Biological, Chemical, and Ecotoxicological Assessments Using Benthos Provide Different and Complementary Measures of Lake Ecological Status
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
- Different weather and climate conditions, belonging to the Alpine (AL) and the Mediterranean (ME) Ecoregions covering the typical Italian climate conditions.
- Different types according to the Italian classification system (WFD requests) (Table 1 [19]): six of the eight lakes belong to the Alps and are in North-Western Italy (Piedmont region), the other two lakes belong to the insular Italy (Sardinia region) (Figure S1); the analyzed lakes cover five groups according to the national classification system in relation to their abiotic characteristics of altitude, surface area, mean depth, and catchment geo-lithology.
- Different origin, with five lakes being natural (mainly of glacial origin) and three representing the results of an artificial impoundment (reservoirs-Table S1). Also, different water uses are represented: the alpine reservoir is used as hydro-power generation plant, and the Mediterranean reservoirs as drinking water supplies.
- Different trophic level covering a gradient from ultra-oligotrophic to eutrophic conditions.
2.2. Sampling Methodology
2.3. Biological Assessment
2.4. POPs Assessment
2.5. Ecotoxicological Assessment
2.6. Statistical Analyses
3. Results
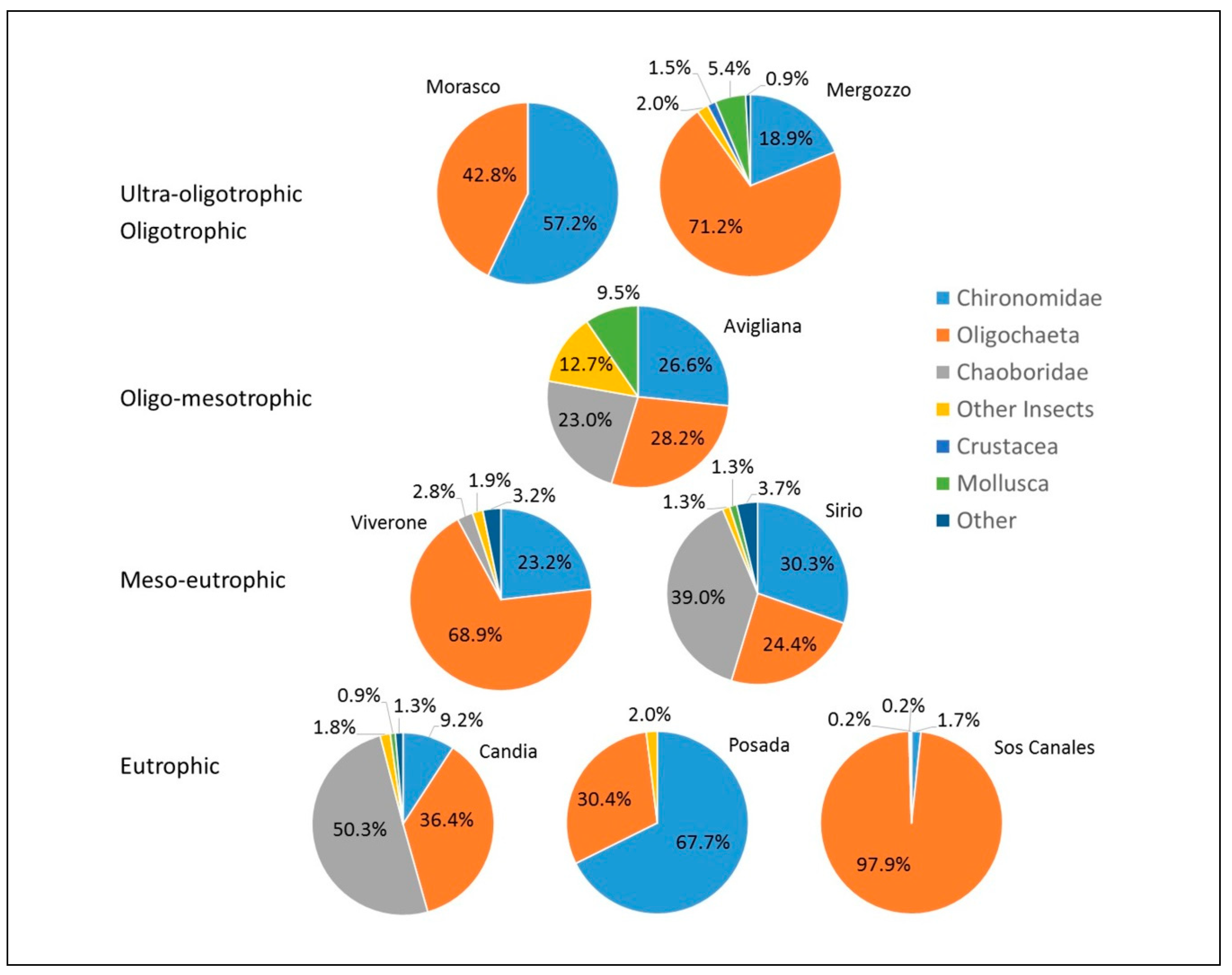
3.1. Biological Assessment
3.2. Micropollutants Assessment
3.3. Ecotoxicological Assessment
3.4. Comparison Between Assessments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Birk, S.; Bonne, W.; Borja, A.; Brucet, S.; Courrat, A.; Poikane, S.; Solimini, A.G.; van de Bund, W.; Zampoukas, N.; Hering, D. Three hundred ways to assess Europe’s surface waters: An almost complete overview of biological methods to implement the Water Framework Directive. Ecol. Indic. 2012, 18, 31–41. [Google Scholar] [CrossRef]
- Belletti, B.; Rinaldi, M.; Gurnell, A.M.; Buijse, A.D.; Mosselman, E. A review of assessment methods for river hydromorphology. Environ. Earth Sci. 2015, 73, 2079–2100. [Google Scholar] [CrossRef]
- Grizzetti, B.; Pistocchi, A.; Liquete, C.; Udias, A.; Bouraoui, F.; van de Bund, W. Human pressures and ecological status of European rivers. Sci. Rep. 2017, 7, 3. [Google Scholar] [CrossRef] [Green Version]
- Boggero, A.; Zaupa, S.; Fontaneto, D.; Bettinetti, R. The Benthic Quality Index to assess water quality of lakes may be affected by confounding environmental features. Water 2020. under review (same issue). [Google Scholar]
- European Union. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off. J. Eur. Union 2000, L327, 1–73. [Google Scholar]
- McFarland, B.; Carse, F.; Sandin, L. Littoral macroinvertebrates as indicators of lake acidification within the UK. Aquat. Conserv. Mar. Freshw. Ecosyst. 2010, 20, S105–S116. [Google Scholar] [CrossRef]
- Poikane, S.; Birk, S.; Böhmer, J.; Carvalho, L.; de Hoyose, C.; Gassner, H.; Hellsten, S.; Kelly, M.; Solheim, A.L.; Olin, M.; et al. A hitchhiker’s guide to European lake ecological assessment and intercalibration. Ecol. Indic. 2015, 52, 533–544. [Google Scholar] [CrossRef]
- Reyjol, Y.; Argillier, C.; Bonne, W.; Borja, A.; Buijse, A.D.; Cardoso, A.C.; Daufresne, M.; Kernan, M.; Ferreira, M.T.; Poikane, S.; et al. Assessing the ecological status in the context of the European Water Framework Directive: Where do we go now? Sci. Total Environ. 2014, 497–498, 332–344. [Google Scholar] [CrossRef]
- Solheim, A.L.; Feld, C.K.; Birk, S.; Phillips, G.; Carvalho, L.; Morabito, G.; Mischke, U.; Willby, N.; Søndergaard, M.; Hellsten, S.; et al. Ecological status assessment of European lakes: A comparison of metrics for phytoplankton, macrophytes, benthic invertebrates and fish. Hydrobiologia 2013, 704, 57–74. [Google Scholar] [CrossRef] [Green Version]
- Rossaro, B.; Zaupa, S.; Lencioni, V.; Marziali, L.; Boggero, A. Indice per la Valutazione della Qualità Ecologica dei Laghi Italiani Basato sulla Comunità Bentonica; Report CNR-ISE 02.13; Consiglio Nazionale delle Ricerche-Istituto per lo Studio degli Ecosistemi: Verbania Pallanza, Italy, 2013; 13p. [Google Scholar]
- Boggero, A.; Zaupa, S.; Cancellario, T.; Lencioni, V.; Marziali, L.; Rossaro, B. Italian Classification Method for Macroinvertebrates in Lakes. Method Summary; Report CNR-ISE 03.16; Consiglio Nazionale delle Ricerche-Istituto per lo Studio degli Ecosistemi: Verbania Pallanza, Italy, 2016; 16p. [Google Scholar]
- Premazzi, G.; Dalmiglio, A.; Cardoso, A.; Chiaudani, G. Lake management in Italy: The implications of the Water Framework Directive. Lakes Reserv. Res. Manag. 2003, 8, 41–59. [Google Scholar] [CrossRef]
- Fornaroli, R.; Cabrini, R.; Zaupa, S.; Bettinetti, R.; Ciampittiello, M.; Boggero, A. Quantile regression analysis as a predictive tool for lake macroinvertebrate biodiversity. Ecol. Indic. 2016, 61, 728–738. [Google Scholar] [CrossRef]
- Wernersson, A.S.; Carere, M.; Maggi, C.; Tusil, P.; Soldan, P.; James, A.; Sanchez, W.; Dulio, V.; Broeg, K.; Reifferscheid, G.; et al. The European technical report on aquatic effect-based monitoring tools under the Water Framework Directive. Environ. Sci. Eur. 2015, 27, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bettinetti, R.; Ponti, B.; Quadroni, S. An ecotoxicological approach to assess the Environmental quality of freshwater basins: A possible implementation of the EU Water Framework Directive? Environments 2014, 1, 92–106. [Google Scholar]
- Bettinetti, R.; Giarei, C.; Provini, A. Chemical analysis and sediment toxicity bioassays to assess the contamination of the river Lambro (Northern Italy). Arch. Environ. Contam. Toxicol. 2003, 45, 72–78. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. Water Quality. Determination of the Inhibition of the Mobility of Daphnia Magna Straus (Cladocera, Crustacea). Acute Toxicity Test; ISO 6341-1996; ISO: Geneva, Switzerland, 1996. [Google Scholar]
- OECD. Guideline for the Testing of Chemicals 218. Sediment-Water Chironomid Toxicity Test Using Spiked Sediment; Organization for Economic Cooperation and Development: Paris, France, 2004. [Google Scholar]
- Buraschi, E.; Salerno, F.; Monguzzi, C.; Barbiero, G.; Tartari, G. Characterization of the Italian lake-types and identification of their reference sites using anthropogenic pressure factors. J. Limnol. 2005, 64, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Boggero, A.; Zaupa, S.; Rossaro, B.; Lencioni, V.; Marziali, L.; Buzzi, F.; Fiorenza, A.; Cason, M.; Giacomazzi, F.; Pozzi, S. Protocollo di Campionamento e Analisi dei Macroinvertebrati Negli Ambienti Lacustri; MATTM-APAT: Roma, Italy, 2013. [Google Scholar]
- Wiederholm, T. (Ed.) Chironomidae of the Holartic region. Keys and Diagnoses. Part I: Larvae. Entomol. Scand. 1983, 19, 1–457. [Google Scholar]
- Timm, T. A Guide to the freshwater Oligochaeta and Polychaeta of Northern and Central Europe. Lauterbornia 2009, 66, 1–235. [Google Scholar]
- Various Authors. Guide per il Riconoscimento delle Specie Animali delle Acque Interne Italiane, Collana del Progetto Finalizzato ‘Promozione della Qualità dell’Ambiente’; Consiglio Nazionale delle Ricerche: Verona, Italy, 1977–1985; Volumes 29.
- Bettinetti, R.; Galassi, S.; Guilizzoni, P.; Quadroni, S. Sediment analysis to support the recent glacial origin of DDT pollution in Lake Iseo (Northern Italy). Chemosphere 2011, 85, 163–169. [Google Scholar] [CrossRef]
- Crawley, M.J. The R Book, 2nd ed.; John Wiley & Sons Ltd.: West Sussex, UK, 2013. [Google Scholar]
- Chapman, D. Water Quality Assessments: A Guide to Use of Biota, Sediments and Water in Environmental Monitoring; World Health Organization; University Press: Cambridge, UK, 1996; 651p. [Google Scholar]
- Mac Donald, D.D.; Ingersoll, C.G.; Berger, T.A. Development and Evaluation of Consensus-Based Sediment Quality Guidelines for Freshwater Systems. Arch. Environ. Contam. Toxicol. 2000, 39, 20–31. [Google Scholar] [CrossRef]
- Bettinetti, R.; Croce, V.; Galassi, S. Ecological risk assessment for the recent case of DDT pollution in Lake Maggiore (Northern Italy). Water Air Soil Pollut. 2005, 162, 385–399. [Google Scholar] [CrossRef]
- Rousch, J.M.; Simmons, T.W.; Kerans, B.L.; Smith, B.P. Relative acute effects of low pH and high iron on the hatching and survival of the water mite (Arrenurus manubriator) and the aquatic insect (Chironomus riparius). Environ. Toxicol. Chem. 1997, 16, 2144–2150. [Google Scholar] [CrossRef]
- Orendt, C. Chironomids as bioindicators in acidified streams: A contribution to the acidity tolerance of chironomid species with a classification in sensitivity classes. Int. Rev. Hydrobiol. 1999, 84, 439–449. [Google Scholar]
- Monda, D.P.; Galat, D.L.; Finger, S.E.; Kaiser, M.S. Acute toxicity of ammonia (NH3-N) in sewage effluent to Chironomus riparius: II. Using a generalized linear model. Arch. Environ. Toxicol. Chem. 1997, 28, 385–390. [Google Scholar] [CrossRef]
- Hynes, H.B.N. The Biology of Polluted Waters; Liverpool University Press: Liverpool, UK, 1960. [Google Scholar]
- Brinkhurst, R.O.; Jamieson, B.G.M. Aquatic Oligochaeta of the World; Oliver & Boyd: Edinburgh, UK, 1971; 860p. [Google Scholar]
- Czechowskia, P.; Stevens, M.I.; Madden, C.; Weinsteing, P. Steps towards a more efficient use of chironomids as bioindicators for freshwater bioassessment: Exploiting eDNA and other genetic tools. Ecol. Indic. 2020, 110, 105868. [Google Scholar] [CrossRef]
- Hering, D.; Carvahlo, L.; Argillier, C. Managing aquatic ecosystems and water resources under multiple stress—An introduction to the MARS project. Sci. Total Environ. 2015, 503–504, 10–21. [Google Scholar] [CrossRef]
- Leavitt, P.R.; Fritz, S.C.; Anderson, N.J. Paleolimnological evidence of the effects on lakes of energy and mass transfer from climate and humans. Limnol. Oceanogr. 2009, 54, 2330–2348. [Google Scholar] [CrossRef]
- Reyjol, Y.; Argillier, C.; Bonne, W.; Borja, A.; Buijse, A.D.; Cardoso, A.C.; Daufresne, M.; Kernan, M.; Ferreira, M.T.; Poikane, S.; et al. Assessing the ecological status in the context of the European Water Framework Directive: Where do we go now? Sci. Total Environ. 2014, 497–498, 332–344. [Google Scholar] [CrossRef]

| Lake Name | BQIESall | BQIESbottom | DDTs | PCBs | DR | ER |
|---|---|---|---|---|---|---|
| Avigliana piccolo | 0.26 | 0.01 | 7.27 | 19.4 | 0.051 | 55 |
| Candia | 0.42 | 0.21 | 14.73 | 29.8 | NA | NA |
| Sirio | 0.22 | 0.01 | 62.84 | 61.5 | 0.052 | 50 |
| Viverone | 0.26 | 0.00 | 25.44 | 38.1 | 0.051 | 70 |
| Mergozzo | 0.52 | 0.14 | 40.15 | 31.9 | 0.041 | 53 |
| Morasco | 0.46 | 0.44 | 0.75 | 2.2 | 0.053 | 51 |
| Posada | 0.37 | 0.37 | 10.36 | 2.7 | 0.049 | 70 |
| Sos Canales | 0.33 | 0.20 | 8.10 | 6.9 | NA | NA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bettinetti, R.; Zaupa, S.; Fontaneto, D.; Boggero, A. Biological, Chemical, and Ecotoxicological Assessments Using Benthos Provide Different and Complementary Measures of Lake Ecological Status. Water 2020, 12, 1140. https://doi.org/10.3390/w12041140
Bettinetti R, Zaupa S, Fontaneto D, Boggero A. Biological, Chemical, and Ecotoxicological Assessments Using Benthos Provide Different and Complementary Measures of Lake Ecological Status. Water. 2020; 12(4):1140. https://doi.org/10.3390/w12041140
Chicago/Turabian StyleBettinetti, Roberta, Silvia Zaupa, Diego Fontaneto, and Angela Boggero. 2020. "Biological, Chemical, and Ecotoxicological Assessments Using Benthos Provide Different and Complementary Measures of Lake Ecological Status" Water 12, no. 4: 1140. https://doi.org/10.3390/w12041140
APA StyleBettinetti, R., Zaupa, S., Fontaneto, D., & Boggero, A. (2020). Biological, Chemical, and Ecotoxicological Assessments Using Benthos Provide Different and Complementary Measures of Lake Ecological Status. Water, 12(4), 1140. https://doi.org/10.3390/w12041140







