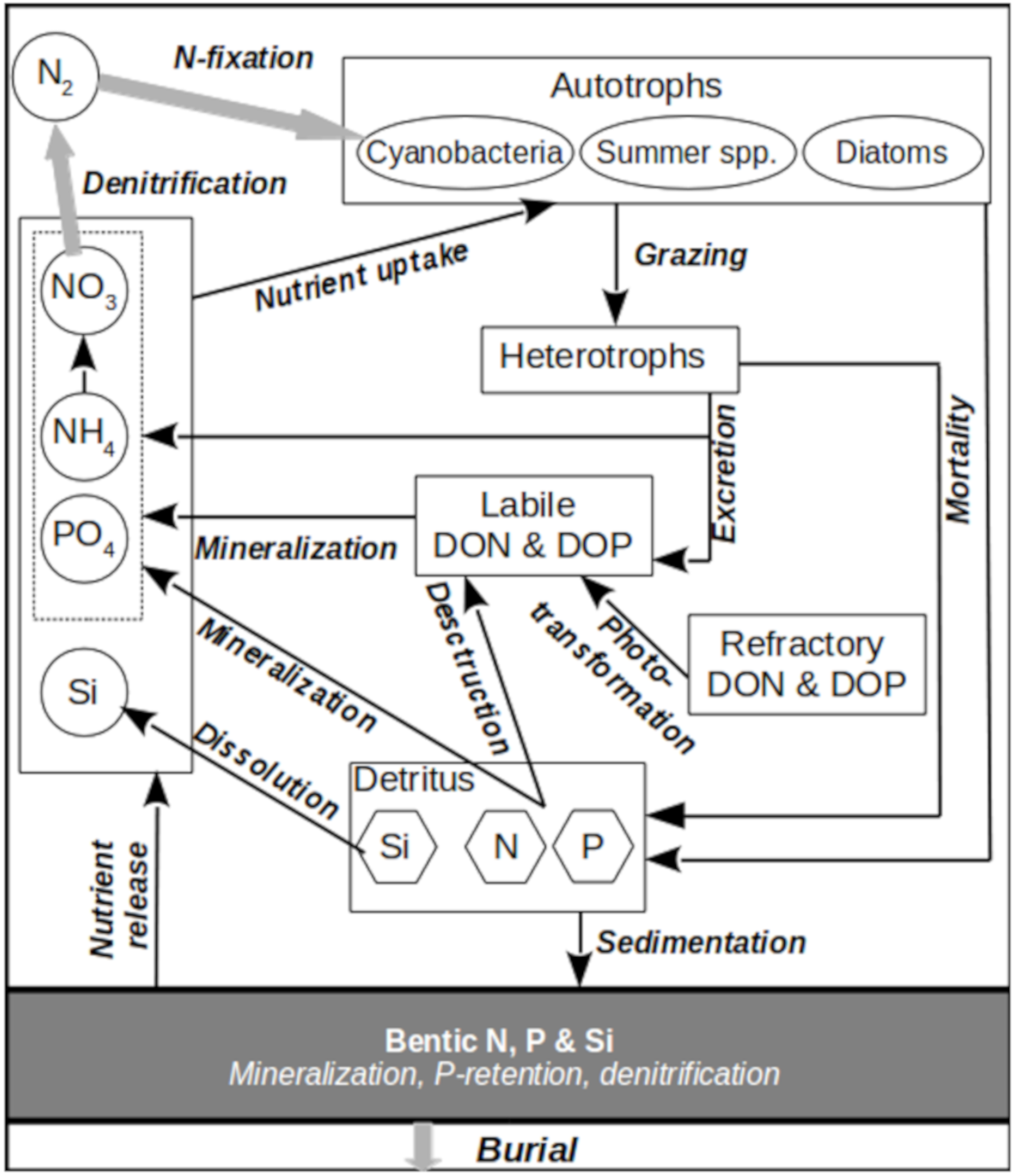
2.1. Modification of the SPBEM-2 Model
The St. Petersburg model of eutrophication (SPBEM) implemented in this study is a further development of the biogeochemical formulations suggested in [
6,
32], which gradually evolved into the 3D SPBEM model [
9,
33,
34,
35]. An updated version of the SPBEM-2 model was presented in [
31], where the hydrodynamic module was based on the MIT general circulation model (MITgcm), with corresponding adjustments. In [
31], the configuration of the MITgcm, used to simulate the hydro-thermodynamics of the Gulf of Finland, was described in detail, including the initial and boundary conditions. In addition, a detailed validation of the hydrodynamic module was performed. In addition to changes in the hydrodynamic module, the same study further presented a modification of the biogeochemical module of the SPBEM family, in which the labile and refractory fractions of dissolved organic nitrogen and phosphorus were introduced as new dependent variables and were described with four additional equations. Here, we further develop the dissolved organic matter sub-model and provide a full description of it.
The original formulation and initial versions of SPBEM intentionally described only the bioavailable fractions of organic nitrogen and phosphorus, both in the external inputs and cycling through the pelagic and sediment subsystems. Moreover, all the “dead” organic matter, both in its particulate and dissolved states, was assumed to be lumped together in detritus variables. Here, we retain variables representing particulate organic nutrients but expand the model with four variables representing the labile and refractory fractions of dissolved organic nitrogen (DON) and phosphorus (DOP) (
Table 1).
In the current version of SPBEM-2, the scheme of biogeochemical fluxes (
Figure 1,
Table 2), described by the biogeochemical translocation functions
(the “right-hand sides”), in a system of advection-diffusion equations is defined as follows:
The equations for the sediment variables are written as:
Most of the parameterizations implemented in Equations (1)–(9) and (14)–(17) were drawn from our previous publications, particularly from [
6]. For the sake of the readers’ convenience, a full mathematical description of these formulations is repeated in
Appendix A, together with new formulations for dissolved organic nutrients, where the initial forms of the formulations were taken from the carbon incarnation of BALTSEM (BALTSEM-C) [
13]. Here, we discuss the assumptions behind the chosen definitions and interpret the newly introduced variables.
The new translocation functions (Equations (10)–(13)), describing biogeochemical transformations of the dissolved organic nitrogen (DON) and phosphorus (DOP), include the following sources and sinks described below. Since our original formulation [
6,
32,
34] does not explicitly resolve diurnal processes, we do not account for dissolved organic substances whose lifetime is a day or less. We are also not interested in the geological scales of thousands of years. Therefore, only two fractions of DON and DOP were introduced into the modified SPBEM-2. The first fraction is considered to be “labile,” with a characteristic lifetime of about 1 year. The sources of labile organic matter are: inputs from land, part of the excretion of heterotrophs (E
N(P)), part of mineralized detritus (W
DN(P)), and influx due to the photochemical transformation of refractory organic matter (W
DRN(P)). A sink for labile organic matter is mineralization (W
DLN(P)) into dissolved inorganic nitrogen and phosphorus.
where
is mineralization rate and
is a temperature constant (see
Table A3).
The second fraction includes all the remaining dissolved organic compounds considered “refractory,” and its transformation is determined only by the process of photochemical oxidation. In the present version of our model, we disregard the autochthonous production of refractory material considered, for instance, in the ERSEM model [
36]. Thus, the only source of refractory organic nutrients here is that part of the land inputs which comes with the river runoff as humic substances and iron-humate complexes [
37,
38]. A small part of the refractory DON and DOP is transferred into a bioavailable labile form by the photochemical transformation (W
DRN(P)).
where
is the photosynthetically active radiation on the z level (see Equation (A6)),
is a rate of photo-transformation, and
is a factor describing faster extinction of the ultraviolet radiation with depth (see
Table A3).
As a result of this modification of the dissolved organic matter sub-model, the directions and interpretations of the biogeochemical fluxes have been changed, compared to [
31]. In the presented version of SPBEM-2, part of the decomposed detritus is immediately mineralized to ammonium and phosphate, and part is hydrolyzed to a labile form of organic matter. Heterotroph excretion fluxes have also been revised. In the new version, some of the catabolic products are excreted in organic form and enter the pool of labile organic matter. However, we still disregard both the extracellular excretion (exudation) and possible utilization of DON and DOP by the phytoplankton.
Adding equations for the dissolved form of organic matter required a revision of the sedimentation and mineralization rates of detritus. Because in the previous model’s version (SPBEM), the variables of particulate nitrogen detritus and particulate phosphorus detritus were the only form of dead organic matter, its sedimentation rate was underestimated in comparison with the observed values in order to account for the DOM implicitly included in detritus variables. The introduction of dissolved organic nutrients made it possible to adjust the detritus sinking velocity closer to the reported ranges [
23,
39,
40], increasing it, for example, from 2.6 to 6.6 m day
−1 (at 10 °C). These values may seem underestimated in comparison to the sinking velocities of diatom aggregates and fecal pellets of 50–100 m day
−1 [
39], which, however, are considered to be a minor component of the total downward flux of the organic matter in the Baltic Sea [
41]. Such an augmentation of the detritus removal from the surface layers required a significant intensification of the detritus destruction rate, from 0.004 to 0.088 day
−1 (at 10 °C). As a result, the nutrient retention capacity in the water column was maintained almost the same as in the comparably well-validated simulation in [
31].
A full mathematical description of the parameterizations used in Equations (1)–(17), including all the coefficients and constants, is given in the
Appendix A.
2.3. Model Set-Up and Initial and Boundary Conditions
The computational grid covers the Gulf of Finland from Neva Bay to the meridian of 24.08° E, where the open boundary is located (
Figure 2). The horizontal resolution of the spherical grid is 2′ in latitude and 4′ in longitude (~2 nautical miles), and the vertical resolution for the z-coordinate is 3 m from the surface to the bottom.
The initial fields of the temperature, salinity, and biogeochemical variables of the model were built from the observed vertical profiles of these oceanographic parameters, which were averaged for the winter months of 2002–2012 by interpolation into the nodes of the computational grid. The values of the sea level elevation and components of the current velocity were initially set equal to zero. The initial fields of benthic variables were taken from the final distributions, simulated from 2009 to 2014 using the previous version of SPBEM-2 and discussed in [
31].
Both the initial conditions and conditions at the western boundary were obtained by processing the data from the international database [
42] and BED. To resolve both the seasonal and interannual variations in the characteristics at the open boundary, the observed vertical profiles from the area between 23° E and 24.7° E longitude were step-wise horizontally averaged over successive 15-day time intervals. Subsequently, the averaged data were interpolated into a continuous time series. In this way, the boundary conditions were generated for the temperature, salinity, inorganic nitrogen, phosphorus, silicon, and organic matter. During the simulations, the boundary conditions for various forms of organic matter (two forms of dissolved organic nutrients, phytoplankton, and detritus) were determined as a fraction of the total organic amount, estimated from observations as the difference between the total amount of nitrogen and phosphorus and their inorganic forms. The further splitting of the organic nutrients between labile and refractory forms was made to be identical to their ratios, which were simulated in the inner grid cell nearest to the boundary.
Atmospheric forcing was set based on the ERA-Interim reanalysis fields (
https://www.ecmwf.int). The model uses fields of pressure, wind speed components, air temperature, humidity, short-wave and long-wave incoming radiation, and precipitation.
Accounting for a water residence time in the Gulf of Finland of 2–3 years [
32], both the pelagic and benthic fields were further adjusted during a spin-up run of three years, performed with the repeating boundary conditions for 2009. Numerical experiments were run for the period from 1 January 2009 to 31 December 2014.
2.4. Model Performance
We demonstrate the model performance by (a) validating it with a quantitative model–data comparison for several variables, and (b) verifying the internal consistency of SPBEM-2 and its capability to reproduce important feedback loops (“the vicious circle” [
43]).
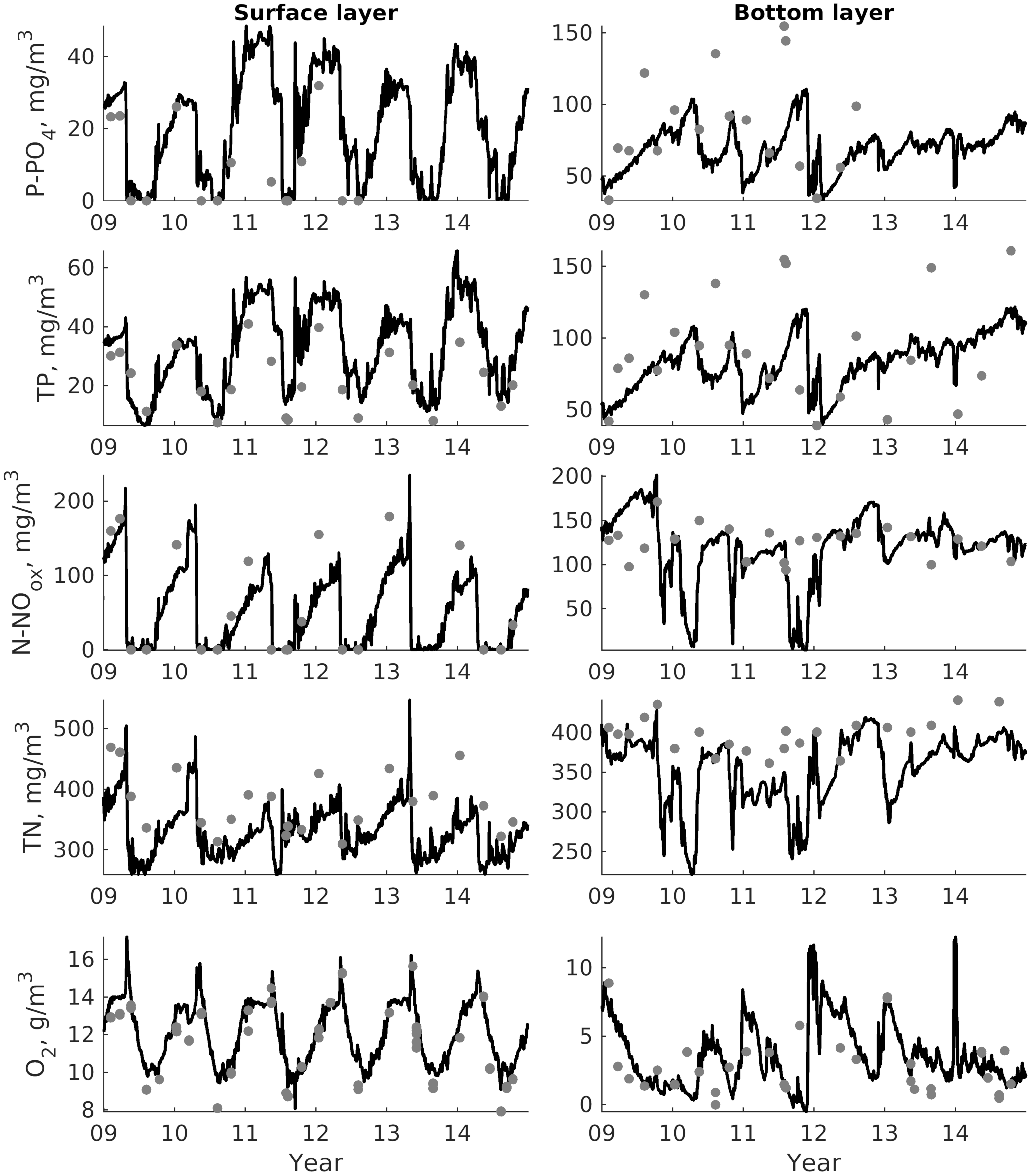
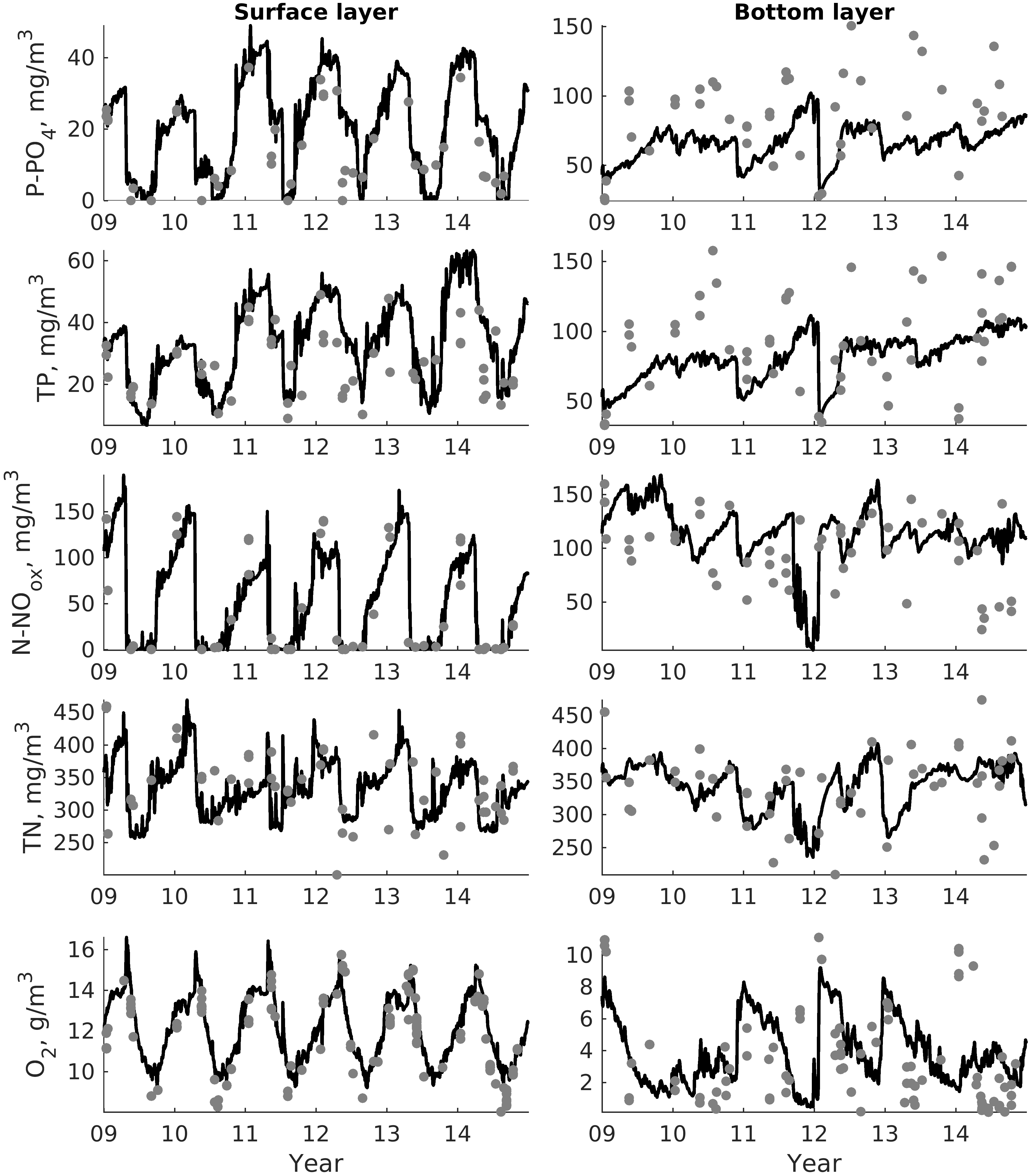
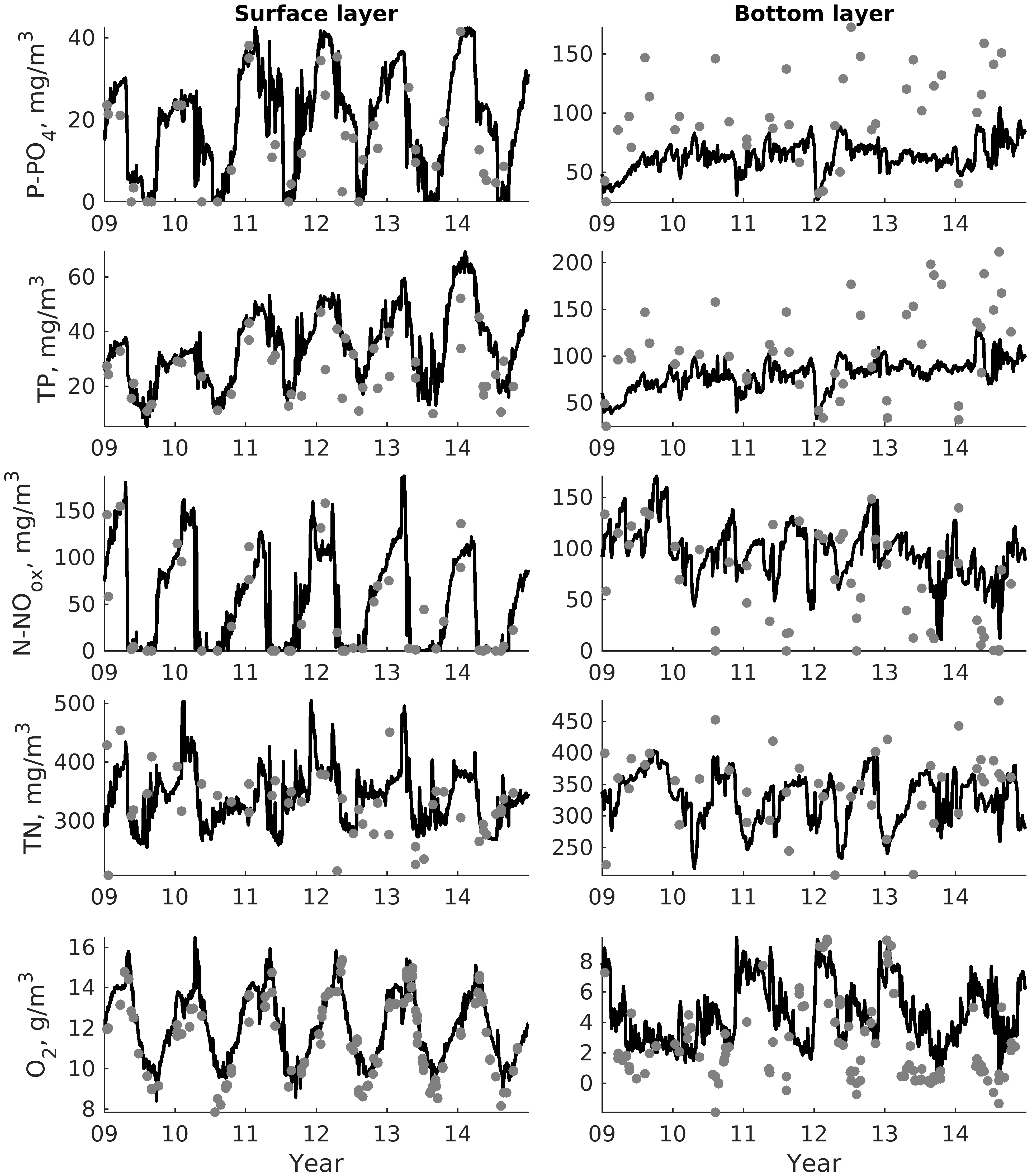
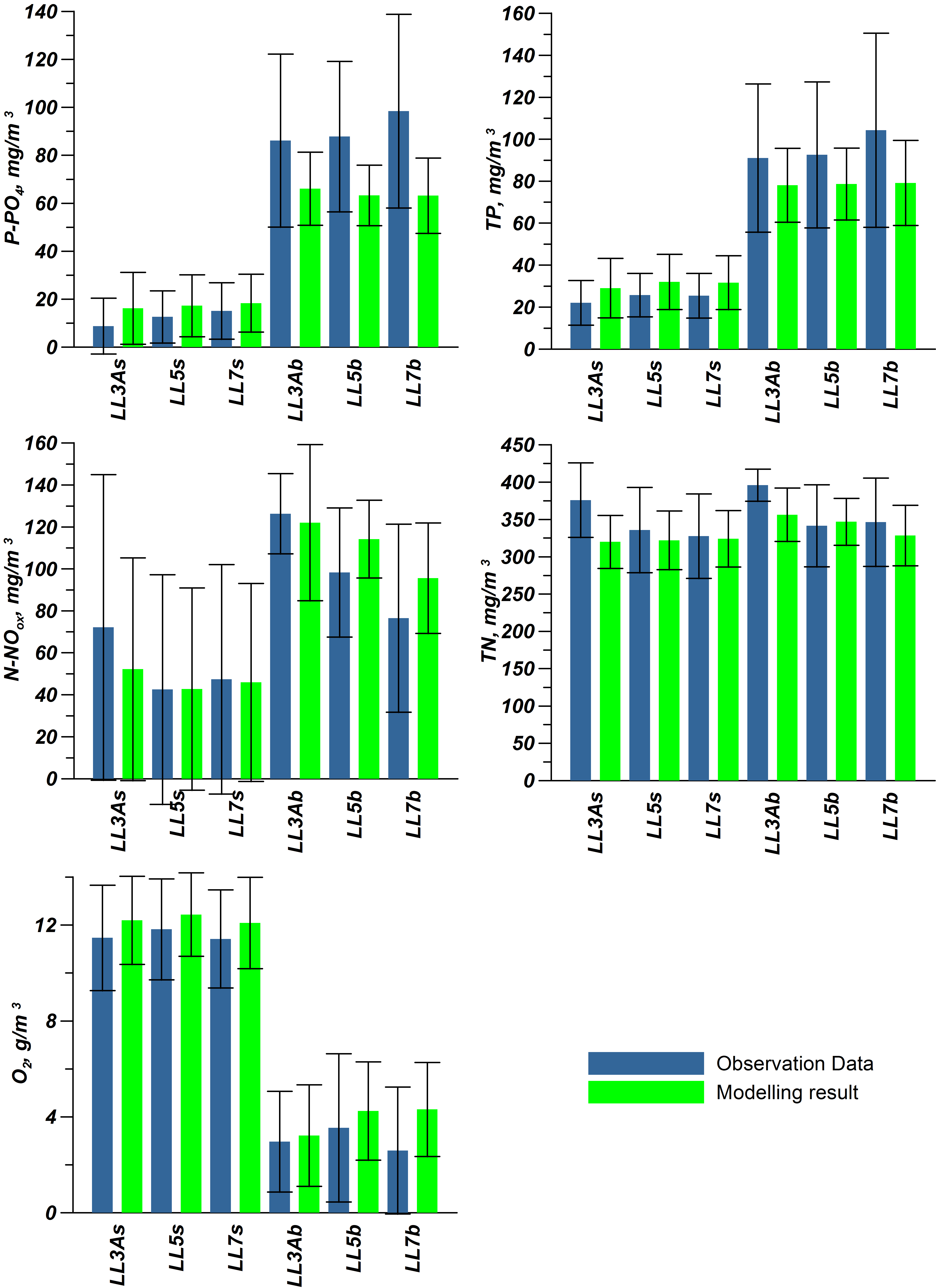
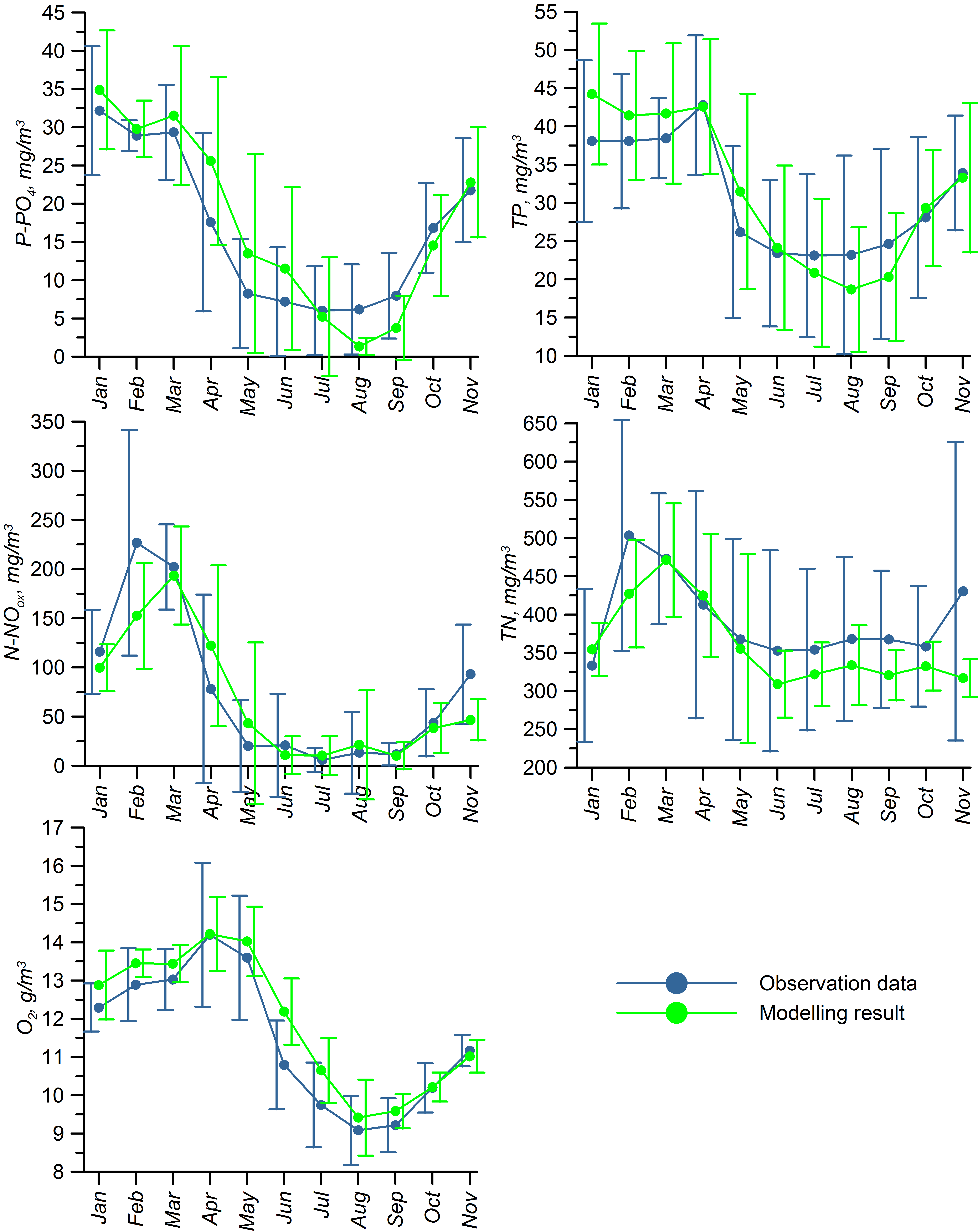
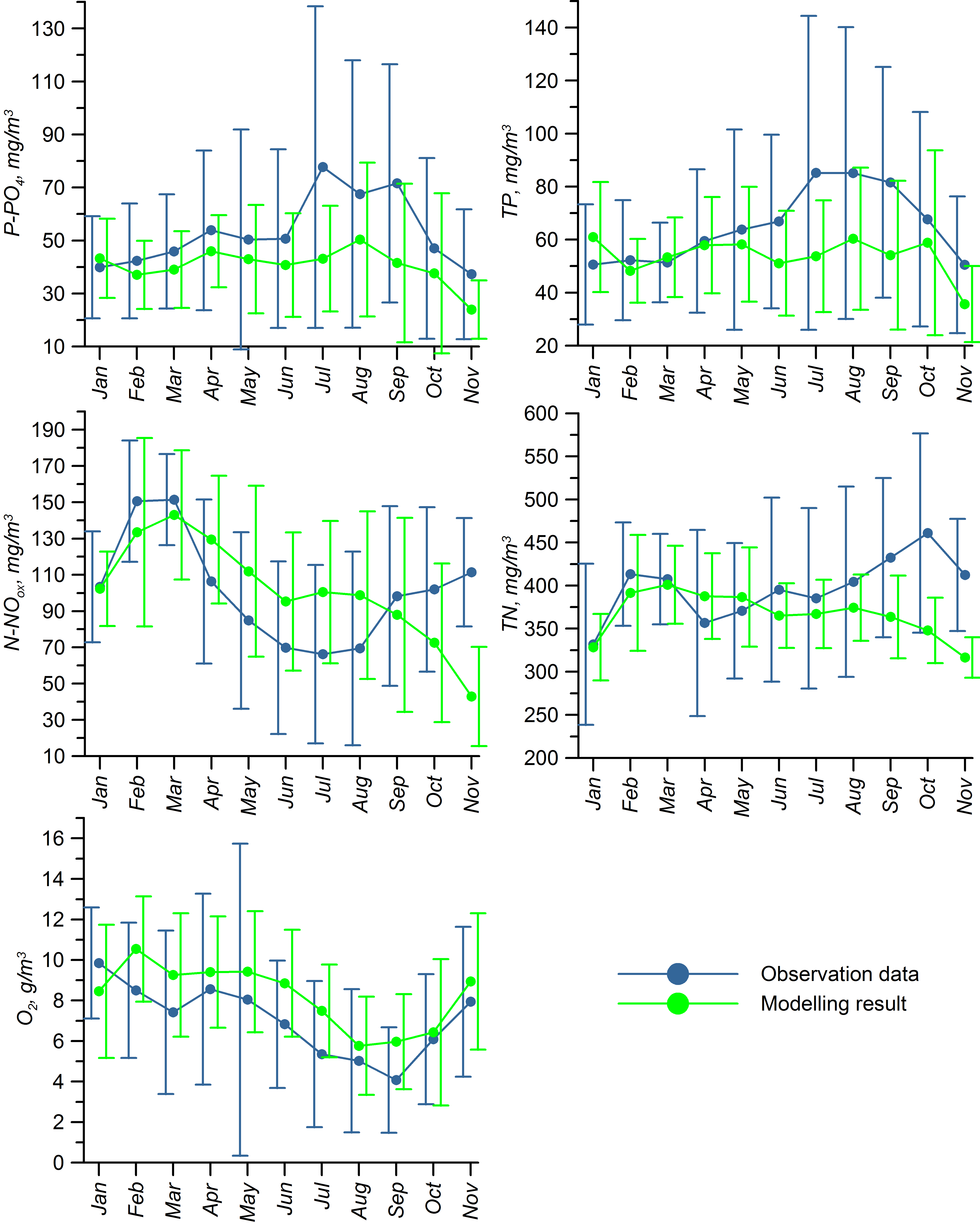
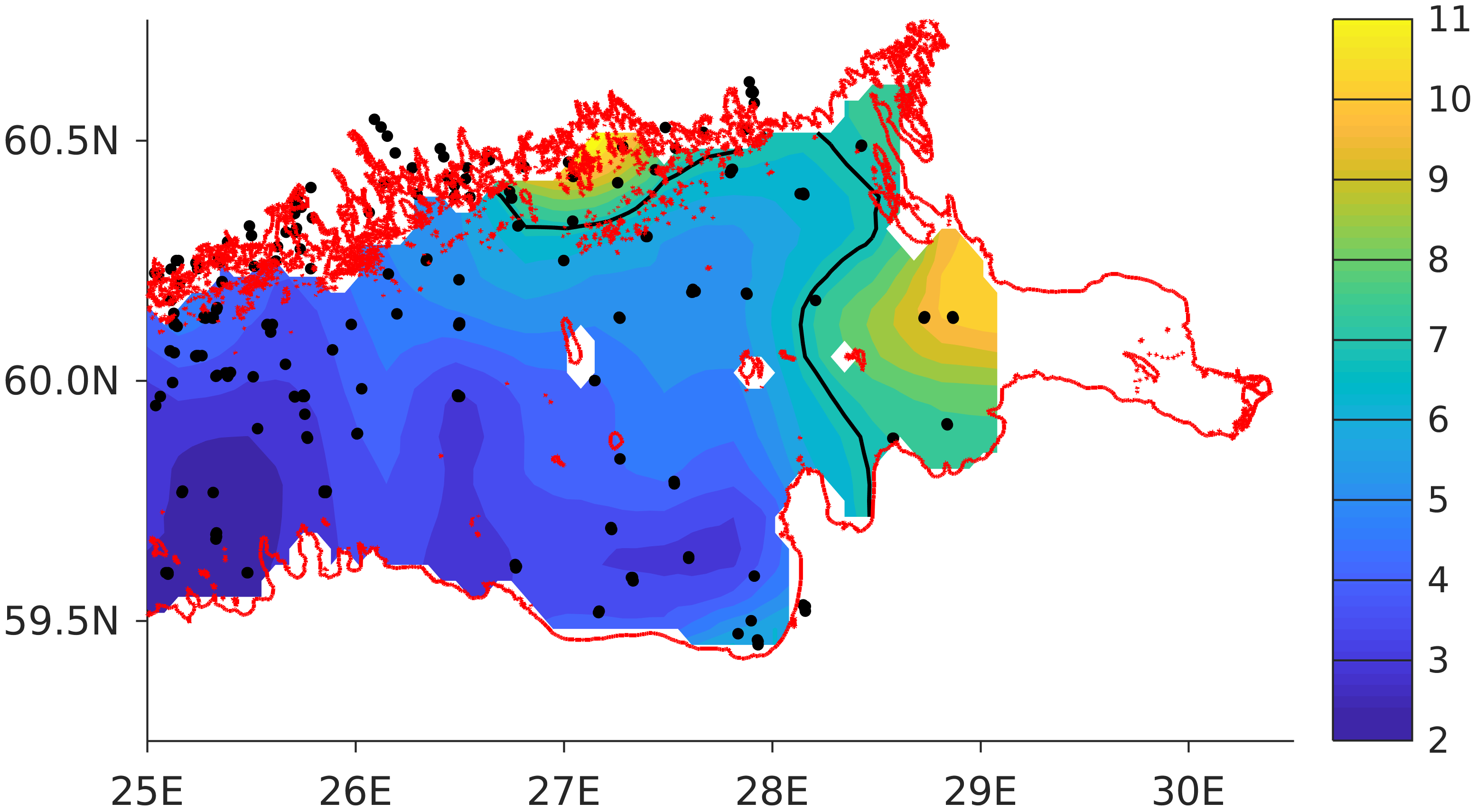
For validation, several methods of demonstrating the capabilities of SPBEM-2 were employed. A direct model–data comparison, with a seasonal and inter-annual resolution, was conducted for the most data-rich international monitoring stations, LL7, LL5, and LL3A (
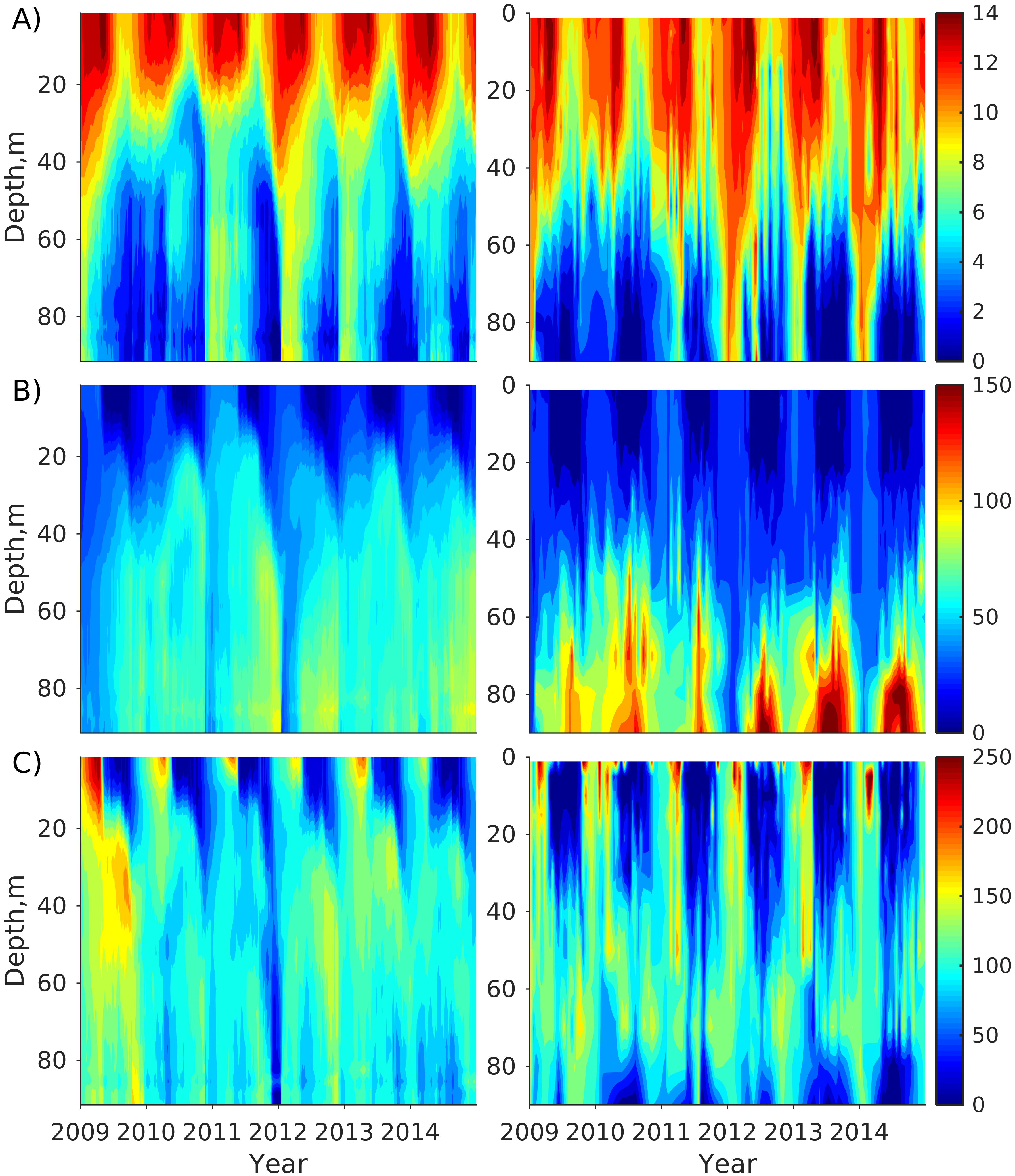
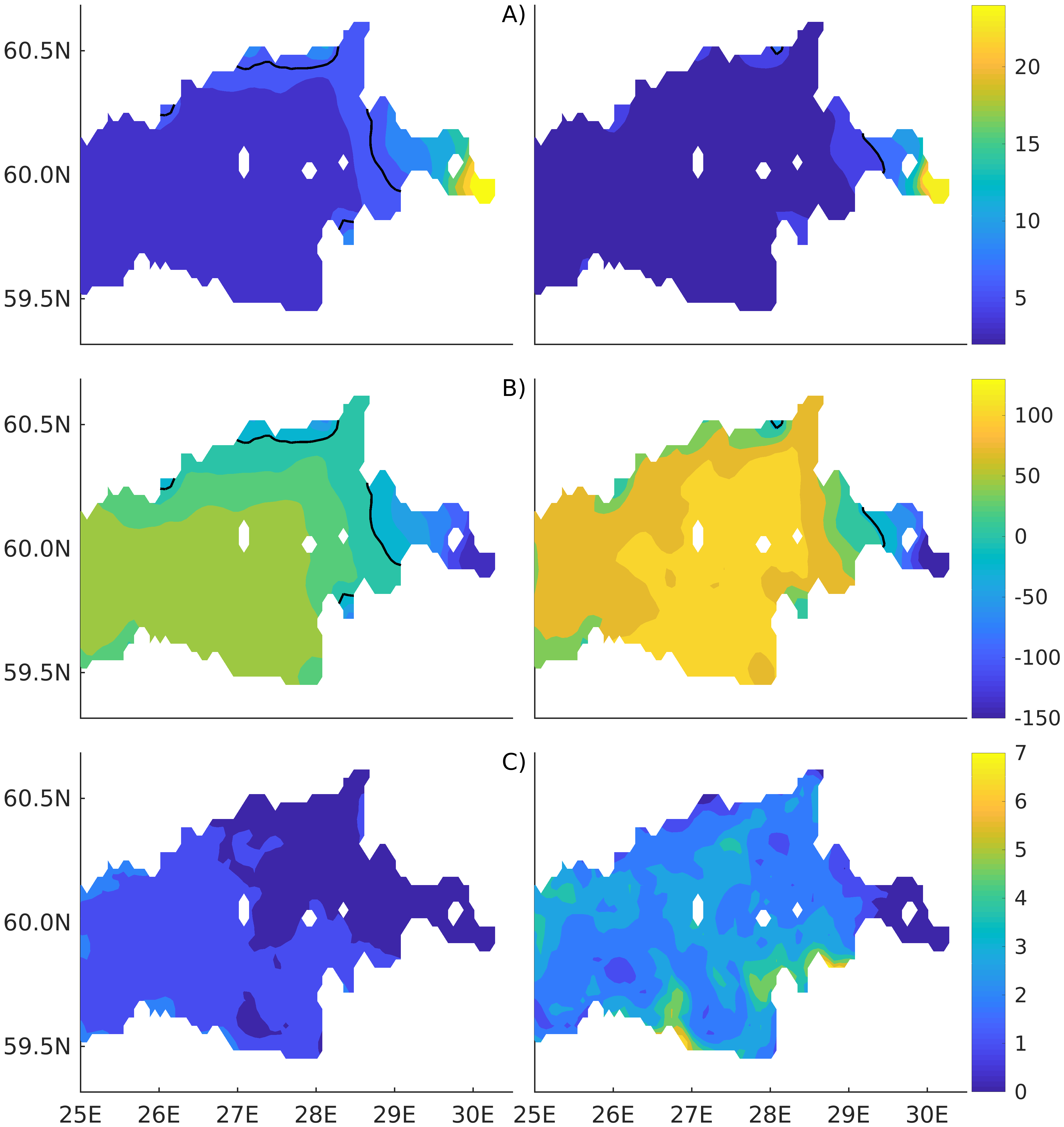
Figure 2). Considering that the real rugged bottom topography is approximated by a two-dimensional step function on a 2 × 2 nautical miles grid, a comparison with simulations was conducted as follows. First, we considered a 3 × 3 square of grid cells, where the central node was chosen as close to the average position of the monitoring station in question as possible. Further, we selected the cell, the depth of which was as close to the depth of the monitoring station as possible, and used the simulated profiles in this cell for comparison with the observed profiles. Unfortunately, all three stations with frequent monitoring are situated in the Western Gulf of Finland. Despite a large volume of observations in the Eastern Gulf of Finland, there are no intensive monitoring stations with an amount of measurements sufficient for making a seasonal to interannual model–data comparison, as described above for the western part of the gulf. Besides, a comparison at the sparse fixed stations gives less information about the quality of the simulation of the horizontal gradients in the distribution of ecosystem components. Therefore, we made a 3D statistical inter-comparison for the surface and bottom layers. For every single observation in the database, a corresponding daily averaged simulated variable was selected for the closest grid point as a match to this observation. The resulting pair-wise datasets were used for the calculation of some simple statistics. Apparently, with a grid size of 2 × 2 nautical miles, a grid cell height of 3 m, and daily averaging, we cannot expect a reproduction of the diurnal extremes in the observed variables, neither in these datasets, nor at the monitoring stations.
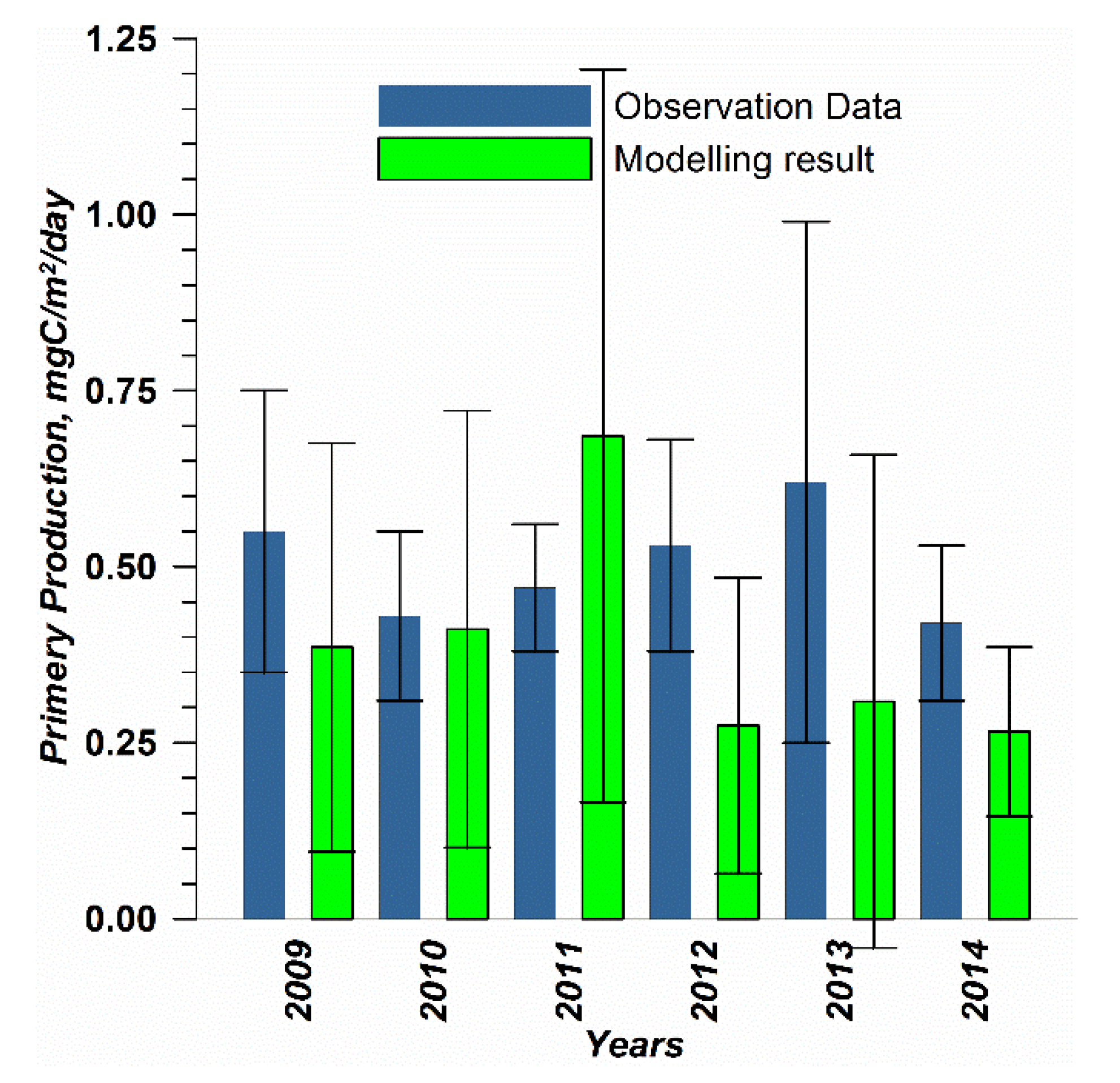
The capability of the model in simulating the biotic part of the ecosystem is demonstrated through a comparison with the measurements of phytoplankton primary production (PP) performed for years by the Russian State Hydrometeorological University in the Eastern Gulf of Finland. Measurements were carried out by the light–dark (or bulk) oxygen evolution method, which has been used for almost a century [
44,
45,
46]. The method consists of the registration of changes in oxygen concentration using the high-precision Winkler method, with a 0.1% precision in oxygen determinations [
47,
48], following the 24 h incubation of natural communities in clear and dark bottles. The primary production is calculated as the sum of the rate of change in the oxygen concentration in clear bottles (equal to the primary production minus respiration) and that in dark bottles, i.e., the respiration. The measurements were conducted at a network of stations (
Figure 2) from the end of July to the beginning of August. The selection of simulated values for comparison was conducted as follows. First, for every experimental estimate of PP, simulated values were compiled from a quadrate of 3 × 3 grid cells surrounding the station of measurements and for three days in a row, enveloping the time of measurements. Then, the simulated phytoplankton growth rates in nitrogen units, as well as the nitrogen fixation by cyanobacteria, were summed up and converted into carbon units, with a factor of 5.7. Finally, this dataset was averaged and used in a statistical model–data comparison for all the measurements in every simulated year. At the same time, we did not consider the routine chlorophyll observations as a good measure for comparison with the simulated phytoplankton biomass, expressed in nitrogen units, when such a comparison is conducted with quota coefficients that are fixed relative to carbon and chlorophyll [
49,
50]. A large intrinsic seasonal and interspecies variation in these quotas [
51,
52,
53] makes this type of model–data comparison too uncertain and unconvincing [
2].
In addition to presenting compiled datasets as the usual graphs (
Figure 3,
Figure 4 and
Figure 5), we used such simple statistics as the average, standard deviation, and coefficient of linear correlation, as well as more integral characteristics, such as the cost function (CF, Equation (18), [
7]):
where Mean
Mod and Mean
Obs are the averages of simulated and observed variables, respectively, and STD
Obs is the standard deviation of observed variables.