Mangrove Forests Evolution and Threats in the Caribbean Sea of Colombia
Abstract
:1. Introduction
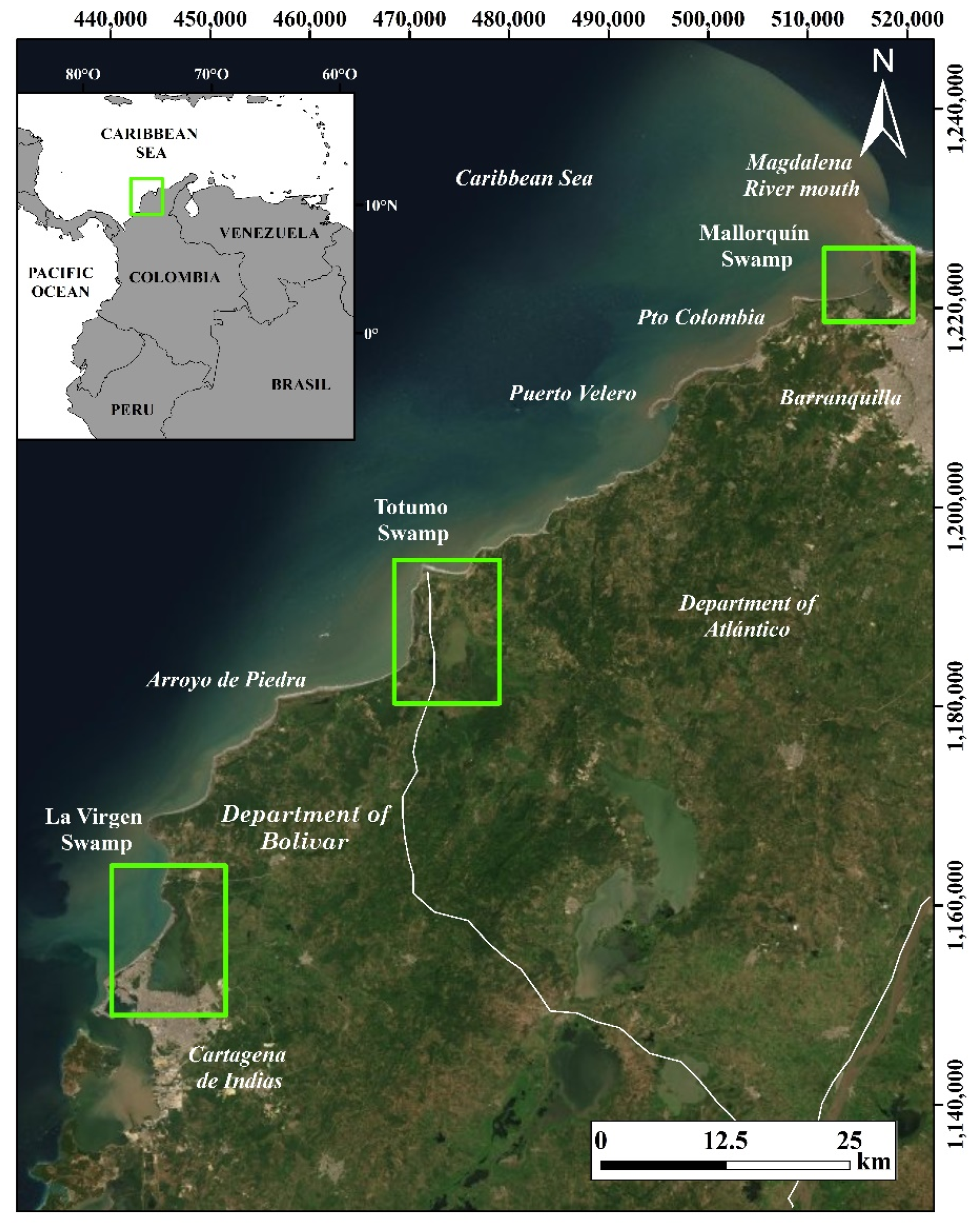
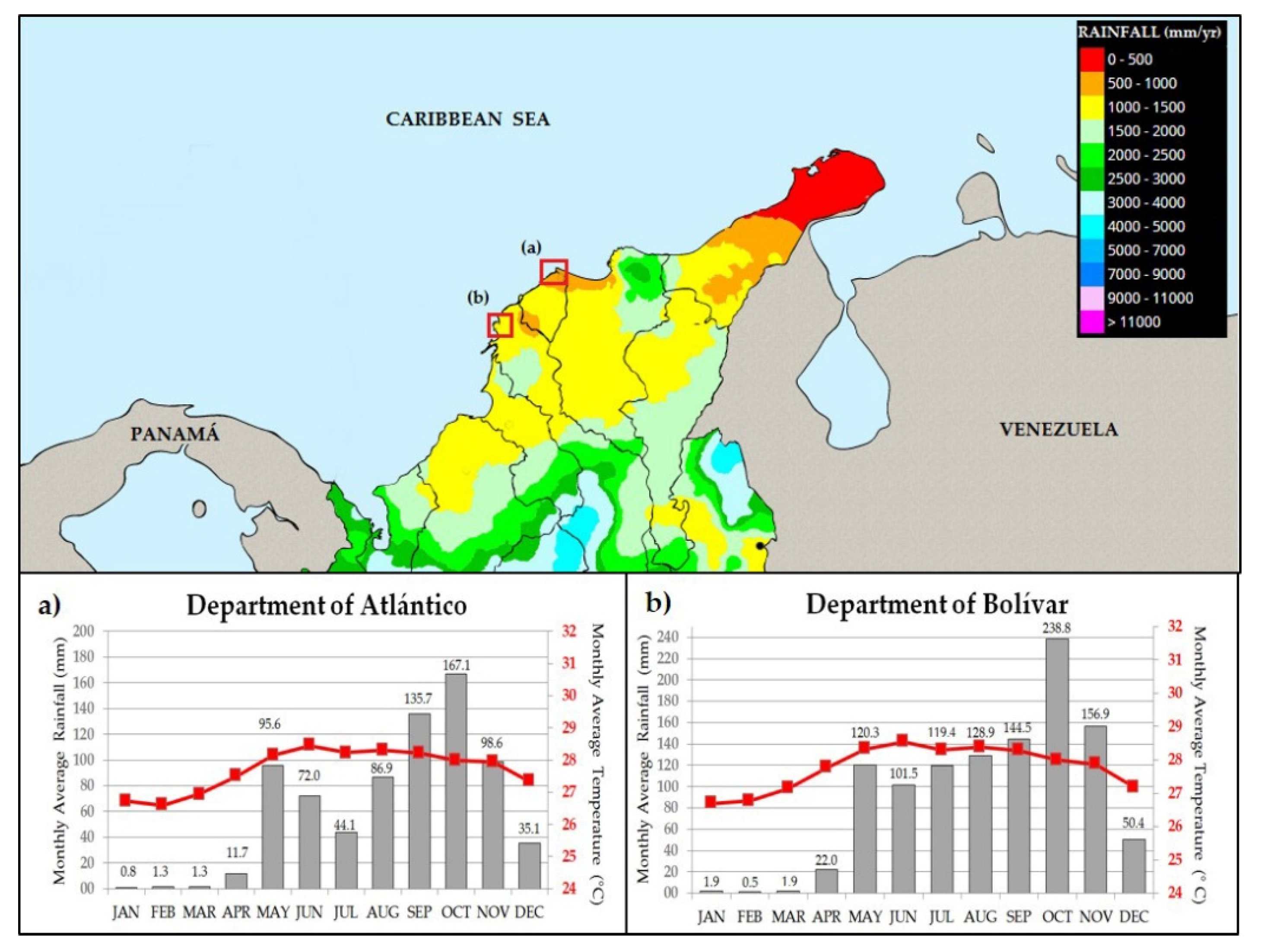
2. Study Area
3. Methodology
3.1. Data Used
3.2. Digital Images Analysis
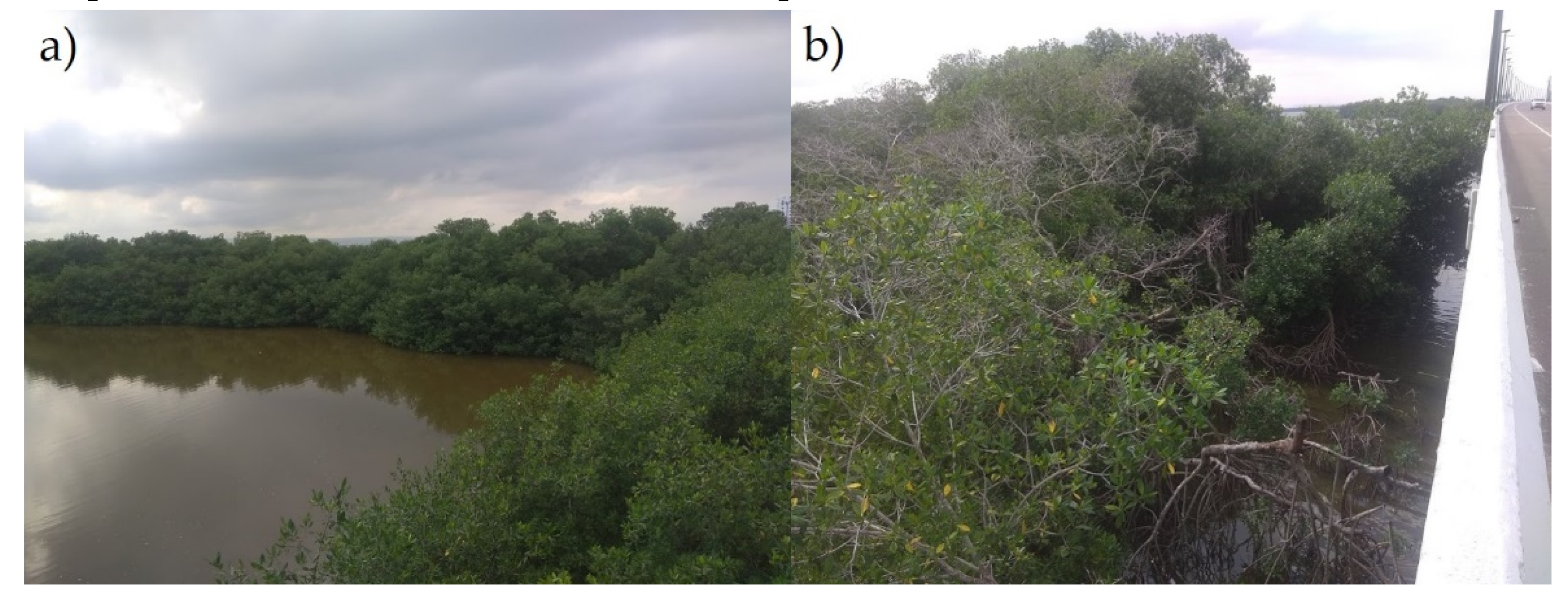
3.3. Field Visits
4. Results and Discussion
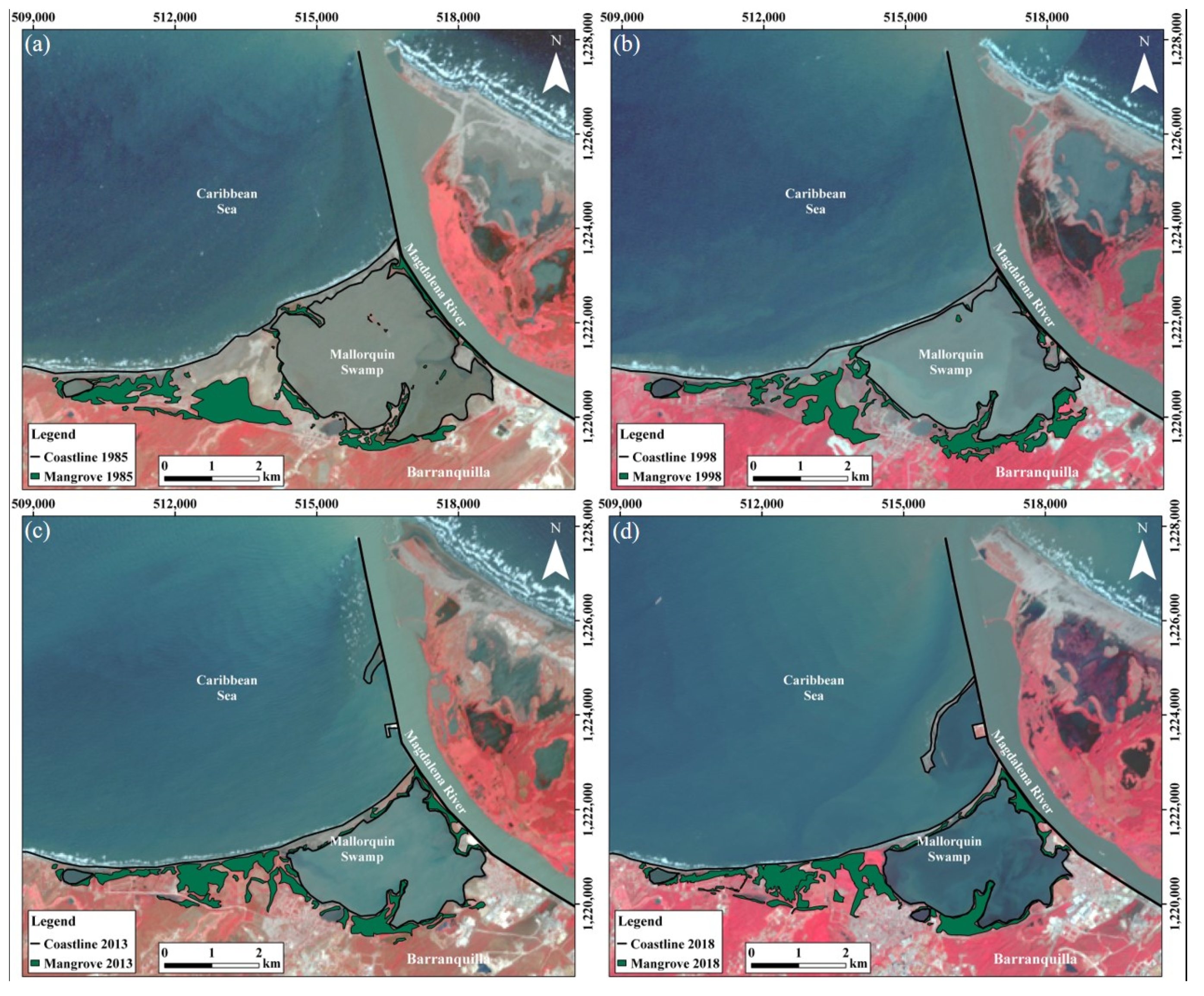
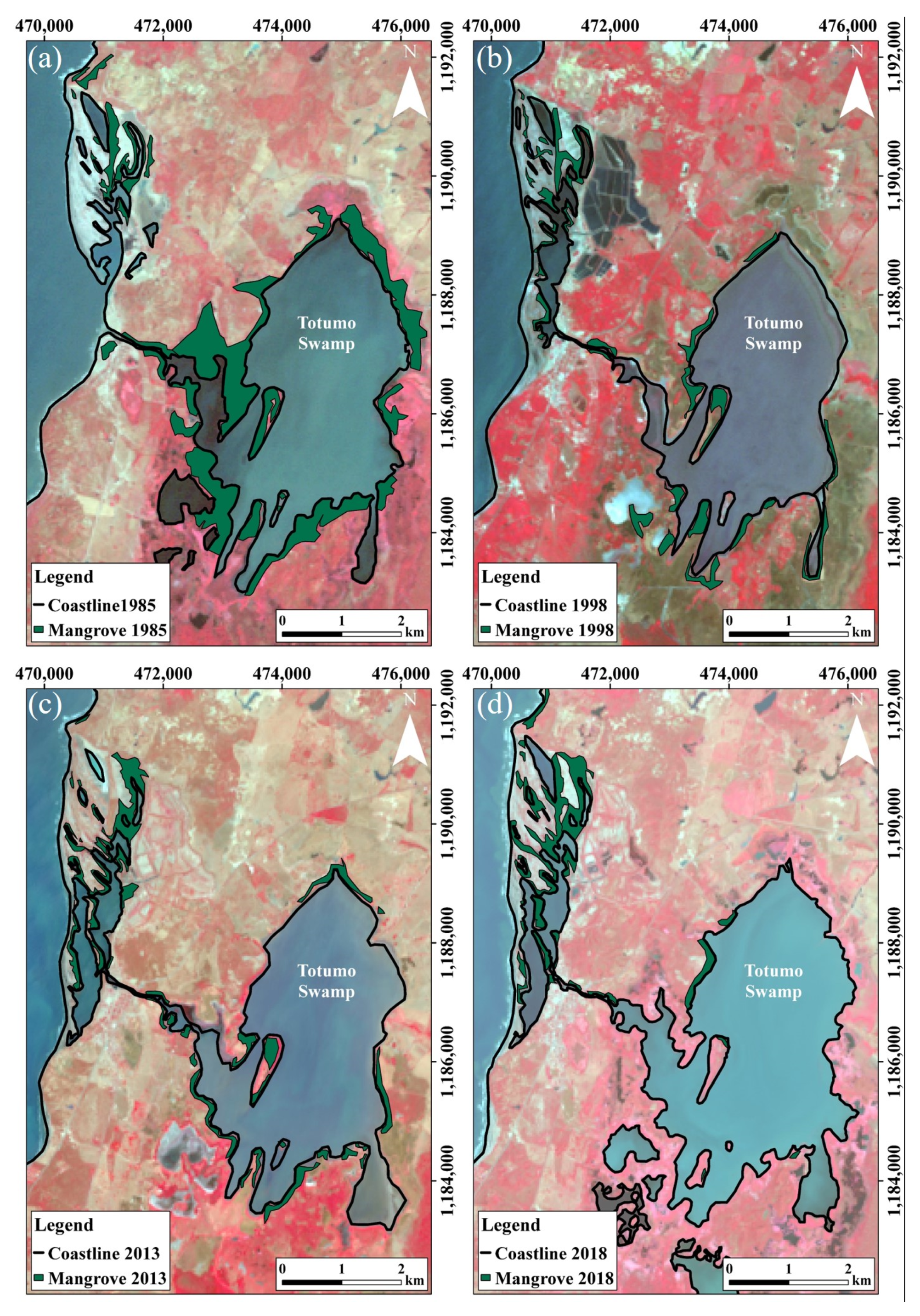
4.1. Mangrove Swamps Evolution
4.1.1. Mallorquín Mangrove Swamp
4.1.2. Totumo Mangrove Swamp
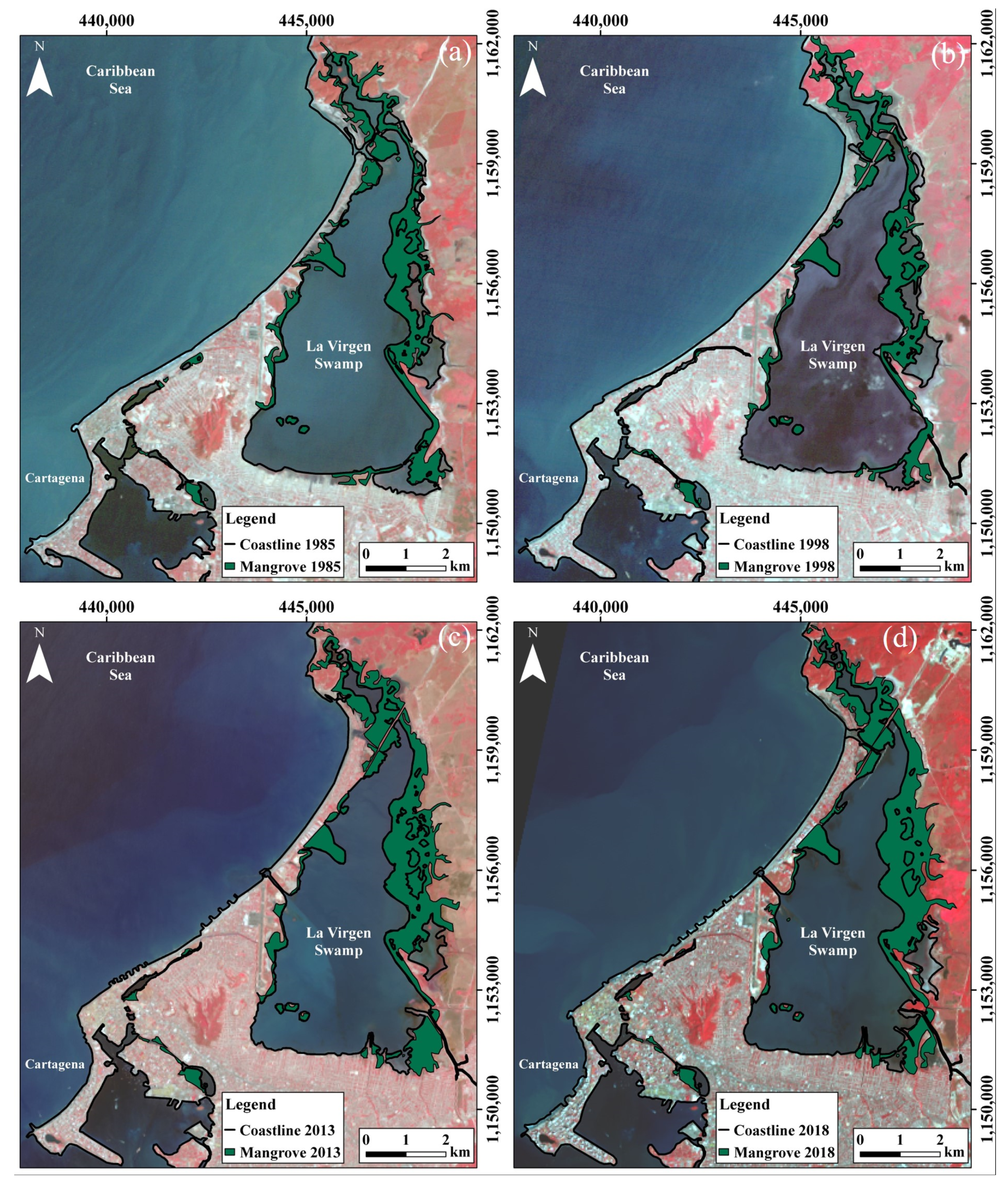
4.1.3. La Virgen Mangrove Swamp
4.2. Environmental and Natural Concerns
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sanderman, J.; Hengl, T.; Fiske, G.; Solvik, K.; Adame, M.F.; Benson, L.; Bukoski, J.J.; Carnell, P.; Cifuentes-Jara, M.; Donato, D.; et al. A global map of mangrove forest soil carbon at 30 m spatial resolution. Environ. Res. Lett. 2018, 13, 055002. [Google Scholar] [CrossRef]
- Carugati, L.; Gatto, B.; Rastelli, E.; Martire, M.L.; Coral, C.; Greco, S.; Danovaro, R. Impact of mangrove forests degradation on biodiversity and ecosystem functioning. Sci. Rep. 2018, 8, 13298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbier, E.B. The protective service of mangrove ecosystems: A review of valuation methods. Mar. Pollut. Bull. 2016, 109, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, H.B. Mangrove Inventory, Monitoring, and Health Assessment. In Coastal Wetlands: Alteration and Remediation; Frinkl, C., Makouski, C., Eds.; Springer: Cham, Switzerland, 2017; pp. 573–630. [Google Scholar]
- Jennerjahn, T.C.; Gilman, E.; Krauss, K.W.; Lacerda, L.D.; Nordhaus, I.; Wolanski, E. Mangrove Ecosystems under Climate Change. In Mangrove Ecosystems: A Global Biogeographic Perspective; Rivera-Monroy, V., Lee, S., Kristensen, E., Twilley, R., Eds.; Springer: Cham, Switzerland, 2017; pp. 211–244. [Google Scholar]
- Osland, M.; Feher, L.; Lopéz-Portillo, J.; Day, R.; Suman, D.; Guzmán, J.; Rivera-Monroy, V. Mangrove forests in a rapidly changing world: Global change impacts and conservation opportunities along the Gulf of Mexico coast. Estuar. Coast. Shelf Sci. 2018, 214, 120–140. [Google Scholar] [CrossRef]
- Thomas, N.; Lucas, R.; Bunting, P.; Hardy, A.; Rosenqvist, A.; Simard, M. Distribution and drivers of global mangrove forest change, 1996–2010. PLoS ONE 2017, 12, e0179302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costanza, R.; de Groot, R.; Sutton, P.; van der Ploeg, S.; Anderson, S.J.; Kubiszewski, I.; Farber, S.; Turner, R.K. Changes in the global value of ecosystem services. Glob. Environ. Chang. 2014, 26, 152–158. [Google Scholar] [CrossRef]
- Jia, M.; Wang, Z.; Zhang, Y.; Mao, D.; Wang, C. Monitoring loss and recovery of mangrove forests during 42 years: The achievements of mangrove conservation in China. Int. J. Appl. Earth Obs. Geoinf. 2018, 73, 535–545. [Google Scholar] [CrossRef]
- Polidoro, B.A.; Carpenter, K.E.; Collins, L.; Duke, N.C.; Ellison, A.M.; Ellison, J.C.; Farnsworth, E.J.; Fernando, E.S.; Kathiresan, K.; Koedam, N.E.; et al. The loss of species: Mangrove extinction risk and geographic areas of global concern. PLoS ONE 2010, 5, e10095. [Google Scholar] [CrossRef]
- Feller, I.C.; Friess, D.A.; Krauss, K.W.; Lewis, R.R. The state of the world’s mangroves in the 21st century under climate change. Hydrobiologia 2017, 803, 1–12. [Google Scholar] [CrossRef] [Green Version]
- De Lacerda, L.D.; Borges, R.; Ferreira, A.C. Neotropical mangroves: Conservation and sustainable use in a scenario of global climate change. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 1347–1364. [Google Scholar] [CrossRef]
- De Almeida, L.T.; Olímpio, J.L.S.; Pantalena, A.F.; de Almeida, B.S.; de Oliveira Soares, M. Evaluating ten years of management effectiveness in a mangrove protected area. Ocean Coast. Manag. 2016, 125, 29–37. [Google Scholar] [CrossRef]
- Lewis, R.; Brown, B.; Flynn, L. Methods and Criteria for Successful Mangrove Forest Rehabilitation. In Coastal Wetlands: An Integrated and Ecosystem Approach; Perillo, G., Wolanski, E., Cahoon, D., Hopkinson, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 863–887. [Google Scholar]
- Palacios, M.L.; Cantera, J.R. Mangrove timber use as an ecosystem service in the Colombian Pacific. Hydrobiologia 2017, 803, 345–358. [Google Scholar] [CrossRef]
- Polanía, J.; Urrego, L.E.; Agudelo, C.M. Recent advances in understanding Colombian mangroves. Acta Oecologica 2015, 63, 82–90. [Google Scholar] [CrossRef]
- Instituto de investigaciones marinas y costeras (INVEMAR). Diagnóstico y Evaluación de la Calidad de Aguas Marinas y Costeras en el Caribe y Pacífico Colombianos; INVEMAR: Santa Marta, Colombia, 2015; pp. 1–315. [Google Scholar]
- Bolívar-Anillo, H.J.; Sánchez, H.; Fernandez, R.; Villate, D.; Anfuso, G. An Overview on Mangrove Forests Distribution in Colombia: An Ecosystem at Risk. J. Aquat. Sci. Mar. Biol. 2019, 2, 16–18. [Google Scholar]
- Instituto de investigaciones marinas y costeras (INVEMAR). Actualizacion y Ajuste del Diagnóstico y Zonificación de los Manglares de la Zona Costera del Departamento del Atlantico, Caribe Colombiano; INVEMAR: Santa Marta, Colombia, 2005; pp. 1–191. [Google Scholar]
- Corporacion Autonoma Regional del Canal del Dique. Zonificación Definitiva de las Áreas de Manglar del Departamento de Bolívar para la Conservación y el Manejo Sostenible; CARDIQUE: Cartagena de Indias, Colombia, 2001; pp. 1–70.
- Corporación Autónoma Regional del Canal del Dique. Diagnostico, Zonificación y Planificación Estrategica de las Areas de Manglar de Bolívar; CARDIQUE: Cartagena de Indias, Colombia, 1998; pp. 1–176.
- Andrade, C. Cambios Recientes del Nivel del Mar en Colombia. In Deltas de Colombia: Morfodinámica y Vulnerabilidad Ante el Cambio Global; Restrepo, J., Ed.; EAFIT Univ. Press: Medellin, Colombia, 2008; pp. 103–122. [Google Scholar]
- Solano, J.M.; Barros Henríquez, J.A.; Roncallo Fandiño, B.; Arrieta Pico, G. Requerimientos hídricos de cuatro gramíneas de corte para uso eficiente del agua en el Caribe seco colombiano. Cienc. y Tecnol. Agropecu. 2015, 15, 83. [Google Scholar] [CrossRef] [Green Version]
- Instituto de Hidrología, Meteorología y Estudios Ambientales (IDEAM). Atlas Climatológico de Colombia. Available online: http://atlas.ideam.gov.co/visorAtlasClimatologico.html (accessed on 19 July 2019).
- Hurtado Montoya, A.F.; Mesa Sánchez, Ó.J. Cambio climatico y variabilidad espacio-temporal de la precipitación en Colombia. Rev. EIA 2015, 11, 131–150. [Google Scholar]
- Andrade Amaya, C.A.; Barton, E.D. Sobre la existencia de una celda de circulación atmosférica sobre el Caribe y su efecto en las corrientes de Ekman del Caribe suroccidental. Boletín Científico CIOH 2013, 31, 73–94. [Google Scholar] [CrossRef] [Green Version]
- Philander, S. Atlantic Ocean Equatorial Currents. In Ocean Currents; Steele, J., Thorpe, S., Turekian, K., Eds.; Elsevier: London, UK, 2001; pp. 188–191. [Google Scholar]
- Instituto de Hidrología, Meteorología y Estudios Ambientales (IDEAM)-Universidad Nacional de Colombia. La Variabilidad Climática y el Cambio Climático en Colombia; IDEAM: Bogotá, Colombia, 2018; pp. 1–53.
- Thomas, Y. Climatología Marina, Presión Atmosférica, Viento y Olas para las Aguas Territoriales Bajo Jurisdicción Colombiana. 8–19 N y 69–84 W.; Datos TOPEXPOSEIDON; Laboratorie de Géographie Physique (CNRS): París, France, 2006; p. 69. [Google Scholar]
- Correa, I.; Morton, R. Coasts of Colombia U.S. Department of the Interior USGS, St. Petersburg, Florida, USA, 2011. Available online: http://coastal.er.usgs.gov/coasts-colombia/ (accessed on 19 July 2019).
- Ortiz-Royero, J.C.; Otero, L.J.; Restrepo, J.C.; Ruiz, J.; Cadena, M. Cold fronts in the Colombian Caribbean Sea and their relationship to extreme wave events. Nat. Hazards Earth Syst. Sci. 2013, 13, 2797–2804. [Google Scholar] [CrossRef] [Green Version]
- Instituto de investigaciones marinas y costeras (INVEMAR). Evaluación de la Calidad Ambiental de los Manglares de La Ciénaga Mallorquín, Departamento del Atlántico; INVEMAR: Santa Marta, Colombia, 2015; pp. 1–32. [Google Scholar]
- Arrieta, L.; de la Rosa, J. Estructura de la comunidad íctica de la ciénaga de Mallorquín, Caribe Colombiano. Bol. Investig. Mar. y Costeras 2003, 32, 231–242. [Google Scholar] [CrossRef]
- Páez, C. Análisis de las dimensiones del desarrollo sostenible en la ciénaga de Mallorquín. Modul. Arquit. 2015, 14, 63–84. [Google Scholar]
- Castro-Rodríguez, E.; León-Luna, I.; Pinedo-Hernández, J. Biogeochemistry of mangrove sediments in the Swamp of Mallorquin, Colombia. Reg. Stud. Mar. Sci. 2018, 17, 38–46. [Google Scholar] [CrossRef]
- Fuentes-Gandara, F.; Pinedo-Hernández, J.; Marrugo-Negrete, J.; Díez, S. Human health impacts of exposure to metals through extreme consumption of fish from the Colombian Caribbean Sea. Environ. Geochem. Health 2018, 40, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Portz, L.; Manzolli, R.; Andrade, C.F.; Villate Daza, D.; Bolivar, D.B.; Alcantara-Carrio, J. Assessment of heavy metals pollution (Hg, Cr, Cd, Ni) in the sediments of Mallorquin lagoon-Barranquilla, Colombia. J. Coast. Res. 2020, in press. [Google Scholar]
- Instituto Colombiano de Desarrollo Rural. Plan de Manejo y Ordenación Pesquera del Humedal Ciénaga del Totumo; Universidad Jorge Tadeo Lozano: Cartagena de Indias, Colombia, 2011; pp. 1–242. [Google Scholar]
- Carvajal, A. Caracterización físico-biótica del litoral del departamento del Atlántico. In Caracterización Físico-Biótica del Litoral Caribe Colombiano; DIMAR-CIOH, Ed.; Dirección General Marítima: Cartagena de Indias, Colombia, 2009; pp. 97–110. [Google Scholar]
- Orejarena Rondón, A.F.; Afanador Franco, F.; Ramos de la Hoz, I.; Conde Frías, M.; Restrepo López, J.C. Evolución morfológica de la espiga de Galerazamba, Caribe colombiano. Boletín Científico CIOH 2015, 123–144. [Google Scholar] [CrossRef]
- Anfuso, G.; Rangel-Buitrago, N.; Correa, I. Evolution of sandspits along the Caribbean coast of Colombia: Natural and human influences. In Sand and Gravel Spits; Randazzo, G., Jackson, D.W.T., Cooper, J.A.G., Eds.; Springer: New York, NY, USA, 2015; pp. 1–19. [Google Scholar]
- Instituto Humboldt-Fundación Omacha. Aplicación de Criterios Biológicos y Ecológicos para la Identificación, Caracterización y Establecimiento de Límites de Humedales en la Ventana de Estudio: Ciénaga de La Virgen; Instituto Humboldt-Fundación Omacha: Bogotá, Colombia, 2015; pp. 1–50. [Google Scholar]
- Agudelo, C. Estructura de los Bosques de Manglar del Departamento de Bolívar y su Relación con Algunos Parametros Abioticos. Bachelor’s Degree Thesis in Marine Biology, Univeridad Jorge Tadeo Lozano, Bogotá, Colombia, 2000. [Google Scholar]
- Maldonado, W.; Baldiris, I.; Díaz, J. Evaluación de la calidad del agua en la Ciénaga de la Virgen (Cartagena, Colombia) durante el período 2006–2010*. Cienc. Exactas y Apl. 2011, 9, 79–87. [Google Scholar]
- Carbal Herrera, A.; Muñoz Carbal, J.; Solar Cumplido, L. Valoración económica integral de los bienes y servicios ambientales ofertados por el ecosistema de manglar ubicado en la Ciénaga de la Virgen. Cartagena-Colombia. Sabercienc. y Lib. 2015, 10, 125–146. [Google Scholar]
- Pavithra, B.; Kalaivani, K.; Ulagapriya, K. Remote sensing techniques for mangrove mapping. Int. J. Eng. Adv. Technol. 2019, 8, 27–30. [Google Scholar]
- Heumann, B.W. Satellite remote sensing of mangrove forests: Recent advances and future opportunities. Prog. Phys. Geogr. 2011, 35, 87–108. [Google Scholar] [CrossRef]
- Khairuddin, B.; Yulianda, F.; Kusmana, C. Degradation Mangrove by Using Landsat 5 TM and Landsat 8 OLI Image in Mempawah Regency, West Kalimantan Province year 1989–2014. Procedia Environ. Sci. 2016, 33, 460–464. [Google Scholar] [CrossRef] [Green Version]
- Lymburner, L.; Bunting, P.; Lucas, R.; Scarth, P.; Alam, I.; Phillips, C.; Ticehurst, C.; Held, A. Mapping the multi-decadal mangrove dynamics of the Australian coastline. Remote Sens. Environ. 2020, 238, 111185. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Available online: https://earthexplorer.usgs.gov (accessed on 15 July 2019).
- Vermote, E.; Justice, C.; Claverie, M.; Franch, B. Preliminary analysis of the performance of the Landsat 8/OLI land surface reflectance product. Remote Sens. Environ. 2016, 185, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Afanador Franco, F.; Carvajal Díaz, A.F.; Franco Arias, D.A.; Orozco Quintero, F.J.; Pacheco Gómez, J.D.; Santos Barrera, Y. Atlas Geomorfológico del Litoral Caribe Colombiano; Dirección General Marítima: Cartagena de Indias, Colombia, 2013; pp. 1–216. [Google Scholar]
- Instituto de investigaciones marinas y costeras (INVEMAR). Ordenamiento Ambiental de la Zona Costera del Departamento del Atlántico; INVEMAR: Santa Marta, Colombia, 2007; pp. 1–583. [Google Scholar]
- Rouse, J.W.; Hass, R.H.; Schell, J.A.; Deering, D.W.; Harlan, J.C. Monitoring The Vernal Advancement and Retrogradation (Greenwave Effect) of Natural Vegetation; Final Report; NASA/GSFC: Greenbelt, MA, USA, 1974; pp. 1–137.
- Thieler, E.R.; Himmelstoss, E.A.; Zichichi, J.L.; Ergul, A. The Digital Shoreline Analysis System (DSAS) Version 4.0-an ArcGIS Extension for Calculating Shoreline Change (ver. 4.4 July 2017); U.S. Geological Survey Open-File Report 2008–1278; USGS: Reston, VA, USA, 2017.
- Instituto de investigaciones marinas y costeras (INVEMAR). El Sistema de Información para la Gestión de los Manglares en Colombia SIGMA. Available online: http://sigma.invemar.org.co/ (accessed on 15 July 2019).
- Grupo de investigación en tecnologías del agua GTA. Análisis Sobre el Manejo Integrado del Recurso Hídrico en la Ciénaga de Mallorquín; GTA: Barranquilla, Colombia, 2005; pp. 1–323. [Google Scholar]
- Corporación Autónoma Regional del Atlántico. Documentación del Estado de las Cuencas Hidrográficas en el Departamento del Atlántico; CRA: Barranquilla, Colombia, 2007; pp. 1–114.
- Martinez, J.O.; Pilkey, O.H.; Neal, W.J. Rapid formation of large coastal sand bodies after emplacement of Magdalena river jetties, northern Colombia. Environ. Geol. Water Sci. 1990, 16, 187–194. [Google Scholar] [CrossRef]
- Galvis, O.; Ramiréz, F.; Vacca, V.; Herrera, O.; Bolaño, F.; Arteta, V.; Rodríguez, L.; Jiménez, M.; Vergara, A. Primera fase del programa para la rehabilitación integral de la ciénaga de Mallorquín. Dugandia 1989, 1, 10–12. [Google Scholar]
- Ulloa-Delgado, G.; Sanchez-Paez, H.; Gil-Torres, W.; Pino-Rengifo, J.; Rodriguez-Cruz, H.; Alvarez-Leon, R. Conservación y Uso Sostenible de los Manglares del Caribe Colombiano; Sanchez-Paez, H., Ulloa-Delgado, G., Alvarez-León, R., Eds.; Ministerio del Medio Ambiente: Bogotá, Colombia, 1998; pp. 1–224.
- Ivar do Sul, J.A.; Costa, M.F.; Silva-Cavalcanti, J.S.; Araújo, M.C.B. Plastic debris retention and exportation by a mangrove forest patch. Mar. Pollut. Bull. 2014, 78, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, J.D.; Kjerfve, B. Magdalena river: Interannual variability (1975–1995) and revised water discharge and sediment load estimates. J. Hydrol. 2000, 235, 137–149. [Google Scholar] [CrossRef]
- Corporación Andina de Fomento. El fenomeno El Niño 1997–1998: Memoria, Retos y Soluciones: Colombia; CAF: Caracas, Venezuela, 2000; pp. 1–242. [Google Scholar]
- Sánchez, H.; Bolívar-Anillo, H.J.; Villate-Daza, D.; Escobar-Olaya, G.; Anfuso, G. Influencia de los impactos antrópicos sobre la evolución del bosque de manglar en Puerto Colombia ( Mar Caribe colombiano ). Rev. Latinoam. Recur. Nat. 2019, 15, 1–16. [Google Scholar]
- Contraloria distrital de Barranquilla. Informe del Estado de los Recursos Naturales y del Ambiente de Barranquilla; Contraloria Distrital: Barranquilla, Colombia, 2018; pp. 1–49.
- Niño, L. Los acuerdos de pesca responsable en el humedal ciénaga del Totumo (Atlántico-Bolívar). In Proceedings of the Biodiversidad y Turismo para un Desarrollo Sostenible; Olivero, J., de León, J., Eds.; AECID: Cartagena de Indias, Colombia, 2011; pp. 111–125. [Google Scholar]
- Narváez, J.; Acero, A.; Blanco, J. Variación morfométrica en poblaciones naturalizadas y domesticadas de la Tilapia del Nilo Oreochromis niloticus (Teleostei: Cichlidae) en el norte de Colombia. Rev. la Acad. Colomb. Cienc. 2005, 29, 383–394. [Google Scholar]
- Anfuso, G.; Rangel-Buitrago, N.; Correa, I. Evolution of four different sandy features along the Caribbean littoral of Colombia. In Sand and Gravel Spits Coastal Research Library; Randazzo, G., Jackson, D., Cooper, A., Eds.; Springer: New York, NY, USA, 2015; pp. 1–21. [Google Scholar]
- Corporación Autónoma Regional del Canal del Dique. Actualización de la Zonificación de Manglares en la Jurisdicción de CARDIQUE.; CARDIQUE: Cartagena de Indias, Colombia, 2007; pp. 1–148.
- Torregroza, E.; Gómez, A.; Borja, F. Aplicación del sistema de información geográfico quantum GIS en la regionalización ecológica de la cuenca ciénaga de la Virgen (Cartagena de Indias-Colombia). RITI J. 2014, 2, 1–13. [Google Scholar]
- Benfield, S.L.; Guzman, H.M.; Mair, J.M. Temporal mangrove dynamics in relation to coastal development in Pacific Panama. J. Environ. Manage. 2005, 76, 263–276. [Google Scholar] [CrossRef]
- Moor, R.; van Maren, M.; van Laarhoven, C. A controlled stable tidal inlet at Cartagena de Indias, Colombia. Terra Aqua 2002, 88, 3–14. [Google Scholar]
- Vélez, S. Estudio de caso: Concesión vial Cartagena-Barranquilla y Circunvalar de la Prosperidad. Análisis de política pública. Derecho y Econ. 2018, 49, 173–207. [Google Scholar]
- Autoridad Nacional de Licencias Ambientales. Resolución 1290 de 2015; ANLA: Bogotá, Colombia, 2015; pp. 1–171.
- Instituto Humboldt-Fundación Omacha. Caracterización Biológica y Ecológica de las Comunidades de Plantas Acuáticas, Plantas Terrrestres y Macroinvertebrados, y Caracterización Físico-Química de las Aguas de la Ventana de Estudio de la Ciénaga de la Virgen; Instituto Humboldt-Fundación Omacha: Bogotá, Colombia, 2015; pp. 1–109. [Google Scholar]
- Mira, J.D.; Urrego, L.E.; Monsalve, K. Determinantes naturales y antrópicos de la distribución, estructura y composición florística de los manglares de la Reserva Natural Sanguaré, Colombia. Rev. Biol. Trop. 2019, 67, 810–824. [Google Scholar] [CrossRef]
- Ball, M.C. Interactive effects of salinity and irradiance on growth: Implications for mangrove forest structure along salinity gradients. Trees-Struct. Funct. 2002, 16, 126–139. [Google Scholar] [CrossRef]
- Sobrado, M.A.; Ewe, S.M.L. Ecophysiological characteristics of Avicennia germinans and Laguncularia racemosa coexisting in a scrub mangrove forest at the Indian River Lagoon, Florida. Trees-Struct. Funct. 2006, 20, 679–687. [Google Scholar] [CrossRef]
- Mckee, K.; Kerrylee, R.; Saintilan, N. Response of salt marsh and mangrove wetlands to changes in atmospheric response of salt marsh and mangrove wetlands to changes in atmospheric CO2, climate, and sea level. In Global Change and the Function and Distribution of Wetlands; Middleton, B., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 63–96. [Google Scholar]
- Cardona, P.; Botero, L. Soil characteristics and vegetation structure in a heavily deteriorated mangrove forest in the Caribbean Coast of Colombia. Biotropica 1998, 30, 24–34. [Google Scholar] [CrossRef]
- Krauss, K.W.; Ball, M.C. On the halophytic nature of mangroves. Trees-Struct. Funct. 2013, 27, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Yan, Z.; You, S.; Zhang, Y.; Chen, L.; Lin, G. Mangroves: Obligate or facultative halophytes? A review. Trees-Struct. Funct. 2011, 25, 953–963. [Google Scholar] [CrossRef]
- Tovilla-Hernández, C.; Espino, G.; Orihuela-Belmonte, D. Impact of logging on a mangrove swamp in South Mexico: Cost/benefit. Rev. Biol. Trop. 2014, 49, 571–580. [Google Scholar]
- Blanco, J.F.; Estrada, E.A.; Ortiz, L.F.; Urrego, L.E. Ecosystem-Wide Impacts of Deforestation in Mangroves: The Urabá Gulf (Colombian Caribbean) Case Study. ISRN Ecol. 2012, 2012, 958709. [Google Scholar] [CrossRef] [Green Version]
- Walters, B.B. Ecological effects of small-scale cutting of Philippine mangrove forests. For. Ecol. Manage. 2005, 206, 331–348. [Google Scholar] [CrossRef]
- Sippo, J.Z.; Lovelock, C.E.; Santos, I.R.; Sanders, C.J.; Maher, D.T. Mangrove mortality in a changing climate: An overview. Estuar. Coast. Shelf Sci. 2018, 215, 241–249. [Google Scholar] [CrossRef]


| Location Area | Sensor and/or Document | Spatial Resolution (m)/or Scale | Year/Month/Day of Acquisition | Source | Cloud Cover (%) |
|---|---|---|---|---|---|
| Mallorquín Swamp | Landsat 5-OLI | 30 | 1985/01/24 | USGS | <5 |
| Landsat 5-OLI | 30 | 1998/05/20 | USGS | <5 | |
| Landsat 8-OLI | 30 | 2013/04/01 | USGS | <5 | |
| Landsat 8-OLI | 30 | 2018/12/05 | USGS | <10 | |
| Totumo Swamp | Landsat 5-OLI | 30 | 1985/01/24 | USGS | <5 |
| Landsat 5-OLI | 30 | 1998/09/20 | USGS | <5 | |
| Landsat 8-OLI | 30 | 2013/05/13 | USGS | <5 | |
| Landsat 8-OLI | 30 | 2018/12/05 | USGS | <5 | |
| La Virgen Swamp | Landsat 5-OLI | 30 | 1985/01/24 | USGS | <5 |
| Landsat 5-OLI | 30 | 1998/09/20 | USGS | <5 | |
| Landsat 8-OLI | 30 | 2013/05/13 | USGS | <5 | |
| Landsat 8-OLI | 30 | 2018/12/05 | USGS | <5 | |
| All areas | Unidentified | 4 | All years | Google Earth Pro | <5 |
| Lidar-Spot 1 | 1:50,000 | All years | DIMAR-CIOH | Unidentified | |
| Layer Colombia 2 Mangrove | 1:5,000,000 | 2005 | INVEMAR | Unidentified | |
| Mallorquín Swamp/Totumo Swamp | Spot-Aster 3 | 30 | 1986 | INVEMAR | Unidentified |
| 2004 | INVEMAR | Unidentified |
| Year | Mangrove Cover (ha) | ||
|---|---|---|---|
| Mallorquín | Totumo | La Virgen | |
| 1985 | 302 | 496 | 725 |
| 1998 | 304 | 215 | 685 |
| 2013 | 253 | 229 | 941 |
| 2018 | 287 | 195 | 910 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villate Daza, D.A.; Sánchez Moreno, H.; Portz, L.; Portantiolo Manzolli, R.; Bolívar-Anillo, H.J.; Anfuso, G. Mangrove Forests Evolution and Threats in the Caribbean Sea of Colombia. Water 2020, 12, 1113. https://doi.org/10.3390/w12041113
Villate Daza DA, Sánchez Moreno H, Portz L, Portantiolo Manzolli R, Bolívar-Anillo HJ, Anfuso G. Mangrove Forests Evolution and Threats in the Caribbean Sea of Colombia. Water. 2020; 12(4):1113. https://doi.org/10.3390/w12041113
Chicago/Turabian StyleVillate Daza, Diego Andrés, Hernando Sánchez Moreno, Luana Portz, Rogério Portantiolo Manzolli, Hernando José Bolívar-Anillo, and Giorgio Anfuso. 2020. "Mangrove Forests Evolution and Threats in the Caribbean Sea of Colombia" Water 12, no. 4: 1113. https://doi.org/10.3390/w12041113
APA StyleVillate Daza, D. A., Sánchez Moreno, H., Portz, L., Portantiolo Manzolli, R., Bolívar-Anillo, H. J., & Anfuso, G. (2020). Mangrove Forests Evolution and Threats in the Caribbean Sea of Colombia. Water, 12(4), 1113. https://doi.org/10.3390/w12041113








