A Forty-Year Karstic Critical Zone Survey (Baget Catchment, Pyrenees-France): Lithologic and Hydroclimatic Controls on Seasonal and Inter-Annual Variations of Stream Water Chemical Composition, pCO2, and Carbonate Equilibrium
Abstract
1. Introduction
2. Materials and Methods
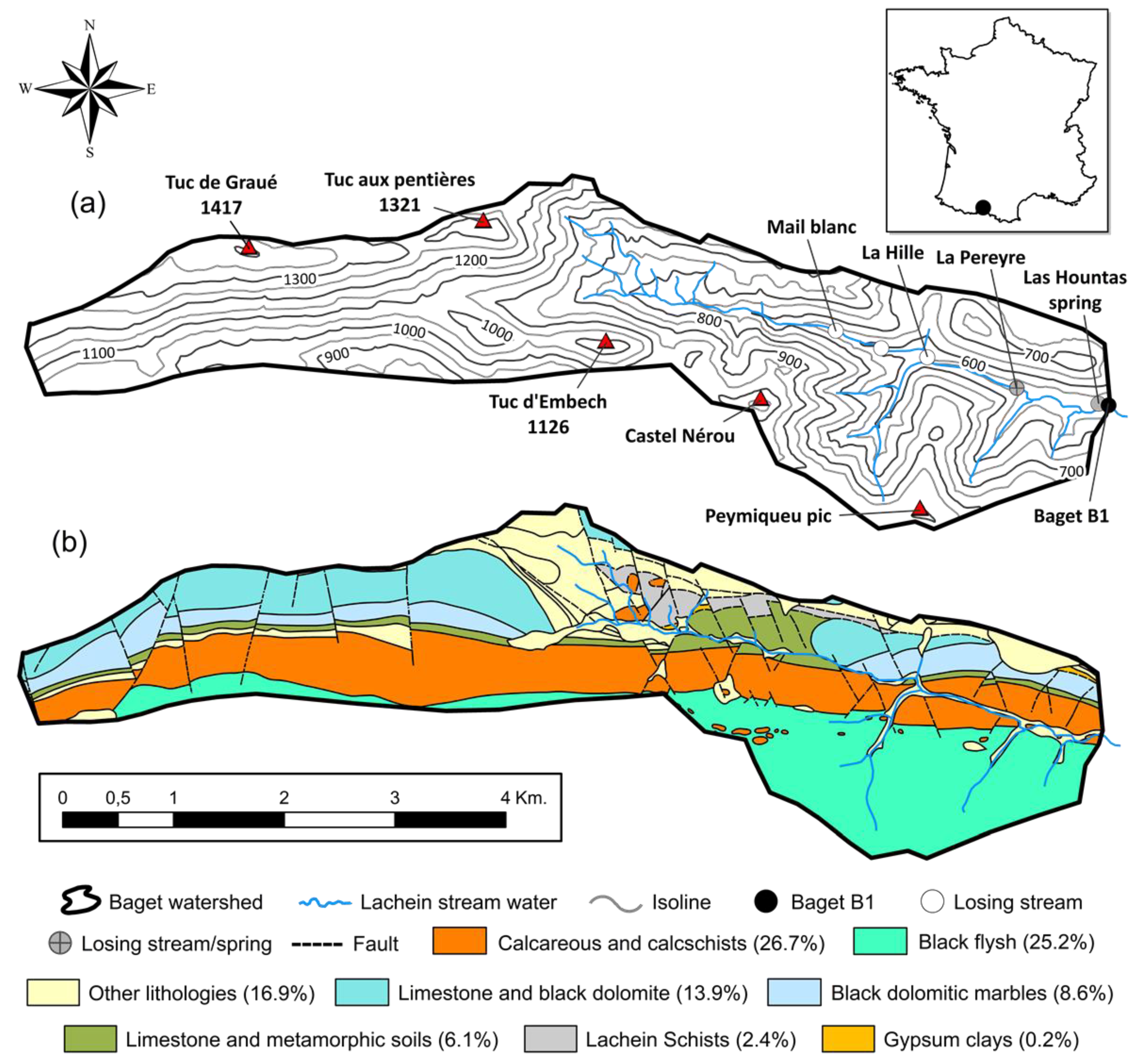
2.1. Site Description
2.2. Sampling and Analytical Methods
2.3. Data Treatment
2.3.1. pCO2 and Calcite Saturation Index (SIc)
2.3.2. Statistics
3. Results
3.1. Stream Water Physico-Chemical Characteristics
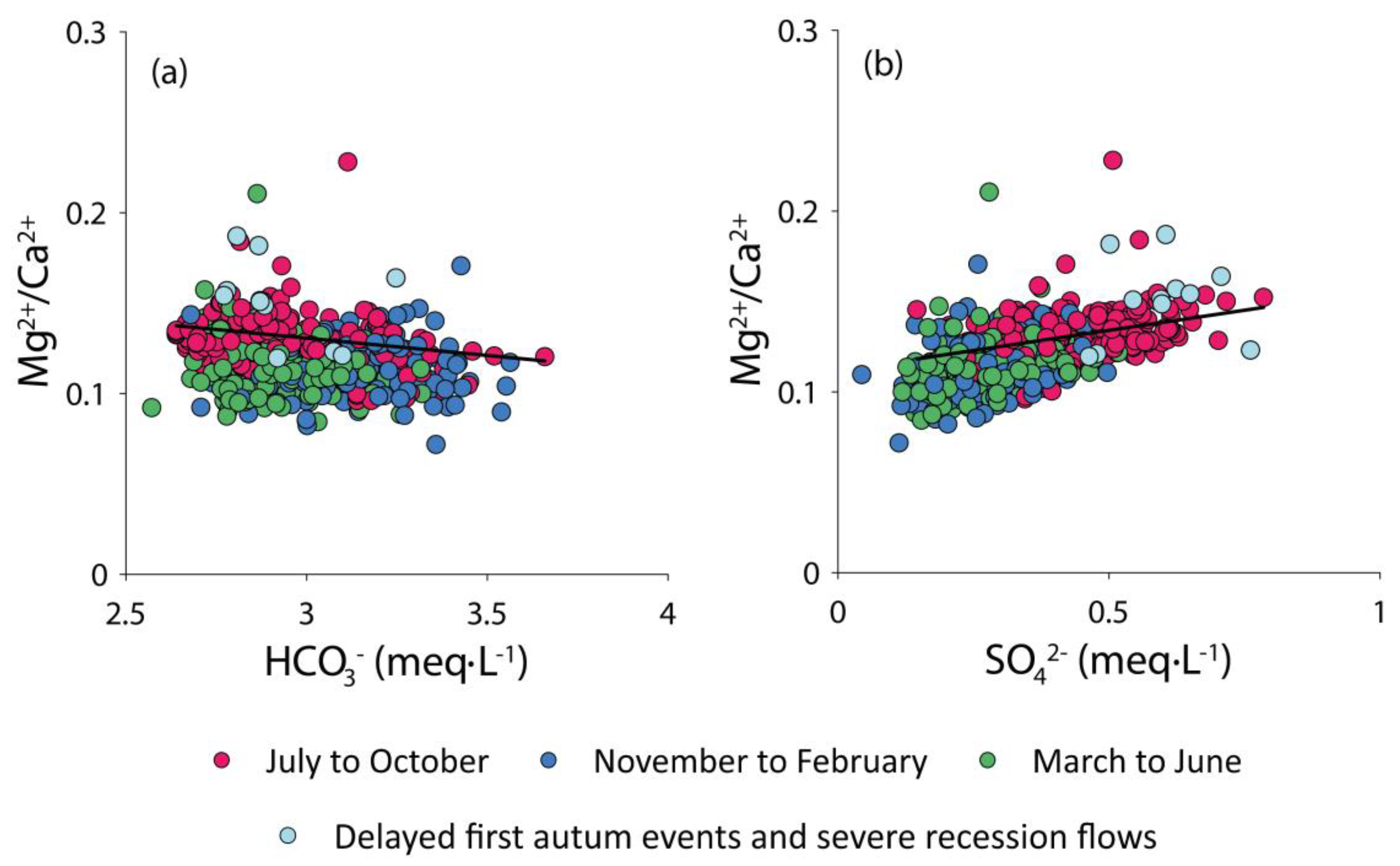
3.1.1. Hydrochemical Composition of the Baget Stream Water
3.1.2. Multivariate Statistical Analysis
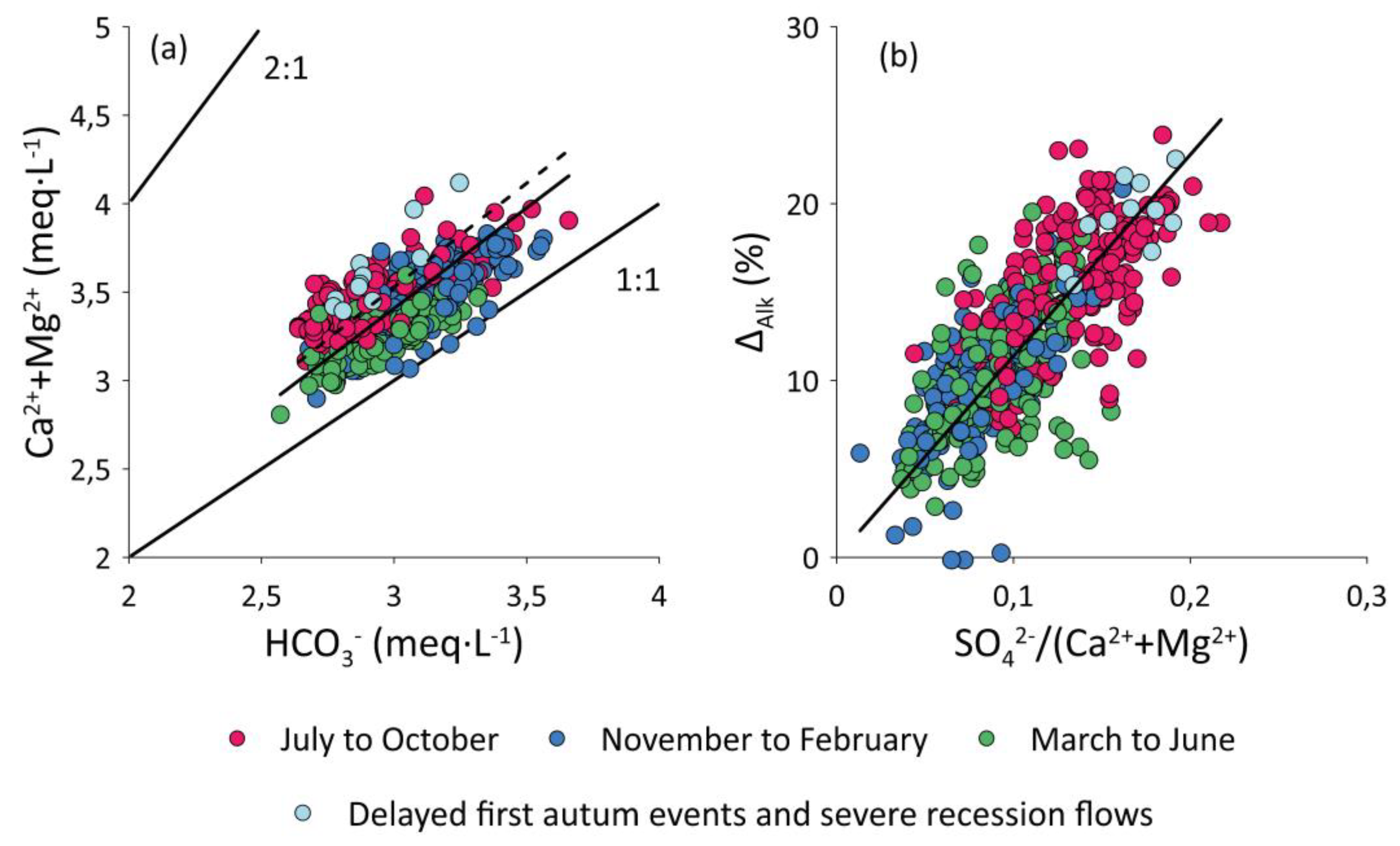
3.1.3. Alkalinity Decrease (∆Alk)
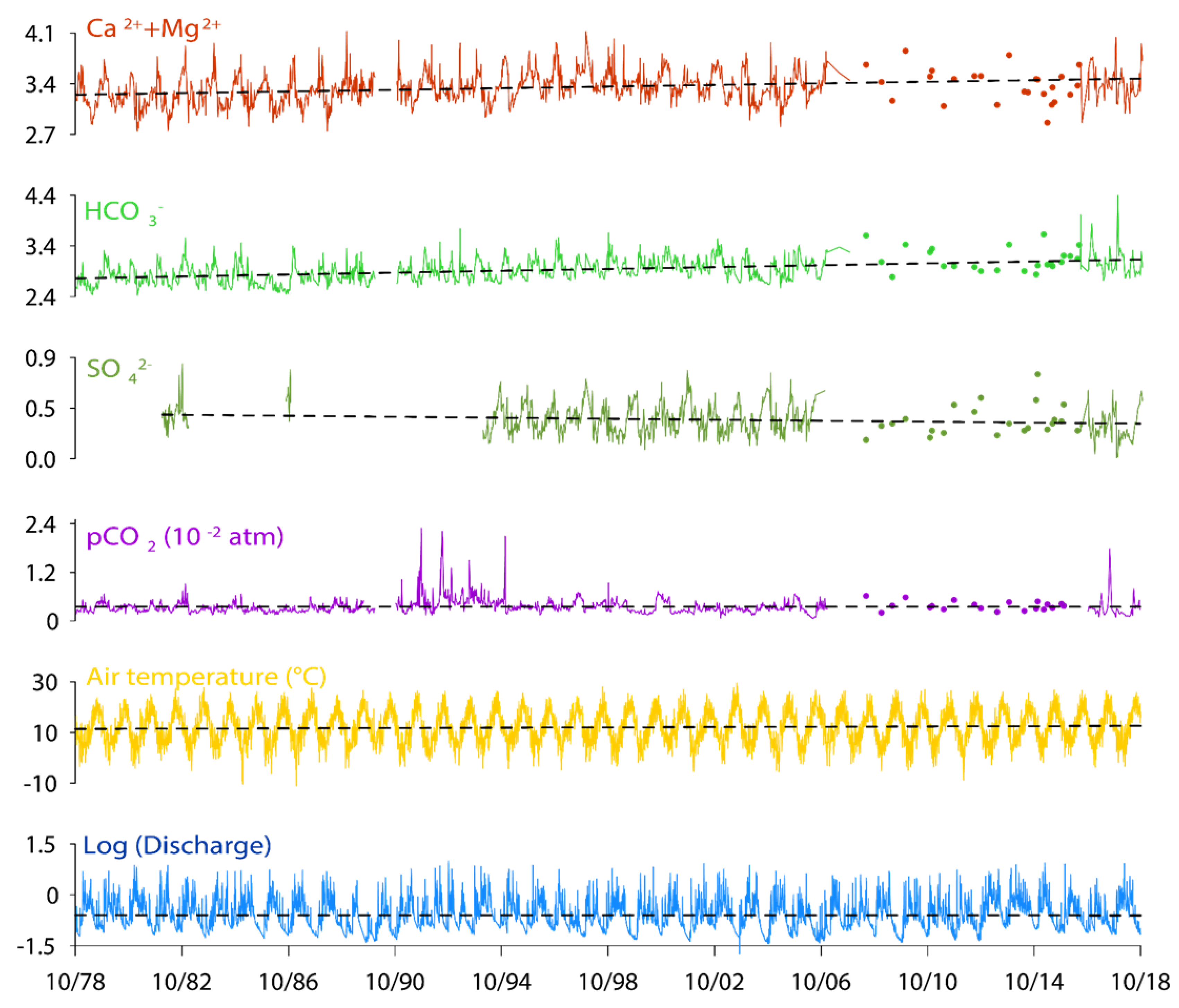
3.2. Long Term Trends in Stream Water Chemistry: Instantaneous Values and Mean Annual Data
3.3. Seasonal Patterns Based on Long Term Data Series in Stream Water Chemistry
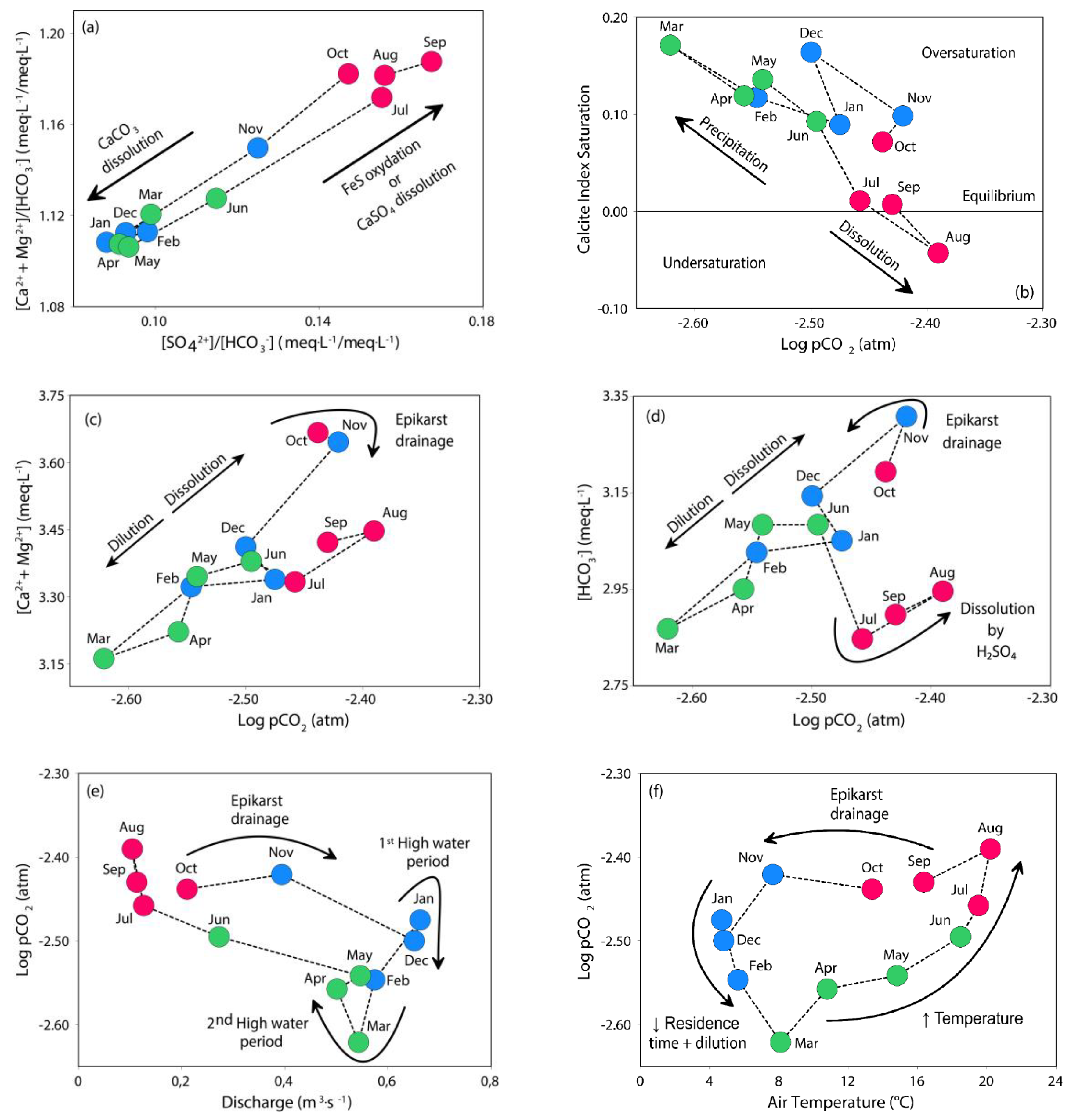
4. Discussion
4.1. Natural vs. Anthropogenic Sources of Elements
4.2. Role of Discharge, Temperature and Vegetation on Stream Water Composition and Trends
4.3. Respective Control of Karst and Epikarst on Stream Water Chemical Composition and Trends
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robertson, G.P.; Paul, E.A.; Harwood, R.R. Greenhouse Gases in Intensive Agriculture: Contributions of Individual Gases to the Radiative Forcing of the Atmosphere. Science 2000, 289, 1922–1926. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhou, C. Greenhouse gas emissions and mitigation measures in Chinese agroecosystems. Agric. For. Meteorol. 2007, 142, 270–277. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, K.; Tao, Y.; Li, Z.; Zhang, G.; Li, S.; Chen, F. Carbon Dioxide Flux from Rice Paddy Soils in Central China: Effects of Intermittent Flooding and Draining Cycles. PLoS ONE 2013, 8, e56562. [Google Scholar] [CrossRef] [PubMed]
- Meybeck, M. Global chemical weathering of surficial rocks estimated from river dissolved loads. Am. J. Sci. 1987, 287, 401–428. [Google Scholar] [CrossRef]
- Gaillardet, J.; Dupre, B.; Louvat, P.; Allegre, C.J. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Ludwig, W.; Probst, J.-L. Predicting the oceanic input of organic carbon by continental erosion. Glob. Biogeochem. Cycles 1996, 10, 23–41. [Google Scholar] [CrossRef]
- Amiotte-Suchet, P.; Probst, J.-L.; Ludwig, W. Worldwide distribution of continental rock lithology: Implications for the atmospheric/soil CO2 uptake by continental weathering and alkalinity river transport to the oceans. Glob. Biogeochem. Cycles 2003, 17. [Google Scholar] [CrossRef]
- Ford, D.; Williams, P. Karst Hydrogeology and Geomorphology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; p. 562. [Google Scholar]
- Chen, Z.; Auler, A.S.; Bakalowicz, M.; Drew, D.; Griger, F.; Hartmann, J.; Jiang, G.; Moosdorf, N.; Richts, A.; Stevanovic, Z.; et al. The World Karst Aquifer Mapping project: Concept, mapping procedure and map of Europe. Hydrogeol. J. 2017, 25, 771–785. [Google Scholar] [CrossRef]
- Meybeck, M. 5.08—Global Occurrence of Major Elements in Rivers. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Pergamon, NY, USA, 2003; Volume 5, pp. 207–223. [Google Scholar]
- Curl, R.L. Carbon Shifted but Not Sequestered. Science 2012, 335, 655. [Google Scholar] [CrossRef]
- Cao, J.; Yuan, D.; Groves, C.; Huang, F.; Yang, H.; Lu, Q. Carbon Fluxes and Sinks: The Consumption of Atmospheric and Soil CO2 by Carbonate Rock Dissolution. Acta Geol. Sin. Engl. Ed. 2012, 86, 963–972. [Google Scholar]
- Blum, J.D.; Gazis, C.A.; Jacobson, A.D.; Page Chamberlain, C. Carbonate versus silicate weathering in the Raikhot watershed within the High Himalayan Crystalline Series. Geology 1998, 26, 411–414. [Google Scholar] [CrossRef]
- Jacobson, A.D.; Blum, J.D.; Walter, L.M. Reconciling the elemental and Sr isotope composition of Himalayan weathering fluxes: Insights from the carbonate geochemistry of stream waters. Geochim. Cosmochim. Acta 2002, 66, 3417–3429. [Google Scholar] [CrossRef]
- Gombert, P. Role of karstic dissolution in global carbon cycle. Glob. Planet. Chang. 2002, 33, 177–184. [Google Scholar] [CrossRef]
- Liu, Z.; Dreybrodt, W.; Liu, H. Atmospheric CO2 sink: Silicate weathering or carbonate weathering? Appl. Geochem. 2011, 26, S292–S294. [Google Scholar] [CrossRef]
- Liu, Z.; Macpherson, G.L.; Groves, C.; Martin, J.B.; Yuan, D.; Zeng, S. Large and active CO2 uptake by coupled carbonate weathering. Earth Sci. Rev. 2018, 182, 42–49. [Google Scholar] [CrossRef]
- Messerli, B.; Viviroli, D.; Weingartner, R. Mountains of the world: Vulnerable water towers for the 21st century. Ambio 2004, 13, 29–34. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2007 Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, P.K., Reisinger, A., Core Writing Team, Eds.; IPCC: Geneva, Switzerland, 2007. [Google Scholar]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Meyer, L.A., Core Writing Team, Eds.; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Viviroli, D.; Archer, D.R.; Buytaert, W.; Fowler, H.J.; Greenwood, G.B.; Hamlet, A.F.; Huang, Y.; Koboltschnig, G.; Litaor, M.I.; López-Moreno, J.I.; et al. Climate change and mountain water resources: Overview and recommendations for research, management and policy. Hydrol. Earth Syst. Sci. 2011, 15, 471–504. [Google Scholar] [CrossRef]
- Beniston, M.; Stoffel, M. Assessing the impacts of climatic change on mountain water resources. Sci. Total Environ. 2014, 493, 1129–1137. [Google Scholar] [CrossRef]
- Morán-Tejeda, E.; Ceballos-Barbancho, A.; Llorente-Pinto, J.M. Hydrological response of Mediterranean headwaters to climate oscillations and land-cover changes: The mountains of Duero River basin (Central Spain). Glob. Planet. Chang. 2010, 72, 39–49. [Google Scholar] [CrossRef]
- López-Moreno, J.I.; Zabalza, J.; Vicente-Serrano, S.M.; Revuelto, J.; Gilaberte, M.; Azorin-Molina, C.; Morán-Tejeda, E.; García-Ruiza, J.M.; Taguec, C. Impact of climate and land use change on water availability and reservoir management: Scenarios in the Upper Aragón River, Spanish Pyrenees. Sci. Total Environ. 2014, 493, 1222–1231. [Google Scholar] [CrossRef]
- López-Moreno, J.I.; Beniston, M.; García-Ruiz, J.M. Environmental change and water management in the Pyrenees: Facts and future perspectives for Mediterranean mountains. Glob. Planet. Chang. 2008, 61, 300–312. [Google Scholar] [CrossRef]
- Szczypta, C.; Gascoin, S.; Houet, T.; Hagolle, O.; Dejoux, J.F.; Vigneau, C.; Fanise, P. Impact of climate and land cover changes on snow cover in a small Pyrenean catchment. J. Hydrol. 2015, 521, 84–99. [Google Scholar] [CrossRef]
- Mokadem, N.; Hamed, Y.; Hfaid, M.; Dhia, H.B. Hydrogeochemical and isotope evidence of groundwater evolution in El Guettar Oasis area, Southwest Tunisia. Carbonates Evaporites 2015, 30, 417–437. [Google Scholar] [CrossRef]
- Bakalowicz, M. Epikarst. In Encyclopedia of Caves, 2nd ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 284–288. [Google Scholar] [CrossRef]
- Bakalowicz, M. Karst groundwater: A challenge for new resources. Hydrogeol. J. 2005, 13, 148–160. [Google Scholar] [CrossRef]
- Bakalowicz, M. The Epikarst, the Skin of Karst; Karst Waters Institute Special Publication 9: Charles Town, WV, USA, 2003; pp. 16–22. [Google Scholar]
- Daher, W.; Pistre, S.; Kneppers, A.; Bakalowicz, M.; Najem, W. Karst and artificial recharge: Theoretical and practical problems. A preliminary approach to artificial recharge assessment. J. Hydrol. 2011, 408, 189–202. [Google Scholar] [CrossRef]
- Raymond, P.A.; Oh, N.-H.; Turner, R.E.; Broussard, W. Anthropogenically enhanced fluxes of water and carbon from the Mississippi River. Nature 2008, 451, 449. [Google Scholar] [CrossRef]
- Jeannin, P.-Y.; Hessenauer, M.; Malard, A.; Chapuis, V. Impact of global change on karst groundwater mineralization in the Jura Mountains. Sci. Total Environ. 2016, 541, 1208–1221. [Google Scholar] [CrossRef]
- Calmels, D.; Gaillardet, J.; François, L. Sensitivity of carbonate weathering to soil CO2 production by biological activity along a temperate climate transect. Chem. Geol. 2014, 390, 74–86. [Google Scholar] [CrossRef]
- Romero-Mujalli, G.; Hartmann, J.; Börker, J.; Gaillardet, J.; Calmels, D. Ecosystem controlled soil-rock pCO2 and carbonate weathering—Constraints by temperature and soil water content. Ann. N. Y. Acad. Sci. 2018, 769, 71–84. [Google Scholar] [CrossRef]
- Meybeck, M. Composition chimique des ruisseaux non pollués en France. Chemical composition of headwater streams in France. Sci. Géol. Bull. 1986, 39, 3–77. [Google Scholar] [CrossRef]
- Amiotte-Suchet, P.; Probst, J.-L. Modelling of atmospheric CO2 consumption by chemical weathering of rocks: Application to the Garonne, Congo and Amazon basins. Chem. Geol. 1993, 107, 205–210. [Google Scholar] [CrossRef]
- Wang, F.S.; Wang, Y.; Zhang, J.; Xu, H.; Wei, X. Human impact on the historical change of CO2 degassing flux in River Changjiang. Geochem. Trans. 2007, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liu, Z.; Huang, H.; Dong, L. Difference in the relationship between soil CO2 concentration and the karst-related carbon cycle under different land use types in southwest China. Carbonates Evaporites 2019, 34, 1569–1581. [Google Scholar] [CrossRef]
- Hartmann, J.; Jansen, N.; Dürr, H.H.; Kempe, S.; Köhler, P. Global CO2-consumption by chemical weathering: What is the contribution of highly active weathering regions? Glob. Planet. Chang. 2009, 69, 185–194. [Google Scholar] [CrossRef]
- Zhong, J.; Li, S.L.; Tao, F.; Yue, F.; Liu, C.Q. Sensitivity of chemical weathering and dissolved carbon dynamics to hydrological conditions in a typical karst river. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Clow, D.W.; Mast, M.A. Mechanisms for chemostatic behavior in catchments: Implications for CO2 consumption by mineral weathering. Chem. Geol. 2010, 269, 40–51. [Google Scholar] [CrossRef]
- Drever, J.I. The Geochemistry of Natural Waters, Surface and Groundwater Environments, 3rd ed.; Prentice-Hal: Upper Saddle River, NJ, USA, 1997. [Google Scholar]
- Parkhurst, D.L.; Appelo, C.A.J. User’s Guide to PHREEQC (Version 2): A Computer Program for Speciation, Batch-reaction, One-dimensional Transport, and Inverse Geochemical Calculations; Geological Survey: Denver, CO, USA, 1999. [Google Scholar]
- Zeebe, R.; Wolf-Gladrow, D. CO2 in Seawater: Equilibrium, Kinetics, Isotopes, 1st ed.; Elservier Oceanography Series: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Troester, J.W.; White, W.B. Seasonal Fluctuations in the Carbon Dioxide Partial Pressure in a Cave Atmosphere. Water Resour. Res. 1984, 20, 153–156. [Google Scholar] [CrossRef]
- Peyraube, N.; Lastennet, R.; Denis, A. Geochemical evolution of groundwater in the unsaturated zone of a karstic massif, using the PCO2–SIc relationship. J. Hydrol. 2012, 430–431, 13–24. [Google Scholar] [CrossRef]
- White, W.B. Carbon fluxes in Karst aquifers: Sources, sinks, and the effect of storm flow. Acta Carsol. 2013, 42, 177–186. [Google Scholar] [CrossRef]
- Probst, J.-L. Géochimie et hydrologie de l’érosion continentale. Mécanismes, bilan global actuel et fluctuations au cours des 500 derniers millions d’années. Sci. Géol. Mem. 1992, 94, 3–164. [Google Scholar]
- Gaillardet, J.; Calmels, D.; Romero-Mujalli, G.; Zakharova, E.; Hartmann, J. Global climate control on carbonate weathering intensity. Chem. Geol. 2019, 527, 118762. [Google Scholar] [CrossRef]
- Reynolds, C.C.; Escobedo, F.J.; Clerici, N.; Zea-Camaño, J. Does “greening” of neotropical cities considerably mitigate carbon dioxide emissions? The case of Medellin, Colombia. Sustainability 2017, 9, 785. [Google Scholar] [CrossRef]
- Rasse, D.P.; François, L.; Aubinet, M.; Kowalski, A.S.; Vande Walle, I.; Laitat, E.; Gérard, J.-C. Modelling short-term CO2 fluxes and long-term tree growth in temperate forests with ASPECTS. Ecol. Modell. 2001, 141, 35–52. [Google Scholar] [CrossRef]
- Li, S.-L.; Liu, C.-Q.; Li, J.; Lang, Y.-C.; Ding, H.; Li, L. Geochemistry of dissolved inorganic carbon and carbonate weathering in a small typical karstic catchment of Southwest China: Isotopic and chemical constraints. Chem. Geol. 2010, 277, 301–309. [Google Scholar] [CrossRef]
- Ek, C.; Godissart, J. Carbon dioxide in cave air and soil air in some karstic areas of Belgium. A prospective view. Geol. Belgica 2014, 17, 102–106. [Google Scholar]
- Raymond, P.A.; Cole, J.J. Increase in the Export of Alkalinity from North America’s Largest River. Science 2003, 301, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, Y.P.; Zhou, G.; Li, S.; Yu, G.; Li, K. Carbon uptake by karsts in the Houzhai Basin, southwest China. J. Geophys. Res. Biogeosci. 2011, 116. [Google Scholar] [CrossRef]
- Stets, E.G.; Kelly, V.J.; Crawford, C.G. Long-term trends in alkalinity in large rivers of the conterminous US in relation to acidification, agriculture, and hydrologic modification. Sci. Total Environ. 2014, 488–489, 280–289. [Google Scholar] [CrossRef]
- Lespinas, F.; Ludwig, W.; Heussner, S. Impact of recent climate change on the hydrology of coastal Mediterranean rivers in Southern France. Clim. Chang. 2010, 99, 425–456. [Google Scholar] [CrossRef]
- García-Ruiz, J.M.; López-Moreno, J.I.; Vicente-Serrano, S.M.; Lasanta–Martínez, T.; Beguería, S. Mediterranean water resources in a global change scenario. Earth Sci. Rev. 2011, 105, 121–139. [Google Scholar] [CrossRef]
- López-Moreno, J.I.; Vicente-Serrano, S.M.; Moran-Tejeda, E.; Zabalza, J.; Lorenzo-Lacruz, J.; García-Ruiz, J.M. Impact of climate evolution and land use changes on water yield in the Ebro basin. Hydrol. Earth Syst. Sci. 2011, 15, 311–322. [Google Scholar] [CrossRef]
- Brunetti, M.; Buffoni, L.; Mangianti, F.; Maugeri, M.; Nanni, T. Temperature, precipitation and extreme events during the last century in Italy. Glob. Planet. Chang. 2004, 40, 141–149. [Google Scholar] [CrossRef]
- Alpert, P.; Krichak, S.O.; Shafir, H.; Haim, D.; Osetinsky, I. Climatic trends to extremes employing regional modeling and statistical interpretation over the E. Mediterranean. Glob. Planet. Chang. 2008, 63, 163–170. [Google Scholar] [CrossRef]
- Ashofteh, P.S.; Bozorg Haddad, O.; Mariño, M.A. Scenario Assessment of Streamflow Simulation and its Transition Probability in Future Periods Under Climate Change. Water Resour. Manag. 2013, 27, 255–274. [Google Scholar] [CrossRef]
- Rabbinge, R.; Van Diepen, C.A. Changes in agriculture and land use in Europe. Eur. J. Agron. 2000, 13, 85–99. [Google Scholar] [CrossRef]
- García-Ruiz, J.M.; Lana-Renault, N. Hydrological and erosive consequences of farmland abandonment in Europe, with special reference to the Mediterranean region—A review. Agric. Ecosyst. Environ. 2011, 140, 317–338. [Google Scholar] [CrossRef]
- Semhi, K.; Amiotte-Suchet, P.; Clauer, N.; Probst, J.-L. Impact of nitrogen fertilizers on the natural weathering-erosion processes and fluvial transport in the Garonne basin. Appl. Geochem. 2000, 15, 865–878. [Google Scholar] [CrossRef]
- Perrin, A.S.; Probst, A.; Probst, J.-L. Impact of nitrogenous fertilizers on carbonate dissolution in small agricultural catchments: Implications for weathering CO2 uptake at regional and global scales. Geochim. Cosmochim. Acta 2008, 72, 3105–3123. [Google Scholar] [CrossRef]
- Brunet, F.; Potot, C.; Probst, A.; Probst, J.L. Stable Carbon isotope evidence for nitrogenous fertilizer impact on carbonate weathering in a small agricultural watershed. Rapid Commun. Mass Spectrom. 2011, 25, 2682–2690. [Google Scholar] [CrossRef]
- Probst, A.; Dambrine, E.; Viville, D.; Fritz, B. Influence of acid atmospheric inputs on surface water chemistry and mineral fluxes in a declining spruce stand within a small granitic catchment (Vosges Massif, France). J. Hydrol. 1990, 116, 101–124. [Google Scholar] [CrossRef]
- Li, S.-L.; Calmels, D.; Han, G.; Gaillardet, J.; Liu, C.Q. Sulfuric acid as an agent of carbonate weathering constrained by δ13CDIC: Examples from Southwest China. Earth Planet. Sci. Lett. 2008, 270, 189–199. [Google Scholar] [CrossRef]
- Ding, H.; Lang, Y.C.; Liu, C.Q.; Liu, T.Z. Chemical characteristics and δ34S-SO42- of acid rain: Anthropogenic sulfate deposition and its impacts on CO2 consumption in the rural karst area of southwest China. Geochem. J. 2013, 47, 625–638. [Google Scholar] [CrossRef]
- Probst, A.; Ambroise, B. Disturbance and resilience of a granitic critical zone submitted to acid atmospheric influence (the Ringelbach catchment, Vosges Mountains, France): Lessons from a hydrogeochemical survey in the nineties. J. Hydrol. 2019, 569, 77–92. [Google Scholar] [CrossRef]
- Fonyuy, E.W.; Atekwana, E.A. Effects of acid mine drainage on dissolved inorganic carbon and stable carbon isotopes in receiving streams. Appl. Geochem. 2008, 23, 743–764. [Google Scholar] [CrossRef]
- Ali, H.N.; Atekwana, E.A. The effect of sulfuric acid neutralization on carbonate and stable carbon isotope evolution of shallow groundwater. Chem. Geol. 2011, 284, 217–228. [Google Scholar] [CrossRef]
- Spence, J.; Telmer, K. The role of sulfur in chemical weathering and atmospheric CO2 fluxes: Evidence from major ions, δ13CDIC, and δ34S SO4 in rivers of the Canadian Cordillera. Geochim. Cosmochim. Acta 2005, 69, 5441–5458. [Google Scholar] [CrossRef]
- Calmels, D.; Gaillardet, J.; Brenot, A.; France-Lanord, C. Sustained sulfide oxidation by physical erosion processes in the Mackenzie River basin: Climatic perspectives. Geology 2007, 35, 1003–1006. [Google Scholar] [CrossRef]
- Lindberg, S.E.; Garten, C.T. Sources of sulphur in forest canopy throughfall. Nature 1988, 336, 148–151. [Google Scholar] [CrossRef]
- Probst, A.; Viville, D.; Fritz, B.; Ambroise, B.; Dambrine, E. Hydrochemical budgets of a small forested granitic catchment exposed to acid deposition: The Strengbach catchment case study (Vosges massif, France). Water Air Soil Pollut. 1992, 62, 337–347. [Google Scholar] [CrossRef]
- Mangin, A. Contribution à L’étude Hydrodynamique des Aquifères Karstiques. Ph.D. Thesis, Université de Dijon, Dijon, France, 1974. (Ann. Spéléo., 1974 29: 283-332; 1974 29: 495-601; 1975 30: 21-124). [Google Scholar]
- Labat, D.; Masbou, J.; Beaulieu, E.; Mangin, A. Scaling behavior of the fluctuations in stream flow at the outlet of karstic watersheds, France. J. Hydrol. 2011, 410, 162–168. [Google Scholar] [CrossRef]
- Sivelle, V.; Labat, D.; Mazzilli, N.; Massei, N.; Jourde, H. Dynamics of the Flow Exchanges between Matrix and Conduits in Karstified Watersheds at Multiple Temporal Scales. Water 2019, 11, 569. [Google Scholar] [CrossRef]
- Binet, S.; Probst, J.L.; Batiot, C.; Seidel, J.L.; Emblanch, C.; Peyraube, N.; Charlier, J.B.; Bakalowicz, M.; Probst, A. Global warming and acid atmospheric deposition impacts on carbonate dissolution and CO2 fluxes in French karst hydrosystems: Evidence from hydrochemical monitoring in recent decades. Geochim. Cosmochim. Acta 2020, 270, 184–200. [Google Scholar] [CrossRef]
- Bakalowicz, M. Contribution de la Géochimie des Eaux a la Connaissance de L’aquifère Karstique et de la Karstification. Ph.D. Thesis, Université Pierre et Marie Curie, Paris, France, 1979. [Google Scholar]
- Mangin, A. Le système karstique du Baget (Ariège). Ann. Spéléol. 1970, 25, 560–580. [Google Scholar]
- Debroas, E. Géologie du bassin versant du Baget (zone nord-pyrénéenne, Ariège, France): Nouvelles observations et conséquences. Assoc. Strata 2009, 46, 1–93. [Google Scholar]
- Info Terre: Téléchargement des Cartes Géologiques. Available online: http://infoterre.brgm.fr/page/telechargement-cartes-geologiques (accessed on 3 March 2020).
- Padilla, A.; Pulido-Bosch, A.; Mangin, A. Relative Importance of Baseflow and Quickflow from Hydrographs of Karst Spring. Groundwater 1944, 32, 267–277. [Google Scholar] [CrossRef]
- Johannet, A.; Vayssade, B.; Bertin, D. Neural Networks: From Black Box towards Transparent Box—Application to Evapotranspiration Modelling. Int. J. Comput. Intell. 2008, 4, 163–170. [Google Scholar]
- Joly, D. Variation spatiale des facteurs qui expliquent le volume des précipitations en France; analyse à échelle locale. In Journées de Climatologie de la Commission "Climat et Société" du CNFG, Climat et Eau; Lyon, France, 2011; p. 16. Available online: hal.archives-ouvertes.fr/hal-00941124 (accessed on 23 April 2020).
- Douguédroit, A.; de Saintignon, M.-F. Les gradients de températures et de précipitations en montagne. Revue Géograph. Alpine 1984, 72, 225–240. [Google Scholar]
- Labat, D.; Ababou, R.; Mangin, A. Rainfall–runoff relations for karstic springs. Part I: Convolution and spectral analyses. J. Hydrol. 2000, 238, 123–148. [Google Scholar] [CrossRef]
- Jourde, H.; Massei, N.; Mazzilli, N.; Binet, S.; Batiot-Guilhe, C.; Labat, D.; Steinmann, M.; Bailly-Comte, V.; Seidel, J.L.; Arfib, B.; et al. SNO KARST: A french network of observatories for the multidisciplinary study of critical zone processes in karst watersheds and aquifers. Vadose Zone J. 2018, 17, 1–18. [Google Scholar] [CrossRef]
- Gaillardet, J.; Braud, I.; Hankard, F.; Anquetin, S.; Bour, O.; Dorfliger, N.; de Dreuzy, J.R.; Galle, S.; Galy, C.; Gogo, S.; et al. OZCAR: The French network of critical zone observatories. Vadose Zone J. 2018, 17, 1–24. [Google Scholar] [CrossRef]
- Parr, T.W.; Ferretti, M.; Simpson, I.C.; Forsius, M.; Kovács-Láng, E. Towards A Long-Term Integrated Monitoring Programme In Europe: Network Design in Theory and Practice. Environ. Monit. Assess 2002, 78, 253–290. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.; Baessler, C.; Schubert, H.; Klotz, S. (Eds.) Long-Term Ecological Research. Between Theory and Application; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- ADES—Eau France: Point d’eau BSS002MAYC (10734X0010/HY) BAGET. Available online: https://ades.eaufrance.fr/Fiche/PtEau?Code=10734X0010/HY (accessed on 3 March 2020).
- Clark, I.D.; Fritz, P. Environmental Isotopes in Hydrogeology; Lewis Publishers: New York, NY, USA, 1997. [Google Scholar]
- Plummer, L.; Busenberg, E. The solubilities of calcite, aragonite and vaterite in CO2-H2O solutions between 0 and 90 °C, and an evaluation of the aqueous model for the system CaCO3-CO2-H2O. Geochim. Cosmochim. Acta 1982, 46, 1011–1040. [Google Scholar] [CrossRef]
- Davies, C.W. The extent of dissociation of salts in water. Part VIII. An equation for the mean ionic activity coefficient of an electrolyte in water, and a revision of the dissociation constants of some sulphates. J. Chem. Soc. 1938, 2093–2098. [Google Scholar] [CrossRef]
- Buishand, T.A. Some methods for testing the homogeneity of rainfall records. J. Hydrol. 1982, 58, 11–27. [Google Scholar] [CrossRef]
- Buishand, T.A. Tests for detecting a shift in the mean of hydrological time series. J. Hydrol. 1984, 73, 51–69. [Google Scholar] [CrossRef]
- Piper, A.M. A graphic procedure in geochemical interpretation of water analyses. Trans. Am. Geophys. Union 1944, 25, 914–923. [Google Scholar] [CrossRef]
- Hamilton. ggtern: An Extension to ‘ggplot2’, for the Creation of Ternary Diagrams. R Package Version 2.2.2. 2018. Available online: https://CRAN.R-project.org/package=ggtern (accessed on 3 March 2020).
- Petelet, E.; Luck, J.-M.; Ben Othman, D.; Negrel, P.; Aquilina, L. Geochemistry and water dynamics of a medium-sized watershed: The Hérault, southern France: 1. Organisation of the different water reservoirs as constrained by Sr isotopes, major, and trace elements. Chem. Geol. 1998, 150, 63–83. [Google Scholar] [CrossRef]
- Donnini, M.; Frondini, F.; Probst, J.-L.; Probst, A.; Cardellini, C.; Marchesini, I.; Guzzetti, F. Chemical weathering and consumption of atmospheric carbon dioxide in the Alpine region. Glob. Planet. Chang. 2016, 136, 65–81. [Google Scholar] [CrossRef]
- El Najjar, P.; Kassouf, A.; Probst, A.; Probst, J.-L.; Ouaini, N.; Daou, C.; El Azzi, D. High-frequency monitoring of surface water quality at the outlet of the Ibrahim River (Lebanon): A multivariate assessment. Ecol. Indic. 2019, 104, 13–23. [Google Scholar] [CrossRef]
- Sarazin, G.; Ciabrini, J.P. Water Geochemistry of Three Mountain Streams from Carbonate Watersheds in the Southern French Alps. Aquatic Geochem. 1997, 3, 233–265. [Google Scholar] [CrossRef]
- Laffitte, P. Traite D’informatique Géologique; Masson: Paris, France, 1972; p. 624. [Google Scholar]
- Kaiser, H.F. The varimax criterion for analytic rotation in factor analysis. Psychometrika 1958, 23, 187–200. [Google Scholar] [CrossRef]
- Nicholson, R.V.; Gillham, R.W.; Reardon, E.J. Pyrite oxidation in carbonate-buffered solution: 1. Experimental kinetics. Geochim. Cosmochim. Acta 1988, 52, 1077–1085. [Google Scholar] [CrossRef]
- Probst, A.; Lelong, F.; Viville, D.; Durand, P.; Ambroise, B.; Fritz, B. Comparative Hydrochemical Behaviour and Element Budgets of the Aubure (Vosges Massif) and Mont-Lozère (Southern Massif Central) Norway Spruce Forested Catchments. In Atmospheric Deposition Effects in the French Mountains; Landmann, G., Bonneau, M., Kaennel, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 203–225. [Google Scholar]
- Kayser, N.; Probst, J.-L.; Cadet, D.; Tardy, Y. Propagation des ondes de sécheresse et d’humidité à travers le monde. Comptes Rendus de l’Academie des Sci. Ser. IIA—Earth Planet. 1990, 310, 757–763. [Google Scholar]
- Mangin, A. Transfer function approach for artificial tracer test interpretation in karstic systems. J. Hydrol. 2015, 529, 866–871. [Google Scholar]
- Viers, J.; Oliva, P.; Dandurand, J.; Dupre, B.; Gaillardet, J. Chemical Weathering Rates, CO2 Consumption, and Control Parameters Deduced from the Chemical Composition of Rivers. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Pergamon, NY, USA, 2014; Volume 7, pp. 175–194. [Google Scholar]
- Probst, J.-L.; Mortatti, J.; Tardy, Y. Carbon river fluxes and weathering CO2 consumption in the Congo and Amazon river basins. Appl. Geochem. 1994, 9, 1–13. [Google Scholar] [CrossRef]
- Amiotte-Suchet, P. Cycle du carbone, érosion chimique des continents et transferts vers les océans. Sci. Géol. Mem. 1995, 97, 3–156. [Google Scholar]
- Amiotte-Suchet, P.; Probst, J.-L. Origines du carbone inorganique dissous dans les eaux de la Garonne. Variations saisonnières et interannuelles. Sci. Géol. Bull. 1996, 49, 101–126. [Google Scholar]
- Mortatti, J.; Probst, J.-L. Silicate rock weathering and atmospheric/soil CO2 uptake in the Amazon basin estimated from river water geochemistry: Seasonal and spatial variations. Chem. Geol. 2003, 197, 177–196. [Google Scholar] [CrossRef]
- Takano, B.; Asano, Y.; Watanuki, K. Characterization of sulfate ion in travertine. Contrib. Mineral. Petrol. 1980, 72, 197–203. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry—Chemical Equilibria and Rates in Natural Waters, 3rd ed.; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Marcé, R.; Obrador, B.; Morguí, J.A.; Lluís Riera, J.; López, P.; Armengol, J. Carbonate weathering as a driver of CO2 supersaturation in lakes. Nat. Geosci. 2015, 8, 107–111. [Google Scholar] [CrossRef]
- Ulloa-Cedamanos, F. Impacts du Changement Climatique et des Activités Anthropiques sur les Cycles Biogéochimiques du Carbone et de L’azote Dans les Hydrosystèmes Karstiques. Ph.D. Thesis, Toulouse University, Toulouse, France, 2021. (In Progress). [Google Scholar]
- Puig, R.; Avila, A.; Soler, A. Sulphur isotopes as tracers of the influence of a coal-fired power plant on a Scots pine forest in Catalonia (NE Spain). Atmos. Environ. 2008, 42, 733–745. [Google Scholar] [CrossRef][Green Version]
- Pierret, M.C.; Viville, D.; Dambrine, E.; Cotel, S.; Probst, A. Twenty-five-year record of chemicals in open field precipitation and throughfall from a medium-altitude forest catchment (Strengbach—NE France): An obvious response to atmospheric pollution trends. Atmos. Environ. 2019, 202, 296–314. [Google Scholar] [CrossRef]
- Schöpp, W.; Posch, M.; Mylona, S.; Johansson, M. Long-term development of acid deposition (1880–2030) in sensitive freshwater regions in Europe. Hydrol. Earth Syst. Sci. 2003, 7, 436–446. [Google Scholar] [CrossRef]
- Pascaud, A.; Sauvage, S.; Coddeville, P.; Nicolas, M.; Croisé, L.; Mezdour, A.; Probst, A. Contrasted spatial and long-term trends in precipitation chemistry and deposition fluxes at rural stations in France. Atmos. Environ. 2016, 146, 28–43. [Google Scholar] [CrossRef]
- Pierret, M.C.; Cotel, S.; Ackerer, P.; Beaulieu, E.; Benarioumlil, S.; Boucher, M.; Boutin, R.; Chabaux, F.; Delay, F.; Fourtet, C.; et al. The Strengbach Catchment: A multidisciplinary environmental sentry for 30 years. Vadose Zone J. 2018, 17, 1–17. [Google Scholar] [CrossRef]
- Meybeck, M. Atmospheric Inputs and River Transport of Dissolved Substances. Available online: http://hydrologie.org/redbooks/a141/iahs_141_0173.pdf (accessed on 23 April 2020).
- Qin, C.; Li, S.; Yue, F.; Xu, S.; Ding, H. Spatiotemporal variations of dissolved inorganic carbon and controlling factors in a small karstic catchment, Southwestern China. Earth Surf. Process. Landf. 2019, 44, 2423–2436. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, J. Contribution of carbonate rock weathering to the atmospheric CO2 sink. Environ. Geol. 1999, 39, 1053–1058. [Google Scholar] [CrossRef]
- Tipper, E.T.; Bickle, M.J.; Galy, A.; West, A.J.; Pomiès, C.; Chapman, H.J. The short-term climatic sensitivity of carbonate and silicate weathering fluxes: Insight from seasonal variations in river chemistry. Geochim. Cosmochim. Acta 2006, 70, 2737–2754. [Google Scholar] [CrossRef]
- Probst, J.-L.; Bazerbachi, A. Transports en solution et en suspension par la Garonne supérieure. Solute and particulate transports by the upstream part of the Garonne river. Sci. Géol. Bull. 1986, 39, 79–98. [Google Scholar] [CrossRef]
- Ladouche, B.; Probst, A.; Viville, D.; Idir, S.; Baqué, D.; Loubet, M.; Probst, J.-L.; Bariac, T. Hydrograph separation using isotopic, chemical and hydrological approaches (Strengbach catchment, France). J. Hydrol. 2001, 242, 255–274. [Google Scholar] [CrossRef]
- Ponnou-Delaffon, V.; Probst, A.; Payre-Suc, V.; Granouillac, F.; Ferrant, S.; Perrin, A.-S.; Probst, J.-L. Long and short-term trends of stream hydrochemistry and high frequency surveys as indicators of the influence of climate change, agricultural practices and internal processes (Aurade agricultural catchment, SW France). Ecol. Indic. 2020, 110, 105894. [Google Scholar] [CrossRef]
- Ilstedt, U.; Nordgren, A.; Malmer, A. Optimum soil water for soil respiration before and after amendment with glucose in humid tropical acrisols and a boreal mor layer. Soil Biol. Biochem. 2000, 32, 1591–1599. [Google Scholar] [CrossRef]
- Drake, J. The effect of soil activity on the chemistry of carbonate groundwaters. Water Resour. Res. 1980, 16, 381–386. [Google Scholar] [CrossRef]
- Zhao, Q.; Chang, D.; Wang, K.; Huang, J. Patterns of nitrogen export from a seasonal freezing agricultural watershed during the thawing period. Sci. Total Environ. 2017, 599–600, 442–450. [Google Scholar] [CrossRef]
- Van Rampelbergh, M.; Verheyden, S.; Allan, M.; Quinif, Y.; Keppens, E.; Claeys, P. Monitoring of a fast-growing speleothem site from the Han-sur-Lesse cave, Belgium, indicates equilibrium deposition of the seasonal δ18O and δ13C signals in the calcite. Clim. Past 2014, 10, 1871–1885. [Google Scholar] [CrossRef][Green Version]
- Telmer, K.; Veizer, J. Carbon fluxes, pCO2 and substrate weathering in a large northern river basin, Canada: Carbon isotope perspectives. Chem. Geol. 1999, 159, 61–86. [Google Scholar] [CrossRef]
- Klimchouk, A.-B. Towards defining, delimiting and classifying epikarst: Its origin, processes and variants of geomorphic evolution. Speleogenesis Evol. Karst Aquifers 2004, 2, 1–13. [Google Scholar]
- Zou, S.; Deng, Z.; Zhu, Y.; Liang, B.; Xia, R.; Tang, J. Hydrologic Features and Eco-Environmental Classification of Epikarst Springs in Luota, West of Hunan, China. Earth Sci. Front. 2008, 15, 190–197. [Google Scholar] [CrossRef]
- Aquilina, L.; Ladouche, B.; Dörfliger, N. Water storage and transfer in the epikarst of karstic systems during high flow periods. J. Hydrol. 2006, 327, 472–485. [Google Scholar] [CrossRef]
- Trček, B. How can the epikarst zone influence the karst aquifer hydraulic behaviour? Environ. Geol. 2007, 51, 761–765. [Google Scholar] [CrossRef]
- Peyraube, N.; Lastennet, R.; Denis, A.; Malaurent, P. Estimation of epikarst air PCO2 using measurements of water δ13CTDIC, cave air PCO2 and δ13C CO2. Geochim. Cosmochim. Acta 2013, 118, 1–17. [Google Scholar] [CrossRef]
- Walling, D.E.; Foster, I.D.L. Variations in the natural chemical concentration of river water during flood flows, and the lag effect: Some further comments. J. Hydrol. 1975, 26, 237–244. [Google Scholar] [CrossRef]
- Mahamat Nour, A.; Vallet-Coulomb, C.; Bouchez, C.; Ginot, P.; Doumnang, J.C.; Sylvestre, F.; Deschamps, P. Geochemistry of the Lake Chad Tributaries Under Strongly Varying Hydro-climatic Conditions. Aquat. Geochem. 2020, 26, 3–29. [Google Scholar] [CrossRef]
- Kämäri, M.; Tattari, S.; Lotsari, E.; Koskiaho, J.; Lloyd, C.-E.-M. High-frequency monitoring reveals seasonal and event-scale water quality variation in a temporally frozen river. J. Hydrol. 2018, 564, 619–639. [Google Scholar] [CrossRef]
- Martínez-Santos, M.; Antigüedad, I.; Ruiz-Romera, E. Hydrochemical variability during flood events within a small forested catchment in Basque Country (Northern Spain). Hydrol. Process. 2014, 28, 5367–5381. [Google Scholar] [CrossRef]
- Bovolin, V.; Cuomo, A.; Guida, D. Monitoring activity at the Middle Bussento Karst System (Cilento Geopark, southern Italy). Eng. Geol. Soc. Territ. 2015, 3, 275–279. [Google Scholar]
- Qin, C.; Li, S.-L.; Waldron, S.; Yue, F.-J.; Wang, Z.-J.; Zhong, J.; Ding, H.; Liu, C.-Q. High-frequency monitoring reveals how hydrochemistry and dissolved carbon respond to rainstorms at a karstic critical zone, Southwestern China. Sci. Total Environ. 2020, 714, 136833. [Google Scholar] [CrossRef]



| Parameter | N | Max. | Min. | Mean | Median | Mode | Std. Dev. (σ) | CV |
|---|---|---|---|---|---|---|---|---|
| Q | 14609/592 | 10.10/5.41 | 0.02/0.04 | 0.44/0.39 | 0.22/0.20 | 0.13/0.15 | 0.67/0.54 | 1.52/1.40 |
| Air T° | 14604/592 | 29.5/28.2 | −11.1/−5.0 | 12.0/12.2 | 11.9/12.0 | 9.2/8.4 | 6.33/6.35 | 0.53/0.52 |
| pH | 1506/592 | 8.4/8.4 | 6.5/6.9 | 7.7/7.7 | 7.7/7.7 | 7.6/7.7 | 0.18/0.18 | 0.02/0.02 |
| Cond | 637/381 | 398/398 | 223/268 | 306/317 | 307/316 | 319/319 | 24.90/18.97 | 0.08/0.06 |
| Ca2+ | 1504/592 | 3.65/3.55 | 2.40/2.57 | 2.97/3.03 | 2.96/3.01 | 3.00/3.15 | 0.18/0.17 | 0.06/0.05 |
| Mg2+ | 1504/592 | 0.75/0.75 | 0.23/0.23 | 0.36/0.37 | 0.36/0.36 | 0.34/0.40 | 0.05/0.06 | 0.13/0.15 |
| Na+ | 880/592 | 0.07/0.06 | 0.03/0.03 | 0.05/0.05 | 0.05/0.05 | 0.05/0.05 | 0.01/0.00 | 0.11/0.10 |
| K+ | 880/592 | 0.19/0.04 | 0.00/0.00 | 0.01/0.01 | 0.01/0.01 | 0.01/0.01 | 0.01/0.00 | 0.57/0.28 |
| HCO3− | 1501/592 | 4.40/3.66 | 2.43/2.57 | 2.90/2.98 | 2.88/2.96 | 2.76/2.76 | 0.22/0.19 | 0.07/0.06 |
| SO42− | 763/592 | 0.84/0.78 | 0.01/0.04 | 0.35/0.35 | 0.34/0.34 | 0.27/0.27 | 0.13/0.13 | 0.37/0.36 |
| Cl− | 893/592 | 0.25/0.13 | 0.00/0.02 | 0.05/0.05 | 0.05/0.05 | 0.04/0.04 | 0.01/0.01 | 0.27/0.23 |
| NO3− | 705/592 | 0.17/0.17 | 0.00/0.00 | 0.03/0.03 | 0.03/0.03 | 0.03/0.03 | 0.02/0.02 | 0.57/0.56 |
| TDS | 642/592 | 331.5/327.5 | 229/229 | 272.8/273.6 | 272.0/272.8 | 264.3/264.3 | 15.02/14.41 | 0.06/0.05 |
| pCO2 | 1498/592 | 1 × 10−1.64 /1 × 10−1.68 | 1 × 10−3.23 /1 × 10−3.23 | 1 × 10−2.45 /1 × 10−2.48 | 1 × 10−2.50 /1 × 10−2.52 | 1 × 10−2.44 /1 × 10−2.50 | 0.002/0.001 | 0.53/0.46 |
| SIc | 1482/592 | 0.78/0.78 | −0.88/0.64 | 0.03/0.09 | 0.03/0.07 | 0.03/0.04 | 0.18/0.18 | 6.09/2.12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulloa-Cedamanos, F.; Probst, J.-L.; Binet, S.; Camboulive, T.; Payre-Suc, V.; Pautot, C.; Bakalowicz, M.; Beranger, S.; Probst, A. A Forty-Year Karstic Critical Zone Survey (Baget Catchment, Pyrenees-France): Lithologic and Hydroclimatic Controls on Seasonal and Inter-Annual Variations of Stream Water Chemical Composition, pCO2, and Carbonate Equilibrium. Water 2020, 12, 1227. https://doi.org/10.3390/w12051227
Ulloa-Cedamanos F, Probst J-L, Binet S, Camboulive T, Payre-Suc V, Pautot C, Bakalowicz M, Beranger S, Probst A. A Forty-Year Karstic Critical Zone Survey (Baget Catchment, Pyrenees-France): Lithologic and Hydroclimatic Controls on Seasonal and Inter-Annual Variations of Stream Water Chemical Composition, pCO2, and Carbonate Equilibrium. Water. 2020; 12(5):1227. https://doi.org/10.3390/w12051227
Chicago/Turabian StyleUlloa-Cedamanos, Francesco, Jean-Luc Probst, Stephane Binet, Thierry Camboulive, Virginie Payre-Suc, Corinne Pautot, Michel Bakalowicz, Sandra Beranger, and Anne Probst. 2020. "A Forty-Year Karstic Critical Zone Survey (Baget Catchment, Pyrenees-France): Lithologic and Hydroclimatic Controls on Seasonal and Inter-Annual Variations of Stream Water Chemical Composition, pCO2, and Carbonate Equilibrium" Water 12, no. 5: 1227. https://doi.org/10.3390/w12051227
APA StyleUlloa-Cedamanos, F., Probst, J.-L., Binet, S., Camboulive, T., Payre-Suc, V., Pautot, C., Bakalowicz, M., Beranger, S., & Probst, A. (2020). A Forty-Year Karstic Critical Zone Survey (Baget Catchment, Pyrenees-France): Lithologic and Hydroclimatic Controls on Seasonal and Inter-Annual Variations of Stream Water Chemical Composition, pCO2, and Carbonate Equilibrium. Water, 12(5), 1227. https://doi.org/10.3390/w12051227








