Comparison of Diatom Paleo-Assemblages with Adjacent Limno-Terrestrial Communities on Vega Island, Antarctic Peninsula
Abstract
1. Introduction
2. Materials and Methods
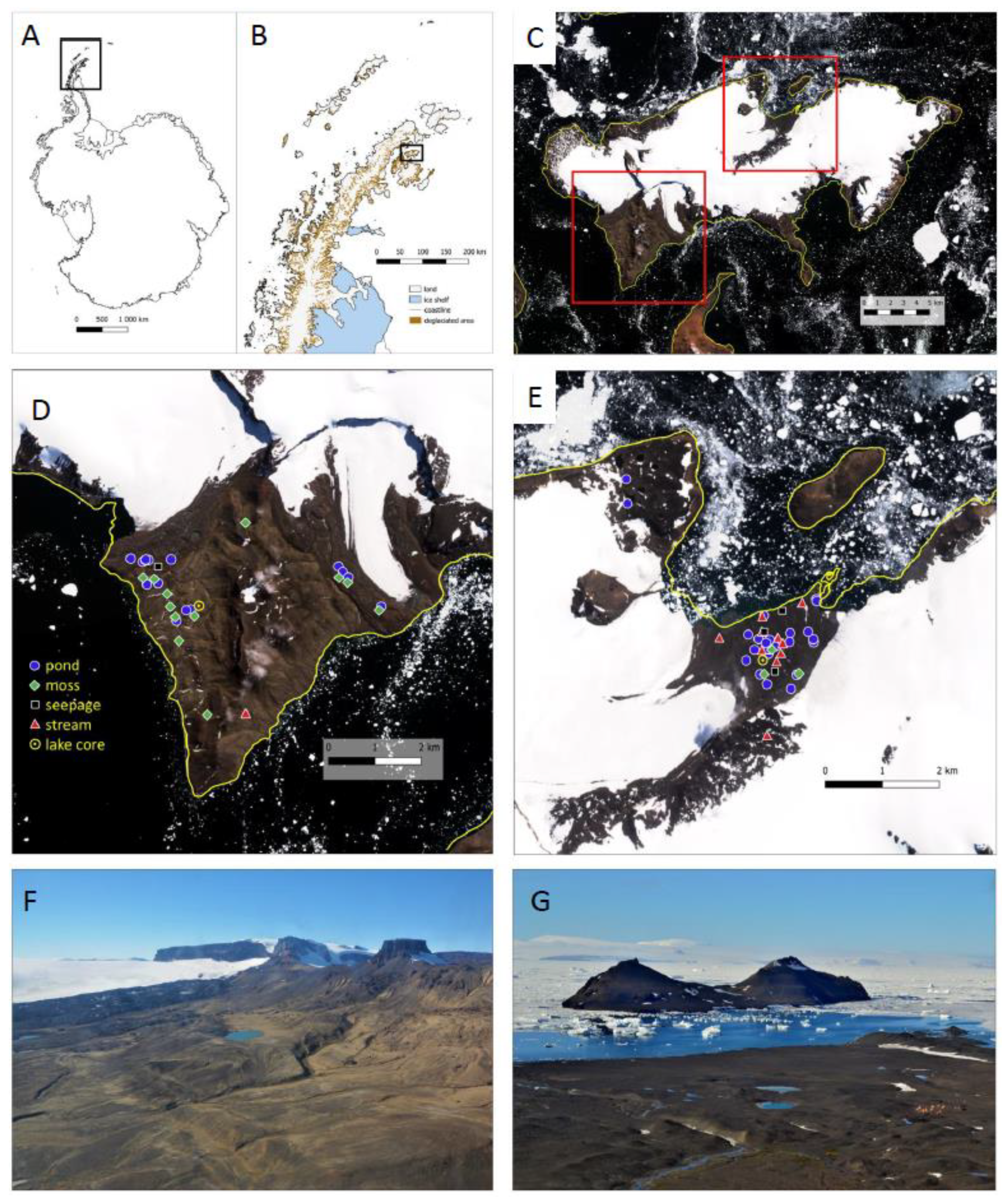
2.1. Site Description
2.2. Sampling of Modern Habitats
2.3. Slide Preparation and Sample Analysis
2.4. Statistical Analysis
3. Results
3.1. Vega Island Diatom Flora and Biogeographic Distributions
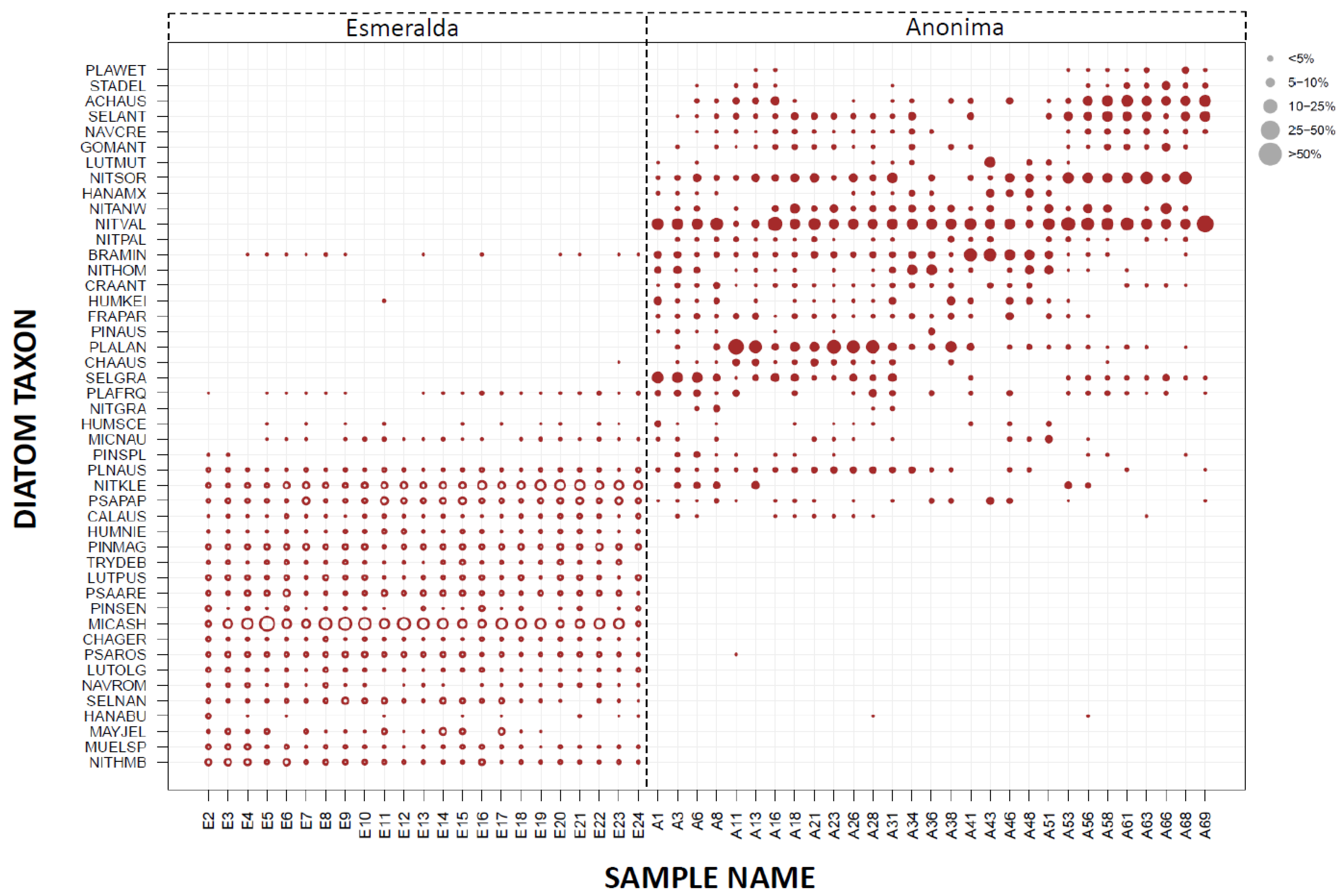
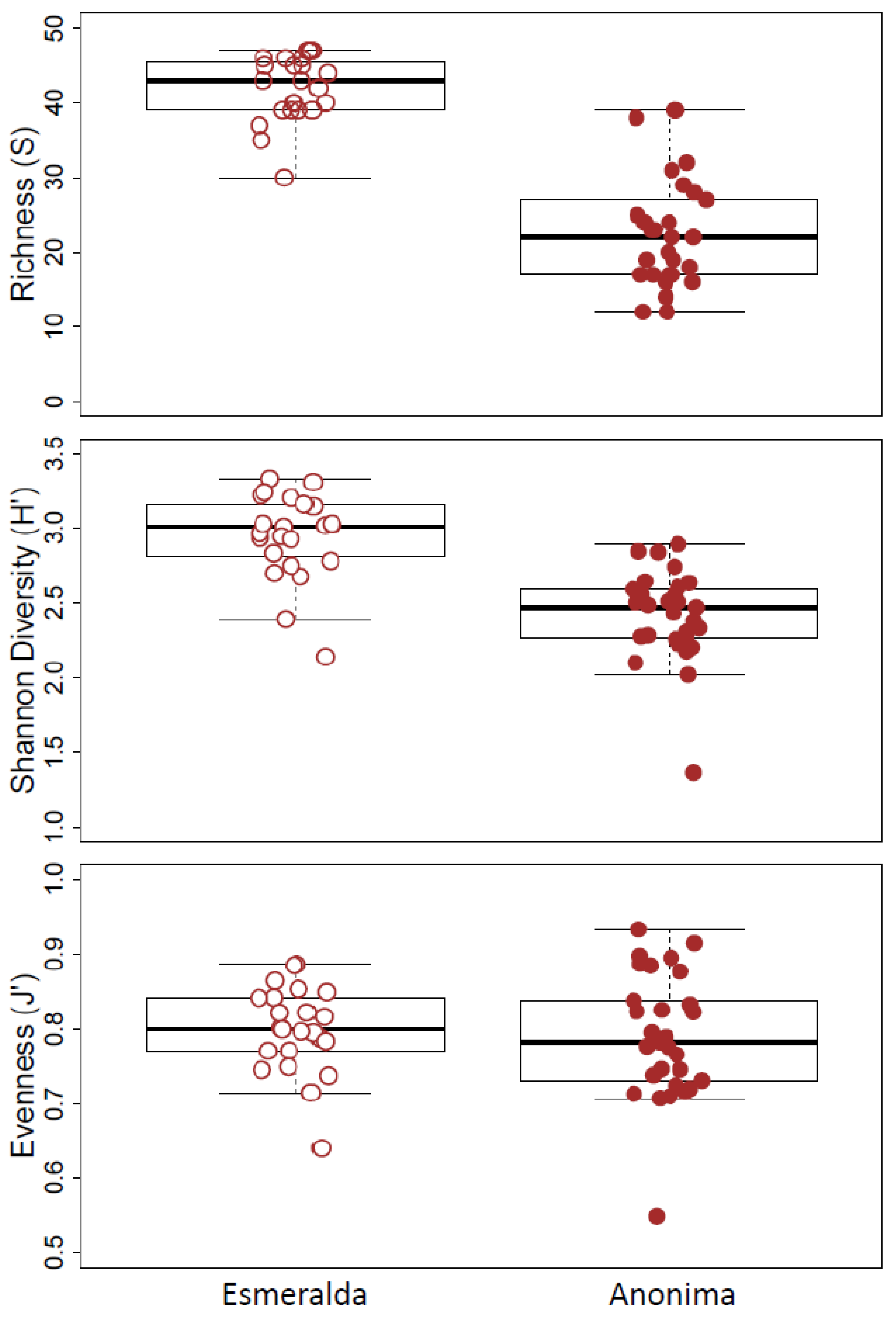
3.2. Paleolimnological Analysis
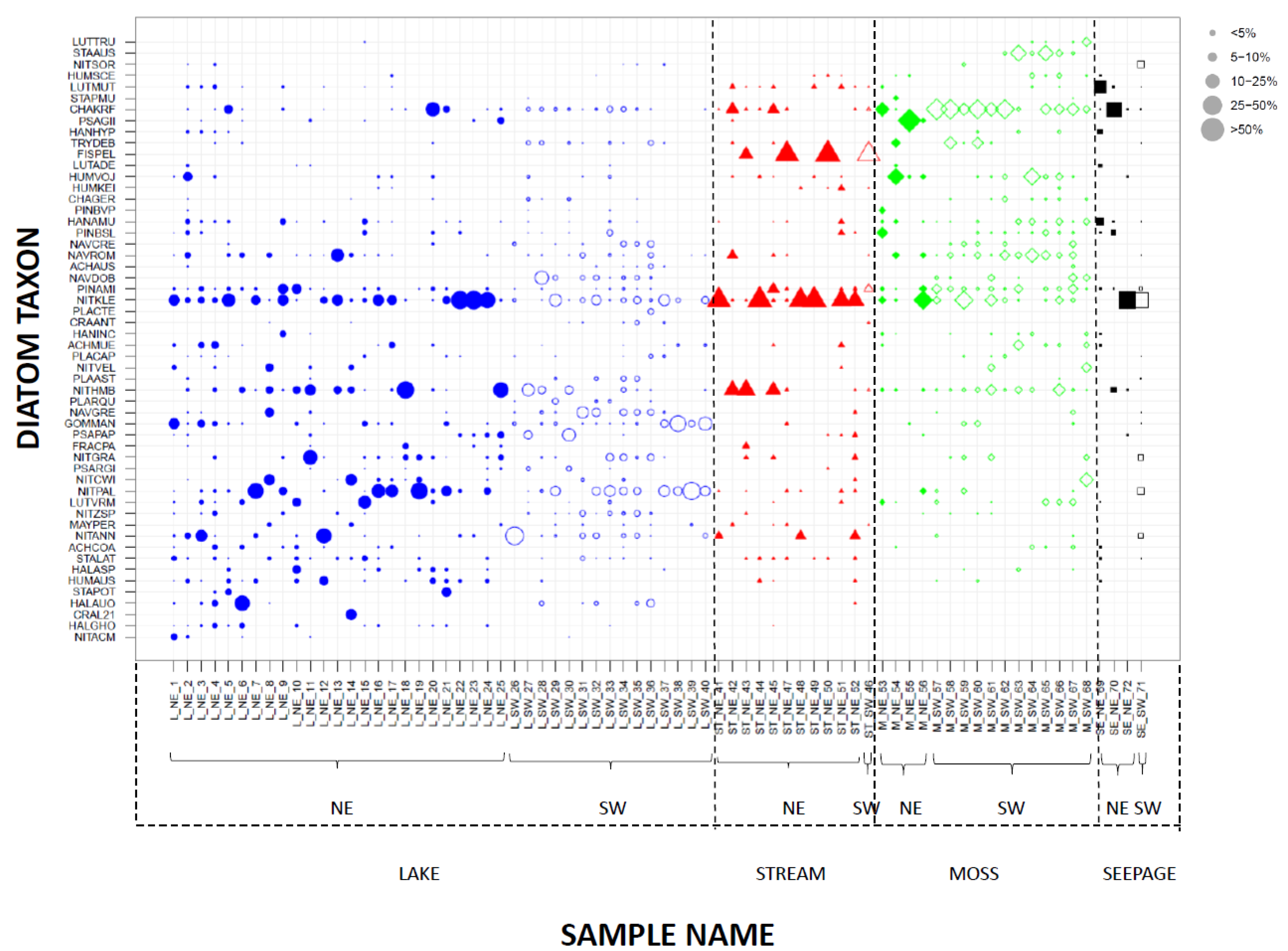
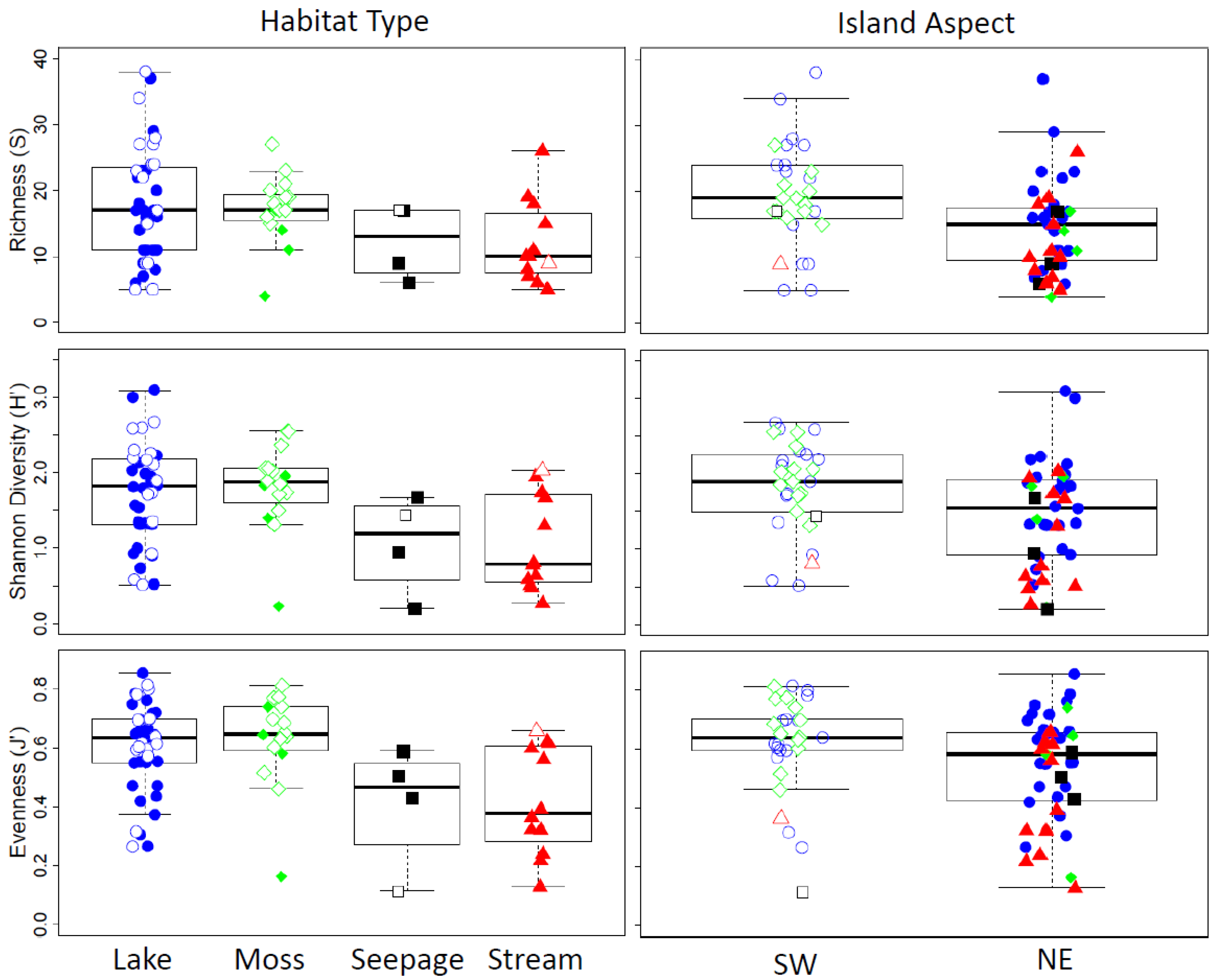
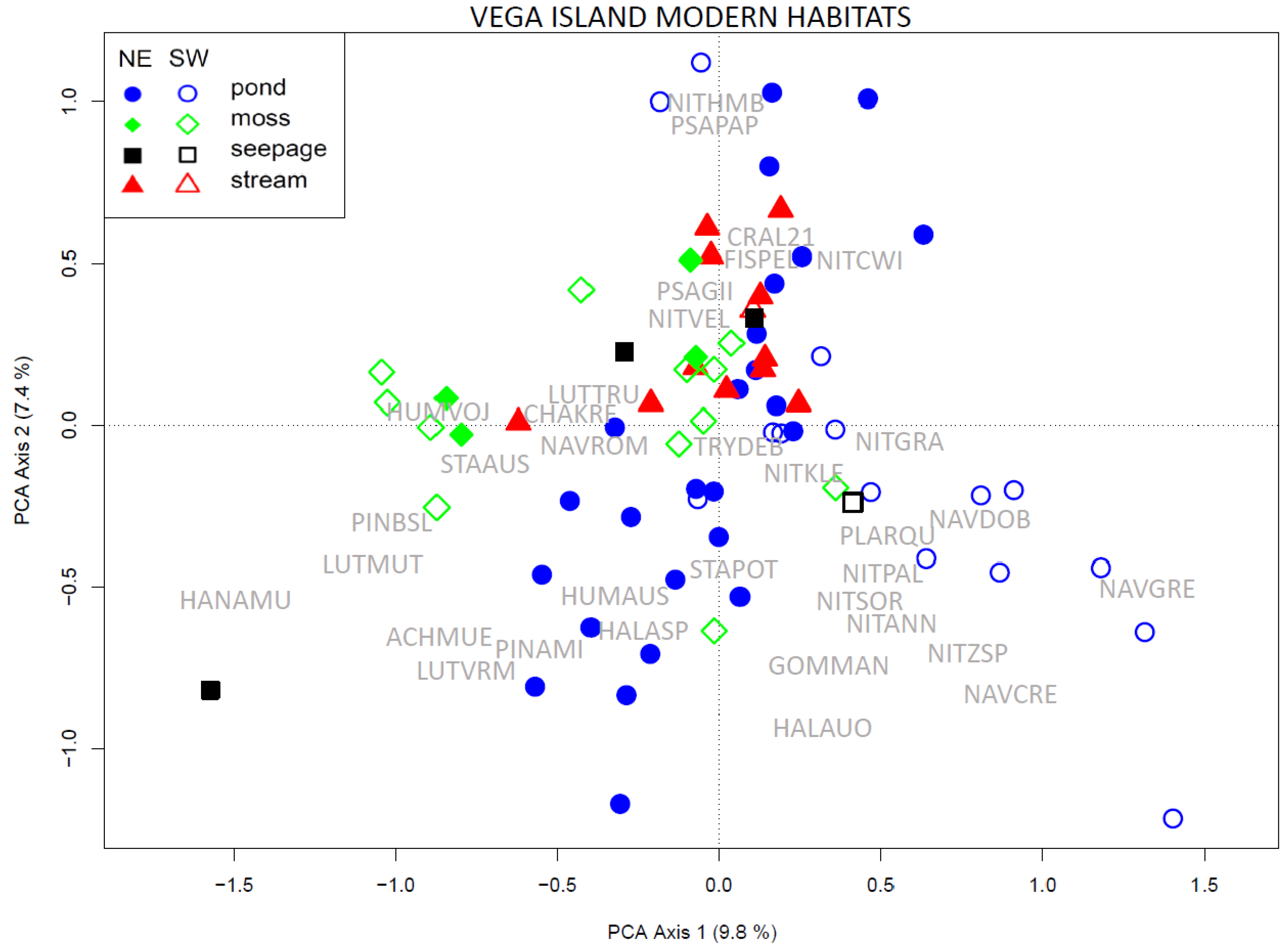
3.3. Species Composition from Modern Habitats
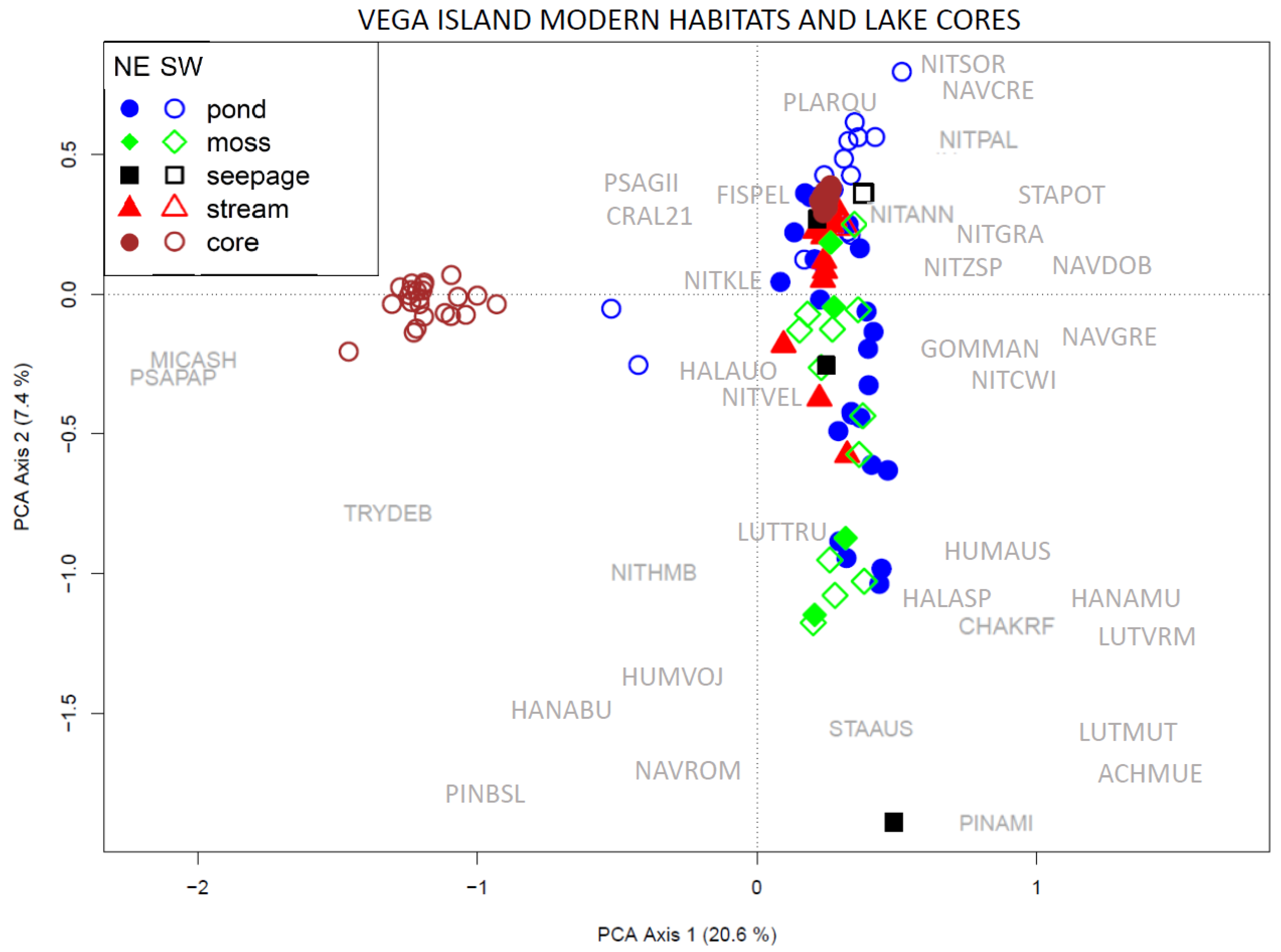
3.4. Comparison of the Entire Vega Island Dataset
4. Discussion
4.1. Comparison of Paleo-Material
4.2. Influence of Habitat Type
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oerlemans, J. Atmospheric science: Extracting a climate signal from 169 glacier records. Science 2005, 308, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Cannone, N.; Diolaiuti, G.; Guglielmin, M.; Smiraglia, C. Accelerating climate change impacts on alpine glacier forefield ecosystems in the European Alps. Ecol. Appl. 2008, 18, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Quayle, W.C.; Peck, L.S.; Peat, H.; Ellis-Evans, J.C.; Harrigan, P.R. Extreme responses to climate change in Antarctic lakes. Science 2002, 295, 645. [Google Scholar] [CrossRef] [PubMed]
- Laybourn-Parry, J.; Pearce, D.A. The biodiversity and ecology of Antarctic lakes: Models for evolution. Philos. Trans. R. Soc. B 2007, 362, 2273–2289. [Google Scholar] [CrossRef]
- Gooseff, M.N.; McKnight, D.M.; Doran, P.; Fountain, A.G.; Lyons, W.B. Hydrological connectivity of the landscape of the McMurdo Dry Valleys, Antarctica. Geogr. Compass 2011, 5, 666–681. [Google Scholar] [CrossRef]
- Roman, M.; Nedbalová, L.; Kohler, T.J.; Lirio, J.M.; Coria, S.H.; Kopáček, J.; Vignoni, P.A.; Kopalová, K.; Lecomte, K.L.; Elster, J.; et al. Lacustrine systems of Clearwater Mesa (James Ross Island, northeastern Antarctic Peninsula): Geomorphological settings and limnological characterization. Antarct. Sci. 2019, 31, 169–188. [Google Scholar] [CrossRef]
- Lee, J.R.; Raymond, B.; Bracegirdle, T.J.; Chades, I.; Fuller, R.A.; Shaw, J.D.; Terauds, A. Climate change drives expansion of Antarctic ice-free habitat. Nature 2017, 547, 49–54. [Google Scholar] [CrossRef]
- Jaros, C.L. Temperature-Elevation Effect on Glacial Meltwater Generation in McMurdo Dry Valleys, Antarctica. Ph.D. Thesis, University of Colorado, Boulder, CO, USA, 2003. [Google Scholar]
- Round, F.E.; Crawford, R.M.; Mann, D.G. Diatoms: Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Smol, J.P.; Stoermer, E.F. The Diatoms: Applications for the Environmental and Earth Sciences, 2nd ed.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Spaulding, S.A.; Van de Vijver, B.; Hodgson, D.A.; McKnight, D.M.; Verleyen, E.; Stanish, L. Diatoms as indicators of environmental change in antarctic and subantarctic freshwaters. In The Diatoms: Applications for the Environmental and Earth Sciences, 2nd ed.; Smol, J.P., Stoermer, E.F., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 267–284. [Google Scholar]
- Chown, S.L.; Convey, P. Spatial and temporal variability across life’s hierarchies in the terrestrial Antarctic. Philos. Trans. R. Soc. B 2007, 362, 2307–2331. [Google Scholar] [CrossRef]
- Zidarova, R.; Kopalová, K.; Van der Vijver, B. Diatoms from the Antarctic Region: Maritime Antarctica. In Iconographia Diatomologica; Lange-Bertalot, H., Ed.; Koeltz Botanical Books: Schmitten, Germany, 2016; Volume 24, pp. 1–509. [Google Scholar]
- Fulford-Smith, S.P.; Sikes, E.L. The evolution of Ace Lake, Antarctica, determined from sedimentary diatom assemblages. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1996, 124, 73–86. [Google Scholar] [CrossRef]
- Roberts, D.; McMinn, A. A weighted-averaging regression and calibration model for inferring lakewater salinity from fossil diatom assemblages in saline lakes of the Vestfold Hills: A new tool for interpreting Holocene lake histories in Antarctica. J. Paleolimnol. 1998, 19, 99–113. [Google Scholar] [CrossRef]
- Roberts, D.; McMinn, A. A diatom-based palaeosalinity history of Ace Lake, Vestfold Hills, Antarctica. Holocene 1999, 9, 401–408. [Google Scholar] [CrossRef]
- Roberts, D.; van Ommen, T.D.; McMinn, A.; Morgan, V.; Roberts, J.L. Late-Holocene East Antarctic climate trends from ice-core and lake-sediment proxies. Holocene 2001, 11, 117–120. [Google Scholar] [CrossRef]
- Roberts, D.; McMinn, A.; Cremer, H.; Gore, D.B.; Melles, M. The Holocene evolution and palaeosalinity history of Beall Lake, Windmill Islands (East Antarctica) using an expanded diatom-based weighted averaging model. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004, 208, 121–140. [Google Scholar] [CrossRef]
- Cremer, H.; Gore, D.; Hultzsch, N.; Melles, M.; Wagner, B. The diatom flora and limnology of lakes in the Amery Oasis, East Antarctica. Polar Biol. 2004, 27, 513–531. [Google Scholar] [CrossRef]
- Hodgson, D.A.; Verleyen, E.; Sabbe, K.; Squier, A.H.; Keely, B.J.; Leng, M.J.; Saunders, K.M.; Vyverman, W. Late Quaternary climate-driven environmental change in the Larsemann Hills, East Antarctica, multi-proxy evidence from a lake sediment core. Quat. Res. 2005, 64, 83–99. [Google Scholar] [CrossRef]
- Björck, S.; Håkansson, H.; Olsson, S.; Barnekow, L.; Janssens, J. Palaeoclimatic studies in South Shetland Islands, Antarctica, based on numerous stratigraphic variables in lake sediments. J. Paleolimnol. 1993, 8, 233–272. [Google Scholar] [CrossRef]
- Sinito, A.M.; Chaparro, M.A.; Irurzun, M.A.; Lirio, J.M.; Nowaczyk, N.R.; Böhnel, H.N.; Nuñez, H. Preliminary palaeomagnetic and rock-magnetic studies on sediments from lake Esmeralda (Vega Island), Anatarctica. Latinmag Lett. 2011, 1, 1–7. [Google Scholar]
- Moreno, L.; Silva-Busso, A.; López-Martínez, J.; Durán-Valsero, J.J.; Martínez-Navarrete, C.; Cuchí, J.A.; Ermolin, E. Hydrogeochemical characteristics at Cape Lamb, Vega Island, Antarctic Peninsula. Antarct. Sci. 2012, 24, 591–607. [Google Scholar] [CrossRef]
- Irurzun, M.A.; Chaparro, M.A.E.; Sinito, A.M.; Gogorza, C.S.G.; Lirio, J.M.; Nuñez, H.; Nowaczyk, N.R.; Böhnel, H.N. Preliminary relative palaeointensity record and chronology on sedimentary cores from Lake Esmeralda (Vega Island, Antarctica). Latinmag Lett. 2013, 3, 1–7. [Google Scholar]
- Irurzun, M.A.; Chaparro, M.A.E.; Sinito, A.M.; Gogorza, C.S.G.; Nuñez, H.; Nowaczyk, N.R.; Böhnel, H.N. Relative palaeointensity and reservoir effect on Lake Esmeralda, Antarctica. Antarct. Sci. 2017, 29, 356–368. [Google Scholar] [CrossRef]
- Píšková, A.; Roman, M.; Bulínová, M.; Pokorný, M.; Sanderson, D.; Cresswell, A.; Lirio, J.M.; Coria, S.H.; Nedbalová, L.; Lami, A.; et al. Late-Holocene palaeoenvironmental changes at Lake Esmeralda (Vega Island, Antarctic Peninsula) based on a multi-proxy analysis of laminated lake sediment. Holocene 2019, 29, 1155–1175. [Google Scholar] [CrossRef]
- Čejka, T.; Nývlt, D.; Kopalová, K.; Bulínová, M.; Kavan, J.; Lirio, J.M.; Coria, S.H.; Van de Vijver, B. Timing of the Neoglacial onset on the north-eastern Antarctic Peninsula based on lacustrine archive from Lake Anónima, Vega Island. Glob. Planet. Chang. 2020, 184, 103050. [Google Scholar] [CrossRef]
- Whittaker, T.E.; Hall, B.L.; Hendy, C.H.; Spaulding, S.A. Holocene depositional environments and surface-level changes at Lake Fryxell, Antarctica. Holocene 2008, 18, 775–786. [Google Scholar] [CrossRef]
- Spaulding, S.A.; McKnight, D.M.; Stoermer, E.F.; Doran, P.T. Diatoms in sediments of perennially ice-covered Lake Hoare, and implications for interpreting lake history in the McMurdo Dry Valleys of Antarctica. J. Paleolimnol. 1997, 17, 403–420. [Google Scholar] [CrossRef]
- Stanish, L.F.; Nemergut, D.R.; McKnight, D.M. Hydrologic processes influence diatom community composition in Dry Valley streams. J. N. Am. Benthol. Soc. 2011, 30, 1057–1073. [Google Scholar] [CrossRef]
- Douglas, M.S.V.; Smol, J.P.; Blake, W., Jr. Marked post-18th century environmental change in high-arctic ecosystems. Science 1994, 226, 416–419. [Google Scholar] [CrossRef]
- Guglielmin, M.; Dalle Fratte, M.; Cannone, N. Permafrost warming and vegetation changes in continental Antarctica. Environ. Res. Lett. 2014, 9, 045001. [Google Scholar] [CrossRef]
- Gooseff, M.N.; Barrett, J.E.; Adams, B.J.; Doran, P.T.; Fountain, A.G.; Lyons, W.B.; McKnight, D.M.; Priscu, J.C.; Sokol, E.R.; Takacs-Vesbach, C.; et al. Decadal ecosystem response to an anomalous melt season in a polar desert in Antarctica. Nat. Ecol. Evol. 2017, 1, 1334–1338. [Google Scholar] [CrossRef]
- Kopalová, K.; Soukup, J.; Kohler, T.J.; Roman, M.; Coria, S.H.; Vignoni, P.A.; Lecomte, K.L.; Nedbalová, L.; Nývlt, D.; Lirio, J.M. Habitat controls on limno-terrestrial diatom communities of Clearwater Mesa, James Ross Island, Maritime Antarctica. Polar Biol. 2019, 42, 1595–1613. [Google Scholar] [CrossRef]
- Van de Vijver, B.; Beyens, L. Biogeography and ecology of freshwater diatoms in Subantarctica: A review. J. Biogeogr. 1999, 26, 993–1000. [Google Scholar] [CrossRef]
- Van de Vijver, B.; Gremmen, N.; Smith, V. Diatom communities from the sub-Antarctic Prince Edward Islands: Diversity and distribution patterns. Polar Biol. 2008, 31, 795–808. [Google Scholar] [CrossRef]
- Kopalová, K.; Veselá, J.; Elster, J.; Nedbalová, L.; Komárek, J.; Van de Vijver, B. Benthic diatoms (Bacillariophyta) from seepages and streams on James Ross Island (NW Weddell Sea, Antarctica). Plant Ecol. Evol. 2012, 145, 190–208. [Google Scholar] [CrossRef]
- Kopalová, K.; Nedbalová, L.; Van de Vijver, B. Moss-inhabiting diatoms from two contrasting Maritime Antarctic islands. Plant Ecol. Evol. 2014, 147, 67–84. [Google Scholar] [CrossRef]
- Kopalová, K.; Nedbalová, L.; Nývlt, D.; Elster, J.; Van de Vijver, B. Diversity, ecology and biogeography of the freshwater diatom communities from Ulu Peninsula (James Ross Island, NE Antarctic Peninsula). Polar Biol. 2013, 36, 933–948. [Google Scholar] [CrossRef]
- Esposito, R.M.M.; Horn, S.L.; McKnight, D.M.; Cox, M.J.; Grant, M.C.; Spaulding, S.A.; Doran, P.T.; Cozzetto, K.D. Antarctic climate cooling and response of diatoms in glacial meltwater streams. Geophys. Res. Lett. 2006, 33, 2–5. [Google Scholar] [CrossRef]
- Stanish, L.F.; Kohler, T.J.; Esposito, R.M.M.; Simmons, B.L.; Nielsen, U.N.; Wall, D.H.; Nemergut, D.R.; McKnight, D.M. Extreme streams: Flow intermittency as a control on diatom communities in meltwater streams in the McMurdo Dry Valleys, Antarctica. In spec issue “A New Hydrology: Inflow Effects on Ecosys”. Can. J. Fish. Aquat. Sci. 2012, 69, 1405–1419. [Google Scholar] [CrossRef]
- Kohler, T.J.; Stanish, L.F.; Crisp, S.W.; Koch, J.C.; Liptzin, D.; Baeseman, J.L.; McKnight, D.M. Life in the Main Channel: Long-Term Hydrologic Control of Microbial Mat Abundance in McMurdo Dry Valley Streams, Antarctica. Ecosystems 2015, 18, 310–327. [Google Scholar] [CrossRef]
- Hrbáček, F.; Nývlt, D.; Láska, K. Active layer thermal dynamics at two lithologically different sites on James Ross Island, Eastern Antarctic Peninsula. Catena 2017, 149, 592–602. [Google Scholar] [CrossRef]
- Ambrožová, K.; Láska, K.; Hrbáček, F.; Kavan, J.; Ondruch, J. Air temperature and lapse rate variation in the icefree and glaciated areas of northern James Ross Island, Antarctic Peninsula, during 2013–2016. Int. J. Climatol. 2019, 39, 643–657. [Google Scholar] [CrossRef]
- Van Lipzig, N.P.M.; King, J.C.; Lachlan-Cope, T.A.; Van den Broeke, M.R. Precipitation, sublimation, and snow drift in the Antarctic Peninsula region from a regional atmospheric model. J. Geophys. Res. Atmos. 2004, 109. [Google Scholar] [CrossRef]
- Turner, J.; Lachlan-Cope, T.A.; Marshall, G.J.; Morris, E.M.; Mulvaney, R.; Winter, W. Spatial variability of Antarctic Peninsula net surface mass balance. J. Geophys. Res. Atmos. 2002, 107. [Google Scholar] [CrossRef]
- Nývlt, D.; Nývltová Fišáková, M.; Barták, M.; Stachoň, Z.; Pavel, V.; Mlčoch, B.; Láska, K. Death age, seasonality, taphonomy and colonization of seal carcasses from Ulu Peninsula, James Ross Island, Antarctic Peninsula. Antarct. Sci. 2016, 28, 3–16. [Google Scholar] [CrossRef]
- Crame, J.A.; Francis, J.E.; Cantrill, D.J.; Pirrie, D. Maastrichtian stratigraphy of Antarctica. Cretaceous. Res. 2004, 25, 411–423. [Google Scholar] [CrossRef]
- Košler, J.; Magna, T.; Mlčoch, B.; Mixa, P.; Nývlt, D.; Holub, F.V. Combined Sr, Nd, Pb and Li isotope geochemistry of alkaline lavas from northern James Ross Island (Antarctic Peninsula) and implications for backarc magma formation. Chem. Geol. 2009, 258, 207–218. [Google Scholar] [CrossRef]
- Altunkaynak, S.; Aldanmaz, E.; Güraslan, I.N.; Caliskanoglu, A.Z.; Ünal, A.; Nývlt, D. Lithostratigraphy and petrology of Lachman Crags and Cape Lachman lava-fed deltas, Ulu Peninsula, James Ross Island, north-eastern Antarctic Peninsula: Preliminary results. Czech Polar Rep. 2018, 8, 60–83. [Google Scholar] [CrossRef]
- Nelson, P.H.H. The James Ross Island Volcanic Group of North-East Graham Land; British Antarctic Survey: Cambridge, UK, 1966; Volume 54. [Google Scholar]
- Smellie, J.L.; Johnson, J.S.; Nelson, A.E. Geological Map of James Ross Island. I. James Ross Island Volcanic Group; British Antarctic Survey: Cambridge, UK, 2013. [Google Scholar]
- Smellie, J.L.; Johnson, J.S.; McIntosh, W.C.; Esser, R.; Gudmundsson, M.T.; Hambrey, M.J.; De Vries, B.V.W. Six million years of glacial history recorded in volcanic lithofacies of the James Ross Island Volcanic Group, Antarctic Peninsula. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 260, 122–148. [Google Scholar] [CrossRef]
- Zale, R.; Karlén, W. Lake sediment cores from the Antarctic Peninsula and surrounding islands. Geogr. Ann. Ser. A Phys. Geogr. 1989, 71, 211–220. [Google Scholar] [CrossRef]
- Robinson, S.A.; Wasley, J.; Tobin, A.K. Living on the edge-plants and global change in continental and maritime Antarctica. Glob. Chang. Biol. 2003, 9, 1681–1717. [Google Scholar] [CrossRef]
- Rinaldi, C.A. The upper cretaceous in the James Ross Island group. In Antarctic Geoscience; University of Wisconsin Press: Madison, WI, USA, 1982; pp. 281–286. [Google Scholar]
- Pirrie, D.; Crame, J.A.; Riding, J.B. Late Cretaceous stratigraphy and sedimentology of Cape Lamb, Vega Island, Antarctica. Cretaceous. Res. 1991, 12, 227–258. [Google Scholar] [CrossRef]
- Lirio, J.M.; Chaparro, A.; Yermolin, E.; Busso, S.A.; Brizuela, M. Carecteristicas batimetricas de las Lagunas Esmeralda y Pan Negro, Cabo Lamb, Isla Vega, Antartida. In VI Simposio Argentino y III Latinoamericano Sobre Investigaciones Antárticas; Dirección Nacional del Antártico/Instituto Antártico Argentino: Buenos Aires, Argentina, 2007. [Google Scholar]
- Marinsek, S.; Ermolin, E. 10-year mass balance by glaciological and geodetic methods of Glaciar Bahía del Diablo, Vega Island, Antarctic Peninsula. Ann. Glaciol. 2015, 56, 141–146. [Google Scholar] [CrossRef]
- Skvarca, P.; De Angelis, H.; Ermolin, E. Mass balance of ´Glaciar Bah9a del Diablo´, Vega Island, Antarctic Peninsula. Ann. Glaciol. 2004, 39, 209–213. [Google Scholar] [CrossRef][Green Version]
- Chaparro, M.A.E.; Chaparro, M.A.E.; Córdoba, F.E.; LeComte, K.L.; Gargiulo, J.D.; Barrios, A.M.; Urán, G.M.; Manograsso Czalbowski, N.T.; Lavat, A.; Böhnel, H.N. Sedimentary analysis and magnetic properties of Lake Anónima, Vega Island. Antarct. Sci. 2017, 29, 1–16. [Google Scholar] [CrossRef]
- Van der Werff, A. A new method of concentrating and cleaning diatoms and other organisms. Verh. Int. Verein. Theor. Angew. Limnol. 1955, 2, 276–277. [Google Scholar] [CrossRef]
- Kopalová, K. Taxonomy, Ecology and Biogeography of Aquatic and Limno-Terrestrial Diatoms (Bacillariophyta) in the Maritime Antarctic Region. Ph.D. Thesis, Charles University, Prague, Czechia, 2013. [Google Scholar]
- Shannon, C.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Pielou, E. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Juggins, S. Rioja: Analysis of Quaternary Science Data. R Package Version 0.9-15.1. 2017. Available online: https://cran.r-project.org/web/packages/rioja/index.html (accessed on 12 October 2017).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-2. 2018. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 12 October 2017).
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Aust. Ecol. 2001, 26, 32–46. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Kohler, T.J.; Chatfield, E.; Gooseff, M.N.; Barrett, J.E.; McKnight, D.M. Recovery of Antarctic stream epilithon from simulated scouring events. Antarct. Sci. 2015, 27, 341–354. [Google Scholar] [CrossRef]
- Smol, J.P.; Cumming, B.F. Tracking long-term changes in climate using algal indicators in lake sediments. J. Phycol. 2000, 36, 986–1011. [Google Scholar] [CrossRef]
- Douglas, M.S.V.; Hamilton, P.B.; Pienitz, R.; Smol, J.P. Algal indicators of environmental change in Arctic and Antarctic lakes and ponds. In Long-Term Environmental Change in Arctic and Antarctic Lakes; Pienitz, R., Douglas, M., Smol, J., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 117–158. [Google Scholar]
- Foreman, C.M.; Wolf, C.F.; Priscu, J.C. Impact of episodic warming events. Aquat. Geochem. 2004, 10, 239–268. [Google Scholar] [CrossRef]
- Hodgson, D.A.; Smol, J.P. High latitude paleolimnology. In Polar Lakes and Rivers—Limnology of Arctic and Antarctic Aquatic Ecosystems; Vincent, W.F., Laybourn-Parry, J., Eds.; Oxford University Press: Oxford, UK, 2008; pp. 43–64. [Google Scholar]
- Chipev, N.; Temniskova-Topalova, D. Diversity, dynamics and distribution of diatom assemblages in land habitats on the Livingston Island (the Antarctic). Bulgarian Antarct. Res. Life Sci. 1999, 2, 32–42. [Google Scholar]
- Van de Vijver, B.; Beyens, L.; Vincke, S.; Gremmen, N.J. Moss-inhabiting diatom communities from Heard Island, sub-Antarctic. Polar Biol. 2004, 27, 532–543. [Google Scholar] [CrossRef]
- Vinocur, A.; Maidana, N.I. Spatial and temporal variations in moss-inhabiting summer diatom communities from Potter Peninsula (King George Island, Antarctica). Polar Biol. 2010, 33, 443–455. [Google Scholar] [CrossRef]
- Jones, V.J.; Juggins, S.; Ellis-Evans, J.C. The relationship between water chemistry and surface sediment diatom assemblages in maritime Antarctic lakes. Antarct. Sci. 1993, 5, 339–348. [Google Scholar] [CrossRef]
- McKnight, D.M.; Runkel, R.L.; Tate, C.M.; Duff, J.H.; Moorhead, D.L. Inorganic N and P dynamics of Antarctic glacial meltwater streams as controlled by hyporheic exchange and benthic autotrophic communities. J. N. Am. Benthol. Soc. 2004, 23, 171–188. [Google Scholar] [CrossRef]
- Sakaeva, A.; Sokol, E.R.; Kohler, T.J.; Stanish, L.F.; Spaulding, S.A.; Howkins, A.; Welch, K.A.; Lyons, W.B.; Barrett, J.E.; McKnight, D.M. Evidence for dispersal and habitat controls on pond diatom communities from the McMurdo Sound Region of Antarctica. Polar Biol. 2016, 39, 2441–2456. [Google Scholar] [CrossRef]
- Kellogg, T.B.; Kellogg, D.E. Non-marine and littoral diatoms from Antarctic and Sub-Antarctic regions. Distribution and updated taxonomy. Diatom Monogr. 2002, 1, 1–795. [Google Scholar]
- Hamsher, S.; Kopalova, K.; Kociolek, J.P.; Zidarova, R.; Van De Vijver, B. The genus Nitzschia on the South Shetland Islands and James Ross Island. Fottea 2007, 16, 79–102. [Google Scholar] [CrossRef]
- Kohler, T.J.; Van Horn, D.J.; Darling, J.P.; Takacs-Vesbach, C.D.; McKnight, D.M. Nutrient treatments alter microbial mat colonization in two glacial meltwater streams from the McMurdo Dry Valleys, Antarctica. FEMS Microbiol. Ecol. 2016, 92, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stabell, B. The development and succession of taxa within the diatom genus Fragilaria Lyngbye as a response to basin isolation from the sea. Boreas 1985, 14, 273–286. [Google Scholar] [CrossRef]
- Denys, L. Fragilaria blooms in the Holocene of the western coastal plain of Belgia. In Proceedings of the 10th International Diatom Symposium, Joensuu, Finland, 28 August–2 September 1988. [Google Scholar]
- Kopalová, K.; Kociolek, J.P.; Lowe, R.L.; Zidarova, R.; Van de Vijver, B. Five new species of the genus Humidophila (Bacillariophyta) from the Maritime Antarctic Region. Diatom Res. 2015, 30, 117–131. [Google Scholar] [CrossRef]
- Cannone, N.; Guglielmin, M.; Convey, P.; Worland, M.R.; Favero Longo, S.E. Vascular plant changes in extreme environments: Effects of multiple drivers. Clim. Chang. 2016, 134, 651–665. [Google Scholar] [CrossRef]
- Cannone, N.; Dalle Fratte, M.; Convey, P.; Worland, M.R.; Guglielmin, M. Ecology of moss banks at Signy Island (maritime Antarctica). Bot. J. Linn. Soc. 2017, 184, 518–533. [Google Scholar] [CrossRef]
| Biogeographic Distribution | Vega Island | Devil’s Bay | Cape Lamb | James Ross Island | Livingston Island |
|---|---|---|---|---|---|
| % | % | % | % | % | |
| Cosmopolitan | 19.08 | 20.93 | 18.18 | 29.37 | 28.37 |
| Maritime Antarctic | 41.62 | 45.74 | 40.56 | 38.89 | 47.52 |
| Unidentified | 21.97 | 16.28 | 23.08 | 10.32 | 9.22 |
| Maritime and Continental Antarctic | 5.78 | 6.98 | 6.29 | 7.14 | 4.26 |
| Maritime and Sub-Antarctic | 2.89 | 3.88 | 2.8 | 7.94 | 9.93 |
| Maritime Antarctic and South America | 2.31 | 0.78 | 2.8 | 0 | 0 |
| Southern Hemisphere | 4.05 | 3.88 | 3.5 | 0 | 0 |
| Continental and Maritime Antarctic and South America | 0.58 | 0.78 | 0.7 | 0 | 0 |
| Maritime, Continental, and Sub-Antarctic (entire Antarctic Region) | 0.58 | 0.78 | 0.7 | 0.79 | 0.71 |
| Marine | 0.58 | 0 | 0.7 | 5.56 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulínová, M.; Kohler, T.J.; Kavan, J.; Van de Vijver, B.; Nývlt, D.; Nedbalová, L.; Coria, S.H.; Lirio, J.M.; Kopalová, K. Comparison of Diatom Paleo-Assemblages with Adjacent Limno-Terrestrial Communities on Vega Island, Antarctic Peninsula. Water 2020, 12, 1340. https://doi.org/10.3390/w12051340
Bulínová M, Kohler TJ, Kavan J, Van de Vijver B, Nývlt D, Nedbalová L, Coria SH, Lirio JM, Kopalová K. Comparison of Diatom Paleo-Assemblages with Adjacent Limno-Terrestrial Communities on Vega Island, Antarctic Peninsula. Water. 2020; 12(5):1340. https://doi.org/10.3390/w12051340
Chicago/Turabian StyleBulínová, Marie, Tyler J. Kohler, Jan Kavan, Bart Van de Vijver, Daniel Nývlt, Linda Nedbalová, Silvia H. Coria, Juan M. Lirio, and Kateřina Kopalová. 2020. "Comparison of Diatom Paleo-Assemblages with Adjacent Limno-Terrestrial Communities on Vega Island, Antarctic Peninsula" Water 12, no. 5: 1340. https://doi.org/10.3390/w12051340
APA StyleBulínová, M., Kohler, T. J., Kavan, J., Van de Vijver, B., Nývlt, D., Nedbalová, L., Coria, S. H., Lirio, J. M., & Kopalová, K. (2020). Comparison of Diatom Paleo-Assemblages with Adjacent Limno-Terrestrial Communities on Vega Island, Antarctic Peninsula. Water, 12(5), 1340. https://doi.org/10.3390/w12051340





