Sensitivity of Hydrangea paniculata Plants to Residual Herbicides in Recycled Irrigation Varies with Plant Growth Stage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatments
2.2. Assessment of Physiological and Morphological Effect of Herbicide
2.3. Statistical Analysis
3. Results
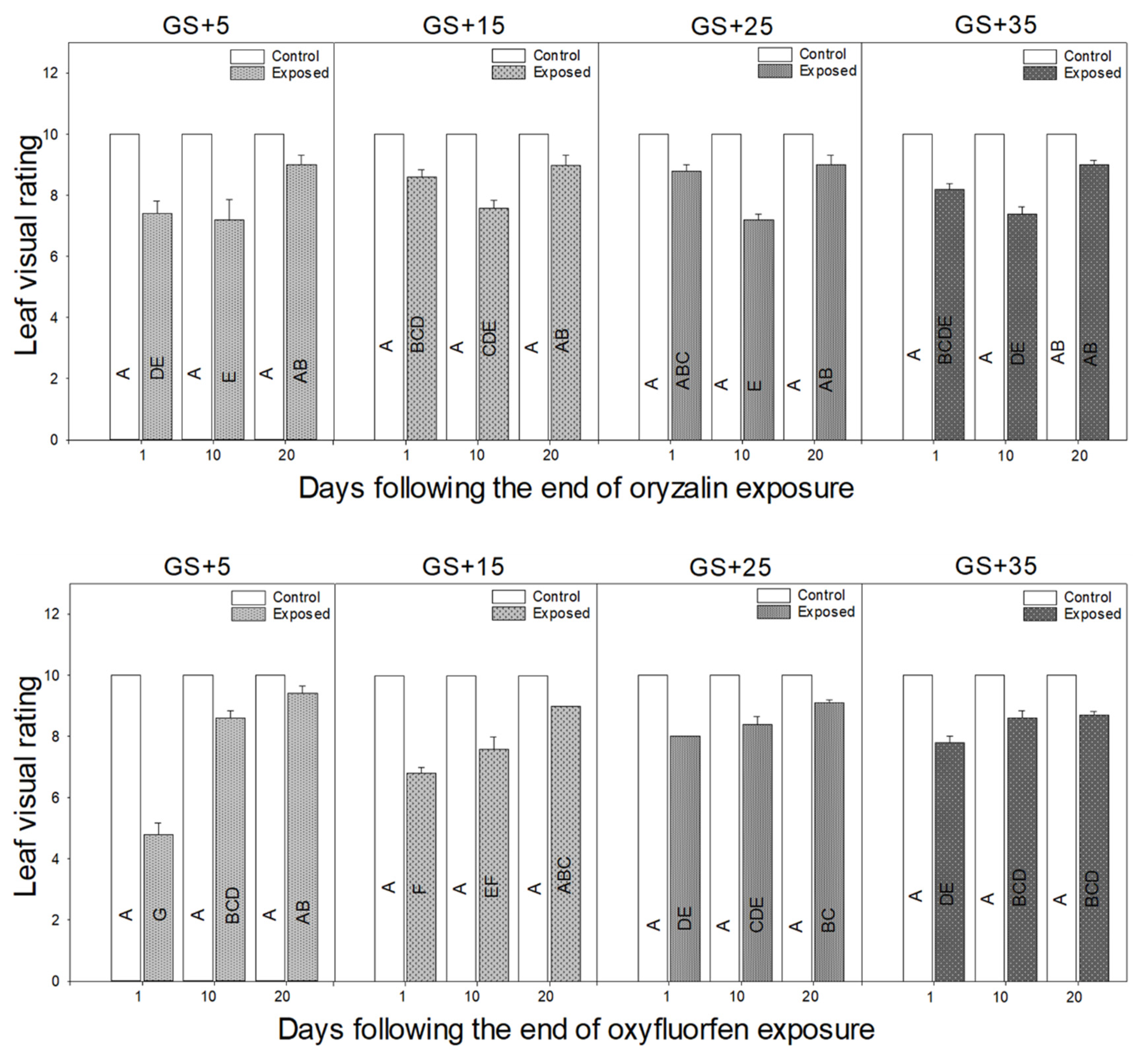
3.1. Morphological Responses to Herbicide Exposure
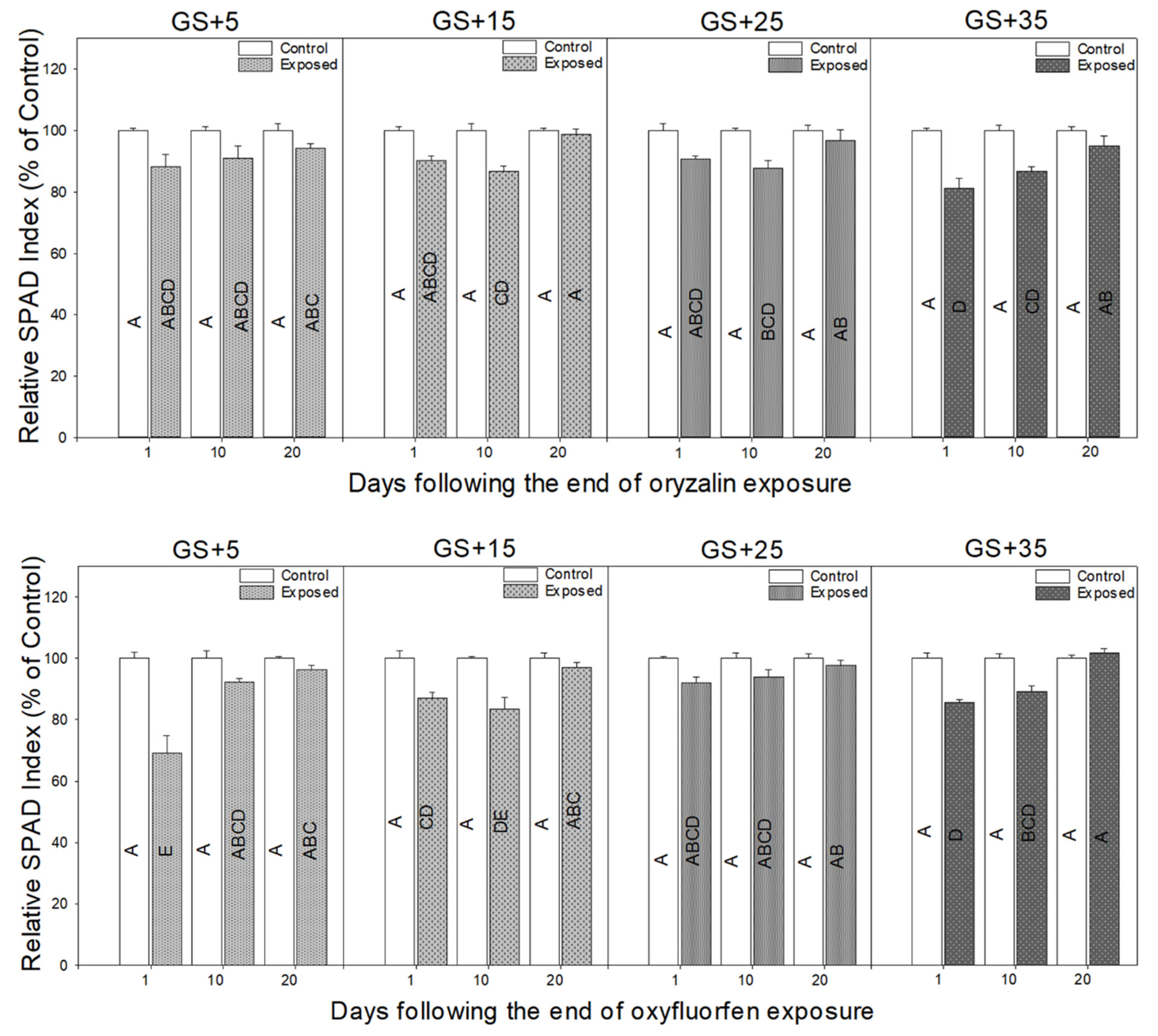
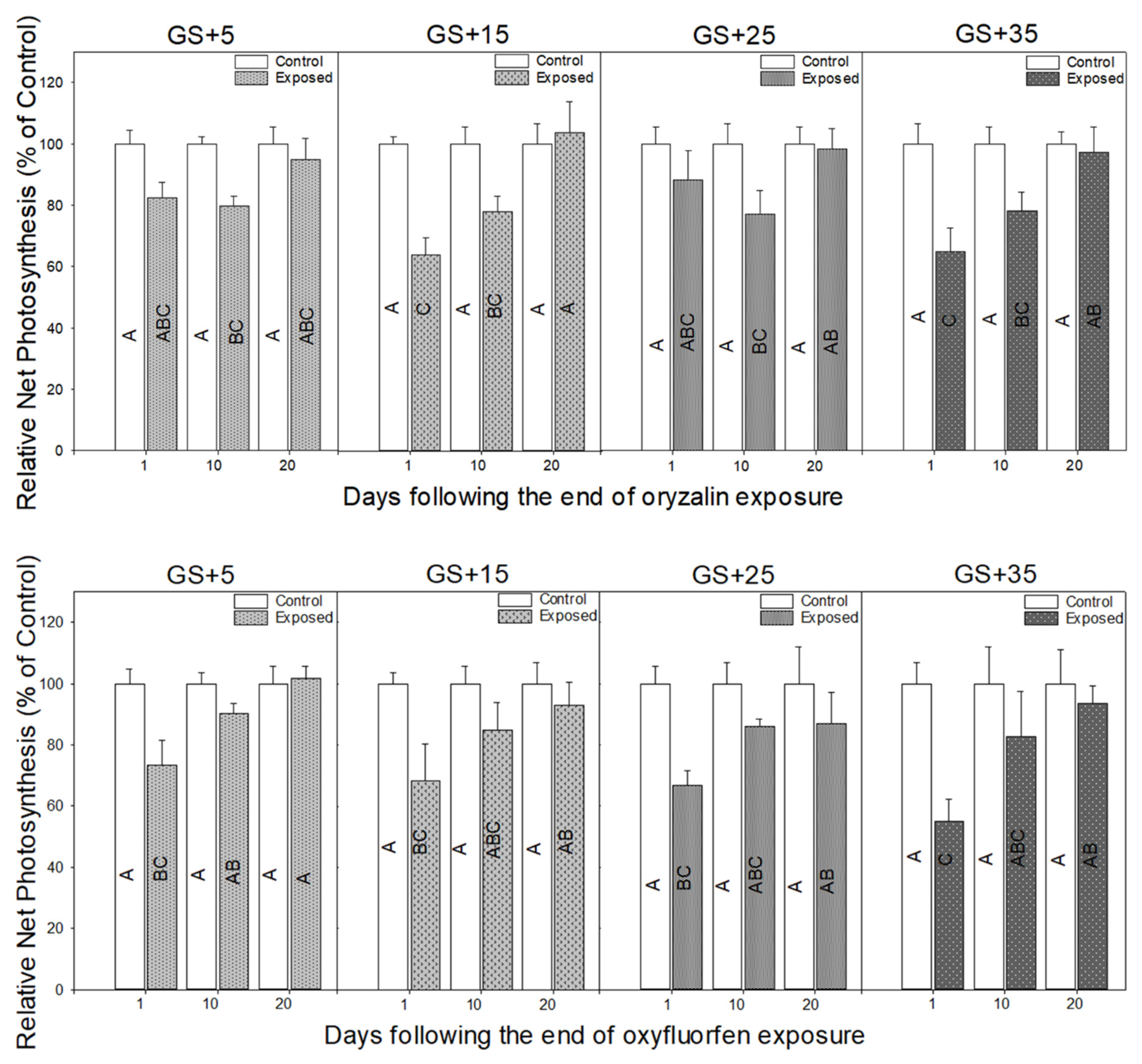
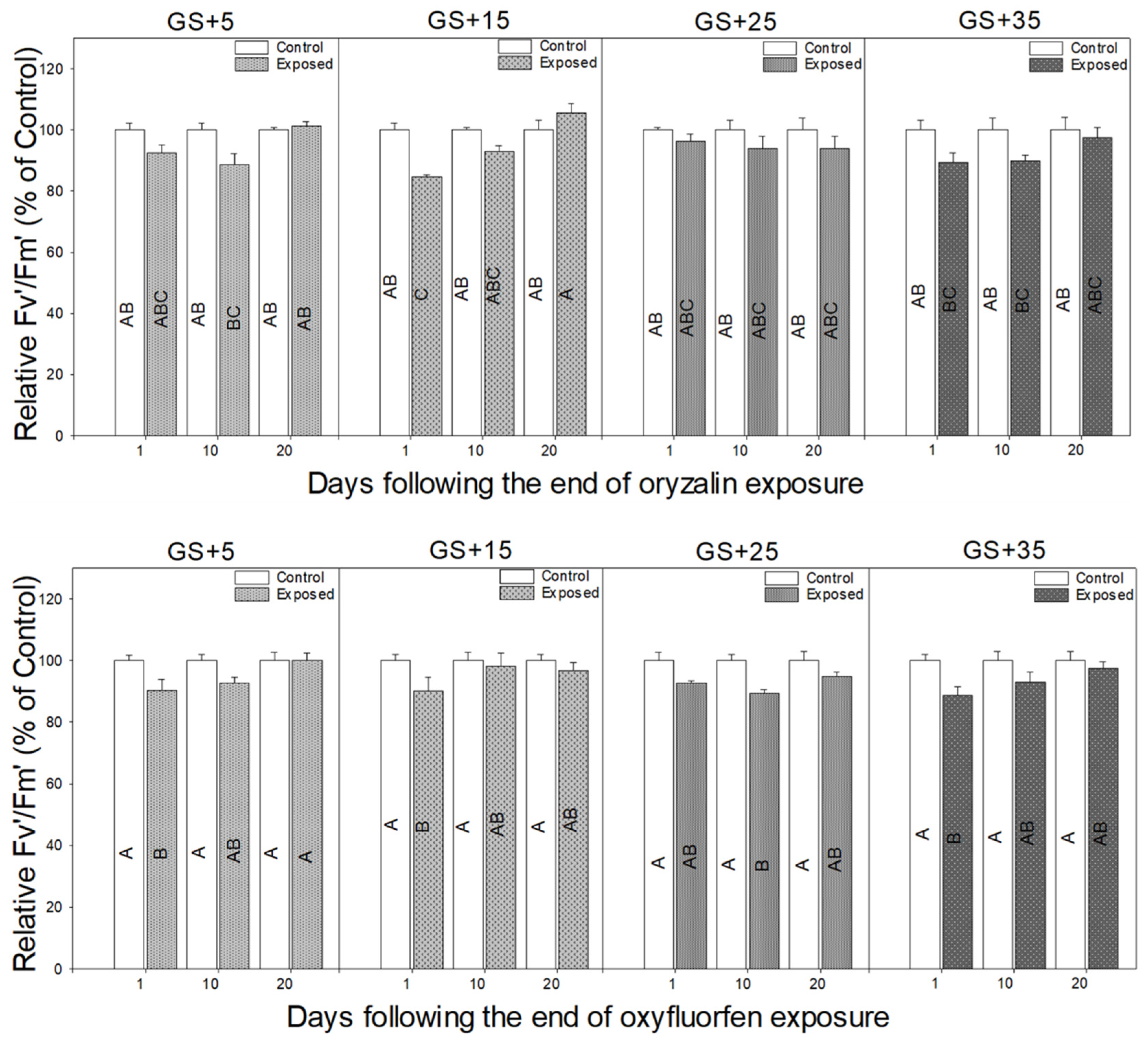
3.2. Physiological Responses to Herbicide Exposure
3.3. Final Evaluation
3.4. Evaluation of Flowers
4. Discussion
4.1. Morphological Response Depends on the Growth Stage of Plant
4.2. Physiological Measurements Provide a Rapid Indicator of Herbicide Damage and Recovery
4.3. Flowers Were Not Damaged by Residual Oryzalin and Oxyfluorfen
4.4. Leaf Visual Injury Takes the Longest to Recover
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beeson, R.C.; Knox, G.W. Analysis of efficiency of overhead irrigation in container production. HortScience 1991, 26, 848–850. [Google Scholar] [CrossRef] [Green Version]
- Fain, G.B.; Gilliam, C.H.; Tilt, K.M.; Olive, J.W.; Wallace, B. Survey of best management practices in container production nurseries. J. Environ. Hortic. 2000, 18, 142–144. [Google Scholar]
- Poudyal, S.; Cregg, B.M. Irrigating nursery crops with recycled run-off: A review of the potential impact of pesticides on plant growth and physiology. HortTechnology 2019, 29, 716–729. [Google Scholar] [CrossRef]
- Oki, L.R.; White, S.A. Ecological approaches used in nurseries to treat water. UCNFA News 2012, 15, 1–6. [Google Scholar]
- Fulcher, A.; LeBude, A.V.; Owen, J.S.; White, S.A.; Beeson, R.C. The next ten years: Strategic vision of water resources for nursery producers. HortTechnology 2016, 26, 121–132. [Google Scholar] [CrossRef] [Green Version]
- Rodell, M.; Famiglietti, J.S.; Wiese, D.N.; Reager, J.T.; Beaudoing, H.K.; Landerer, F.W.; Lo, M.H. Emerging trends in global freshwater availability. Nature 2018, 557, 651–659. [Google Scholar] [CrossRef]
- De Amorim, W.S.; Valduga, I.B.; Ribeiro, J.M.P.; Williamson, V.G.; Krauser, G.E.; Magtoto, M.K.; de Andrade Guerra, J.B.S.O. The nexus between water, energy, and food in the context of the global risks: An analysis of the interactions between food, water, and energy security. Environ. Impact Assess. Rev. 2018, 72, 1–11. [Google Scholar] [CrossRef]
- Pitton, B.J.L.; Hall, C.R.; Haver, D.L.; White, S.A.; Oki, L.R. A cost analysis for using recycled irrigation runoff water in container nursery production: A Southern California nursery case study. Irrig. Sci. 2018, 36, 217–226. [Google Scholar] [CrossRef]
- Ferraro, N.; Bosch, D.; Pease, J.; Owen, J.S. Costs of capturing and recycling irrigation water in container nurseries. HortScience 2017, 52, 258–263. [Google Scholar] [CrossRef]
- Poudyal, S.; Cregg, B.; Fernandez, T.R.; Owen, J. Dose-Dependent Phytotoxicity of Pesticides in Simulated Nursery Runoff on Landscape Nursery Plants. Water 2019, 11, 2354. [Google Scholar] [CrossRef] [Green Version]
- Regan, R.; Ticknor, R. Application of isoxaben and pendimethalin to container-grown broadleaved evergreen shrubs. Ornam. Northwest Arch. 1987, 11, 12–14. [Google Scholar]
- Tesfamariam, T.; Bott, S.; Cakmak, I.; Römheld, V.; Neumann, G. Glyphosate in the rhizosphere-Role of waiting times and different glyphosate binding forms in soils for phytotoxicity to non-target plants. Eur. J. Agron. 2009, 31, 126–132. [Google Scholar] [CrossRef]
- Isbister, K.M.; Lamb, E.G.; Stewart, K.J. Herbicide toxicity testing with non-target boreal plants: The sensitivity of Achillea millefolium L. and Chamerion angustifolium L. to triclopyr and imazapyr. Environ. Manag. 2017, 60, 136–156. [Google Scholar] [CrossRef]
- Follak, S.; Hurle, K. Recovery of non-target plants affected by airborne bromoxynil-octanoate and metribuzin. Weed Res. 2004, 44, 142–147. [Google Scholar] [CrossRef]
- Jursik, M.; Hamouzova, K.; Andr, J.; Soukup, J. Effect of different adjuvants on phytotoxicity of flumioxazin to sunflower in different growth stages. Rom. Agric. Res. 2013, 30, 365–372. [Google Scholar]
- Rouse, C.E.; Dittmar, P.J. Factors affecting herbicide use in fruits and vegetables. EDIS 2013, HS1219, 1–4. [Google Scholar]
- Richardson, F.E. Critical Growth Stages For 2,4-D Phytotoxicity To Sugarcane In South Africa. Proc. S. Afr. Sugar Technol. Assoc. 1972, 6, 168–176. [Google Scholar]
- Lourens, A.F.; Lange, A.H.; Calitz, F.J. Phytotoxicity of pre-emergence herbicides to peach seedlings (Prunus persica). S. Afr. J. Plant Soil 1989, 6, 97–102. [Google Scholar] [CrossRef]
- Gonzalez, M.P.; Karlik, J. Evaluation of herbicides for phytotoxicity to rose plants and efficacy. J. Environ. Hortic. 1999, 17, 164–167. [Google Scholar]
- Keese, R.J.; Camper, N.D.; Whitwell, T.; Riley, M.B.; Wilson, P.C. Herbicide runoff from ornamental container nurseries. J. Environ. Qual. 1994, 23, 320–324. [Google Scholar] [CrossRef]
- Goodwin, P.B.; Beach, S. Oxadiazon, oryzalin, and oxyfluorfen residues in container plant nurseries. HortScience 2001, 36, 900–904. [Google Scholar] [CrossRef] [Green Version]
- Riley, M.B.; Keese, R.J.; Camper, N.D.; Whitwell, T.; Chris, P.; Riley, M.B.; Keese, R.J.; Camper, N.D.; Whitwell, T.E.D.; Wilson, P.C. Pendimethalin and oxyfluorfen residues in pond water and sediment from container. Weed Technol. 1994, 8, 299–303. [Google Scholar] [CrossRef]
- Wasteneys, G.O.; Williamson, R.E. Reassembly of microtubules in Nitella tasmanica: Assembly of cortical microtubules in branching clusters and its relevance to steady-state microtubule assembly. J. Cell Sci. 1989, 93, 705–714. [Google Scholar]
- Sharma, D.; Bhardwaj, R.; Maheshwari, V. Inhibition of photosynthesis by oxyfluorfen. Curr. Sci. 1989, 58, 1334–1336. [Google Scholar]
- Dragoeva, A.; Koleva, V.; Hasanova, N.; Slanev, S. Cytotoxic and genotoxic effects of diphenyl-ether herbicide GOAL (Oxyfluorfen) using the Allium cepa test. Res. J. Mutagen. 2012, 2, 1–9. [Google Scholar] [CrossRef]
- Priya, S.R.; Chinnusamy, C.; Arthanar, M.P.; Janaki, P. Carryover effect and plant injury from oxyfluorfen herbicide applied in transplanted rice. Int. J. Chem. Stud. 2017, 5, 535–539. [Google Scholar]
- Nosratti, I.; Mahdavi-Rad, S.; Heidari, H.; Saeidi, M. Differential tolerance of pumpkin species to bentazon, metribuzin, trifluralin, and oxyfluorfen. Planta Daninha 2017, 35. [Google Scholar] [CrossRef]
- Akey, W.C.; Machado, V.S. Response of onion (Allium cepa) to oxyfluorfen during early seedling development. Can. J. Plant Sci. 1985, 65, 357–362. [Google Scholar] [CrossRef]
- Baker, N.R. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef] [Green Version]
- Krugh, B.W.; Miles, D. Monitoring the effects of five “nonherbicidal” pesticide chemicals on terrestrial plants using chlorophyll fluorescence. Environ. Toxicol. Chem. 1996, 15, 495–500. [Google Scholar] [CrossRef]
- Moreland, D.E.; Farmer, F.S.; Hussey, G.G. Inhibition of photosynthesis and respiration by substituted 2,6-dinitroaniline herbicides. I. Effects on Chloroplast and Mitochondrial Activities. Pestic. Biochem. Physiol. 1972, 2, 342–353. [Google Scholar] [CrossRef]
- Pan, H.; Li, X.; Xu, X.; Gao, S. Phytotoxicity of four herbicides on Ceratophyllum demersum, Vallisneria natans and Elodea nuttallii. J. Environ. Sci. 2009, 21, 307–312. [Google Scholar] [CrossRef]
- Odom, J. Hydrangeas’ Renaissance Gains Momentum in 2016. Available online: https://www.totallandscapecare.com/landscaping/hot-hydrangeas/ (accessed on 17 February 2020).
- Shrubs: In-Demand. Available online: https://www.gardencentermag.com/article/shrubs-in-demand-plants-june-2018 (accessed on 17 February 2020).
- Briggs, J.A.; Whitwell, T.; Riley, M.B. Effect of delayed irrigation on isoxaben and oryzalin runoff from a container nursery. Weed Sci. 2003, 51, 463–470. [Google Scholar] [CrossRef]
- Roe, E.; Buchman, R. Effect of herbicide, dosage, and volume on hazel brush at different foliar stages. For. Sci. 1963, 9, 477–484. [Google Scholar]
- Klingaman, T.E.; King, C.A.; Oliver, L.R. Effect of application rate, weed species, and weed stage of growth on imazethapyr activity. Weed Sci. 1992, 40, 227–232. [Google Scholar] [CrossRef]
- Shim, S.I.; Lee, B.M.; Ryu, E.I.; Kang, B.H. Response of leaf acetolactate synthase from different leaf positions and seedling ages to sulfonylurea herbicide. Pestic. Biochem. Physiol. 2003, 75, 39–46. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Shin, J.S.; Shin, D.Y.; Hyun, K.H.; Burgos, N.R.; Lee, S.; Kuk, Y.I. Tolerance to paraquat-mediated oxidative and environmental stresses in squash (Cucurbita spp.) leaves of various ages. Pestic. Biochem. Physiol. 2011, 99, 65–76. [Google Scholar] [CrossRef]
- Kuk, Y.I.; Shin, J.-S.; Jung, H.; Guh, J.O.; Jung, S.; Burgos, N.R. Mechanism of paraquat tolerance in cucumber leaves of various ages. Weed Sci. 2006, 54, 6–15. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Du, Z.; Chen, X.; Zhou, X.; Kong, X.; Sun, W.; Chen, Z.; Chen, C.; Chen, M. Tender leaf and fully-expanded leaf exhibited distinct cuticle structure and wax lipid composition in Camellia sinensis cv Fuyun 6. Sci. Rep. 2018, 8, 14944. [Google Scholar] [CrossRef] [Green Version]
- Sellers, B.A.; Smeda, R.J.; Johnson, W.G. Diurnal fluctuations and leaf angle reduce glufosinate efficacy. Weed Technol. 2003, 17, 302–306. [Google Scholar] [CrossRef]
- Moustaka, J.; Tanou, G.; Adamakis, I.D.; Eleftheriou, E.P.; Moustakas, M. Leaf age-dependent photoprotective and antioxidative response mechanisms to paraquat-induced oxidative stress in Arabidopsis thaliana. Int. J. Mol. Sci. 2015, 16, 13989–14006. [Google Scholar] [CrossRef] [Green Version]
- Nobossé, P.; Fombang, E.N.; Mbofung, C.M.F. Effects of age and extraction solvent on phytochemical content and antioxidant activity of fresh Moringa oleifera L. leaves. Food Sci. Nutr. 2018, 6, 2188–2198. [Google Scholar] [CrossRef]
- McCullough, P.E.; de Barreda, D.G.; Sidhu, S.; Yu, J. Dithiopyr behavior in smooth crabgrass (Digitaria ischaemum) as influenced by growth stage and temperature. Weed Sci. 2014, 62, 11–21. [Google Scholar] [CrossRef]
- Chun, J.C.; Lee, H.J.; Lim, S.J.; Kim, S.E.; Guh, J.O. Comparative absorption, translocation, and metabolism of foliar-applied oxyfluorfen in wheat and barley. Pestic. Biochem. Physiol. 2001, 70, 118–125. [Google Scholar] [CrossRef]
- Sterling, T.M. Mechanisms of herbicide absorption across plant membranes and accumulation in plant cells. Weed Sci. 1994, 42, 263–276. [Google Scholar] [CrossRef]
- Appleby, A.P.; Valverde, B.E. Behavior of dinitroaniline herbicides in plants. Weed Technol. 1989, 3, 198–206. [Google Scholar] [CrossRef]
- Kunert, K.J.; Homrighausen, C.; Böhme, H.; Böger, P. Oxyfluorfen and lipid peroxidation: Protein damage as a phytotoxic consequence. Weed Sci. 1985, 33, 766–770. [Google Scholar] [CrossRef]
- Anatra-Cordone, M.; King, C.; Klotzbach, J.; Durkin, P.R. Oxyfluorfen-Human Health and Ecological Risk Assessment; Final Report USDA No. SERA TR 05-43-26-03b; Syracuse Environmental Research Associates: Fayetteville, NY, USA, 2005.
- Hugdahl, J.D.; Morejohn, L.C. Rapid and reversible high-affinity binding of the dinitroaniline herbicide oryzalin to tubulin from Zea mays L. Plant Physiol. 1993, 102, 725–740. [Google Scholar] [CrossRef] [Green Version]
- Boutin, C.; Strandberg, B.; Carpenter, D.; Mathiassen, S.K.; Thomas, P.J. Herbicide impact on non-target plant reproduction: What are the toxicological and ecological implications? Environ. Pollut. 2014, 185, 295–306. [Google Scholar] [CrossRef] [Green Version]
- Scholtes, A.B.; Sperry, B.P.; Reynolds, D.B.; Irby, J.T.; Eubank, T.W.; Barber, L.T.; Dodds, D.M. Effect of soybean growth stage on sensitivity to sublethal rates of dicamba and 2,4-D. Weed Technol. 2019, 33, 555–561. [Google Scholar] [CrossRef] [Green Version]
- Van De Sande-Bakhuyzen, H.L.; Alsberg, C.L. The growth curve in annual plants. Physiol. Rev. 1927, 7, 151–187. [Google Scholar] [CrossRef]
- Goudriaan, J.; Van Laar, H.H. Modelling Potential Crop Growth Processes; Current Issues in Production Ecology; Springer: Dordrecht, The Netherlands, 1994; Volume 2, ISBN 978-0-7923-3220-6. [Google Scholar]
- Wang, P.; Li, H.; Jia, W.; Chen, Y.; Gerhards, R. A fluorescence sensor capable of real-time herbicide effect monitoring in greenhouses and the field. Sensors 2018, 18, 3771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanniccari, M.; Tambussi, E.; Istilart, C.; Castro, A.M. Glyphosate effects on gas exchange and chlorophyll fluorescence responses of two Lolium perenne L. biotypes with differential herbicide sensitivity. Plant Physiol. Biochem. 2012, 57, 210–217. [Google Scholar] [CrossRef]
- Bhandary, R.; Whitwell, T.; Briggs, J.A.; Fernandez, R.T. Influence of Surflan ( Oryzalin ) Concentrations in irrigation water on growth and physiological processes of Gardenia jasminoides radicans and Pennisetum rupelli. J. Environ. Hortic. 1997, 15, 169–172. [Google Scholar]
- Lysenko, V.; Varduny, T. Anthocyanin-dependent anoxygenic photosynthesis in coloured flower petals? Sci. Rep. 2013, 3, 3373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomson, W.W.; Whatley, J.M. Development of Nongreen Plastids. Annu. Rev. Plant Physiol. 1980, 31, 375–394. [Google Scholar] [CrossRef]
- Zhang, F.P.; Carins Murphy, M.R.; Cardoso, A.A.; Jordan, G.J.; Brodribb, T.J. Similar geometric rules govern the distribution of veins and stomata in petals, sepals and leaves. New Phytol. 2018, 219, 1224–1234. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; Dang, X.; Cai, X.; Yu, P.; Li, Y.; Zhang, S.; Liu, M.; Chen, B.; Lin, D. Spatio-temporal orientation of microtubules controls conical cell shape in Arabidopsis thaliana petals. PLOS Genet. 2017, 13, e1006851. [Google Scholar] [CrossRef]
- Daugovish, O.; Fennimore, S.A.; Mochizuki, M.J. Integration of oxyfluorfen into strawberry (Fragaria×ananassa) weed management programs. Weed Technol. 2008, 22, 685–690. [Google Scholar] [CrossRef]
- Chaudhari, S.; Jennings, K.M.; Meyers, S.L. Response of sweetpotato to oryzalin application rate and timing. Weed Technol. 2018, 32, 722–725. [Google Scholar] [CrossRef]
- Grabowski, J.M.; Hopen, H.J. Phytotoxic effect of oxyfluorfen vaporization. Weed Sci. 1985, 33, 306–309. [Google Scholar] [CrossRef]
- Farnham, M.W.; Harrison, H.F. Response of broccoli (Brassica oleracea) cultivars to post-transplant oxyfluorfen. Weed Technol. 1995, 9, 385–391. [Google Scholar] [CrossRef]


| Treatment Groups | control | No Herbicide exposure | No Herbicide exposure | No Herbicide exposure | No Herbicide exposure | No Herbicide exposure | No Herbicide exposure | No Herbicide exposure |
|---|---|---|---|---|---|---|---|---|
| GS +5 | Before Herbicide exposure | Herbicide exposure | Recovery phase | Recovery phase | Recovery phase | Recovery phase | Recovery phase | |
| GS +15 | Before Herbicide exposure | Before Herbicide exposure | Herbicide exposure | Recovery phase | Recovery phase | Recovery phase | Recovery phase | |
| GS +25 | Before Herbicide exposure | Before Herbicide exposure | Before Herbicide exposure | Herbicide exposure | Recovery phase | Recovery phase | Recovery phase | |
| GS +35 | Before Herbicide exposure | Before Herbicide exposure | Before Herbicide exposure | Before Herbicide exposure | Herbicide exposure | Recovery phase | Recovery phase | |
| Day 0 | Day 0–5 | Day 5–15 | Day 15–25 | Day 25–35 | Day 35–45 | Day 45–55 | Day 55–65 | |
| Oryzalin Exposure. | ||||||
|---|---|---|---|---|---|---|
| GS | TDB (g) | VR | SPAD | GI (cm) | A (µmol m−2 s−1) | Fv’/Fm’ |
| Control | 143 ± 10 | 10 ± 0a | 36 ± 0.48 | 96 ± 1.1 | 15.9 ± 0.7 | 0.6 ± 0.03 |
| GS+5 | 124 ± 8 | 9.7 ± 0.13ab | 37 ± 0.59 | 92 ± 2.1 | 16.1 ± 0.6 | 0.59 ± 0.01 |
| GS+15 | 126 ± 5 | 9.5 ± 0.23abc | 35 ± 0.57 | 91 ± 0.8 | 14.7 ± 1.4 | 0.6 ± 0.02 |
| GS+25 | 119 ± 8 | 9.3 ± 0.2bc | 36 ± 1.33 | 89 ± 2.1 | 15.2 ± 1.6 | 0.6 ± 0.03 |
| GS+35 | 127 ± 6 | 9 ± 0.16c | 35 ± 1.23 | 90 ± 3.2 | 15.5 ± 1.4 | 0.58 ± 0.02 |
| p-value | NS | <0.0005 | NS | NS | NS | NS |
| Oxyfluorfen exposure | ||||||
| GS | TDB (g) | VR | SPAD | GI (cm) | A (µmol m−2 s−1) | Fv’/Fm’ |
| Control | 128 ± 5a | 10 ± 0a | 37 ± 0.4 | 91 ± 1.1 | 16.5 ± 1.9 | 0.56 ± 0.02 |
| GS+5 | 88 ± 13b | 10 ± 0a | 36 ± 0.61 | 88 ± 2.3 | 15.9 ± 1.9 | 0.53 ± 0.02 |
| GS+15 | 104 ± 7ab | 9.6 ± 0.1b | 35 ± 0.61 | 91 ± 2.8 | 15.2 ± 1.7 | 0.55 ± 0.02 |
| GS+25 | 108 ± 8ab | 9.2 ± 0.13c | 36 ± 0.56 | 89 ± 1.2 | 15.7 ± 1.8 | 0.52 ± 0.02 |
| GS+35 | 128 ± 9ab | 8.7 ± 0.13d | 37 ± 0.42 | 92 ± 1.6 | 15.4 ± 1 | 0.54 ± 0.02 |
| p-value | <0.05 | <0.0005 | NS | NS | NS | NS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poudyal, S.; Owen, J.S., Jr.; Fernandez, R.T.; Cregg, B. Sensitivity of Hydrangea paniculata Plants to Residual Herbicides in Recycled Irrigation Varies with Plant Growth Stage. Water 2020, 12, 1402. https://doi.org/10.3390/w12051402
Poudyal S, Owen JS Jr., Fernandez RT, Cregg B. Sensitivity of Hydrangea paniculata Plants to Residual Herbicides in Recycled Irrigation Varies with Plant Growth Stage. Water. 2020; 12(5):1402. https://doi.org/10.3390/w12051402
Chicago/Turabian StylePoudyal, Shital, James S. Owen, Jr., R. Thomas Fernandez, and Bert Cregg. 2020. "Sensitivity of Hydrangea paniculata Plants to Residual Herbicides in Recycled Irrigation Varies with Plant Growth Stage" Water 12, no. 5: 1402. https://doi.org/10.3390/w12051402
APA StylePoudyal, S., Owen, J. S., Jr., Fernandez, R. T., & Cregg, B. (2020). Sensitivity of Hydrangea paniculata Plants to Residual Herbicides in Recycled Irrigation Varies with Plant Growth Stage. Water, 12(5), 1402. https://doi.org/10.3390/w12051402







