Dynamics of Biocorrosion in Copper Pipes under Actual Drinking Water Conditions
Abstract
:1. Introduction
2. Materials and Methods
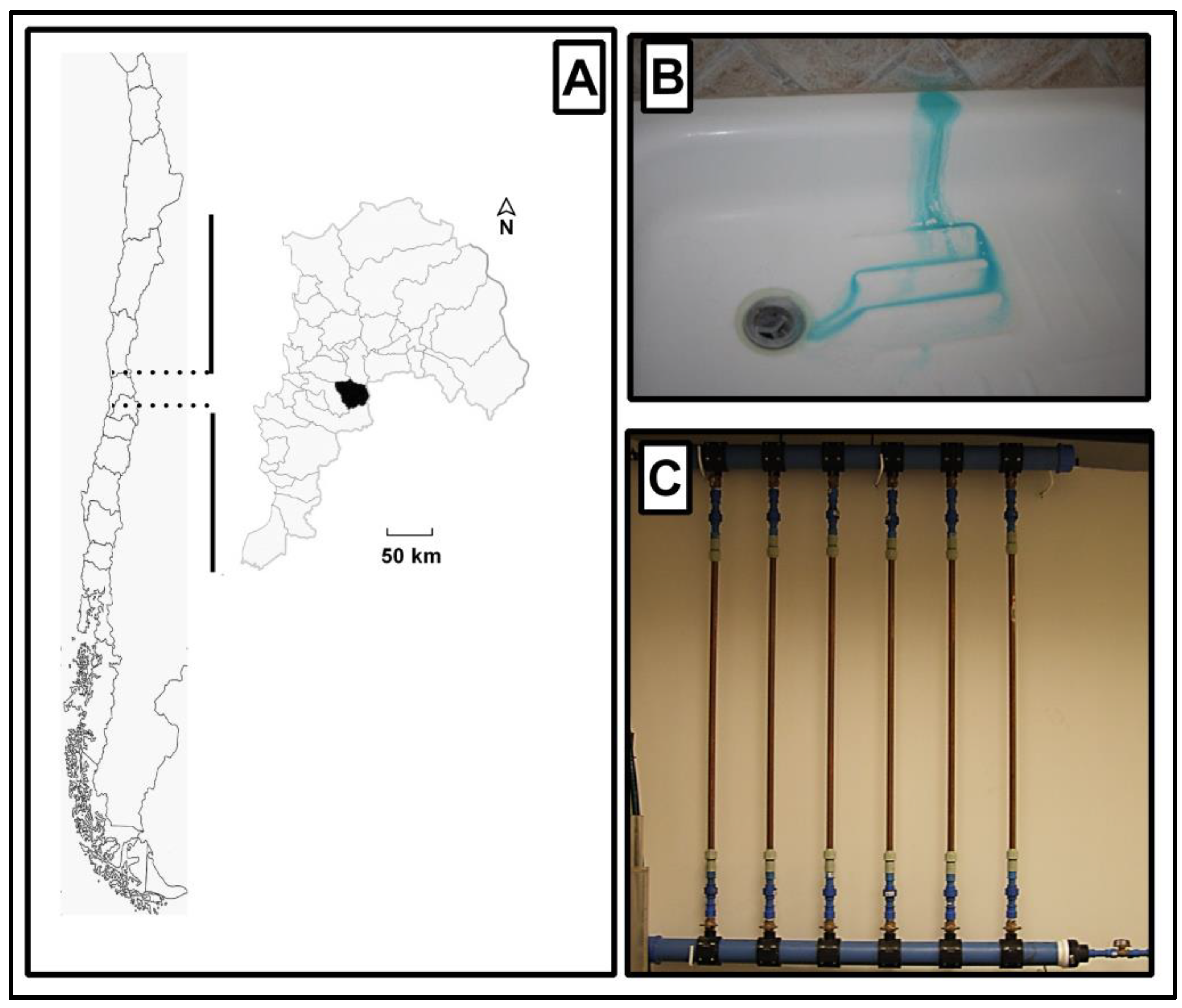
2.1. Sample Collection
2.2. Flushing Experiments
2.3. Water Analysis
2.4. DNA Extraction
2.5. Denaturing Gradient Gel Electrophoresis
2.6. Sequence Analysis
2.7. Scanning Electron Microscopy
2.8. Electrochemical Test
3. Results and Discussion
3.1. Water Quality
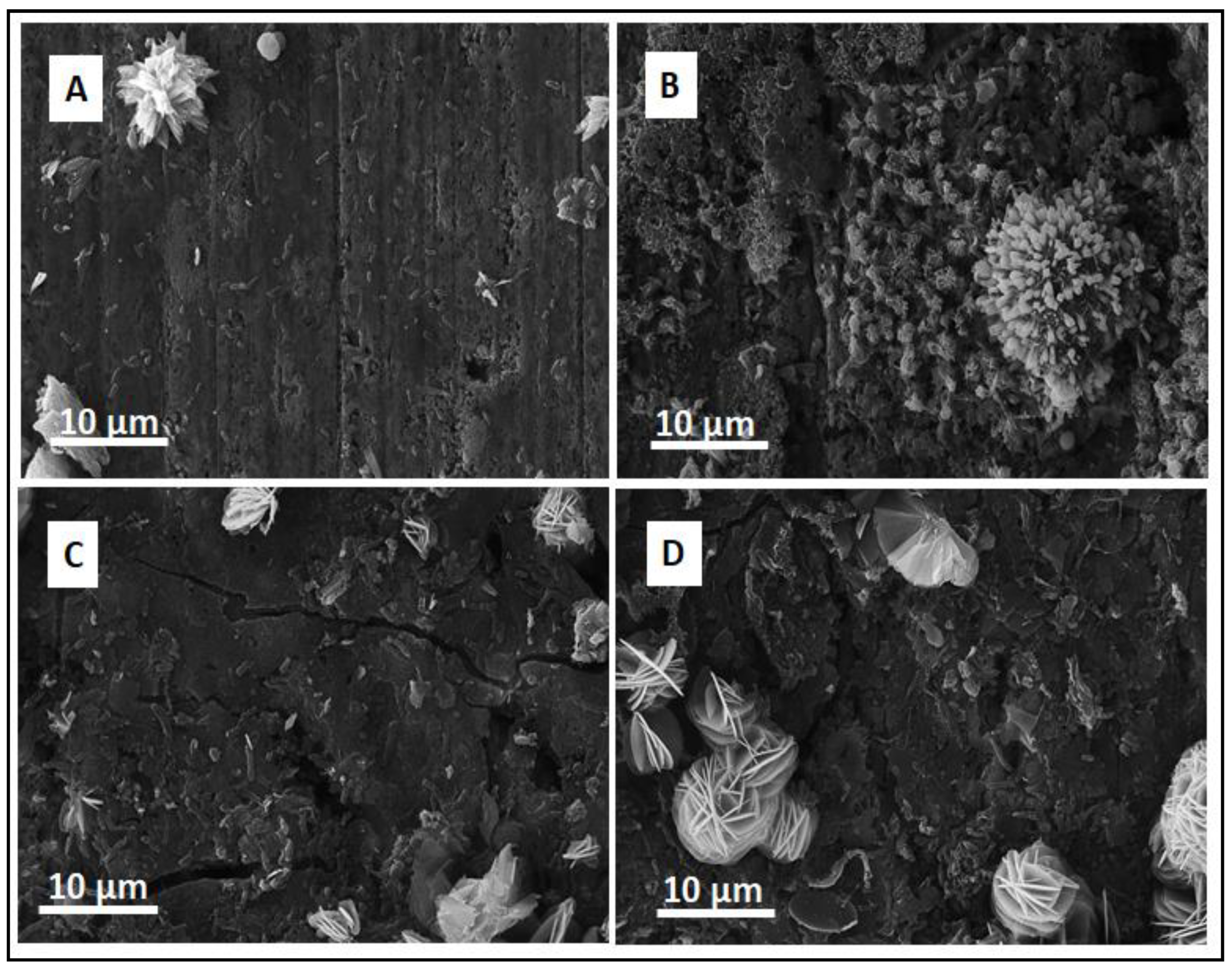
3.2. Scanning Electron Microscopy
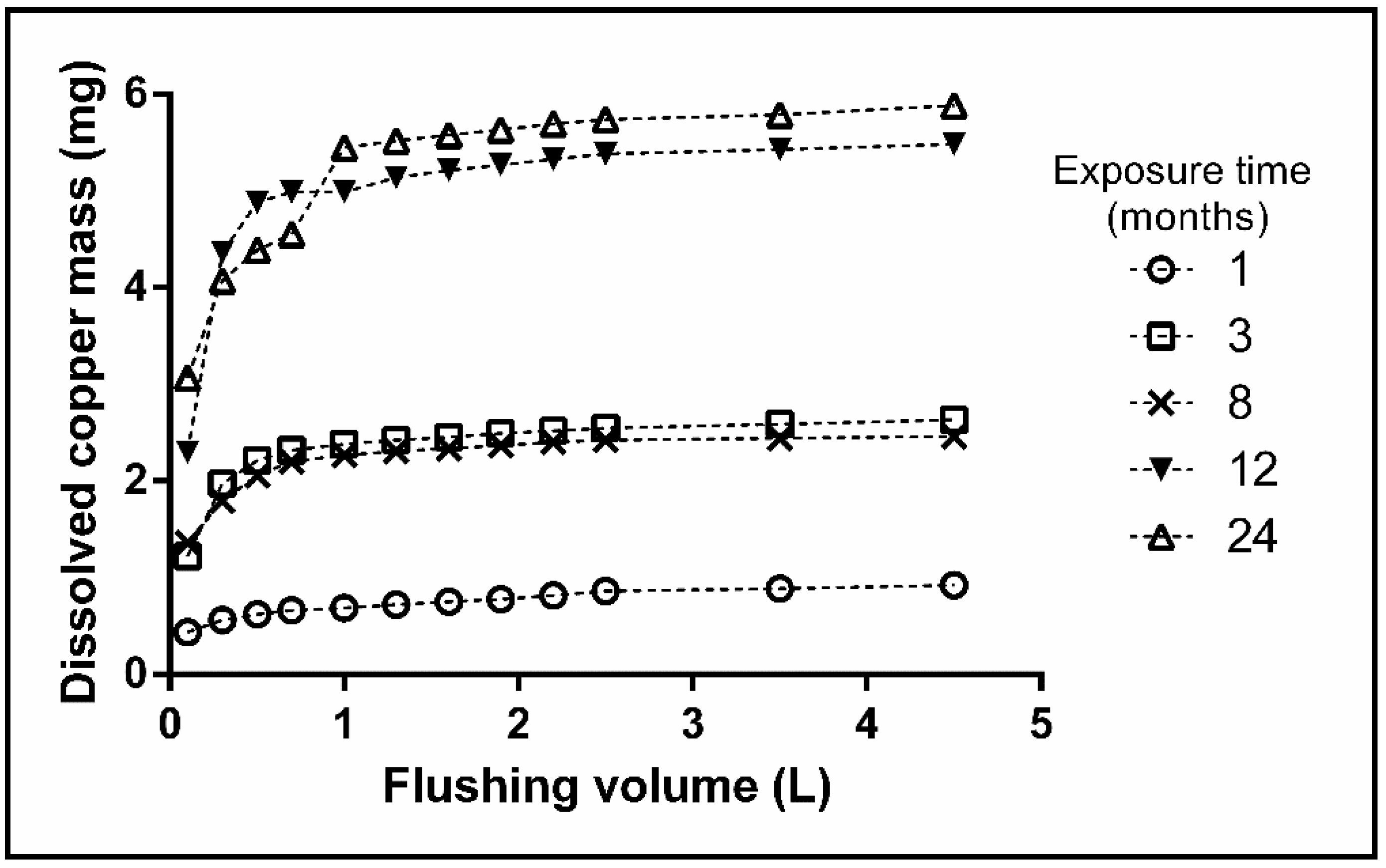
3.3. Flushing Experiments
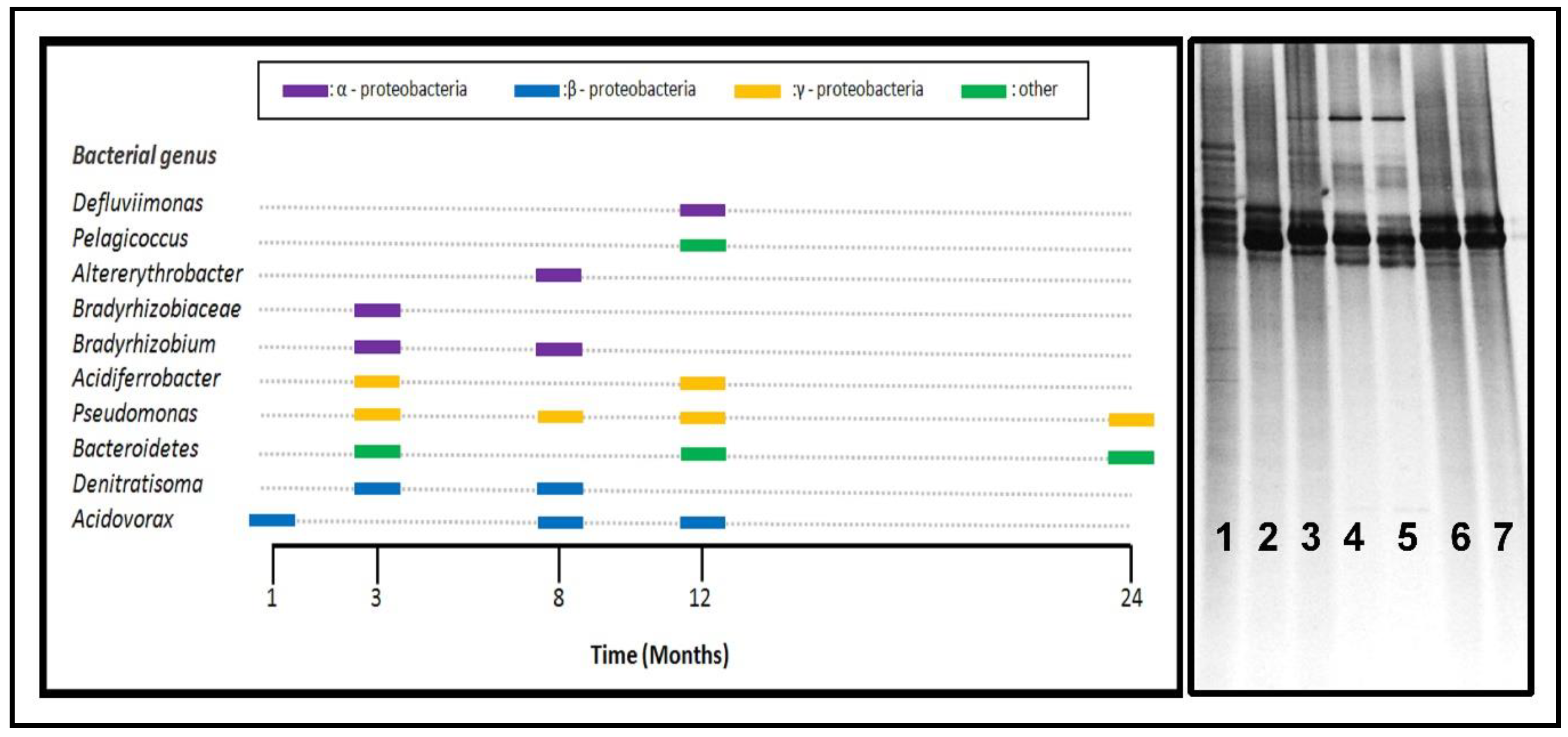
3.4. Microbial Community Analyses
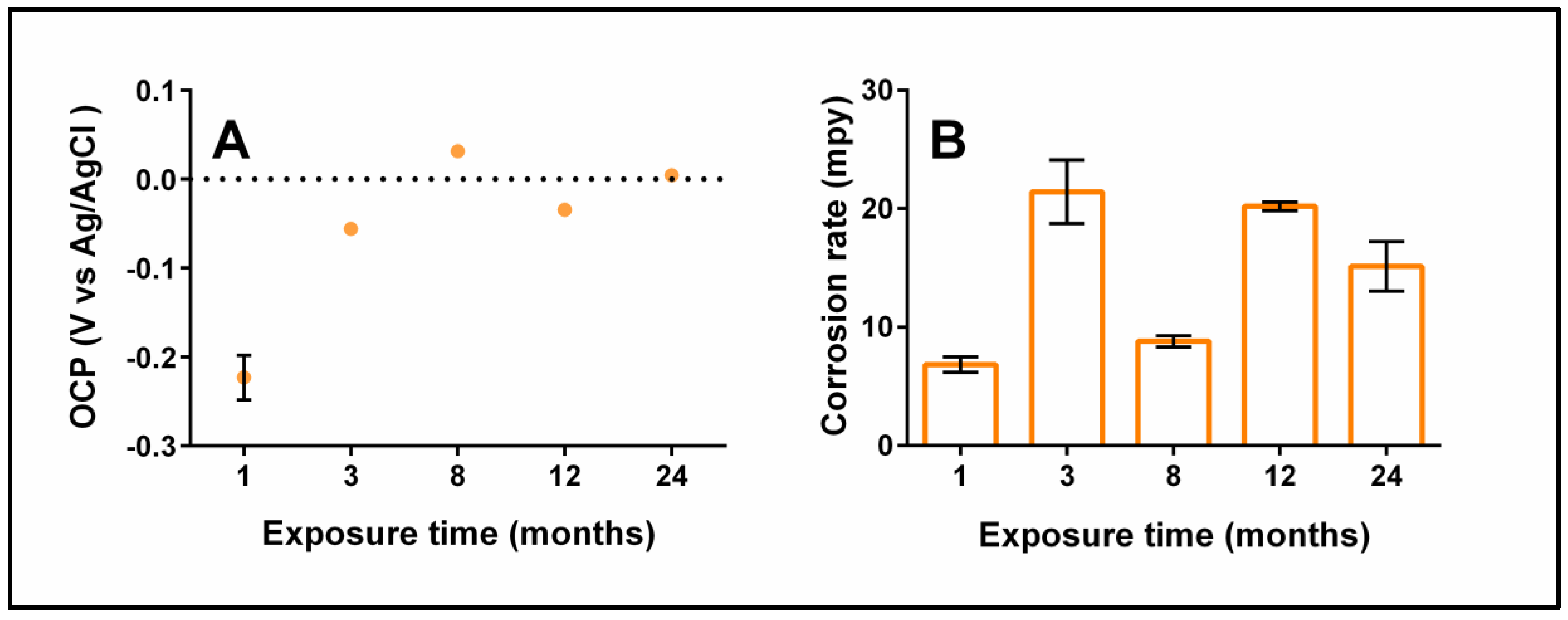
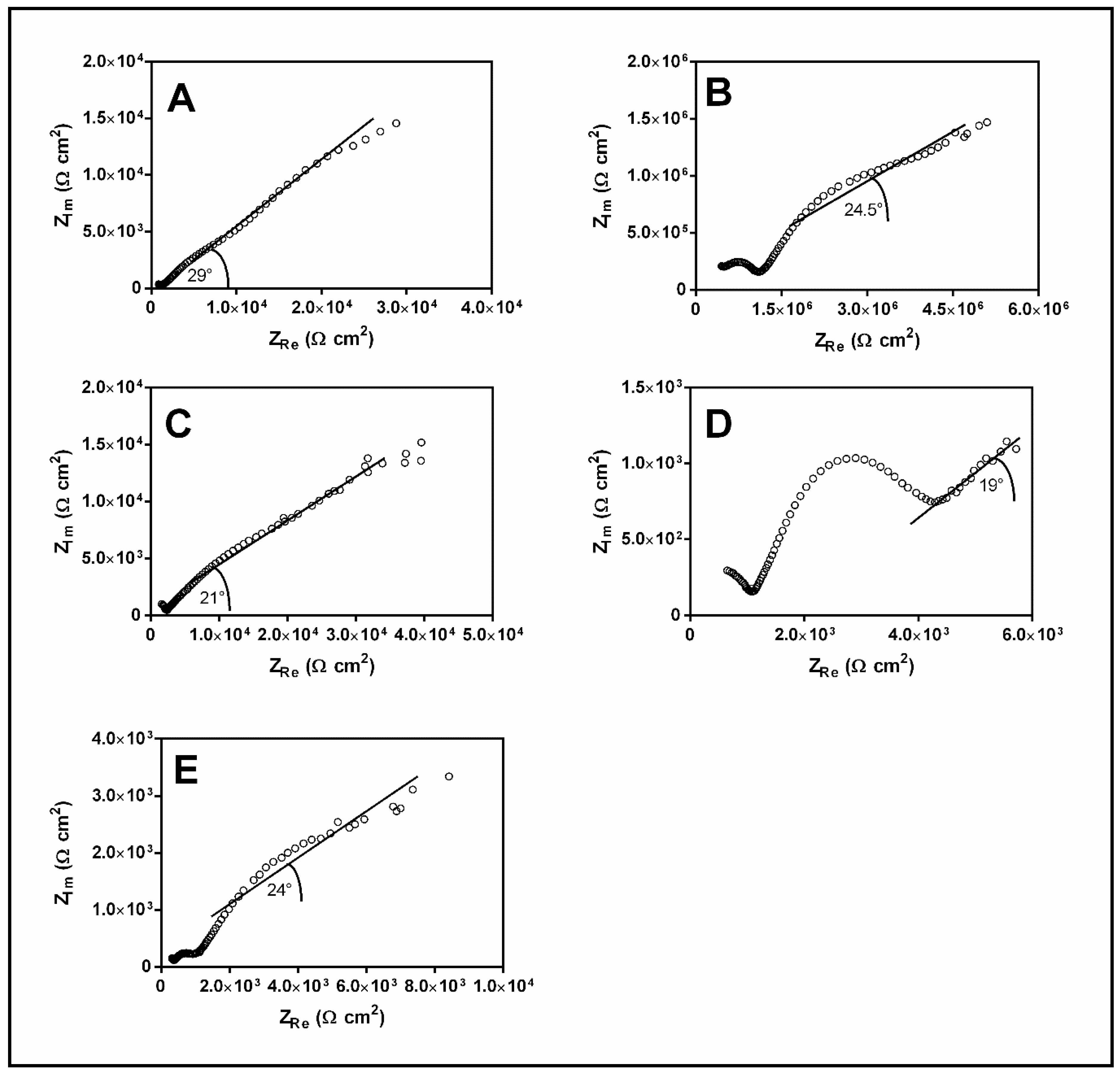
3.5. Electrochemical Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Douterelo, I.; Jackson, M.; Solomon, C.; Boxall, J. Microbial analysis of in situ biofilm formation in drinking water distribution systems: Implications for monitoring and control of drinking water quality. Appl. Microbiol. Biotechnol. 2016, 100, 3301–3311. [Google Scholar] [CrossRef] [Green Version]
- Douterelo, I.; Jackson, M.; Solomon, C.; Boxall, J. Spatial and temporal analogies in microbial communities in natural drinking water biofilms. Sci. Total Environ. 2017, 581, 277–288. [Google Scholar] [CrossRef]
- Kelly, J.J.; Minalt, N.; Culotti, A.; Pryor, M.; Packman, A. Temporal Variations in the Abundance and Composition of Biofilm Communities Colonizing Drinking Water Distribution Pipes. PLoS ONE 2014, 9, e98542. [Google Scholar] [CrossRef] [Green Version]
- Videla, H.A.; Herrera, L.K. Microbiologically influenced corrosion: Looking to the future. Int. Microbiol. 2005, 8, 169–180. [Google Scholar]
- Pavissich, J.P.; Vargas, I.T.; González, B.; Pastén, P.A.; Pizarro, G.E. Culture dependent and independent analyses of bacterial communities involved in copper plumbing corrosion. J. Appl. Microbiol. 2010, 109, 771–782. [Google Scholar] [CrossRef]
- Beech, I.B.; Sunner, J. Biocorrosion: Towards understanding interactions between biofilms and metals. Curr. Opin. Biotechnol. 2004, 15, 181–186. [Google Scholar] [CrossRef]
- Vargas, I.T.; Fischer, D.A.; Alsina, M.A.; Pavissich, J.P.; Pasten, P.; Pizarro, G.E. Copper Corrosion and Biocorrosion Events in Premise Plumbing. Materials 2017, 10, 1036. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, G.E. The effect of corrosion inhibitor on corrosion control of copper pipe and green water problem. Environ. Eng. Res. 2012, 17, 17–25. [Google Scholar] [CrossRef]
- Keevil, C.W. The physico-chemistry of biofilm-mediated pitting corrosion of copper pipe supplying potable water. Water Sci. Technol. 2004, 49, 91–98. [Google Scholar] [CrossRef]
- Luo, J.; Hein, C.; Mücklich, F.; Solioz, M. Killing of bacteria by copper, cadmium, and silver surfaces reveals relevant physicochemical parameters. Biointerphases 2017, 12, 20301. [Google Scholar] [CrossRef] [Green Version]
- Reyes, A.; Letelier, M.V.; De la Iglesia, R.; Gonzalez, B.; Lagos, G. Microbiologically induced corrosion of copper pipes in low-pH water. Int. Biodeterior. Biodegrad. 2008, 61, 135–141. [Google Scholar] [CrossRef]
- Lehtola, M.J.; Miettinen, I.T.; Keinänen, M.M.; Kekki, T.K.; Laine, O.; Hirvonen, A.; Vartiainen, T.; Martikainen, P.J. Microbiology, chemistry and biofilm development in a pilot drinking water distribution system with copper and plastic pipes. Water Res. 2004, 38, 3769–3779. [Google Scholar] [CrossRef]
- Liu, S.; Gunawan, C.; Barraud, N.; Rice, S.A.; Harry, E.J.; Amal, R. Understanding, Monitoring, and Controlling Biofilm Growth in Drinking Water Distribution Systems. Environ. Sci. Technol. 2016, 50, 8954–8976. [Google Scholar] [CrossRef]
- Beech, I.B. Corrosion of technical materials in the presence of biofilms—Current understanding and state-of-the art methods of study. Int. Biodeterior. Biodegrad. 2004, 53, 177–183. [Google Scholar] [CrossRef]
- Kip, N.; A van Veen, J. The dual role of microbes in corrosion. ISME J. 2015, 9, 542–551. [Google Scholar] [CrossRef]
- Bremer, P.J.; Webster, B.J.; Brett Wells, D. Biocorrosion of copper in potable water. J. Am. Water Work. Assoc. 2001, 93, 82–91. [Google Scholar] [CrossRef]
- Videla, H.A.; Herrera, L.K. Understanding microbial inhibition of corrosion. A comprehensive overview. Int. Biodeterior. Biodegrad. 2009, 63, 896–900. [Google Scholar] [CrossRef]
- Vargas, I.T.; Pavissich, J.P.; Olivares, T.E.; Jeria, G.A.; Cienfuegos, R.A.; Pastén, P.A.; Pizarro, G.E. Increase of the concentration of dissolved copper in drinking water systems due to flow-induced nanoparticle release from surface corrosion by-products. Corros. Sci. 2010, 52, 3492–3503. [Google Scholar] [CrossRef]
- Stern, B.R.; Solioz, M.; Krewski, D.; Aggett, P.; Aw, T.-C.; Baker, S.; Crump, K.; Dourson, M.; Haber, L.; Hertzberg, R. Copper and human health: Biochemistry, genetics, and strategies for modeling dose-response relationships. J. Toxicol. Environ. Health Part B 2007, 10, 157–222. [Google Scholar] [CrossRef]
- Zietz, B. Copper concentrations in tap water and possible effects on infant’s health—Results of a study in Lower Saxony, Germany. Environ. Res. 2003, 92, 129–138. [Google Scholar] [CrossRef]
- Mohod, C.V.; Dhote, J. Review of heavy metals in drinking water and their effect on human health. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 2992–2996. [Google Scholar]
- Ehi-Eromosele, C.; Okiei, W. Heavy Metal Assessment of Ground, Surface and Tap Water Samples in Lagos Metropolis Using Anodic Stripping Voltammetry. Resour. Environ. 2012, 2, 82–86. [Google Scholar]
- Gorchev, H.G.; Ozolins, G. WHO guidelines for drinking-water quality. WHO Chron. 2011, 38, 104–108. [Google Scholar]
- Edwards, M.; Jacobs, S.; Taylor, R.J. The blue water phenomenon. J. Am. Water Work. Assoc. 2000, 92, 72–82. [Google Scholar] [CrossRef]
- Vargas, I.T.; Alsina, M.A.; Pavissich, J.P.; Jeria, G.A.; Pastén, P.A.; Walczak, M.; Pizarro, G.E. Multi-technique approach to assess the effects of microbial biofilms involved in copper plumbing corrosion. Bioelectrochemistry 2014, 97, 15–22. [Google Scholar] [CrossRef]
- Calle, G.R.; Vargas, I.T.; Alsina, M.A.; Pastén, P.A.; Pizarro, G.E. Enhanced copper release from pipes by alternating stagnation and flow events. Environ. Sci. Technol. 2007, 41, 7430–7436. [Google Scholar] [CrossRef]
- Dunne, W.M. Bacterial Adhesion: Seen Any Good Biofilms Lately? Clin. Microbiol. Rev. 2002, 15, 155–166. [Google Scholar] [CrossRef] [Green Version]
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial biofilms: Development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb. Perspect. Med. 2013, 3, a010306. [Google Scholar] [CrossRef] [Green Version]
- Landoulsi, J.; El Kirat, K.; Richard, C.; Féron, D.; Pulvin, S. Enzymatic Approach in Microbial-Influenced Corrosion: A Review Based on Stainless Steels in Natural Waters. Environ. Sci. Technol. 2008, 42, 2233–2242. [Google Scholar] [CrossRef]
- Olivares, T.E.; Cienfuegos, R.; Vargas, I.T.; Pizarro, G.E. Experimental evidence for enhanced copper release from domestic copper plumbing under hydrodynamic control. Corros. Sci. 2014, 80, 473–481. [Google Scholar] [CrossRef]
- Rojas, C.; Vargas, I.T.; Bruns, M.A.; Regan, J.M. Electrochemically active microorganisms from an acid mine drainage-affected site promote cathode oxidation in microbial fuel cells. Bioelectrochemistry 2017, 118, 139–146. [Google Scholar] [CrossRef]
- Muyzer, G.; Smalla, K. Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie Van Leeuwenhoek 1998, 73, 127–141. [Google Scholar] [CrossRef]
- Diéz, B.D.; Pedrós-Alió, C.; Marsh, T.L.; Massana, R. Application of Denaturing Gradient Gel Electrophoresis (DGGE) To Study the Diversity of Marine Picoeukaryotic Assemblages and Comparison of DGGE with Other Molecular Techniques. Appl. Environ. Microbiol. 2001, 67, 2942–2951. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Teo, W.K.; Siow, K.S.; Tan, K.L.; Hsieh, A.K. The corrosion behaviour of copper in neutral tap water. Part I: Corrosion mechanisms. Corros. Sci. 1996, 38, 369–385. [Google Scholar] [CrossRef]
- Jungfer, C.; Friedrich, F.; Varela Villarreal, J.; Brändle, K.; Gross, H.-J.; Obst, U.; Schwartz, T. Drinking water biofilms on copper and stainless steel exhibit specific molecular responses towards different disinfection regimes at waterworks. Biofouling 2013, 29, 891–907. [Google Scholar] [CrossRef]
- Yu, D.; Tian, J.; Dai, J.; Wang, X.; Birnhack, L.; Lahav, O.; Rios, E.C.; Zimer, A.M.; Pereira, E.C.; Mascaro, L.H.; et al. Effect of pH and chloride on the micro-mechanism of pitting corrosion for high strength pipeline steel in aerated NaCl solutions. Water Res. 2015, 124, 22–31. [Google Scholar]
- Lin, W.; Yu, Z.; Chen, X.; Liu, R.; Zhang, H. Molecular characterization of natural biofilms from household taps with different materials: PVC, stainless steel, and cast iron in drinking water distribution system. Appl. Microbiol. Biotechnol. 2012, 97, 8393–8401. [Google Scholar] [CrossRef]
- Hwang, C.; Ling, F.; Andersen, G.L.; LeChevallier, M.W.; Liu, W.T. Microbial community dynamics of an urban drinking water distribution system subjected to phases of chloramination and chlorination treatments. Appl. Environ. Microbiol. 2012, 78, 7856–7865. [Google Scholar] [CrossRef] [Green Version]
- Serrano, J.; Leiva, E. Removal of Arsenic Using Acid/Metal-Tolerant Sulfate Reducing Bacteria: A New Approach for Bioremediation of High-Arsenic Acid Mine Waters. Water 2017, 9, 994. [Google Scholar] [CrossRef] [Green Version]
- Ling, F.; Hwang, C.; LeChevallier, M.W.; Andersen, G.L.; Liu, W.-T. Core-satellite populations and seasonality of water meter biofilms in a metropolitan drinking water distribution system. ISME J. 2016, 10, 582–595. [Google Scholar] [CrossRef] [Green Version]
- Qian, J.; Horn, H.; Tarchitzky, J.; Chen, Y.; Katz, S.; Wagner, M. Water quality and daily temperature cycle affect biofilm formation in drip irrigation devices revealed by optical coherence tomography. Biofouling 2017, 33, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Galarce, C.; Pineda, F.; Fischer, D.A.; Flores, M.; Vargas, I.T.; Sancy, M.; Pizarro, G.E. Effect of hazardous bacteria isolated from copper plumbing system on microbiologically influenced corrosion of copper. Int. J. Electrochem. Sci. 2019, 14, 2305–2320. [Google Scholar] [CrossRef]
- Edwards, M.; Powers, K.; Hidmi, L.; Schock, M.R. The role of pipe ageing in copper corrosion by-product release. Water Sci. Technol. Water Supply 2001, 1, 25–32. [Google Scholar] [CrossRef]
- Webster, B.J.; Werner, S.E.; Wells, D.B.; Bremer, P.J. Microbiologically Influenced Corrosion of Copper in Potable Water Systems—pH Effects. Corrosion 2000, 56, 942–950. [Google Scholar] [CrossRef]
- Feng, Y.; Teo, W.-K.; Siow, K.-S.; Hsieh, A.-K. The corrosion behaviour of copper in neutral tap water. Part II: Determination of corrosion rates. Corros. Sci. 1996, 38, 387–395. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (EPA). National Primary Drinking Water Regulations; (EPA 816-F-09-004); Office of Ground Water and Drinking Water: Washington, DC, USA, 2009; Volume 1, p. 7.
- Lautenschlager, K.; Boon, N.; Wang, Y.; Egli, T.; Hammes, F. Overnight stagnation of drinking water in household taps induces microbial growth and changes in community composition. Water Res. 2010, 44, 4868–4877. [Google Scholar] [CrossRef]
- Gomez-Alvarez, V.; Revetta, R.P.; Domingo, J.W.S. Metagenomic analyses of drinking water receiving different disinfection treatments. Appl. Environ. Microbiol. 2012, 78, 6095–6102. [Google Scholar] [CrossRef] [Green Version]
- Revetta, R.P.; Pemberton, A.; Lamendella, R.; Iker, B.; Santo Domingo, J.W. Identification of bacterial populations in drinking water using 16S rRNA-based sequence analyses. Water Res. 2010, 44, 1353–1360. [Google Scholar] [CrossRef]
- Buse, H.Y.; Lu, J.; Lu, X.; Mou, X.; Ashbolt, N.J. Microbial diversities (16S and 18S rRNA gene pyrosequencing) and environmental pathogens within drinking water biofilms grown on the common premise plumbing materials unplasticized polyvinylchloride and copper. FEMS Microbiol. Ecol. 2014, 88, 280–295. [Google Scholar] [CrossRef]
- Lu, J.; Buse, H.Y.; Gomez-Alvarez, V.; Struewing, I.; Santo Domingo, J.; Ashbolt, N.J. Impact of drinking water conditions and copper materials on downstream biofilm microbial communities and Legionella pneumophila colonization. J. Appl. Microbiol. 2014, 117, 905–918. [Google Scholar] [CrossRef]
- Gomez-Alvarez, V.; Humrighouse, B.W.; Revetta, R.P.; Santo Domingo, J.W. Bacterial composition in a metropolitan drinking water distribution system utilizing different source waters. J. Water Health 2015, 13, 140–151. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Zhang, Y.; Knibbe, W.-J.; Feng, C.; Liu, W.; Medema, G.; van der Meer, W. Potential impacts of changing supply-water quality on drinking water distribution: A review. Water Res. 2017, 116, 135–148. [Google Scholar] [CrossRef]
- Baeza, S.; Vejar, N.; Gulppi, M.; Azocar, M.; Melo, F.; Monsalve, A.; Pérez-Donoso, J.; Váquez, C.C.; Pavez, J.; Zagal, J.H.; et al. New Evidence on the Role of Catalase in Escherichia coli Mediated Biocorrosion. Corros. Sci. 2013, 67, 32–41. [Google Scholar] [CrossRef]
- Hallberg, K.B.; Hedrich, S.; Johnson, D.B. Acidiferrobacter thiooxydans, gen. nov. sp. nov.; an acidophilic, thermo-tolerant, facultatively anaerobic iron- and sulfur-oxidizer of the family Ectothiorhodospiraceae. Extremophiles 2011, 15, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Busalmen, J.P.; Vázquez, M.; De Sánchez, S.R. New evidences on the catalase mechanism of microbial corrosion. Electrochim. Acta 2002, 47, 1857–1865. [Google Scholar] [CrossRef]
- Nercessian, D.; Duville, F.B.; Desimone, M.; Simison, S.; Busalmen, J.P. Metabolic turnover and catalase activity of biofilms of Pseudomonas fluorescens (ATCC 17552) as related to copper corrosion. Water Res. 2010, 44, 2592–2600. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Wang, W.; Liu, Y.; Liu, S.; Lou, L.; Cheng, D.; He, X.; Zhou, X.; Qiu, S.; Fu, L.; et al. Pyrosequencing analysis of bacterial communities in biofilms from different pipe materials in a city drinking water distribution system of East China. Appl. Microbiol. Biotechnol. 2015, 24, 10713–10724. [Google Scholar] [CrossRef]
- Revetta, R.P.; Gomez-Alvarez, V.; Gerke, T.L.; Curioso, C.; Santo Domingo, J.W.; Ashbolt, N.J. Establishment and early succession of bacterial communities in monochloramine-treated drinking water biofilms. FEMS Microbiol. Ecol. 2013, 86, 404–414. [Google Scholar] [CrossRef] [Green Version]
- Cha, J.; Cooksey, D. Copper resistance in Pseudomonas syringae mediated by periplasmic and outer membrane proteins. Proc. Natl. Acad. Sci. USA 1991, 88, 8915–8919. [Google Scholar] [CrossRef] [Green Version]
- Fahrbach, M.; Kuever, J.; Meinke, R.; Kämpfer, P.; Hollender, J. Denitratisoma oestradiolicum gen. nov., sp. nov., a 17beta-oestradiol-degrading, denitrifying betaproteobacterium. Int. J. Syst. Evol. Microbiol. 2006, 56, 1547–1552. [Google Scholar] [CrossRef] [Green Version]
- Foesel, B.U.; Drake, H.L.; Schramm, A. Defluviimonas denitrificans gen. nov., sp. nov., and Pararhodobacter aggregans gen. nov., sp. nov., non-phototrophic Rhodobacteraceae from the biofilter of a marine aquaculture. Syst. Appl. Microbiol. 2011, 34, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Giongo, A.; Ambrosini, A.; Vargas, L.K.; Freire, J.R.J.; Bodanese-Zanettini, M.H.; Passaglia, L.M.P. Evaluation of genetic diversity of bradyrhizobia strains nodulating soybean [Glycine max (L.) Merrill] isolated from South Brazilian fields. Appl. Soil Ecol. 2008, 38, 261–269. [Google Scholar] [CrossRef]
- Marcondes de Souza, J.A.; Carareto Alves, L.M.; de Mello Varani, A.; de Macedo Lemos, E.G. The Family Bradyrhizobiaceae. In The Prokaryotes; Springer: Berlin/Heidelberg, Germany, 2014; pp. 135–154. [Google Scholar]
- White, C.; Tancos, M.; Lytle, D.A. Microbial community profile of a lead service line removed from a drinking water distribution system. Appl. Environ. Microbiol. 2011, 77, 5557–5561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, S. Microbial extracellular electron transfer and its relevance to iron corrosion. Microb. Biotechnol. 2016, 9, 141–148. [Google Scholar] [CrossRef]
- Melchers, R.E. Bi-modal trends in the long-term corrosion of copper and high copper alloys. Corros. Sci. 2015, 95, 51–61. [Google Scholar] [CrossRef]
- Melchers, R.E. Long-term immersion corrosion of steels in seawaters with elevated nutrient concentration. Corros. Sci. 2014, 81, 110–116. [Google Scholar] [CrossRef]
- Kahlisch, L.; Henne, K.; Groebe, L.; Draheim, J.; Höfle, M.G.; Brettar, I. Molecular analysis of the bacterial drinking water community with respect to live/dead status. Water Sci. Technol. 2010, 61, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Noya, Y.E.; Suárez-Arriaga, M.C.; Rojas-Valdes, A.; Montoya-Ciriaco, N.M.; Gómez-Acata, S.; Fernández-Luqueño, F.; Dendooven, L. Pyrosequencing Analysis of the Bacterial Community in Drinking Water Wells. Microb. Ecol. 2013, 66, 19–29. [Google Scholar] [CrossRef]
- Cao, S.; Du, R.; Li, B.; Ren, N.; Peng, Y. High-throughput profiling of microbial community structures in an ANAMMOX-UASB reactor treating high-strength wastewater. Appl. Microbiol. Biotechnol. 2016, 100, 6457–6467. [Google Scholar] [CrossRef]
- Fallowfield, H.; Critchley, M.; Bolton, N.; Cromar, N. Microbiologically influenced corrosion of copper and galvanised steel pipe. In Proceedings of the Australasian Corrosion Association Inc, Symposium on Microbial Influenced Corrosion, Melbourne, Australia, 10–11 August 2011. [Google Scholar]
- Bellenberg, S.; Díaz, M.; Noël, N.; Sand, W.; Poetsch, A.; Guiliani, N.; Vera, M. Biofilm formation, communication and interactions of leaching bacteria during colonization of pyrite and sulfur surfaces. Res. Microbiol. 2014, 165, 773–781. [Google Scholar] [CrossRef]
- Douterelo, I.; Boxall, J.B.; Deines, P.; Sekar, R.; Fish, K.E.; Biggs, C.A. Methodological approaches for studying the microbial ecology of drinking water distribution systems. Water Res. 2014, 65, 134–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douterelo, I.; Sharpe, R.L.; Boxall, J.B. Influence of hydraulic regimes on bacterial community structure and composition in an experimental drinking water distribution system. Water Res. 2013, 47, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Fish, K.E.; Collins, R.; Green, N.H.; Sharpe, R.L.; Douterelo, I.; Osborn, A.M.; Boxall, J.B. Characterisation of the physical composition and microbial community structure of biofilms within a model full-scale drinking water distribution system. PLoS ONE 2015, 10, e0115824. [Google Scholar] [CrossRef] [PubMed]
- Martiny, A.C.; Jørgensen, T.M.; Albrechtsen, H.-J.; Arvin, E.; Molin, S. Long-term succession of structure and diversity of a biofilm formed in a model drinking water distribution system. Appl. Environ. Microbiol. 2003, 69, 6899–6907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dexter, S.C.; Duquette, D.J.; Siebert, O.W.; Videla, H.A. Use and limitations of electrochemical techniques for investigating microbiological corrosion. Corrosion 1991, 47, 308–318. [Google Scholar] [CrossRef]
- Cornwell, F.J.; Wildsmith, G.; Gilbert, P.T. Pitting Corrosion in Copper Tubes in Cold Water Service. Br. Corros. J. 1973, 8, 202–209. [Google Scholar] [CrossRef]
- Frateur, I.; Deslouis, C.; Orazem, M.E.; Tribollet, B. Modeling of the cast iron/drinking water system by electrochemical impedance spectroscopy. Electrochim. Acta 1999, 44, 4345–4356. [Google Scholar] [CrossRef]
| Parameter | Simulated Tap Water Concentration |
|---|---|
| Chloride | 11.4 mg/L |
| Sulfate () | 90 mg/L |
| 98 mg/L | |
| pH | 7.5 |
| Conductivity | 680 µS/cm |
| Temperature | 23 °C |
| Exposure Time (Month)/Parameter (Unit) | 1 | 3 | 8 | 12 | 24 |
|---|---|---|---|---|---|
| Fluoride (mg/L) | 0.019 | 0.035 | 0.152 | 0.035 | -- 1 |
| Chloride (mg/L) | 21.53 | 21.45 | 23.94 | 3.23 | 19.43 |
| Alkalinity (mg/L as CaCO3) | 200 | 193 | 219 | 144.6 | 51.47 |
| Total hardness (mg/L as CaCO3) | 145.7 | 142.29 | 139.2 | 150.2 | 147.74 |
| Sulfate (mg/L) | 155.2 | 155 | 163.6 | 34 | 149.46 |
| Nitrite (mg/L) | 0.016 | 0.009 | 0.015 | 0 | -- 1 |
| Nitrate (mg/L) | 10.22 | 11.1 | 9.44 | 1.32 | 10.07 |
| Phosphate (mg/L) | 0.71 | 0.3 | 0.36 | 0.17 | 0.41 |
| TOC (mg/L) | 2.1 | 1.67 | 3.04 | 0.57 | 0.35 |
| DO (mg/L) | 8.1 | 8 | 8.2 | 8.8 | 8.86 |
| pH | 6.84 | 7 | 6.96 | 7 | 6.93 |
| Conductivity | 704 | 673 | 708 | 736 | 629 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galarce, C.; Fischer, D.; Díez, B.; Vargas, I.T.; Pizarro, G.E. Dynamics of Biocorrosion in Copper Pipes under Actual Drinking Water Conditions. Water 2020, 12, 1036. https://doi.org/10.3390/w12041036
Galarce C, Fischer D, Díez B, Vargas IT, Pizarro GE. Dynamics of Biocorrosion in Copper Pipes under Actual Drinking Water Conditions. Water. 2020; 12(4):1036. https://doi.org/10.3390/w12041036
Chicago/Turabian StyleGalarce, Carlos, Diego Fischer, Beatriz Díez, Ignacio T. Vargas, and Gonzalo E. Pizarro. 2020. "Dynamics of Biocorrosion in Copper Pipes under Actual Drinking Water Conditions" Water 12, no. 4: 1036. https://doi.org/10.3390/w12041036
APA StyleGalarce, C., Fischer, D., Díez, B., Vargas, I. T., & Pizarro, G. E. (2020). Dynamics of Biocorrosion in Copper Pipes under Actual Drinking Water Conditions. Water, 12(4), 1036. https://doi.org/10.3390/w12041036





