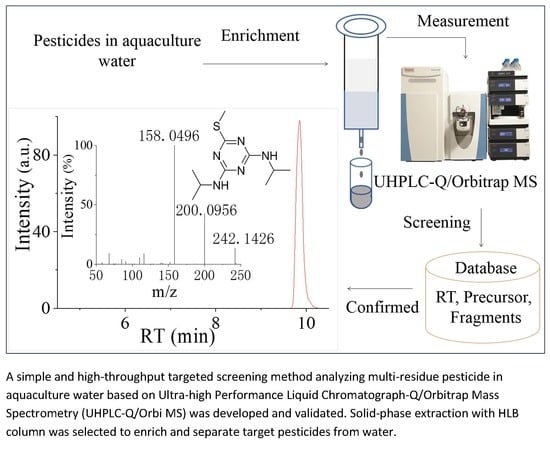
Multi-Residue Screening of Pesticides in Aquaculture Waters through Ultra-High-Performance Liquid Chromatography-Q/Orbitrap Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Samples
2.3. Sample Preparation
2.4. Instrument
2.5. Identification and Validation
3. Results and Discussion
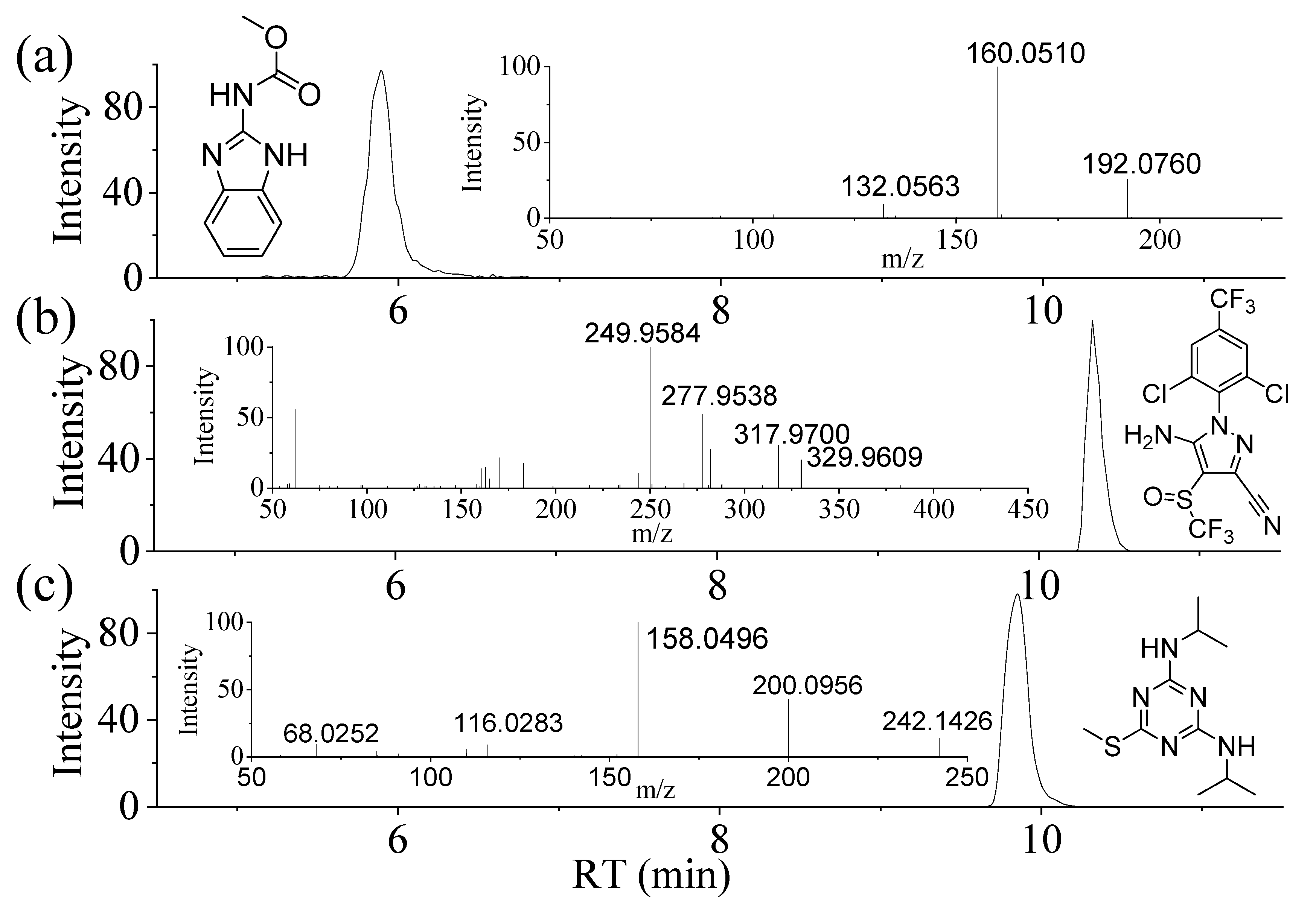
3.1. Data Acquisition with UHPLC-Q/Orbi-MS and the Ionization of Compounds
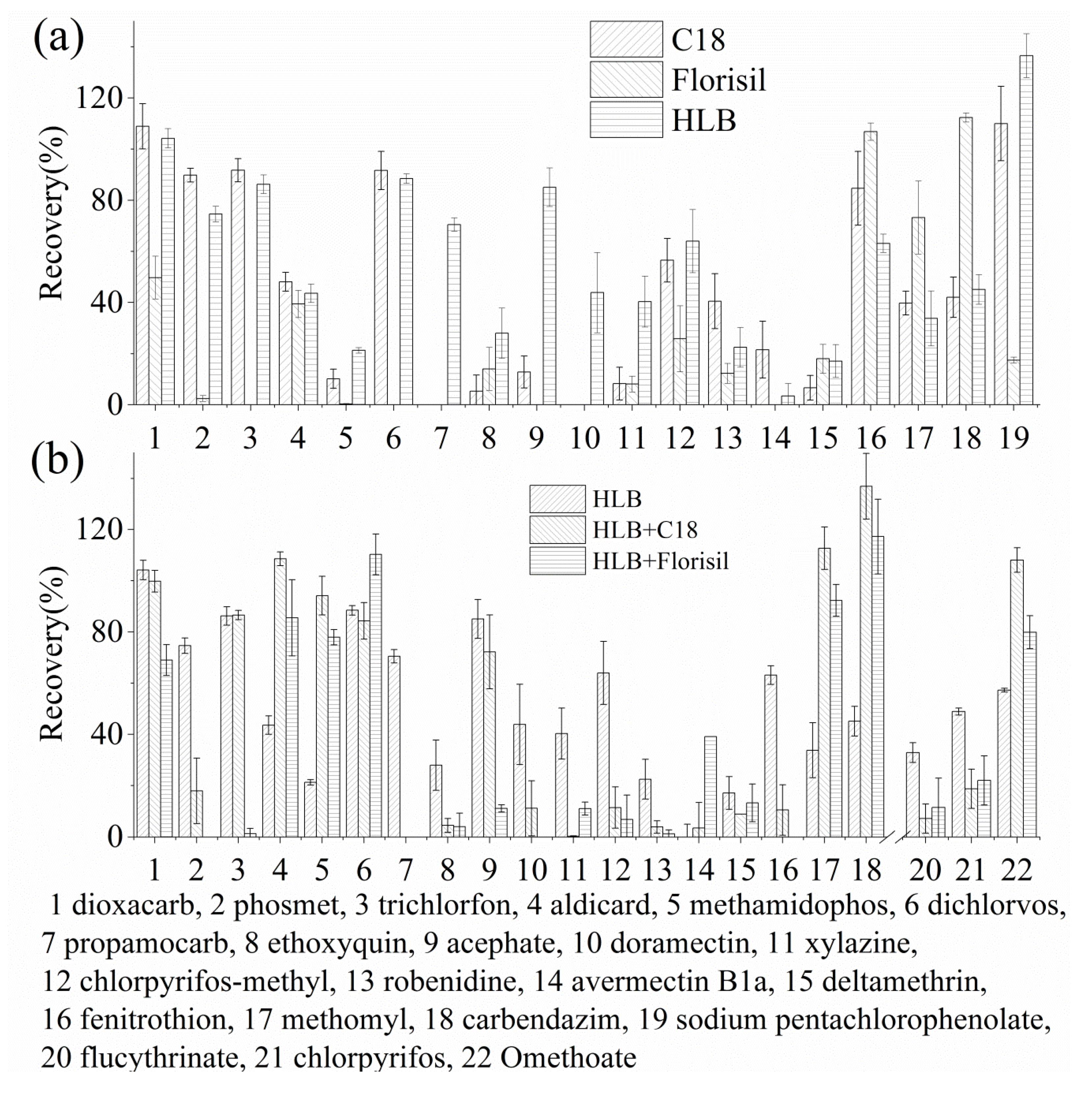
3.2. Selection of Extraction Cartridges
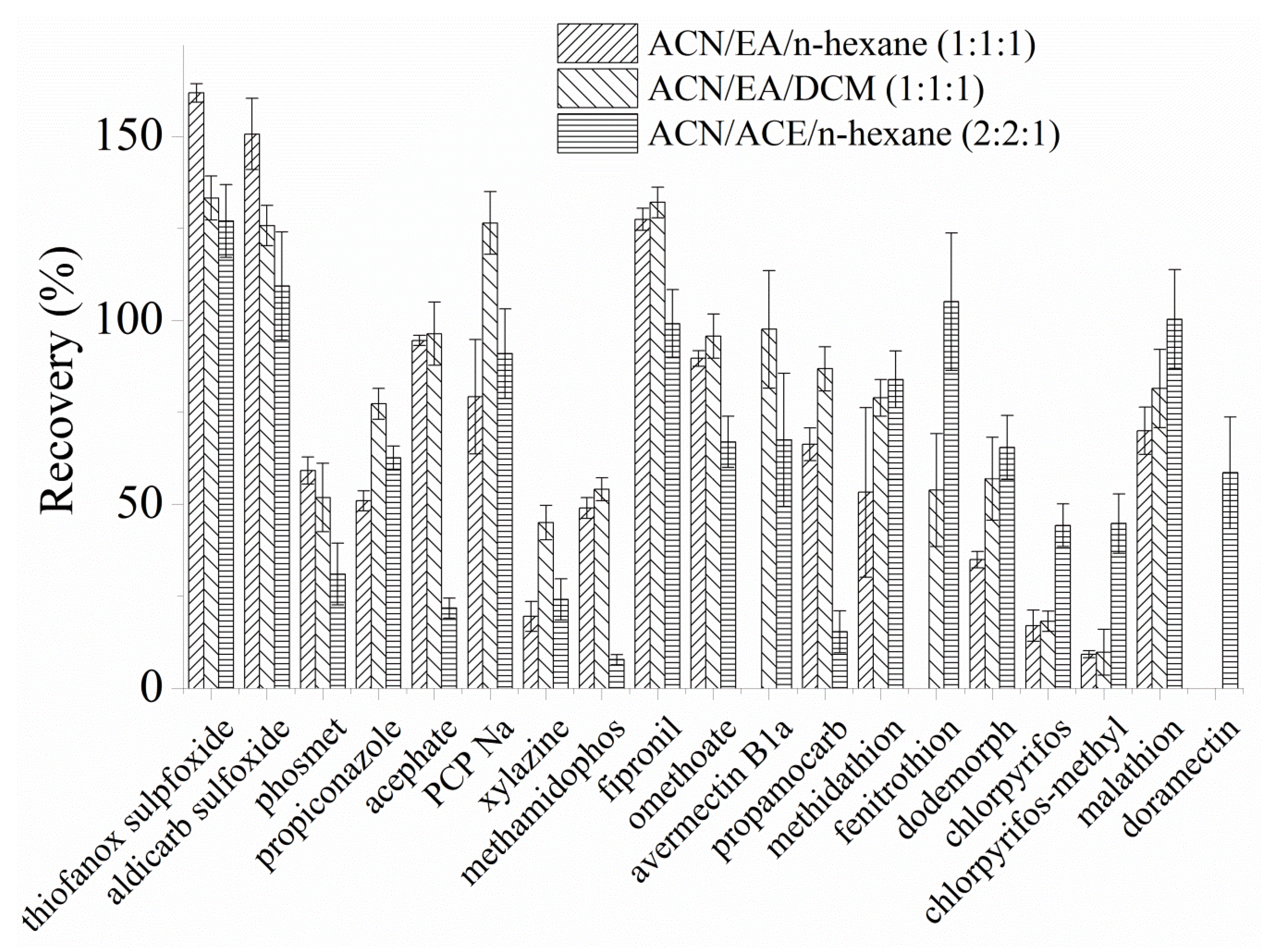
3.3. Optimazation of Eluents
3.4. Effect of the Cartridge Capacity on Pesticide Recovery
3.5. Method Performance and Matrix Effect
3.6. Application to Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Farajzadeh, M.A.; Mogaddam, M.R.; Aghdam, A.A. Comparison of air-agitated liquid-liquid microextraction technique and conventional dispersive liquid-liquid micro-extraction for determination of triazole pesticides in aqueous samples by gas chromatography with flame ionization detection. J. Chromatogr. A 2013, 1300, 70–78. [Google Scholar] [CrossRef]
- Yipel, M.; Kürekci, C.; Tekeli, İ.O.; Metli, M.; Sakin, F. Determination of selected antibiotics in farmed fish species using LC-MS/MS. Aquacult. Res. 2017, 48, 3829–3836. [Google Scholar] [CrossRef]
- Golge, O.; Kabak, B. Determination of 115 pesticide residues in oranges by high-performance liquid chromatography–triple-quadrupole mass spectrometry in combination with QuEChERS method. J. Food Compos. Anal. 2015, 41, 86–97. [Google Scholar] [CrossRef]
- Samsidar, A.; Siddiquee, S.; Shaarani, S.M. A review of extraction, analytical and advanced methods for determination of pesticides in environment and foodstuffs. Trends Food Sci. Technol. 2018, 71, 188–201. [Google Scholar] [CrossRef]
- Yusà, V.; Coscollà, C.; Millet, M. New screening approach for risk assessment of pesticides in ambient air. Atmos. Environ. 2014, 96, 322–330. [Google Scholar] [CrossRef]
- He, X.; Ma, Y.F.; Zhao, H.X.; Nie, X.J. Simultaneous Determination of 24 Pesticide Residues in Environmental Water Using Solid-phase Extraction and High Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Inst. Anal. 2017, 36, 1487–1493. [Google Scholar] [CrossRef]
- Montesdeoca-Esponda, S.; Checchini, L.; Del Bubba, M.; Sosa-Ferrera, Z.; Santana-Rodriguez, J.J. Analytical approaches for the determination of personal care products and evaluation of their occurrence in marine organisms(Review). Sci. Total Environ. 2018, 633, 405–425. [Google Scholar] [CrossRef]
- Abdel Ghani, S.; Hanafi, A. QuEChERS method combined with GC‒MS for pesticide residues determination in water. J. Anal. Chem. 2016, 71, 508–512. [Google Scholar] [CrossRef]
- Bulgurcuoğlu, A.E.; Yılmaz, B.; Chormey, D.S.; Bakırdere, S. Simultaneous determination of estrone and selected pesticides in water medium by GC-MS after multivariate optimization of microextraction strategy. Environ. Monit. Assess. 2018, 190, 252. [Google Scholar] [CrossRef]
- Taghani, A.; Goudarzi, N.; Bagherian, G. Application of multiwalled carbon nanotubes for the preconcentration and determination of organochlorine pesticides in water samples by gas chromatography with mass spectrometry. J. Sep. Sci. 2016, 39, 4219–4226. [Google Scholar] [CrossRef]
- Schwanz, T.G.; Carpilovsky, C.K.; Weis, G.C.C.; Costabeber, I.H. Validation of a multi-residue method and estimation of measurement uncertainty of pesticides in drinking water using gas chromatography–mass spectrometry and liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2019, 1585, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Pellicer-Castell, E.; Belenguer-Sapina, C.; Amoros, P.; ElHaskouri, J.; Herrero-Martinez, J.; Mauri-Aucejo, A. Study of silica-structured materials as sorbents for organophosphorus pesticides determination in environmental water samples. Talanta 2018, 189, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Shu, B.; Yang, Z.; Lee, H.; Qiu, B.; Li, H. Development of ultrasound-assisted emulsification microextraction based on solidification of a floating organic droplet for determination of organochlorine pesticides in water samples. J. Sep. Sci. 2016, 39, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Yazdanfar, N.; Yamini, Y.; Ghambarian, M. Homogeneous Liquid–Liquid Microextraction for Determination of Organochlorine Pesticides in Water and Fruit Samples. Chromatographia 2014, 77, 329–336. [Google Scholar] [CrossRef]
- Ma, J.; Wu, G.; Li, S.; Tan, W.; Wang, X.; Li, J.; Chen, L. Magnetic solid-phase extraction of heterocyclic pesticides in environmental water samples using metal-organic frameworks coupled to high performance liquid chromatography determination. J. Chromatogr. A 2018, 1553, 57–66. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Marei, A.E.S.M.; El-Nouby, M.A.M. Preparation and characterization of chitosan-siloxane magnetic nanoparticles for the extraction of pesticides from water and determination by HPLC. Sep. Sci. Plus 2018, 1, 506–519. [Google Scholar] [CrossRef]
- Bazrafshan, A.A.; Ghaedi, M.; Rafiee, Z.; Hajati, S.; Ostovan, A. Nano-sized molecularly imprinted polymer for selective ultrasound-assisted microextraction of pesticide Carbaryl from water samples: Spectrophotometric determination(Article). J. Colloid Interface Sci. 2017, 498, 313–322. [Google Scholar] [CrossRef]
- Raks, V.A.; Turchin, V.A.; Zaitsev, V.N. Chromatographic determination of pesticide 2,4-D in water bodies. J. Water Chem. Technol. 2015, 37, 295–298. [Google Scholar] [CrossRef]
- Smedes, F. Silicone–water partition coefficients determined by cosolvent method for chlorinated pesticides, musks, organo phosphates, phthalates and more. Chemosphere 2018, 210, 662–671. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Ferreira, J.M.S.; Talamini, V.; Facco, J.D.F.; Rizzetti, T.M.; Prestes, O.D.; Adaime, M.B.; Zanella, R.; Bottoli, C.B.G. Determination of pesticides in coconut (Cocos nucifera Linn.) water and pulp using modified QuEChERS and LC–MS/MS. Food Chem. 2016, 213, 616–624. [Google Scholar] [CrossRef]
- Amelin, V.G.; Bol’shakov, D.S.; Andoralov, A.M. Screening and Determination of Pesticides from Various Classes in Natural Water without Sample Preparation by Ultra HPLC–High-Resolution Quadrupole Time-of-Flight Mass Spectrometry. J. Anal. Chem. 2018, 73, 257–265. [Google Scholar] [CrossRef]
- Arsand, J.B.; Hoff, R.B.; Jank, L.; Dallegrave, A.; Galeazzi, C.; Barreto, F.; Pizzolato, T.M. Wide-Scope Determination of Pharmaceuticals and Pesticides in Water Samples: Qualitative and Confirmatory Screening Method Using LC-qTOF-MS. Water Air Soil Pollut. 2018, 229. [Google Scholar] [CrossRef]
- Amelin, V.G.; Saun’kina, M.A.; Andoralov, A.M. Direct Analysis of Natural Waters by Electron Spray Ionization and High-Resolution Time-of-Flight Mass Spectrometry Detection. Determination of Pesticides of Various Classes. Moscow Univ. Chem. Bull. 2017, 72, 315–321. [Google Scholar] [CrossRef]
- Zhang, Z.; Lefebvre, T.; Kerr, C.; Osprey, M. Simultaneous extraction and determination of various pesticides in environmental waters. J. Sep. Sci. 2014, 37, 3699–3705. [Google Scholar] [CrossRef]
- Ahmadkhaniha, R.; Rastkari, N. Development of a carbon nanotube-coated stir bar for determination of organophosphorus pesticides in water. Asia Pac. J. Chem. Eng. 2016, 11, 893–900. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Kamalian, S.; Shayeghi, M.; Yousefi, M.; Heidarinejad, Z.; Agarwal, S.; Gupta, V.K. High-performance removal of diazinon pesticide from water using multi-walled carbon nanotubes. Microchem. J. 2019, 145, 486–491. [Google Scholar] [CrossRef]
- Salemi, A.; Khaleghifar, N.; Mirikaram, N. Optimization and comparison of membrane-protected micro-solid-phase extraction coupled with dispersive liquid-liquid microextraction for organochlorine pesticides using three different sorbents. Microchem. J. 2019, 144, 215–220. [Google Scholar] [CrossRef]
- Hossain, M.S.; Chowdhury, M.A.Z.; Pramanik, K.; Rahman, M.A.; Fakhruddin, A.N.M.; Alam, M.K. Determination of selected pesticides in water samples adjacent to agricultural fields and removal of organophosphorus insecticide chlorpyrifos using soil bacterial isolates. Appl. Water Sci. 2015, 5, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Anjos, J.P.; Andrade, J.B. Determination of nineteen pesticides residues (organophosphates, organochlorine, pyrethroids, carbamate, thiocarbamate and strobilurin) in coconut water by SDME/GC–MS. Microchem. J. 2014, 112, 119–126. [Google Scholar] [CrossRef]
- Villaverde, J.J.; Sevilla-Morán, B.; López-Goti, C.; Alonso-Prados, J.L.; Sandín-España, P. Trends in analysis of pesticide residues to fulfil the european regulation (ec) no. 1107/2009. TrAC Trends Anal. Chem. 2016, 80, 568–580. [Google Scholar] [CrossRef]
- Masiá, A.; Ibáñez, M.; Blasco, C.; Sancho, J.V.; Picó, Y.; Hernández, F. Combined use of liquid chromatography triple quadrupole mass spectrometry and liquid chromatography quadrupole time-of-flight mass spectrometry in systematic screening of pesticides and other contaminants in water samples. Anal. Chim. Acta 2013, 761, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Del Mar Gomez-Ramos, M.; Rajski, L.; Heinzen, H.; Fernandez-Alba, A.R. Liquid chromatography orbitrap mass spectrometry with simultaneous full scan and tandem ms/ms for highly selective pesticide residue analysis. Anal. Bioanal. Chem. 2015, 407, 6317–6326. [Google Scholar] [CrossRef] [PubMed]
- Fenik, J.; Tankiewicz, M.; Biziuk, M. Properties and determination of pesticides in fruits and vegetables. TrAC Trends Anal. Chem. 2011, 30, 814–826. [Google Scholar] [CrossRef]
- Kong, C.; Wang, Y.; Huang, Y.; Yu, H. Multiclass screening of> 200 pharmaceutical and other residues in aquatic foods by ultrahigh-performance liquid chromatography–quadrupole-Orbitrap mass spectrometry. Anal. Bioanal. Chem. 2018, 410, 5545–5553. [Google Scholar] [CrossRef]
- Trufelli, H.; Palma, P.; Famiglini, G.; Cappiello*, A. An overview of matrix effects in liquid chromatography-mass spectrometry. Mass Spectrom. Rev. 2011, 30, 491–509. [Google Scholar] [CrossRef]
- Mekebri, A.; Crane, D.B.; Blondina, G.J.; Oros, D.R.; Rocca, J.L. Extraction and analysis methods for the determination of pyrethroid insecticides in surface water, sediments and biological tissues at environmentally relevant concentrations. Bul. Environ. Contam. Toxicol. 2008, 80, 455–460. [Google Scholar] [CrossRef]
- Moloney, M.; Tuck, S.; Ramkumar, A.; Furey, A.; Danaher, M. Determination of pyrethrin and pyrethroid residues in animal fat using liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. B 2018, 1077, 60–70. [Google Scholar] [CrossRef]
- European Commission. Guidance Document on Analytical Quality Control and Validation Procedures for Pesticide Residues Analysis in Food and Feed. Sante/11813/2017. Available online: https://ec.europa.eu/food/sites/food/files/plant/docs/pesticides_mrl_guidelines_wrkdoc_2017-11813.pdf (accessed on 1 March 2018).
- Aminot, Y.; Sayfritz, S.J.; Thomas, K.V.; Godinho, L.; Botteon, E.; Ferrari, F.; Boti, V.; Albanis, T.; Köck-Schulmeyer, M.; Diaz-Cruz, M.S.; et al. Environmental risks associated with contaminants of legacy and emerging concern at European aquaculture areas. Environ. Pollut. 2019, 252, 1301–1310. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Duarte, K.R.; Freitas, A.C.; Panteleitchouk, T.S.L.; Duarte, A.C.; Rocha-Santos, T.A.P. Contaminants in aquaculture: Overview of analytical techniques for their determination(Review). TrAC Trends Anal. Chem. 2016, 80, 293–310. [Google Scholar] [CrossRef] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-Y.; Fodjo, E.K.; Kong, C.; Yu, H.-J. Multi-Residue Screening of Pesticides in Aquaculture Waters through Ultra-High-Performance Liquid Chromatography-Q/Orbitrap Mass Spectrometry. Water 2020, 12, 1238. https://doi.org/10.3390/w12051238
Wang S-Y, Fodjo EK, Kong C, Yu H-J. Multi-Residue Screening of Pesticides in Aquaculture Waters through Ultra-High-Performance Liquid Chromatography-Q/Orbitrap Mass Spectrometry. Water. 2020; 12(5):1238. https://doi.org/10.3390/w12051238
Chicago/Turabian StyleWang, Shou-Ying, Essy Kouadio Fodjo, Cong Kong, and Hui-Juan Yu. 2020. "Multi-Residue Screening of Pesticides in Aquaculture Waters through Ultra-High-Performance Liquid Chromatography-Q/Orbitrap Mass Spectrometry" Water 12, no. 5: 1238. https://doi.org/10.3390/w12051238
APA StyleWang, S.-Y., Fodjo, E. K., Kong, C., & Yu, H.-J. (2020). Multi-Residue Screening of Pesticides in Aquaculture Waters through Ultra-High-Performance Liquid Chromatography-Q/Orbitrap Mass Spectrometry. Water, 12(5), 1238. https://doi.org/10.3390/w12051238