Effect of Feed Delivery Rate and Pellet Size on Rearing Performance, Feed Wastage and Economic Profitability in Gilthead Seabream (Sparus Aurata) Ongrowing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Conditions and Procedures
| Ongrowing performance: | |
| Specific Growth Rate (SGR): | SGR (% BWi ∙ day−1) = (LnBWf − LnBWi) ∙ 100/t |
| Coefficient of Variation of weight (CV): | CV (% sd) = 100 ∙ (sd/BWm) |
| Waste production: | |
| Feed Lost by Chewing (LbC): | LbC (% Fs) = 100 ∙ Ff/Fs |
| Wasted Feed Ratio (WFR): | |
| As a function of daily ration (WFRDR): | WFRDR (% DR) = 100 − (100 ∙ Fi/DR) |
| As a function of feed supplied (WFRFs): | WFRFs (% Fs) = 100 − (100 ∙ Fi/Fs) |
| Economic analysis: | |
| Economic Feed Conversion Ratio (eFCR): | eFCR (kg feed/kg biomass)= Fs/(Bf − Bi) |
| Economic Conversion Ratio (ECR): | |
| As a function of daily ration: | ECRDR (€ kg−1) = DC ∙ [DR/(Bf − Bi + Bd)] |
| As a function of feed supplied: | ECRFs (€ kg−1) = DC ∙ [Fs/(Bf − Bi + Bd)] |
| As a function of feed intake: | ECRFi (€ kg−1) = DC ∙ [Fi/(Bf − Bi + Bd)] |
| As a function of truly ingested feed: | ECRTFi (€ kg−1) = DC ∙ [TFi/(Bf − Bi+ Bd)] |
| As a function of daily ration: | EPIDR (€ fish−1) = (BWf ∙ SSP) − (ECRDR ∙ ∆BW) |
| As a function of supplied feed: | EPIFs (€ fish−1) = (BWf ∙ SSP) − (ECRFs ∙ ∆BW) |
| As a function feed intake: | EPIFi (€ fish−1) = (BWf ∙ SSP) − (ECRFi ∙ ∆BW) |
| As a function of truly ingested feed: | EPITFi (€ fish−1) = (BWf ∙ SSP) − (ECRTFi ∙ ∆BW) |
2.2. Statistical Analyses
3. Results
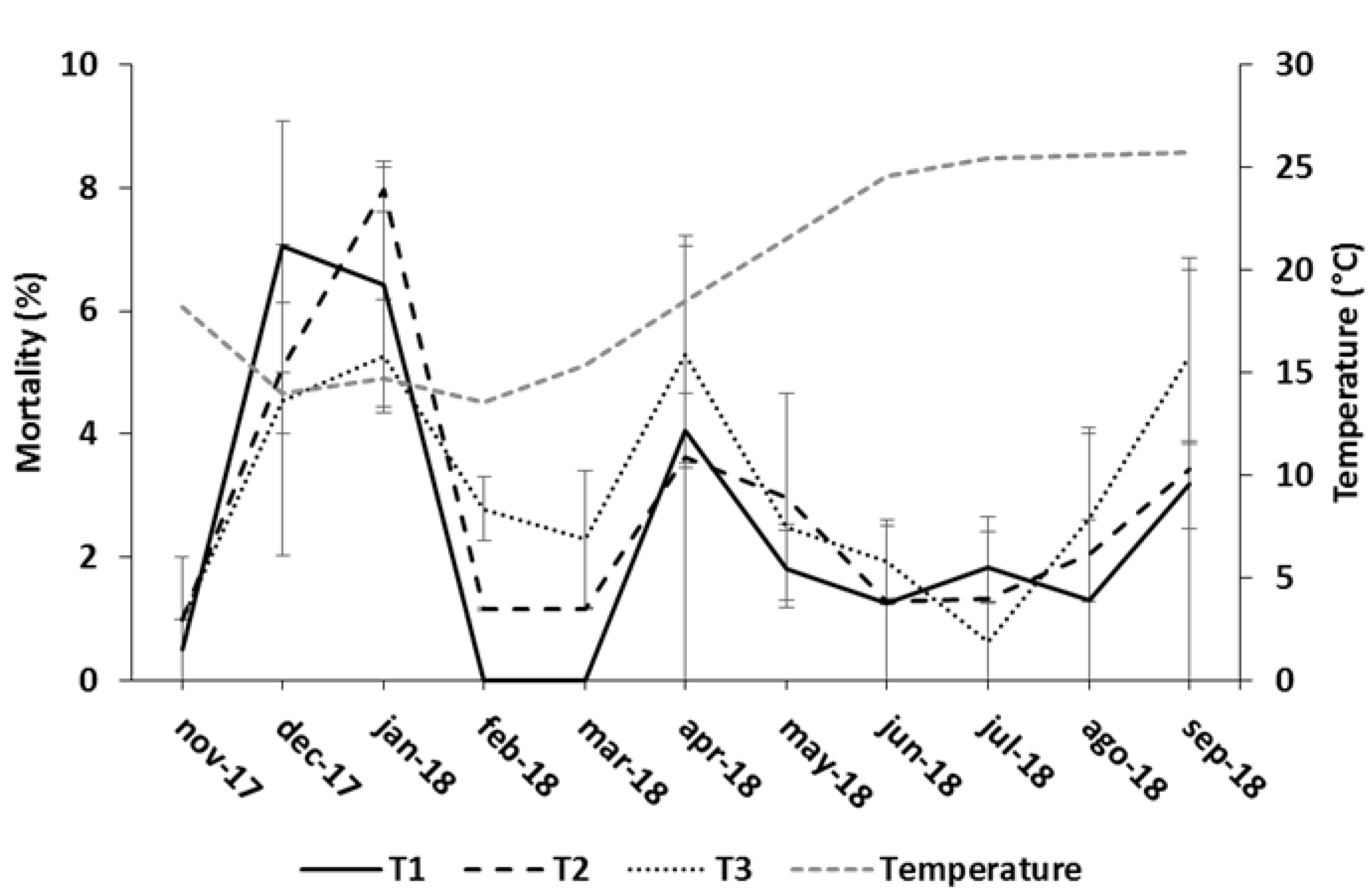
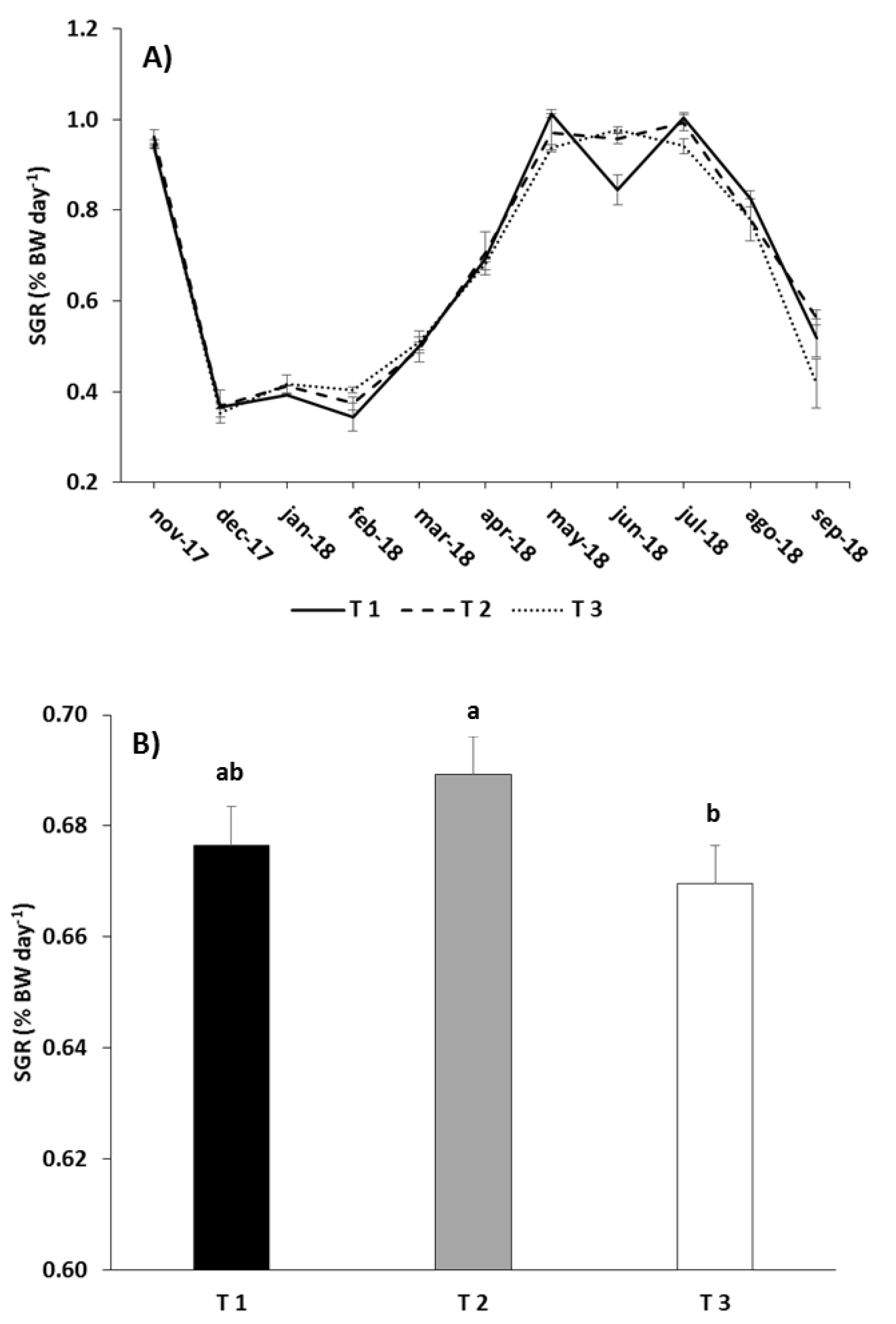
3.1. Growth Performance
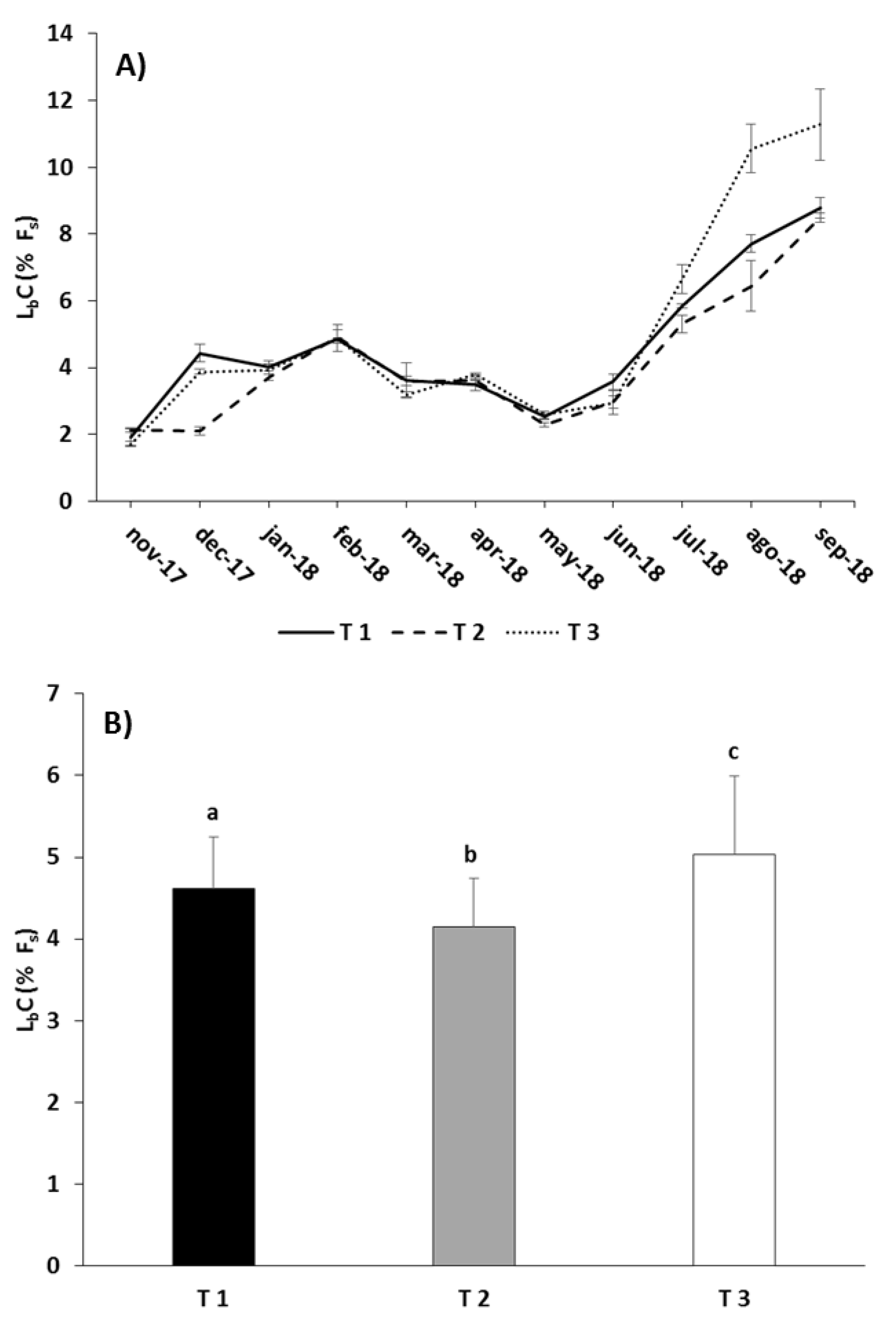
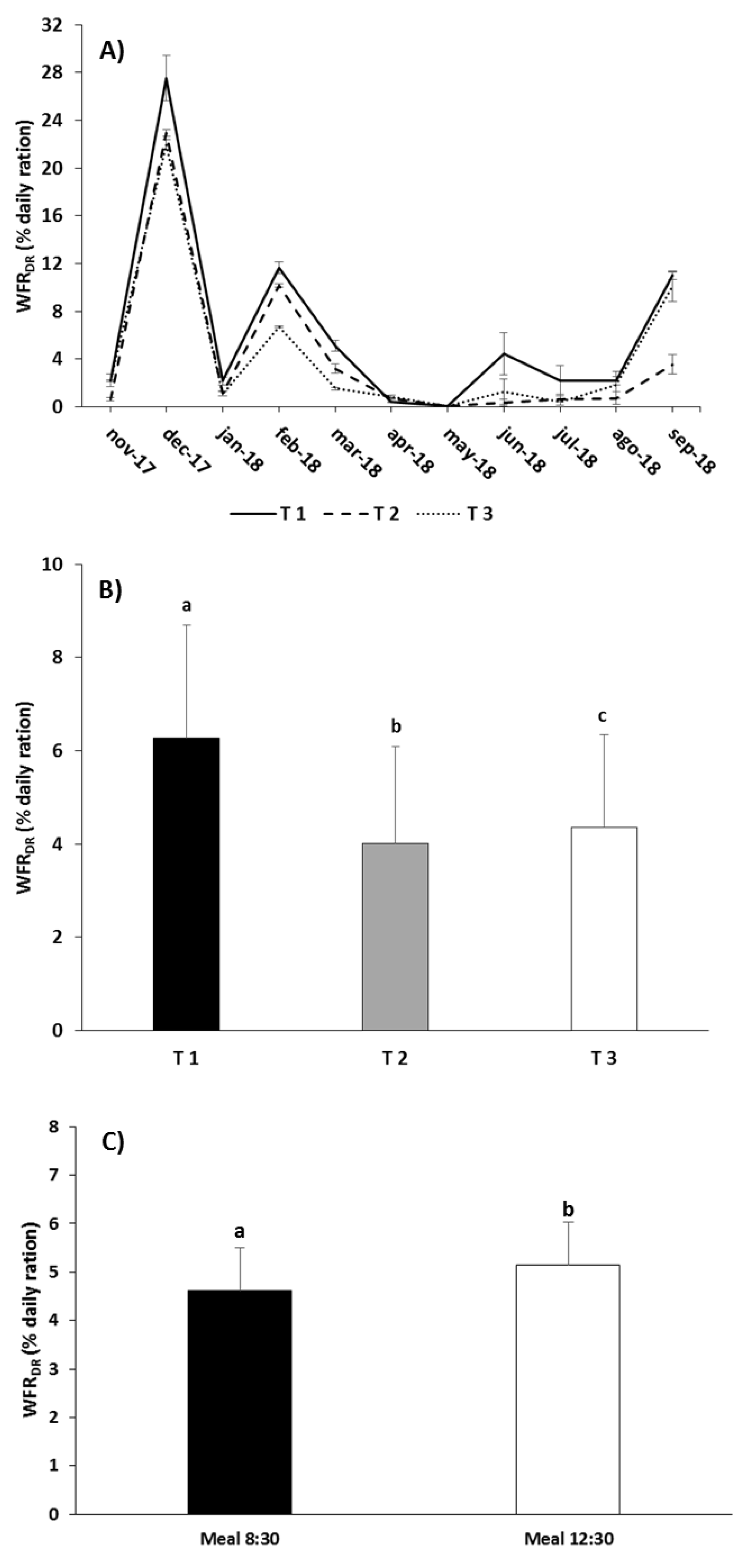
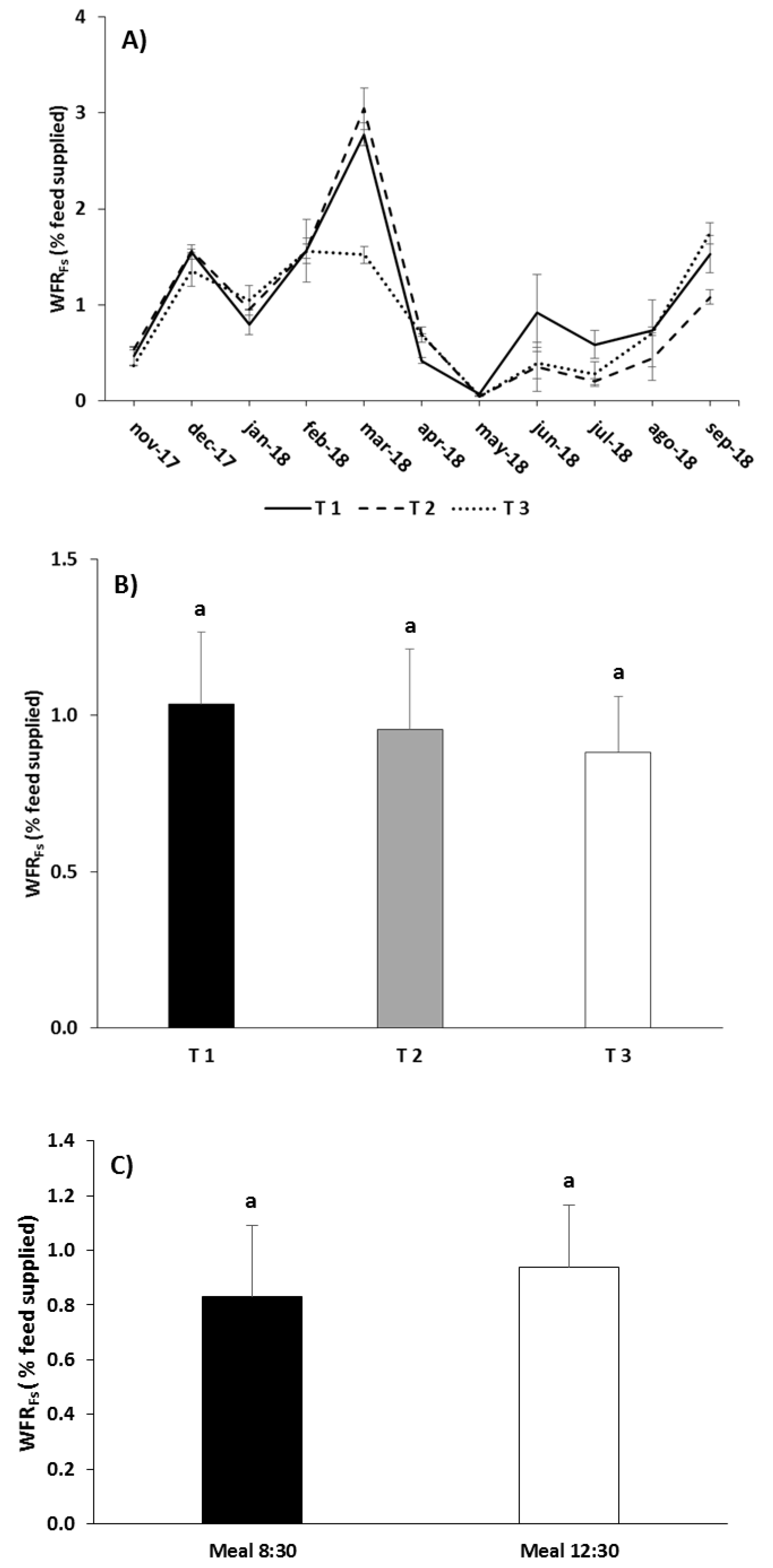
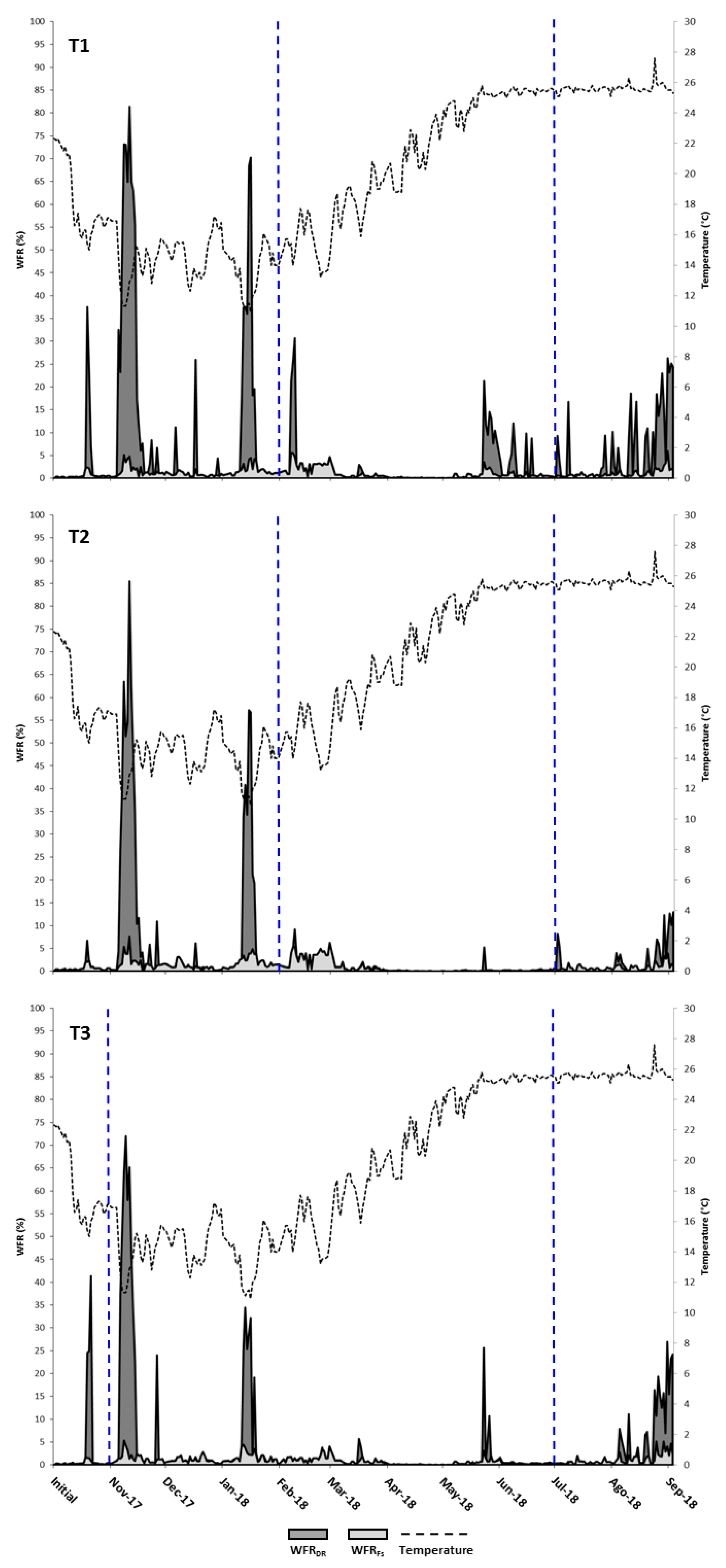
3.2. Waste Production
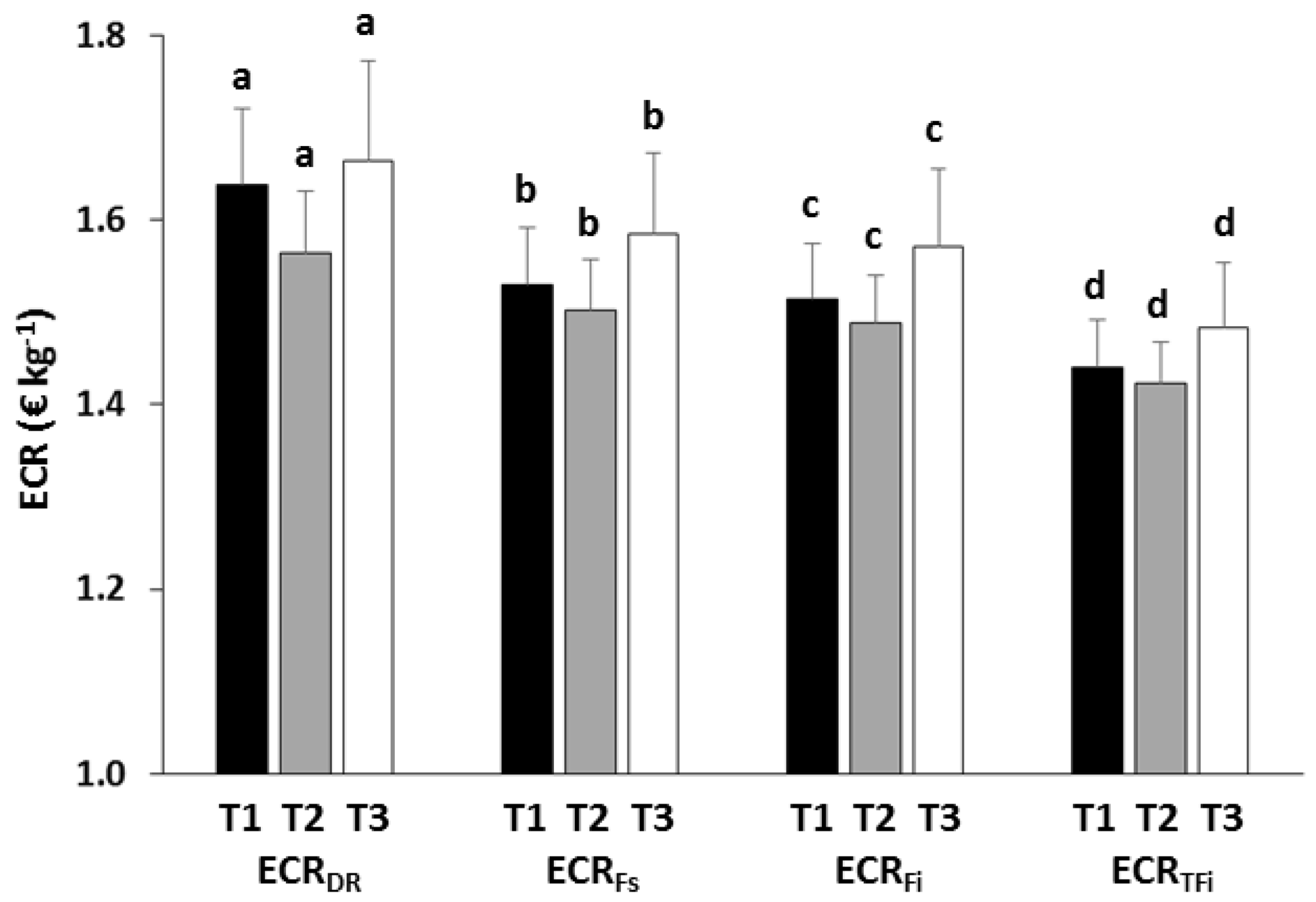
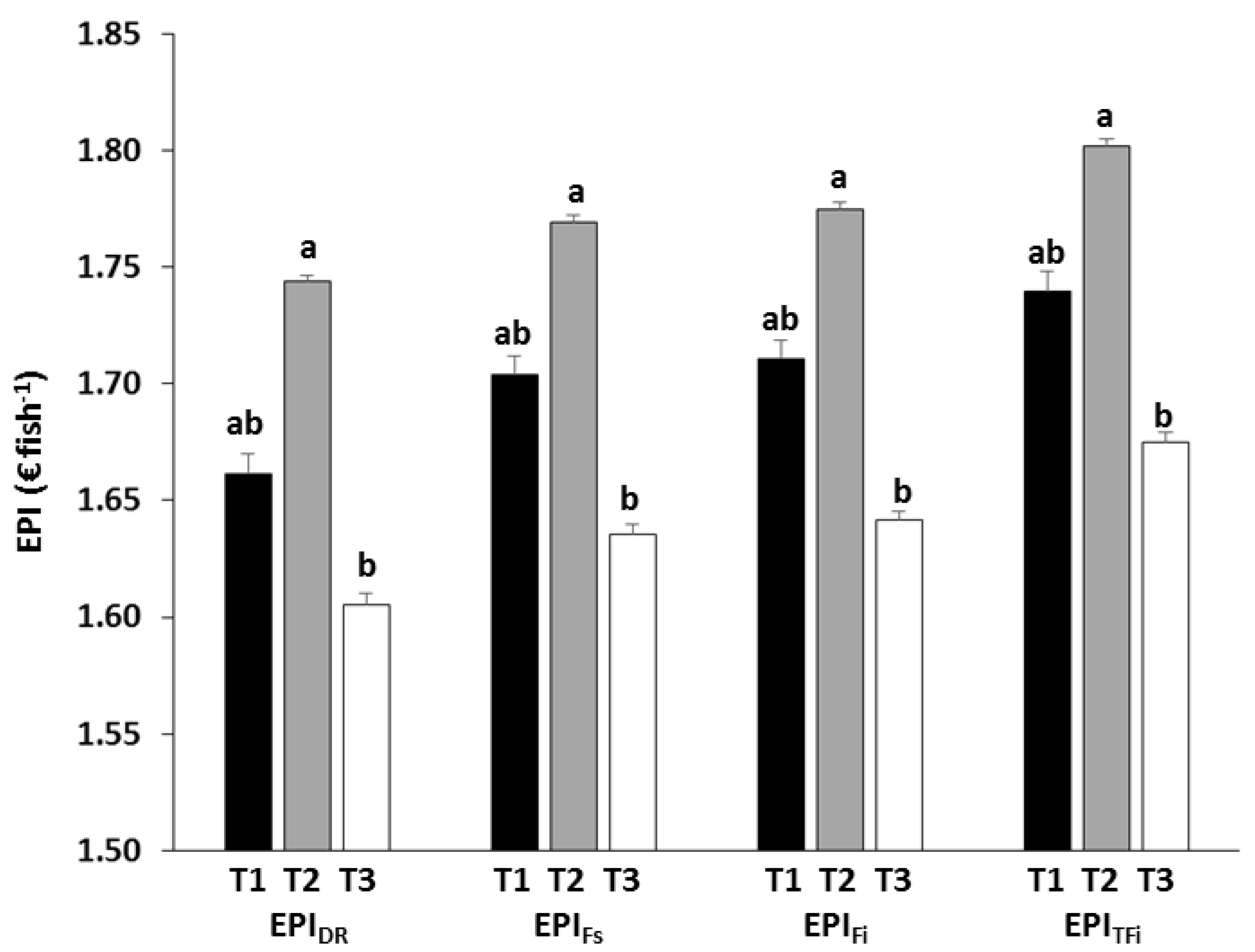
3.3. Economic Analysis
4. Discussion
4.1. Growth Performance
4.2. Waste Production
4.3. Economic Implications
Funding
Acknowledgments
Conflicts of Interest
References
- APROMAR. La Acuicultura en España 2018. Asociación Empresarial de Acuicultura de España. Available online: http://www.apromar.es (accessed on 20 March 2020).
- Colloca, F.; Cerasi, S. Sparus Aurata. In Cultured Aquatic Species Fact Sheets; Crespi, V., New, M., Eds.; FAO: Rome, Italy, 2009; Available online: http://www.fao.org/tempref/FI/DOCUMENT/aquaculture/CulturedSpecies/file/en/en_giltheadseabr.htm (accessed on 20 March 2020).
- Goldan, O.; Popper, D.; Karplus, I. Food competition in small groups of juvenile gilthead seabream (Sparus aurata). Isr. J. Aquac. Bamidgeh 2003, 55, 94–106. [Google Scholar]
- Saoud, I.P.; Davies, D.A.; Roy, L.A.; Phelps, R.P. Evaluating the benefits of size-sorting tilapia fry before stocking. J. Appl. Aquac. 2005, 17, 73–85. [Google Scholar] [CrossRef]
- Brown, R.C. Genetic Management and Selective Breeding in Farmed Populations of Gilthead Seabream (Sparus Aurata). Ph.D. Thesis, University of Stirling, Stirling, Scotland, 2003. [Google Scholar]
- Kuhl, H.; Sarrapoulou, E.; Tine, M.; Kotoulas, G.; Magoulas, A.; Reinhart, R. A comparative BAC map for the gilthead seabream (Sparus aurata L.). J. Biomed. Biotech. 2010, 2011, 329025. [Google Scholar]
- Baras, E.; Dugué, R.; Legendre, M. Do cannibalistic fish forage optimally? An experimental study of prey size preference, bioenergetics of cannibalism and their ontogenic variation in the African catfish Heterobranchus longifilis. Aquat. Living Resour. 2014, 27, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Magnuson, J.J. An analysis of aggressive behaviour, growth and competition for food and space in medaka (Oryzias latipes). Can. J. Zool. 1962, 40, 313–363. [Google Scholar] [CrossRef]
- Hernández, J.; Gasca-Leyva, E. A Size Distribution Model Applied to Fish Farming. In Numerical Methods and Applications. Lecture Notes in Computer Science; Dimov, I., Lirkov, I., Margenov, S., Zlatev, Z., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; Volume 2542, pp. 221–229. [Google Scholar]
- Cammarata, M.; Vazzana, M.; Accardi, D.; Parrinello, N. Seabream (Sparus aurata) long-term dominant-subordinate interplays affects phagocytosis by peroneal cavity cells. Brain Behav. Immun. 2012, 26, 580–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, I.D.; Carter, C.G.; Houlihan, D.F. The effect of feeding hierarchy on individual variability in daily feeding of rainbow trout, Oncorhynchus mykiis (Walbaum). J. Fish Biol. 1992, 41, 257–263. [Google Scholar] [CrossRef]
- Montero, D.; Lalumera, G.; Izquierdo, M.S.; Caballero, M.J.; Saroglia, M.; Tort, L. Establishment of dominance relationships in gilthead seabream Sparus aurata juveniles during feeding: Effects on feeding behaviour, feed utilization and fish health. J. Fish Biol. 2009, 74, 790–805. [Google Scholar] [CrossRef]
- Damsgård, B.; Huntingford, F. Fighting and aggression. In Aquaculture and Behaviour; Huntingford, F., Jobling, M., Kadri, S., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2012; pp. 248–285. [Google Scholar]
- Jobling, M.; Reinsnes, T.G. Physiological and social constraints on growth of Arctic charr, Salvelinus alpinus L.: An investigation of factors leading to stunting. J. Fish Biol. 1986, 28, 379–384. [Google Scholar] [CrossRef]
- Goldan, O.; Popper, D.; Karplus, I. Management of size variation in juvenile gilthead seabream (Sparus aurata) I: Particle size and frequency of feeding dry and live food. Aquaculture 1997, 152, 181–190. [Google Scholar] [CrossRef]
- Ruzzante, D.E. Domestication effects on aggressive and schooling behavior in fish. Aquaculture 1994, 120, 1–24. [Google Scholar] [CrossRef]
- Barki, A.; Harpaz, S.; Hulata, G.; Karplus, I. Effect of larger fish and size grading on growth and size variation in fingerling silver perch. Aquac. Int. 2000, 8, 391–401. [Google Scholar] [CrossRef]
- Jobling, M. Effect of feeding frequency on food intake and growth of Artic charr, Salvelinus alpinus L. J. Fish Biol. 1983, 23, 177–185. [Google Scholar] [CrossRef]
- Ryer, C.H.; Olla, B.L. The influence of food distribution upon the development of aggresive and competitive behaviour in juvenile chum salmon, Oncorhynchus keta. J. Fish Biol. 1995, 46, 264–272. [Google Scholar] [CrossRef]
- Hakoyama, H.; Iguchi, K. Why is competition more intense if food is supplied more slowly? Behav. Ecol. Sociobiol. 1997, 40, 159–168. [Google Scholar] [CrossRef]
- Papadakis, V.M.; Glaropoulos, A.; Alvanopoulou, M.; Kentouri, M. A behavioural approach of dominance establishment in tank-held seabream (Sparus aurata L.) under different feeding conditions. Aquac. Res. 2016, 47, 4015–4023. [Google Scholar] [CrossRef]
- Andrew, J.E.; Holm, J.; Kadri, S.; Huntingford, F.A. The effect of competition on the feeding efficiency and feed handling behaviour in gilthead seabream (Sparus aurata L.) held in tanks. Aquaculture 2004, 232, 317–331. [Google Scholar] [CrossRef]
- Linnér, J.; Brännäs, E. Growth in Arctic charr and rainbow trout fed temporally concentrated of spaced daily meals. Aquac. Int. 2001, 9, 35–44. [Google Scholar] [CrossRef]
- Juell, J.-E.; Lekang, O.I. The effect of feed supply rate on growth of juvenile perch (Perca fluviatilis). Aquac. Res. 2002, 32, 459–464. [Google Scholar] [CrossRef]
- Dwyer, K.S.; Brown, J.A.; Parrish, C.; Lall, S.P. Feeding frequency affects food consumption, feeding pattern and growth of juvenile yellowtail flounder (Limanda ferruginea). Aquaculture 2002, 213, 279–292. [Google Scholar] [CrossRef]
- Artigas, E.G. Feeding Policy for Marine Fish; Pro Aqua Nutrición: Burgos, Spain, 1999; pp. 21–22. [Google Scholar]
- Andrew, J.E.; Anras, M.L.B.; Kadri, S.; Holm, J.; Huntingford, F.A. Feeding responses of hatchery-reared Gilthead Sea bream (Sparus aurata L.) to a commercial diet and natural prey items. Mar. Freshw. Behav. Physiol. 2003, 36, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Ballester-Moltó, M.; Sanchez-Jerez, P.; García-García, J.; Cerezo-Valverde, J.; Aguado- Giménez, F. Controlling feed losses by chewing in gilthead sea bream (Sparus aurata) ongrowing may improve the fish farming environmental sustainability. Aquaculture 2016, 464, 111–116. [Google Scholar] [CrossRef]
- Koçak, Ö.; Tathdil, F.F. Cost analysis in gilthead seabream (Sparus aurata Linnaeus, 1758) and seabass (Dicentrarchus labrax Linnaeus, 1758) production in Milas District—Muğla province, Turkey. Turk. J. Fish. Aquat. Sci. 2004, 4, 33–38. [Google Scholar]
- Rana, K.J.; Siriwardena, S.; Mohammed, R.H. Impact of Rising Feed Ingredient Prices on Aquafeeds and Aquaculture Production; FAO Fisheries and Aquaculture Technical Paper: Rome, Italy, 2009; Volume 541, pp. 1–78. [Google Scholar]
- Robb, D.H.F.; Crampton, V.O. On-farm feeding and feed management: Perspectives from the fish feed industry. In On-Farm, Feeding and Feed Management in Aquaculture; Hasan, M.R., New, M.B., Eds.; FAO Fisheries and Aquaculture Technical Paper: Rome, Italy, 2013; Volume 583, pp. 489–518. [Google Scholar]
- Amirkolaie, A.K. Reduction in the environmental impact of waste discharged by fish farms through feed and feeding. Rev. Aquac. 2011, 3, 19–26. [Google Scholar] [CrossRef]
- White, P. Environmental consequences of poor feed quality and feed management. In On-Farm, Feeding and Feed Management in Aquaculture; Hasan, M.R., New, M.B., Eds.; FAO Fisheries and Aquaculture Technical Paper: Rome, Italy, 2013; Volume 583, pp. 553–564. [Google Scholar]
- García-García, B.; Rosique-Jiménez, C.; Aguado-Giménez, F.; García-García, J. Life cycle assessment of gilthead seabream (Sparus aurata) production in offshore fish farms. Sustainability 2016, 8, 1228–1237. [Google Scholar] [CrossRef] [Green Version]
- Ortega, A. Cultivo de Dorada (Sparus Aurata), 1st ed.; Serie Cuadernos de Acuicultura nº 1; Fundación Observatorio Español de Acuicultura, Consejo Superior de Investigaciones Científicas, Ministerio de Medio Ambiente y Medio Rural y Marino: Madrid, Spain, 2008; pp. 1–44. [Google Scholar]
- Tucker, J.W. Marine Fish. In Culture, 1st ed.; Kluwer Academic Publishers: Massachusetts, MA, USA, 1998; pp. 72–81. [Google Scholar]
- Mayer, P.; Estruch, V.D.; Jover, M. A two-stage growth model for gilthead sea bream (Sparus aurata) based on the thermal growth coefficient. Aquaculture 2012, 358–359, 6–13. [Google Scholar] [CrossRef]
- Cho, C.Y.; Bureau, D.P. Development of bioenergetics models and the Fish-PrFEQ software to estimate production, feeding ration and waste output in aquaculture. Aquat. Living Res. 1998, 11, 199–210. [Google Scholar] [CrossRef]
- Mayer, P.; Estruch, V.D.; Blasco, J.; Jover, M. Predicting growth of gilthead sea bream (Sparus aurata) in marine farms under real productions conditions using temperature and time-dependent models. Aquac. Res. 2008, 39, 1046–1052. [Google Scholar] [CrossRef]
- Martínez-Llorens, S.; Tomás, A.; Jover, M. A new tool for determining the optimum fish meal and vegetable meals in diets for maximizing the economic profitability of gilthead seabream (Sparus aurata L.) feeding. Aquac. Res. 2011, 43, 1697–1709. [Google Scholar] [CrossRef]
- Weatherley, A.H.; Gill, H.S. The Biology of Fish. In Growth, 1st ed.; Academic Press: London, UK, 1987; pp. 256–262. [Google Scholar]
- Wedemeyer, G.A. Physiology of Fish. In Intensive Culture Systems, 1st ed.; Chapman & Hall, International Thompson Publishing: New York, NY, USA, 1996; pp. 166–170. [Google Scholar]
- Lall, S.P.; Tibbetts, S.M. Nutrition, feeding and behavior of fish. Vet. Clin. Exot. Anim. 2009, 12, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Karplus, I.; Popper, D.; Goldan, O. The effect of food competition and relative size of group members on growth of juvenile gilthead seabream, Sparus aurata. Fish Physiol. Biochem. 2000, 22, 119–123. [Google Scholar] [CrossRef]
- Okinomidou, E.; Batzina, A.; Karakatsouli, N. Effects of food quantity and distribution on aggressive behaviour of gilthead seabream and European seabass. Appl. Anim. Behav. Sci. 2019, 213, 124–130. [Google Scholar] [CrossRef]
- Alanärä, A.; Kadri, S.; Paspatis, M. Feeding management. In Food Intake in Fish; Houlihan, D., Boujard, T., Jobling, M., Eds.; Blackwell Science Ltd.: London, UK, 2001; pp. 332–353. [Google Scholar]
- Smith, I.P.; Metcalfe, N.B.; Huntingford, F.A. The effect of food pellet dimensions on feeding responses by Atlantic salmon (Salmo salar L.) in a marine net pen. Aquaculture 1995, 130, 167–175. [Google Scholar] [CrossRef]
- Aguado-Giménez, F. Final Report of the Project Reduction of Production Costs due to Feeding, and the Environmental Impact of Gilthead Seabream (Sparus Aurata) Farming. Inclusion of Microalgae in the Diet and Establishment of Optimal Feeding Strategies; Murcian Institute for Agricultural and Food Research and Development (IMIDA), European Fund for Regional Development (EFRD; 80%) and Council for Water, Agriculture, Forestry and Fisheries of the Autonomous Government of the Region of Murcia: Murcia, Spain, 2018; pp. 1–68. [Google Scholar]
- Aguado-Giménez, F. Training and Technology Transfer Service, Council of Water, Agriculture, Forestry and Fisheries. In Practical Recommendations to Improve Feeding of Gilthead Seabream (Sparus Aurata) Reared in Floating Cages, 1st ed.; Autonomous Government of the Region of Murcia: Murcia, Spain, 2019; pp. 1–49. [Google Scholar]
- Juell, J.-E.; Bjordal, A.; Fernö, A.; Huse, I. Effect of feeding intensity on food intake and growth of Atlantic salmon, Salmo salar L., in sea cages. Aquac. Fish. Manag. 1994, 25, 453–464. [Google Scholar] [CrossRef]
- Bailey, J.; Alanärä, A.; Crampton, V. Do delivery rate and pellet size affect growth rate in Atlantic salmon (Salmo salar L.) raised under semi-commercial farming conditions? Aquaculture 2003, 224, 79–88. [Google Scholar] [CrossRef]
- Cho, C.Y.; Bureau, D.P. A review of diet formulation strategies and feeding systems to reduce excretory and feed wastes in aquaculture. Aquac. Res. 2001, 32, 349–360. [Google Scholar] [CrossRef]
- Ballester-Moltó, M.; Sanchez-Jerez, P.; Cerezo-Valverde, J.; Aguado- Giménez, F. Particulate waste outflow from fish-farming cages. How much is uneaten feed? Mar. Pollut. Bull. 2017, 119, 23–30. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, D.; Lin, K.; Sun, C.; Yang, X. Intelligent feeding control methods in aquaculture with an emphasis on fish: A review. Rev. Aquac. 2018, 10, 975–993. [Google Scholar] [CrossRef]
- Jobling, M.; Baardvik, B.M. Patterns of growth of maturing and immature Arctic charr, Salvelinus alpinus, in a hatchery population. Aquaculture 1991, 94, 343–354. [Google Scholar] [CrossRef]
- Carter, C.; Houlihan, D.; Kiessling, A.; Médale, F.; Jobling, M. Physiological effects of feeding. In Food Intake in Fish; Houlihan, D., Boujard, T., Jobling, M., Eds.; Blackwell Science Ltd.: London, UK, 2001; pp. 297–331. [Google Scholar]
- Grant, J.W.A. Whether or not to defend? The influence of resource distribution. Mar. Behav. Physiol. 1993, 23, 137–153. [Google Scholar] [CrossRef]
- Sánchez, J.A.; López-Olmeda, J.F.; Blanco-Vives, B.; Sánchez-Vázquez, F.J. Effects of feeding schedule on locomotor activity rhythms and stress response in seabream. Physiol. Behav. 2009, 98, 125–129. [Google Scholar] [CrossRef]
- Glencross, B.D.; Baily, J.; Berntssen, M.H.G.; Hardy, R.; MacKenzie, S.; Tocher, D.R. Risk assessment of the use of alternative animal and plant raw material resources in aquaculture feeds. Rev. Aquac 2019. [Google Scholar] [CrossRef] [Green Version]
- Naylor, R.L.; Goldburg, R.J.; Primavera, J.H.; Kautsky, N.; Beveridge, M.C. Effect of aquaculture on world fish supplies. Nature 2000, 405, 1017–1024. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Xu, L.; Liu, H. Detection of uneaten fish food pellets in underwater images for aquaculture. Aquac. Eng. 2017, 78, 85–94. [Google Scholar] [CrossRef]
- Føre, M.; Frank, K.; Norton, T.; Svendsen, E.; Alfredsen, J.A.; Dempster, T.; Eguiraun, H.; Watson, W.; Stahl, A.; Sunde, L.M.; et al. Precision fish farming: A new framework to improve production in aquaculture. Biosyst. Eng. 2017, 173, 176–193. [Google Scholar] [CrossRef]
- Tveteras, S. Norwegian salmon aquaculture and sustainability: The relationship between environmental quality and industry growth. Mar. Res. Econ. 2002, 17, 131–132. [Google Scholar] [CrossRef] [Green Version]
- Marine Harvest. The Marine Harvest Salmon Farming Industry Handbook; Marine Harvest ASA: Norway, 2019; pp. 1–114. Available online: https://ml.globenewswire.com/Resource/Download/1766f220-c83b-499a-a46e-3941577e038b (accessed on 20 March 2020).


| Feeding Regime | Pellet Size/Fish Weight | Feeding Rate (g min−1) | BWi (kg) | CVi (%) | Di (kg m−3) |
|---|---|---|---|---|---|
| T1 | 2 mm: from 50 to 100 g | 20 | 0.049 ± 0.004 | 10.36 ± 0.43 | 2.07 ± 0.02 |
| 4 mm: from 100 to 450 g | |||||
| T2 | 2 mm: from 50 to 100 g | 10 | 0.049 ± 0.005 | 10.71 ± 0.49 | 2.08 ± 0.03 |
| 4 mm: from 100 to 450 g | |||||
| T3 | 2 mm: from 50 to 70 g | 20 | 0.050 ± 0.005 | 10.83 ± 0.63 | 2.10 ± 0.03 |
| 4 mm: from 70 to 220 g | |||||
| 6 mm from 220 to 450 g |
| Pellet Size: | 2 mm A | 2 mm B/4 mm A | 4 mm B/6 mm |
|---|---|---|---|
| Crude proteins (%) | 48.5 | 46.0 | 44.0 |
| Crude lipids (%) | 18.0 | 19.0 | 21.0 |
| Ash (%) | 6.4 | 5.9 | 6.3 |
| Fibre (%) | 2.7 | 4.5 | 3.0 |
| Moisture (%) | 8.7 | 7.9 | 8.2 |
| N°. pellets g−1 | 90 | 90/23 | 23/9 |
| Feed price (€ kg−1) | 1.09 | 1.09/1.00 | 1.00/0.96 |
| Pellet Size | ||||||
|---|---|---|---|---|---|---|
| Feeding Regime | 2 mm A | 2 mm B | 4 mm A | 4 mm B | 6 mm | Diet Cost (€ kg−1) |
| T1 Months delivering | 1 | 3 | 4 | 3 | - | 1.012 |
| Feeding rate: g min−1 | 20 | 20 | 20 | 20 | - | |
| Pellets fish−1 min−1: | 36–54 | 36–54 | 10–12 | 10–12 | - | |
| Meal duration (min): | 0.9–2 | 0.9–2 | 1.5–9 | 1.5–9 | - | |
| T2 Months delivering | 1 | 3 | 4 | 3 | - | 1.012 |
| Feeding rate: g min−1 | 10 | 10 | 10 | 10 | - | |
| Pellets fish−1 min−1: | 18–27 | 18–27 | 5–6 | 5–6 | - | |
| Meal duration (min): | 1.2–4 | 1.2–4 | 3–23 | 3–23 | - | |
| T3 Months delivering | 1 | - | 7 | - | 3 | 0.979 |
| Feeding rate: g min−1 | 20 | - | 20 | - | 20 | |
| Pellets fish−1 min−1: | 36–54 | - | 10–12 | - | 4–6 | |
| Meal duration (min): | 0.9–2 | - | 1.5–9 | - | 6–10 | |
| T1 | a | a | a | a | a | a | ab | a |
|---|---|---|---|---|---|---|---|---|
| Month | SFR | DR | Of | Fs | Uf | Fi | LbC | TFi |
| November-17 | 1.15 ± 0.00 | 2143 ± 3 | 37 ± 3 | 2106 ± 6 | 10 ± 2 | 2096 ± 4 | 40 ± 5 | 2056 ± 1 |
| December-17 | 0.69 ± 0.00 | 1368 ± 0 | 361 ± 27 | 1007 ± 27 | 16 ± 1 | 991 ± 26 | 44 ± 2 | 947 ± 28 |
| January-18 | 0.54 ± 0.00 | 1144 ± 14 | 16 ± 4 | 1128 ± 18 | 9 ± 1 | 1119 ± 17 | 45 ± 2 | 1074 ± 15 |
| February-18 | 0.56 ± 0.00 | 1198 ± 0 | 123 ± 5 | 1075 ± 5 | 17 ± 1 | 1058 ± 6 | 51 ± 0 | 1007 ± 6 |
| March-18 | 0.73 ± 0.01 | 1618 ± 0 | 39 ± 6 | 1579 ± 6 | 44 ± 2 | 1535 ± 7 | 56 ± 8 | 1480 ± 15 |
| April-18 | 0.86 ± 0.01 | 2400 ± 0 | 0 ± 0 | 2400 ± 0 | 10 ± 1 | 2390 ± 1 | 84 ± 5 | 2306 ± 6 |
| May-18 | 1.18 ± 0.02 | 4727 ± 31 | 0 ± 0 | 4727 ± 31 | 3 ± 0 | 4724 ± 31 | 119 ± 3 | 4605 ± 34 |
| June-18 | 1.47 ± 0.05 | 5610 ± 138 | 201 ± 83 | 5409 ± 55 | 50 ± 22 | 5359 ± 33 | 191 ± 14 | 5168 ± 19 |
| July-18 | 1.40 ± 0.03 | 9950 ± 242 | 164 ± 119 | 9786 ± 123 | 58 ± 15 | 9728 ± 108 | 567 ± 0 | 9161 ± 109 |
| August-18 | 1.29 ± 0.03 | 10,987 ± 319 | 162 ± 40 | 10825 ± 279 | 80 ± 5 | 10,745 ± 274 | 828 ± 9 | 9917 ± 282 |
| September-18 | 1.26 ± 0.01 | 10,719 ± 361 | 1030 ± 20 | 9689 ± 341 | 148 ± 14 | 9958 ± 819 | 837 ± 1 | 8705 ± 353 |
| Total | 51,864 ± 1102 | 2132 ± 224 | 49,732 ± 878 | 443 ± 27 | 49,288 ± 851 | 2861 ± 5 | 46,427 ± 857 | |
| 4.11% DR | 95.89% DR | 0.89% Fs | 99.11% Fs | 5.81% Fi | 89.51% DR | |||
| T2 | a | a | b | a | a | a | a | a |
| November-17 | 1.14 ± 0.00 | 2146 ± 0 | 3 ± 3 | 2143 ± 3 | 12 ± 0 | 2132 ± 3 | 45 ± 1 | 2086 ± 4 |
| December-17 | 0.69 ± 0.01 | 1368 ± 0 | 297 ± 4 | 1071 ± 4 | 17 ± 0 | 1054 ± 4 | 22 ± 1 | 1032 ± 5 |
| January-18 | 0.52 ± 0.00 | 1138 ± 20 | 2 ± 2 | 1136 ± 22 | 11 ± 0 | 1125 ± 22 | 42 ± 2 | 1083 ± 20 |
| February-18 | 0.56 ± 0.00 | 1205 ± 13 | 105 ± 3 | 1100 ± 18 | 17 ± 4 | 1082 ± 14 | 53 ± 3 | 1029 ± 11 |
| March-18 | 0.73 ± 0.01 | 1618 ± 0 | 3 ± 3 | 1615 ± 3 | 49 ± 3 | 1566 ± 6 | 56 ± 2 | 1510 ± 4 |
| April-18 | 0.86 ± 0.01 | 2400 ± 0 | 0 ± 0 | 2400 ± 0 | 17 ± 2 | 2383 ± 2 | 86 ± 1 | 2297 ± 2 |
| May-18 | 1.16 ± 0.03 | 4649 ± 131 | 0 ± 0 | 4649 ± 131 | 2 ± 0 | 4647 ± 131 | 107 ± 0 | 4540 ± 131 |
| June-18 | 1.43 ± 0.02 | 5427 ± 183 | 0 ± 0 | 5427 ± 183 | 20 ± 14 | 5407 ± 169 | 160 ± 5 | 5248 ± 173 |
| July-18 | 1.36 ± 0.01 | 9701 ± 377 | 42 ± 42 | 9659 ± 335 | 20 ± 4 | 9636 ± 331 | 510 ± 8 | 9129 ± 339 |
| August-18 | 1.26 ± 0.02 | 10,684 ± 622 | 32 ± 32 | 10652 ± 590 | 49 ± 28 | 10,603 ± 562 | 687 ± 116 | 9916 ± 446 |
| September-18 | 1.22 ± 0.01 | 10,318 ± 762 | 250 ± 74 | 10068 ± 836 | 110 ± 17 | 9958 ± 819 | 847 ± 83 | 9111 ± 736 |
| Total | 50,654 ± 2108 | 735 ± 5 | 49,919 ± 2113 | 322 ± 69 | 49,597 ± 2045 | 2616 ± 189 | 46,981 ± 1855 | |
| 1.45% DR | 98.55% DR | 0.65% Fs | 99.35% Fs | 5.27% Fi | 92.74% DR | |||
| T3 | a | a | ab | a | a | a | b | a |
| November-17 | 1.13 ± 0.01 | 2146 ± 0 | 40 ± 11 | 2106 ± 11 | 8 ± 0 | 2099 ± 11 | 36 ± 2 | 2063 ± 9 |
| December-17 | 0.69 ± 0.01 | 1368 ± 0 | 286 ± 4 | 1082 ± 4 | 15 ± 2 | 1068 ± 6 | 41 ± 1 | 1026 ± 6 |
| January-18 | 0.53 ± 0.00 | 1157 ± 27 | 1 ± 1 | 1157 ± 28 | 12 ± 2 | 1144 ± 29 | 45 ± 2 | 1100 ± 31 |
| February-18 | 0.54 ± 0.00 | 1229 ± 11 | 64 ± 0 | 1165 ± 11 | 18 ± 1 | 1147 ± 11 | 56 ± 4 | 1091 ± 15 |
| March-18 | 0.70 ± 0.00 | 1618 ± 0 | 0 ± 0 | 1618 ± 0 | 25 ± 1 | 1593 ± 1 | 51 ± 1 | 1542 ± 2 |
| April-18 | 0.84 ± 0.01 | 2400 ± 0 | 4 ± 4 | 2396 ± 4 | 16 ± 0 | 2380 ± 3 | 90 ± 1 | 2290 ± 4 |
| May-18 | 1.16 ± 0.02 | 4620 ± 62 | 0 ± 0 | 4620 ± 62 | 2 ± 0 | 4618 ± 62 | 119 ± 3 | 4498 ± 66 |
| June-18 | 1.42 ± 0.03 | 5410 ± 82 | 47 ± 47 | 5363 ± 129 | 21 ± 8 | 5342 ± 137 | 156 ± 15 | 5186 ± 152 |
| July-18 | 1.37 ± 0.03 | 9572 ± 248 | 13 ± 13 | 9559 ± 235 | 27 ± 12 | 9532 ± 247 | 632 ± 25 | 8901 ± 272 |
| August-18 | 1.27 ± 0.02 | 10,577 ± 179 | 124 ± 84 | 10,453 ± 95 | 74 ± 37 | 10,379 ± 58 | 1096 ± 82 | 9283 ± 24 |
| September-18 | 1.27 ± 0.00 | 10,283 ± 333 | 882 ± 172 | 9401 ± 161 | 164 ± 8 | 9237 ± 168 | 1040 ± 79 | 8197 ± 247 |
| Total | 50,380 ± 866 | 1459 ± 211 | 48,921 ± 655 | 382 ± 15 | 48,539 ± 640 | 3362 ± 31 | 45,177 ± 671 | |
| 2.90% DR | 97.10% DR | 0.78% Fs | 99.22% Fs | 6.93% Fi | 89.67% DR |
| Feeding Regime | BWf (kg) | ∆BW (kg) | eFCR | CVf (%) | Df (kg m−3) | Cum. Mort. (%) |
|---|---|---|---|---|---|---|
| T1 | 0.447 ± 0.004 | 0.397 ± 0.004 | 1.70 ± 0.01 | 14.78 ± 0.36 | 14.23 ± 0.12 | 23.50 ± 2.50 |
| T2 | 0.460 ± 0.003 | 0.410 ± 0.003 | 1.71 ± 0.07 | 15.94 ± 0.44 | 14.29 ± 0.22 | 25.50 ± 4.00 |
| T3 | 0.440 ± 0.002 | 0.389 ± 0.002 | 1.82 ± 0.03 | 17.51 ± 0.31 | 13.80 ± 0.43 | 27.50 ± 2.50 |
| Variables | Factors | F | P |
|---|---|---|---|
| CV | Treatment | 12.663 | 0.034 |
| SGR | Treatment | 9.594 | 0.049 |
| LbC | Treatment | 36.561 | 0.008 |
| WFRDR | Meal | 9.996 | 0.020 |
| Treatment | 68.818 | 0.001 | |
| Treatment × Meal | 3.760 | 0.087 | |
| WFRFs | Treatment | 1.882 | 0.232 |
| Meal | 1.703 | 0.240 | |
| Treatment × Meal | 0.134 | 0.878 | |
| eFCR | Treatment | 0.408 | 0.697 |
| ECRDR | Treatment | 1.086 | 0.442 |
| ECRFs | Treatment | 0.347 | 0.732 |
| ECRFi | Treatment | 4.882 | 0.114 |
| ECRRFi | Treatment | 0.887 | 0.498 |
| EPIDR | Treatment | 12.426 | 0.035 |
| EPIFs | Treatment | 12.384 | 0.036 |
| EPIFi | Treatment | 13.072 | 0.033 |
| EPIRFi | Treatment | 11.352 | 0.040 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguado-Giménez, F. Effect of Feed Delivery Rate and Pellet Size on Rearing Performance, Feed Wastage and Economic Profitability in Gilthead Seabream (Sparus Aurata) Ongrowing. Water 2020, 12, 954. https://doi.org/10.3390/w12040954
Aguado-Giménez F. Effect of Feed Delivery Rate and Pellet Size on Rearing Performance, Feed Wastage and Economic Profitability in Gilthead Seabream (Sparus Aurata) Ongrowing. Water. 2020; 12(4):954. https://doi.org/10.3390/w12040954
Chicago/Turabian StyleAguado-Giménez, Felipe. 2020. "Effect of Feed Delivery Rate and Pellet Size on Rearing Performance, Feed Wastage and Economic Profitability in Gilthead Seabream (Sparus Aurata) Ongrowing" Water 12, no. 4: 954. https://doi.org/10.3390/w12040954
APA StyleAguado-Giménez, F. (2020). Effect of Feed Delivery Rate and Pellet Size on Rearing Performance, Feed Wastage and Economic Profitability in Gilthead Seabream (Sparus Aurata) Ongrowing. Water, 12(4), 954. https://doi.org/10.3390/w12040954





