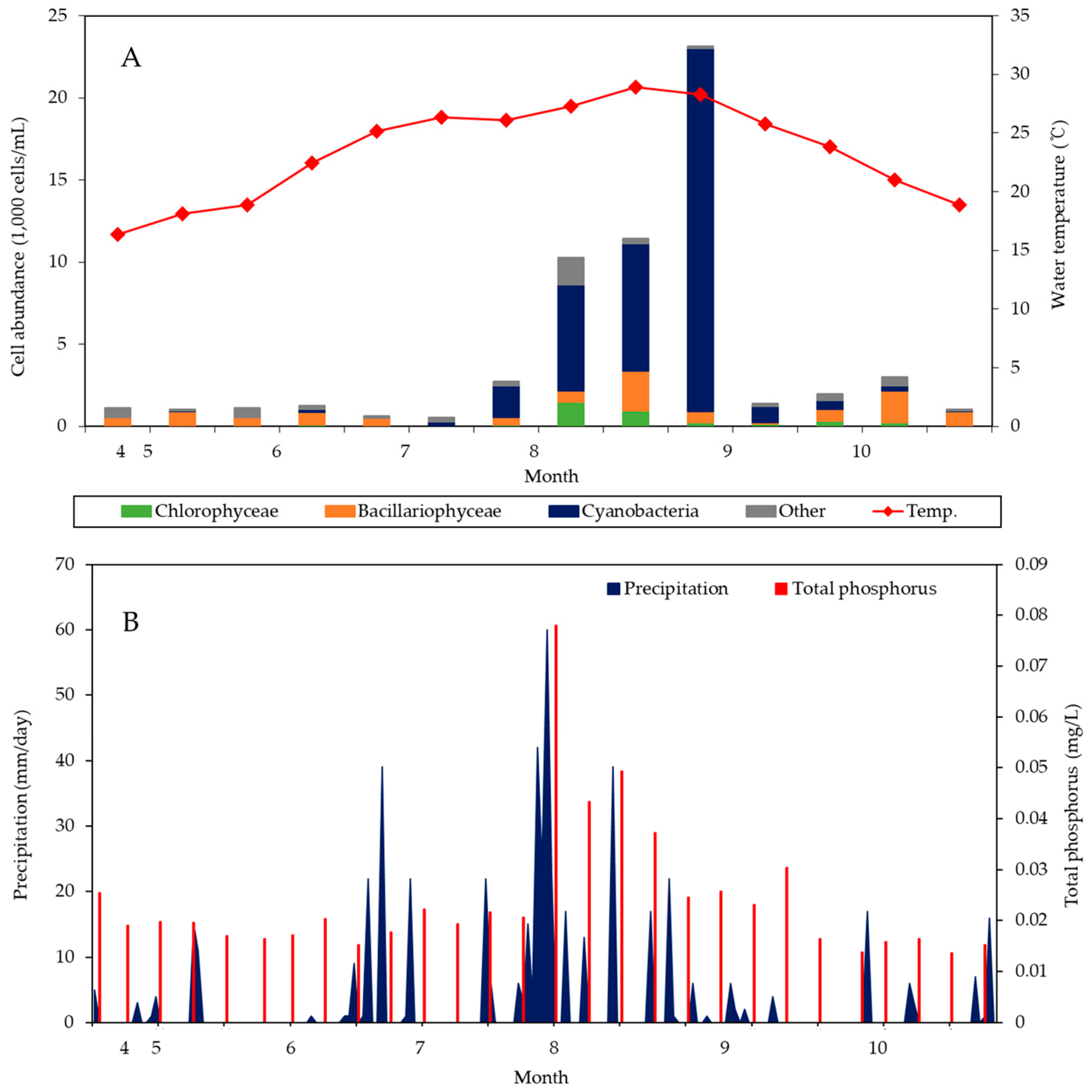
3.1. Fluctuation of Phytoplankton Cell Abundance in the Raw Water Source
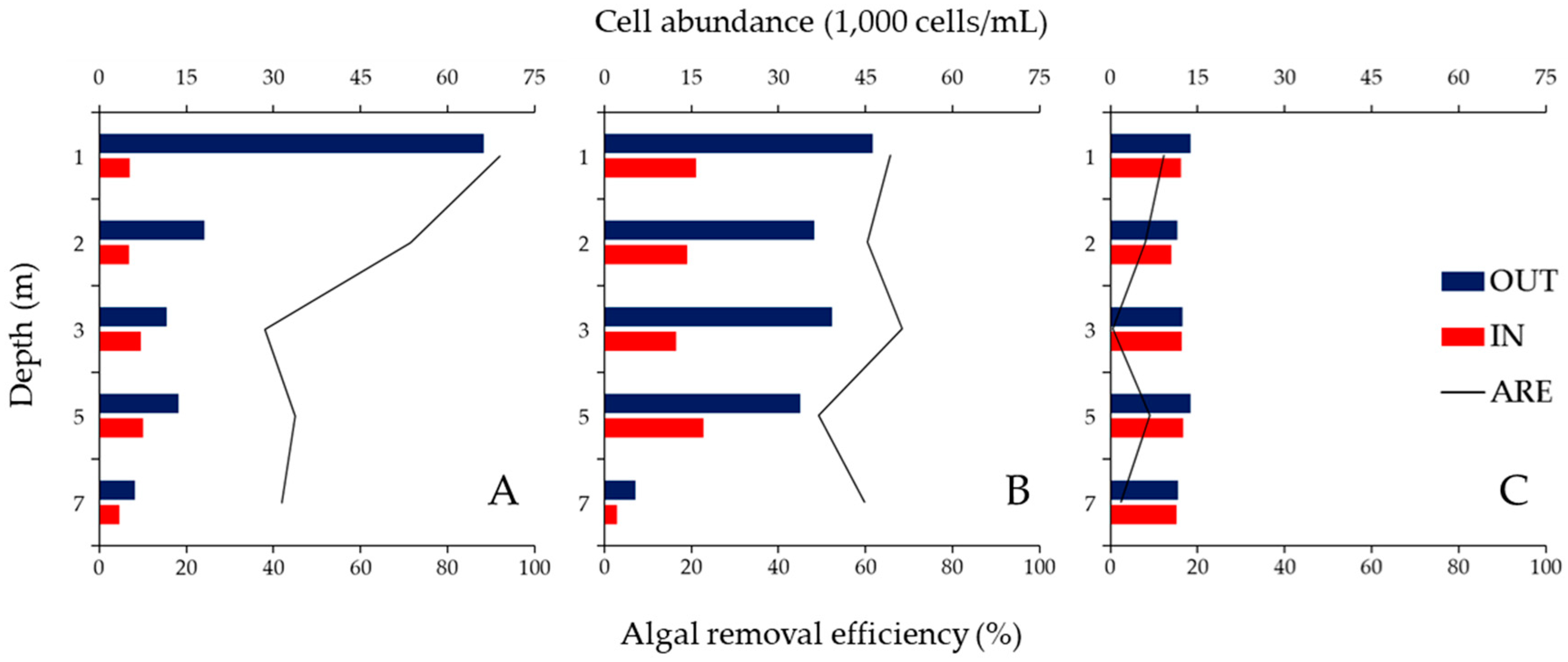
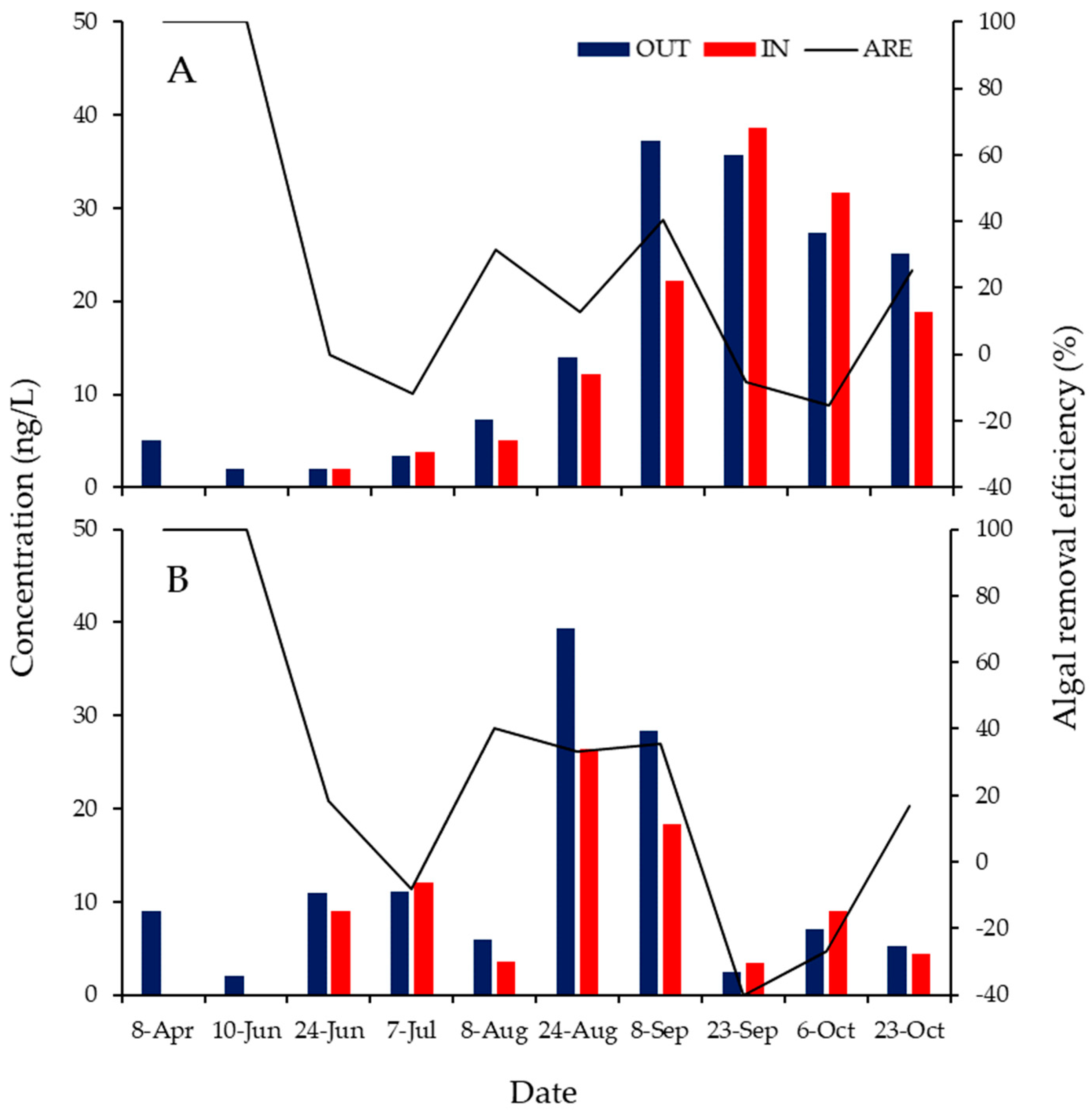
3.2. ARE of the ABM at Each Water Depth
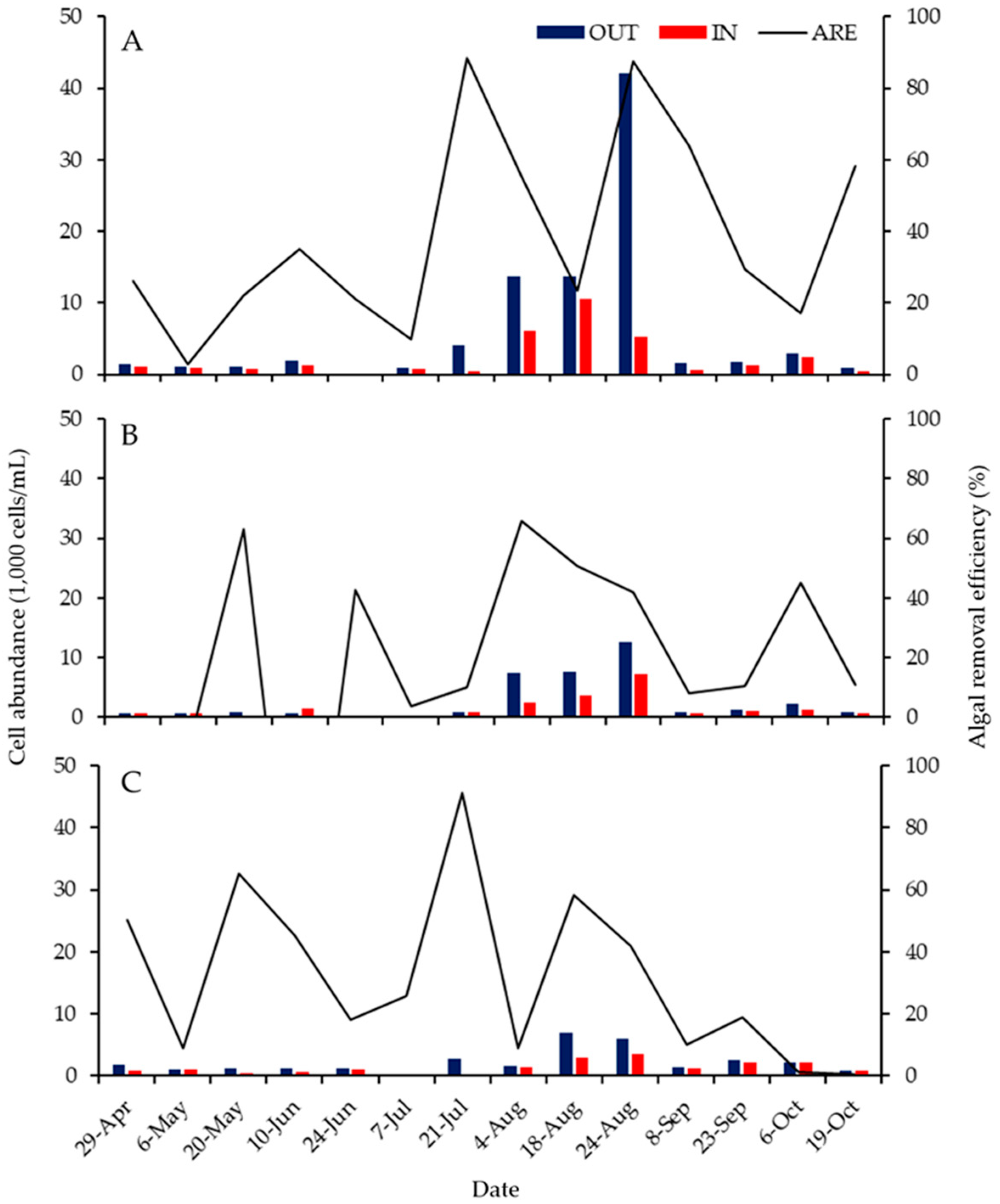
3.3. ABM Effects over Time
3.4. Algae-Related Odor Removal Efficiency of the ABM
3.5. Effects of the ABM and Its Operation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Srivastava, A.; Ahn, C.-Y.; Asthana, R.K.; Lee, H.-G.; Oh, H.-M. Status, Alert System, and Prediction of Cyanobacterial Bloom in South Korea. BioMed Res. Int. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schindler, D.W. Eutrophication and Recovery in Experimental Lakes: Implications for Lake Management. Science 1974, 184, 897–899. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Hall, N.S.; Calandrino, E.S. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci. Total. Environ. 2011, 409, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.-H.; Hwang, S.-J.; Kim, B.-H.; Park, J.-R.; Lee, J.-K.; Lim, B.-J. Relationship between a Dense Population of Cyanobacteria and Odorous Compounds in the North Han River System in 2014 and 2015. Korean J. Ecol. Environ. 2015, 48, 263–271. [Google Scholar] [CrossRef]
- Michalak, A.M.; Anderson, E.J.; Beletsky, D.; Boland, S.; Bosch, N.S.; Bridgeman, T.B.; Chaffin, J.D.; Cho, K.; Confesor, R.; Daloglu, I.; et al. Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6448–6452. [Google Scholar] [CrossRef]
- Wines, M. Behind Toledo’s water crisis, a long-troubled Lake Erie. New York Times, 5 August 2014. [Google Scholar]
- Park, C.H.; Park, M.H.; Choi, D.H.; Lee, J.H.; Lee, M.H.; Hwang, S.J. The removal of algae and phosphorus in eutrophic waters using various filter media. Korean J. Ecol. Environ. 2012, 45, 102–109. [Google Scholar]
- Yoo, H.; Lee, B.; Rhee, S.W. Correlation between Paldang reservoir discharge and causes of algal blooming. J. Korean Organic Resour. Recyc. Assoc. 2013, 21, 93–98. [Google Scholar]
- Srinivasan, R.; Sorial, G.A. Treatment of taste and odor causing compounds 2-methyl isoborneol and geosmin in drinking water: A critical review. J. Environ. Sci. 2011, 23, 1–13. [Google Scholar] [CrossRef]
- Jahn, S.A.A. Using Moringa Seeds as Coagulants in Developing Countries. J. Am. Water Work. Assoc. 1988, 80, 43–50. [Google Scholar] [CrossRef]
- Sigee, D.C.; Glenn, R.; Andrews, M.J.; Bellinger, E.G.; Butler, R.D.; Epton, H.A.S.; Hendry, R.D. Biological control of cyanobacteria: Principles and possibilities. In The Ecological Bases for Lake and Reservoir Management; Springer: Amsterdam, The Netherlands, 1999; pp. 161–172. [Google Scholar]
- Kim, N.-Y.; Park, M.-H.; Hwang, S.-O.; Kim, B.-H.; Hwang, S.-J. Combined Effects of Filter-feeding Bivalve and Zooplankton on the Growth Inhibition of Cyanobacterium Microcystis aeruginosa. Korean J. Ecol. Environ. 2015, 48, 108–114. [Google Scholar] [CrossRef]
- Hong, N.S. Investigation of reducing characteristics for the spreading of dredging soil and the diffusion of contaminant by silt protector curtain through three dimensional numerical model experiment. J. Ocean Eng. Technol. 2010, 24, 78–85. [Google Scholar]
- Asaeda, T.; Pham, H.; Priyantha, D.N.; Manatunge, J.; Hocking, G. Control of algal blooms in reservoirs with a curtain: A numerical analysis. Ecol. Eng. 2001, 16, 395–404. [Google Scholar] [CrossRef]
- Asaeda, T.; Priyantha, D.; Saitoh, S.; Gotoh, K. A new technique for controlling algal blooms in the withdrawal zone of reservoirs using vertical curtains. Ecol. Eng. 1996, 7, 95–104. [Google Scholar] [CrossRef]
- Joo, G.J.; Jang, M.H.; Park, S.B.; Jung, J.M.; Roh, J.S.; Jeong, G.S. The application of an algal fence for the reduction of algal intake into the water intake facility. Korean J. Limnol. 2003, 36, 467–472. [Google Scholar]
- Kong, D.S. Limnological and ecological characteristics of a river-reservoir (Paldang), Korea. Korean J. Limnol. 1997, 30, 524–535. [Google Scholar]
- Kim, B.-H.; Hwang, S.-J.; Han, M.-S. Bacterium and Fish-Mediated Morphological Changes of the Toxic Cyanobacterium Microcystis aeruginosa. J. Freshw. Ecol. 2008, 23, 613–622. [Google Scholar] [CrossRef][Green Version]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA-AWWA-WEF: Washington, DC, USA, 2005. [Google Scholar]
- Watanabe, M.F.; Oishi, S. Effects of Environmental Factors on Toxicity of a Cyanobacterium (Microcystis aeruginosa) under Culture Conditions. Appl. Environ. Microbiol. 1985, 49, 1342–1344. [Google Scholar] [CrossRef]
- Ha, K.; Kim, H.-W.; Jeong, K.-S.; Joo, G.-J. Vertical distribution of Microcystis population in the regulated Nakdong River, Korea. Limnology 2000, 1, 225–230. [Google Scholar] [CrossRef]
- Chien, Y.C.; Wu, S.C.; Chen, W.C.; Chou, C.C. Model Simulation of Diurnal Vertical Migration Patterns of Different-Sized Colonies of Microcystis Employing a Particle Trajectory Approach. Environ. Eng. Sci. 2013, 30, 179–186. [Google Scholar] [CrossRef]
- Wallace, B.B.; Bailey, M.C.; Hamilton, D.P. Simulation of vertical position of buoyancy regulating Microcystis aeruginosa in a shallow eutrophic lake. Aquat. Sci. 2000, 62, 320–333. [Google Scholar] [CrossRef]
- Hunter, P.; Tyler, A.; Willby, N.J.; Gilvear, D. The spatial dynamics of vertical migration by Microcystis aeruginosa in a eutrophic shallow lake: A case study using high spatial resolution time-series airborne remote sensing. Limnol. Oceanogr. 2008, 53, 2391–2406. [Google Scholar] [CrossRef]
- Sugiura, N.; Yagi, O.; Sudo, R. Musty odor from blue-green alga, Phormidium tenue in Lake Kasumigaura. Environ. Technol. Lett. 1986, 7, 77–86. [Google Scholar] [CrossRef]
- Sugiura, N.; Nakano, K. Causative microorganisms for musty odor occurrence in the eutrophic Lake Kasumigaura. Hydrobiology 2000, 434, 145–150. [Google Scholar] [CrossRef]
- Xu, L.; Xiong, B.; Pan, Y.; Wang, J.; Cao, H.; Zhao, W. Relationship between concentrations of odorous compounds and biomass of phytoplankton and actinomycetes in freshwater ponds of Beijing, China. Aquac. Int. 2009, 18, 245–254. [Google Scholar] [CrossRef]
- Parinet, J.; Rodriguez, M.J.; Sérodes, J. Influence of water quality on the presence of off-flavor compounds (geosmin and 2-methylisoborneol). Water Res. 2010, 44, 5847–5856. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.W.; Piedrahita, R.H. The relation between phytoplankton and dissolved oxygen in fish ponds. Aquaculture 1988, 68, 249–265. [Google Scholar] [CrossRef]
- Lee, K.; Choi, M.J.; Park, H.K.; Lee, J.H. Characteristics of Release Rate of Nutrients from Sediment in Lake Paldang and Lake Cheongpyeong. J. Korean Soc. Water Qual. 2009, 25, 742–749. [Google Scholar]
- Kim, B.-H.; Lee, J.-H.; Hwang, S.-J. Inter- and intra-specific differences in filtering activities between two unionids, Anodonta woodiana and Unio douglasiae, in ambient eutrophic lake waters. Ecol. Eng. 2011, 37, 1957–1967. [Google Scholar] [CrossRef]
- Kim, B.-H.; Hwang, S.-J.; Han, M.-S.; Sang, M. In situ bacterial mitigation of the toxic cyanobacterium Microcystis aeruginosa: Implications for biological bloom control. Limnol. Oceanogr. Methods 2008, 6, 513–522. [Google Scholar] [CrossRef]
- Teixeira, M.R.; Rosa, M.J. Comparing dissolved air flotation and conventional sedimentation to remove cyanobacterial cells of Microcystis aeruginosa. Sep. Purif. Technol. 2006, 52, 84–94. [Google Scholar] [CrossRef]
- Na, G.H.; Nam, J.B.; Park, K.D.; Lee, J.A. Experimental elimination of cyanobacteria Microcystis sp. by loess suspension in column test. J. Korean Soc. Water Qual. 1998, 14, 399–404. [Google Scholar]
- Park, M.-H.; Chung, I.-M.; Ahmad, A.; Kim, B.-H.; Hwang, S.-J. Growth inhibition of unicellular and colonial Microcystis strains (Cyanophyceae) by compounds isolated from rice (Oryza sativa) hulls. Aquat. Bot. 2009, 90, 309–314. [Google Scholar] [CrossRef]
- Kim, S.; Jeon, M.S.; Kim, J.Y.; Sim, S.J.; Choi, J.-S.; Kwon, J.; Choi, Y.-E. Adsorptive removal of harmful algal species Microcystis aeruginosa directly from aqueous solution using polyethylenimine coated polysulfone-biomass composite fiber. Biodegradation 2018, 29, 349–358. [Google Scholar] [CrossRef]


| Specification | SBM | AFM |
|---|---|---|
| Material | Polypropylene | Polypropylene fiber |
| Water intake volume (Q, m3/day) | 1,500,000 | 1,500,000 |
| Thickness (cm) | 2 | 2 |
| Vertical length (m) | 2 | 3 |
| Pore size (μm) | <9 | <100 |
| Targets | Water and most suspended solids | Phytoplankton |
| Mat surface structure of ABM and AFM fiber | 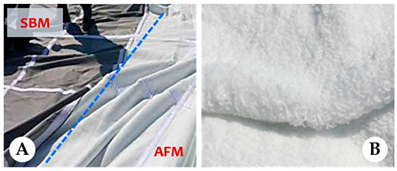 | |
| Sampling Time * (YYYY/MM/DD) | Dominant Species | |
|---|---|---|
| OUT | IN | |
| 2015/04/29 | Rhodomonas lacustris | Rhodomonas lacustris |
| 2015/05/06 | Cyclotella meneghiniana | Pediastrum boryanum |
| 2015/0/520 | Fragilaria crotonensis | Asterionella formosa |
| 2015/06/10 | Microcystis flos-aquae | Fragilaria crotonensis |
| 2015/06/24 | Aulacoseira granulata | Fragilaria crotonensis |
| 2015/07/07 | Dolichospermum crassum | Dolichospermum crassum |
| 2015/07/21 | Dolichospermum crassum | Fragilaria crotonensis |
| 2015/08/04 | Merismopedia tenuissima | Merismopedia tenuissima |
| 2015/08/18 | Microcystis aeruginosa | Microcystis aeruginosa |
| 2015/08/24 (2:00 p.m.) ** | Microcystis aeruginosa | Microcystis aeruginosa |
| 2015/08/24 (8:00 p.m.) | Microcystis aeruginosa | Microcystis aeruginosa |
| 2015/08/25 (8:00 a.m.) | Microcystis aeruginosa | Microcystis aeruginosa |
| 2015/09/08 | Microcystis wesenbergii | Microcystis aeruginosa |
| 2015/09/23 | Aulacoseira granulata | Aulacoseira granulata |
| 2015/10/06 | Aulacoseira granulata | Aulacoseira granulata |
| 2015/10/19 | Aulacoseira granulata | Dictyosphaerium pulchellum |
| Date (YYYY/MM/DD) | Temp (°C) | Cond (μS/cm) | pH | Turb (NTU) | DO (mg/L) | Chl-a (μg/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OUT | IN | OUT | IN | OUT | IN | OUT | IN | OUT | IN | OUT | IN | |
| 2015/04/29 | 16.38 ± 1.14 | 15.14 ± 0.65 | 219.76 ± 17.46 | 202.33 ± 7.30 | 8.95 ± 0.12 | 8.83 ± 0.32 | 2.14 ± 0.27 | 1.57 ± 1.29 | 13.33 ± 0.69 | 12.36 ± 1.43 | 7.41 ± 2.10 | 6.35 ± 2.11 |
| 2015/05/06 | 18.11 ± 1.05 | 16.92 ± 1.21 | 218.33 ± 24.78 | 196.13 ± 24.22 | 8.71 ± 0.17 | 8.43 ± 0.34 | 1.71 ± 0.56 | 2.71 ± 1.21 | 11.39 ± 0.54 | 10.82 ± 0.83 | 4.36 ± 1.33 | 6.88 ± 3.43 |
| 2015/05/20 | 18.89 ± 0.08 | 18.58 ± 0.48 | 244.60 ± 2.33 | 215.66 ± 28.38 | 8.19 ± 0.11 | 8.25 ± 0.14 | 1.42 ± 0.04 | 2.05 ± 0.75 | 10.22 ± 0.04 | 9.679 ± 0.63 | 6.70 ± 0.36 | 6.89 ± 1.30 |
| 2015/06/10 | 22.43 ± 1.10 | 21.26 ± 0.99 | 191.93 ± 47.57 | 145.90 ± 22.20 | 8.34 ± 0.07 | 8.05 ± 0.10 | 0.62 ± 0.33 | 1.14 ± 0.38 | 9.796 ± 0.20 | 9.476 ± 0.53 | 4.43 ± 1.26 | 3.83 ± 2.30 |
| 2015/06/24 | 25.12 ± 1.26 | 23.46 ± 1.42 | 212.40 ± 42.68 | 170.60 ± 39.50 | 8.11 ± 0.08 | 8.06 ± 0.15 | 0.54 ± 0.86 | 1.60 ± 1.28 | 8.873 ± 0.25 | 8.297 ± 0.57 | ND | ND |
| 2015/07/07 | 26.36 ± 0.34 | 25.71 ± 0.66 | 259.10 ± 1.35 | 234.20 ± 39.16 | 8.00 ± 0.08 | 7.86 ± 0.04 | 0.67 ± 0.55 | 1.17 ± 0.46 | 8.021 ± 0.70 | 7.179 ± 0.62 | 4.00 ± 0.18 | 3.71 ± 0.35 |
| 2015/07/21 | 26.09 ± 0.41 | 25.63 ± 0.53 | 228.10 ± 10.90 | 219.50 ± 34.15 | 8.12 ± 0.08 | 8.02 ± 0.09 | 1.98 ± 0.81 | 1.34 ± 0.64 | 8.333 ± 0.83 | 7.525 ± 0.57 | 14.10 ± 7.35 | 6.94 ± 1.29 |
| 2015/08/04 | 27.31 ± 1.33 | 25.89 ± 0.95 | 178.9 ± 20.05 | 156.50 ± 14.26 | 8.57 ± 0.15 | 8.02 ± 0.14 | 3.31 ± 0.31 | 3.38 ± 0.81 | 10.09 ± 1.28 | 7.816 ± 0.76 | 29.00 ± 10.70 | 21.3 ± 5.66 |
| 2015/08/18 | 28.89 ± 0.54 | 28.01 ± 0.78 | 216.36 ± 16.57 | 230.80 ± 15.43 | 9.18 ± 0.19 | 8.77 ± 0.23 | 5.09 ± 1.06 | 7.15 ± 4.38 | 11.21 ± 1.35 | 8.49 ± 1.27 | 34.80 ± 7.14 | 28.3 ± 3.29 |
| 2015/08/24 (2:00 p.m.) | 28.31 ± 0.42 | 27.69 ± 0.34 | 204.80 ± 6.01 | 207.93 ± 9.60 | 9.41 ± 0.16 | 8.92 ± 0.18 | 8.48 ± 2.40 | 4.72 ± 0.58 | 13.04 ± 1.37 | 9.394 ± 0.96 | 55.30 ± 20.10 | 36.0 ± 8.36 |
| 2015/08/24 (8:00 p.m.) | 28.53 ± 0.36 | 27.67 ± 0.51 | 208.25 ± 3.90 | 204.56 ± 2.34 | 9.47 ± 0.10 | 9.05 ± 0.24 | 9.62 ± 2.10 | 5.30 ± 0.45 | 13.56 ± 0.78 | 10.12 ± 1.69 | 55.00 ± 15.40 | 45.3 ± 14.9 |
| 2015/08/25 (8:00 a.m.) | 27.67 ± 0.01 | 27.68 ± 0.00 | 217.00 ± 0.00 | 217.00 ± 0.00 | 9.00 ± 0.00 | 9.01 ± 0.00 | 5.73 ± 0.45 | 6.02 ± 0.21 | 10.02 ± 0.02 | 9.967 ± 0.01 | 53.30 ± 1.61 | 53.3 ± 0.66 |
| 2015/09/08 | 25.75 ± 0.11 | 25.61 ± 0.10 | 222.40 ± 3.87 | 227.73 ± 1.37 | 7.68 ± 0.04 | 7.77 ± 0.01 | 3.28 ± 0.18 | 3.15 ± 0.23 | 7.91 ± 0.31 | 7.200 ± 0.45 | 10.9 ± 1.68 | 13.2 ± 0.44 |
| 2015/09/23 | 23.85 ± 0.09 | 23.75 ± 0.05 | 204.80 ± 5.11 | 204.80 ± 5.11 | 7.56 ± 0.22 | 8.10 ± 0.01 | 1.51 ± 0.31 | 1.47 ± 0.11 | 9.41 ± 0.38 | 9.076 ± 0.07 | 7.94 ± 3.12 | 9.95 ± 0.90 |
| 2015/10/19 | 18.84 ± 0.05 | 18.73 ± 0.00 | 183.20 ± 1.60 | 181.10 ± 0.20 | 7.65 ± 0.00 | 7.49 ± 0.02 | 1.31 ± 0.05 | 1.86 ± 0.22 | 8.993 ± 0.04 | 8.842 ± 0.02 | 7.18 ± 1.01 | 7.53 ± 0.95 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-H.; Gwon, E.-M.; Kim, H.-K.; Cho, I.-H.; Lee, H.; Kim, B.-H. Control of Nuisance Cyanobacteria in Drinking Water Resources Using Alternative Algae-Blocking Mats. Water 2020, 12, 1576. https://doi.org/10.3390/w12061576
Kim Y-H, Gwon E-M, Kim H-K, Cho I-H, Lee H, Kim B-H. Control of Nuisance Cyanobacteria in Drinking Water Resources Using Alternative Algae-Blocking Mats. Water. 2020; 12(6):1576. https://doi.org/10.3390/w12061576
Chicago/Turabian StyleKim, Young-Hyo, En-Mi Gwon, Ha-Kyung Kim, In-Hwan Cho, Hyuk Lee, and Baik-Ho Kim. 2020. "Control of Nuisance Cyanobacteria in Drinking Water Resources Using Alternative Algae-Blocking Mats" Water 12, no. 6: 1576. https://doi.org/10.3390/w12061576
APA StyleKim, Y.-H., Gwon, E.-M., Kim, H.-K., Cho, I.-H., Lee, H., & Kim, B.-H. (2020). Control of Nuisance Cyanobacteria in Drinking Water Resources Using Alternative Algae-Blocking Mats. Water, 12(6), 1576. https://doi.org/10.3390/w12061576






