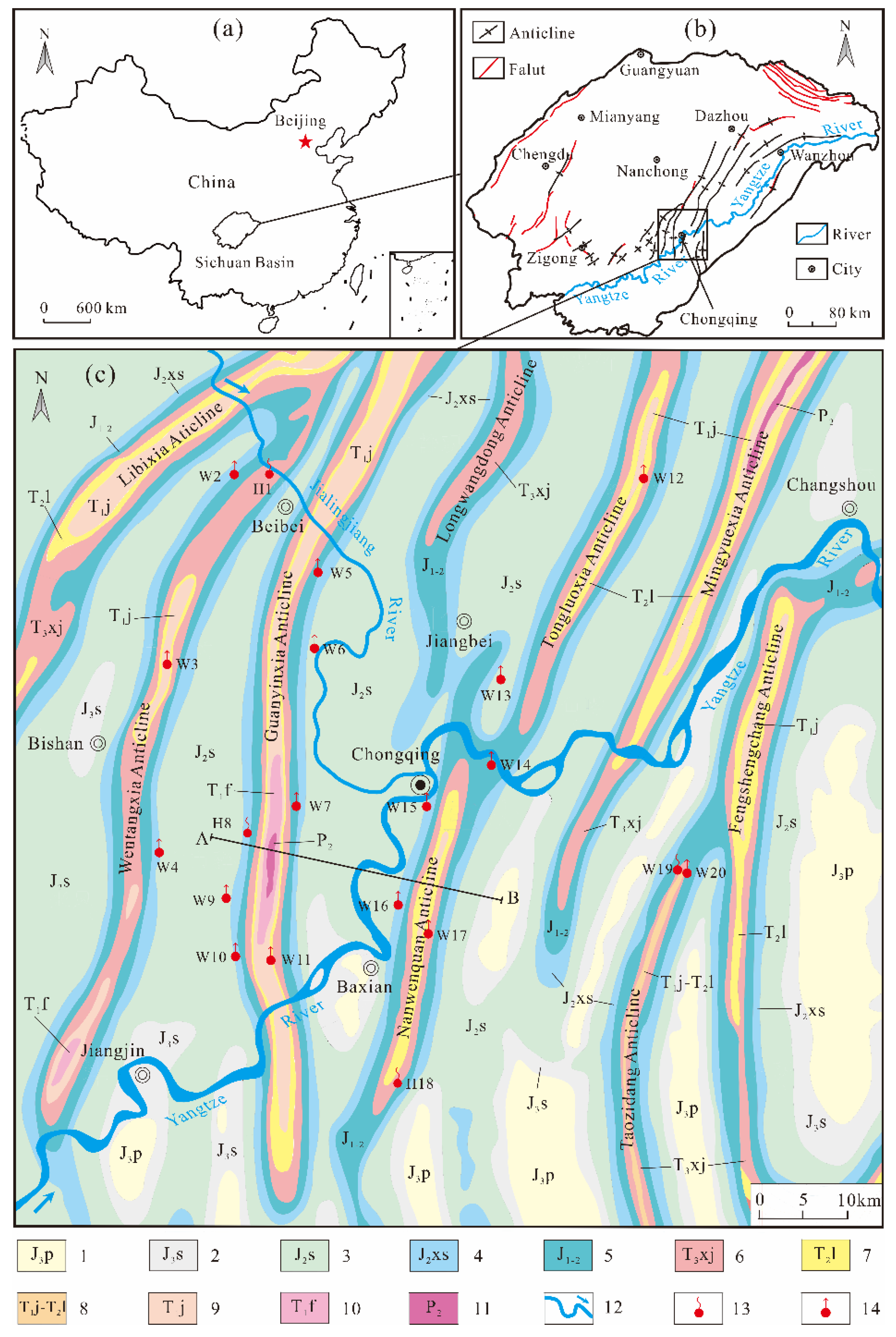
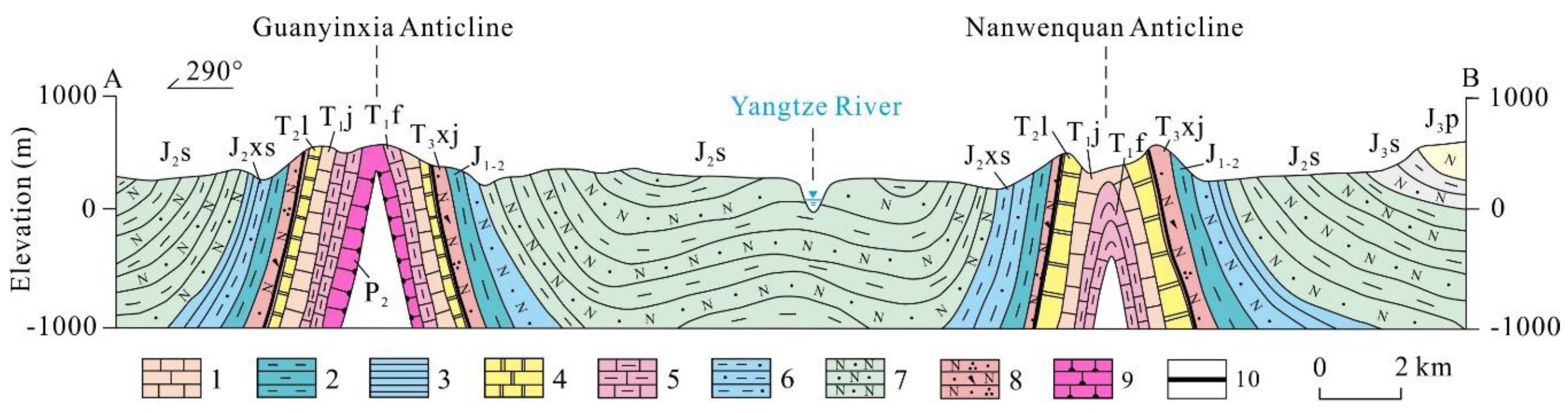
4.2.1. Hierarchical Cluster Analysis (HCA)
Based on the 11 main hydrochemical parameters (T, pH, K
+, Na
+, Ca
2+, Mg
2+, Cl
−, SO
42−, HCO
3−, SiO
2, and TDS), Q-mode HCA was carried out on results from the thermal groundwater. The results of cluster analysis are represented using a dendrogram (
Figure 6). Classification is clearest when the distance is 10 (
Figure 6) and the 28 samples can be divided into three clusters: A, B, and C. Observations of the dendrogram, provides information on the degree of similarity of the three categories. The link distances between clusters A and B are relatively small (16), indicating that the hydrogeochemical characteristics of these clusters are similar, whereas the link distances between cluster C and the others are up to 25. Thus, the hydrogeochemical characteristics are quite different from those of the other clusters. The Piper diagram (
Figure 7) shows that the waters in all three clusters are of SO
4-Ca type. Among the clusters, the milliequivalent of SO
42− decreases gradually between A, B, and C, with the range of 91.5% to 93.8%, 87.3% to 92%, and 84% to 84.8%, respectively, while that of Ca
2+ is 71.4% to 80.2%, 68.1% to 75.1%, and 70.7% to 77.7%. The milliequivalent of Mg
2+ is higher, with an average of 22.115%, 22.76%, and 22.12%, respectively—and even 24.8% in sample W4.
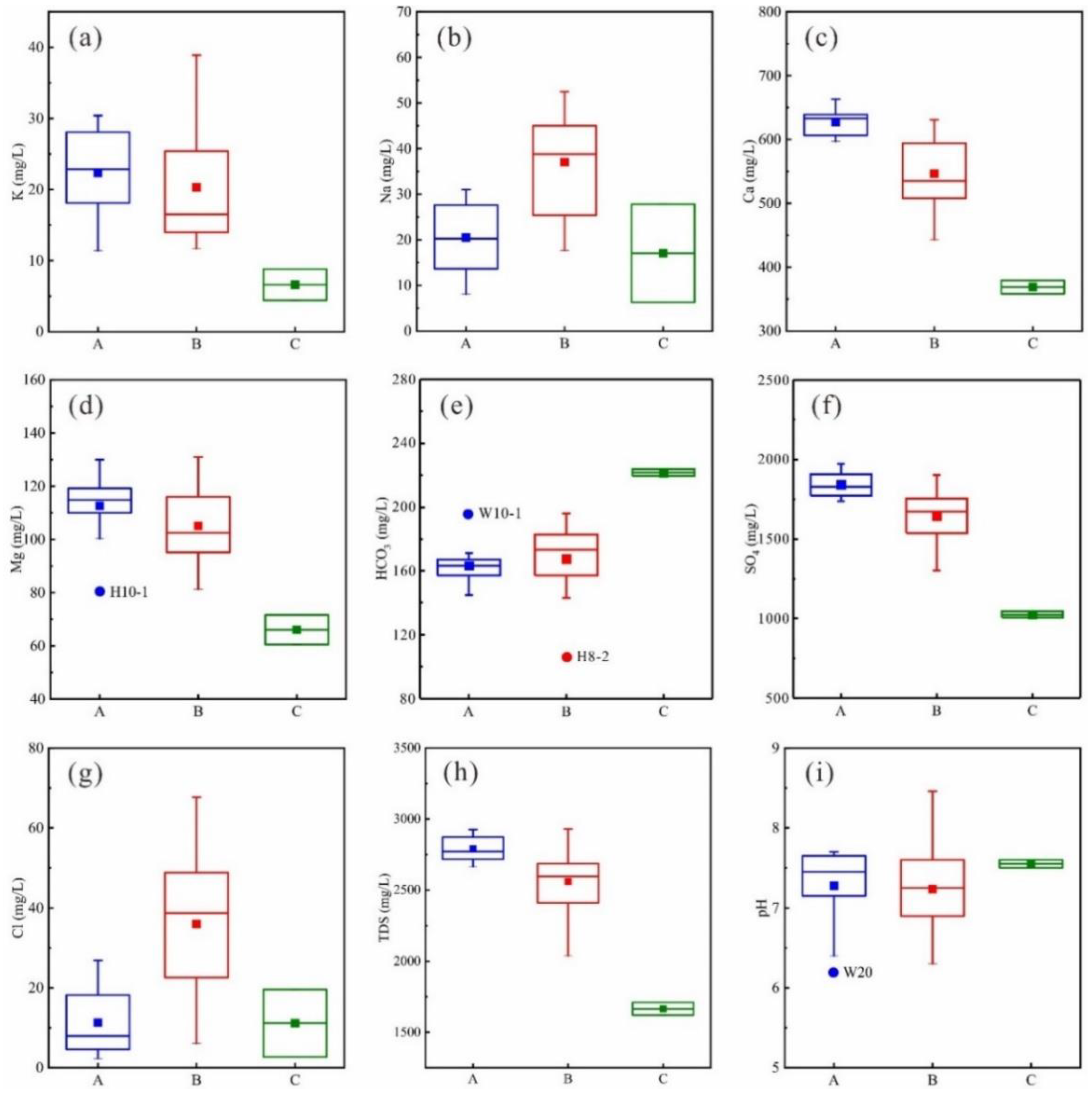
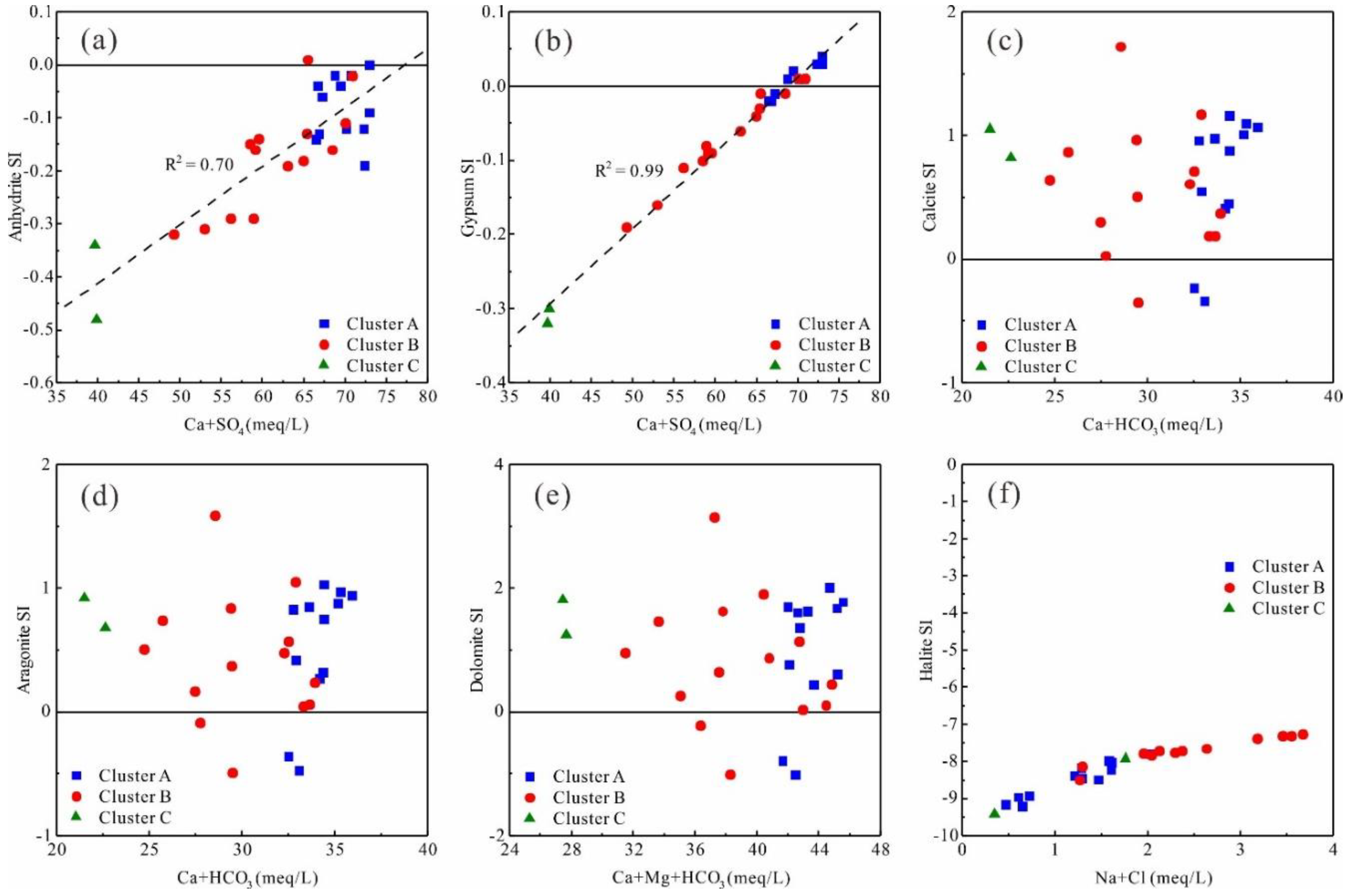
Cluster A contains 12 samples, accounting for 42.9% of the total. Compared with other clusters the TDS of this cluster is obviously higher (up to 2789.3 g/L), and the concentrations of K
+, Ca
2+, Mg
2+, and SO
42− are highest in cations and anions, respectively, while the contents of HCO
3− are the lowest (
Table 1,
Figure 8a,c,d,f,h). Sample H10-1 was the lowest Mg
2+ concentration and the highest HCO
3− concentration—80.4 mg/L and 195.9 mg/L—respectively. Based on the stratigraphy lithology of the study area, it can be inferred that cluster A waters may dissolve more evaporite (gypsum and anhydrite) and silicate rocks, but relatively less carbonate. Cluster B contains 14 samples, accounting for 50% of the total. The concentrations of each parameter in this cluster lies between those of the other two, with the exception of Na
+ and Cl
−, where the concentrations of these ions are the maximum of all the water samples, at 37.0 mg/L and 36.0 mg/L, respectively (
Table 1,
Figure 8b,g). This may reflect the dissolution of more halite. Cluster C contains only two samples, accounting for about 7.1% of the total. However, the concentration of HCO
3− and pH of these samples are the highest (221.6 mg/L and 7.6, respectively), of all samples, whereas other parameters are the smallest, indicating that more carbonate was dissolved (
Figure 8e,i). Thus, cluster A has high TDS, high Ca
2+ and SO
42−, cluster B is medium TDS, and high Na
+ and Cl
−, and cluster C has low TDS and high HCO
3−.
Comparison of the TDS, Ca
2+, SO
42−, and HCO
3− contents in the three clusters of water samples show that TDS, Ca
2+, and SO
42− gradually decrease, whereas HCO
3− gradually increases. According to the dissolution order of anions in crustal rocks (Cl
− > SO
42− > HCO
3−), and combined with the hydrochemical characteristics of the salty hot spring (Cl-Na type, TDS of 13.37 g/L) in Wenquanzhenn, eastern Sichuan basin, which is formed in the same geothermal reservoir [
3], the geothermal groundwater in the study area (SO
4-Ca type) is undergoing gradual desalination, but it has not yet reached the stage of ‘fresh water’ dominated by HCO
3−.
4.2.2. Factor Analysis
The correlation coefficient matrix is the basis of factor analysis.
Table 2 illustrates the statistics of correlation among the hydrochemical parameters of hot water in the study area. There is a significant correlation among SO
42−, Cl
−, Ca
2+, Mg
2+, Na
+ and TDS (correlation coefficients are greater than 0.7), which indicates that there is information overlap among variables, and data dimensionality reduction analysis is needed.
PCA is used to extract the eigenvalues from physico-chemical parameters of the 28 water samples in the study area. The eigenvalues of the extracted factors, their percentage of variance, and cumulative percentage of variance of the hydrochemical parameters are presented in
Table 3. In particular, four factors are extracted based on the Kaiser criterion, which explain 83.86% of the total variance. These are considered to reflect the basic information of the original data. The loadings for varimax rotated factor matrix in the four factors model are compiled in
Table 4. It is considered that the corresponding hydrochemical components are closely related to the hydrogeochemical processes represented by the factor when the absolute value of factor loading is more than 0.5 (marked in bold). The scores of each factor reflecting the degree of influence on each water sample are calculated, and the scatter plots of factor scores are drawn (
Figure 9).
Factor 1 has the largest proportion of total variance (38.2%) with high positive loadings for SO
42 (0.98), Ca
2+ (0.95), Mg
2+ (0.88), and TDS (0.98) (
Table 4). The high value points of factor 1 scores are mostly from cluster A, indicating that in this cluster is greatly affected by Factor 1 (
Figure 9a). The scores of samples corresponding to Factor 1 gradually decrease in the three clusters, implying that the effects of Factor 1 are gradually weakened. There is a good linear correlation between SO
42 and Ca
2+ in the geothermal water (R
2 = 0.92,
Figure 10a), indicating that the dissolution of gypsum and anhydrite in the T
1j
2 and T
2l rocks is the main source of these components.
Figure 10a shows that the trend lines of SO
42 and Ca
2+ lie below the dissolution line of CaSO
4, demonstrates that other hydrogeochemical processes have increased the SO
42− contents. The lithology suggests that the dissolution of the coal-bearing formation (pyrite and marcasite) in T
3xj may increase the SO
42 content in the groundwater [
53]. The values of Ca
2+/HCO
3− and (Ca
2+ + Mg
2+)/HCO
3− are much greater than 1 (
Figure 10b,c), suggesting that the dissolution of carbonate rocks is not the main source of Ca
2+ and Mg
2+. The dissolution of gypsum also increases the content of Ca
2+ in the hot water and inhibits the dissolution of carbonate rocks due to the common ion effect, consistent with the negative factor loading of HCO
3− (−0.57). Mg
2+ may originate from the dissolution of magnesium sulfate minerals in the geothermal reservoirs, indicated by the relatively good correlation between Mg
2+ and SO
42 (R
2 = 0.69) (
Figure 10d). Factor 1 therefore, reflects the dissolution of gypsum and anhydrite, magnesium sulfate minerals and pyrite-bearing formation rocks.
Factor 2, accounting for 18.1% of the total variance, contains HCO
3−, T and SiO
2 with positive loadings of 0.6, 0.78 and 0.86, respectively. The high points are uniformly distributed, corresponding to the relative consistency of the influence of this factor (
Figure 9a). As one of the characteristic components of geothermal water, SiO
2 has a good correlation with temperature (
Table 2), and SiO
2 geothermometers are widely used to estimate the temperature of reservoirs waters. The positive loadings for HCO
3− and SiO
2 indicate that the increasing SiO
2 content of geothermal waters may promote an increase in HCO
3− concentration. Factor 2 reflects the dissolution of quartz or chalcedony and other silicates.
Factor 3, with high positive loadings for Cl
− (0.89) and Na
+ (0.93), accounts for 16.76% of the total variance. The high value points of the scores are mainly from cluster B, which shows that these water samples are greatly affected this factor (
Figure 9b). The ionic ratios of Cl
− and Na
+ (
Figure 10e) show that most of the water falls above the dissolution line for halite, indicating that Na
+ has other sources apart from the dissolution of halite. The ratio of (Ca
2+ + Mg
2+)/(SO
42− + HCO
3−) is close to 1 (
Figure 10f), suggesting that the dissolution of dolomite, calcite, gypsum, and anhydrite is responsible for the production of Ca
2+, Mg
2+, SO
42− and HCO
3−, thus eliminating the cation exchange between Na
+, Ca
2+, and Mg
2+ [
50,
54,
55]. The incongruent dissolution of albite may contribute to the excess Na
+. Thus, Factor 3 reflects the dissolution of halite and albite.
Factor 4 corresponds to a variance portion of 10.8% with positive loadings for pH (0.62) and K+ (0.76). The high value points of the scores are mainly from clusters A and B, and the trend is similar to that of Factor 1. The Factor 4 score gradually decreases from cluster A to cluster C, indicating that the influence of this factor gradually weakens. The lithology and factor analyses results indicate that the main source of K+ is in dissolution of silicate rocks (K-feldspar). Thus, Factor 4 therefore reflects the dissolution of K-feldspar related to pH.