Reactive Barriers for Renaturalization of Reclaimed Water during Soil Aquifer Treatment
Abstract
:1. Introduction
2. Materials and Methods
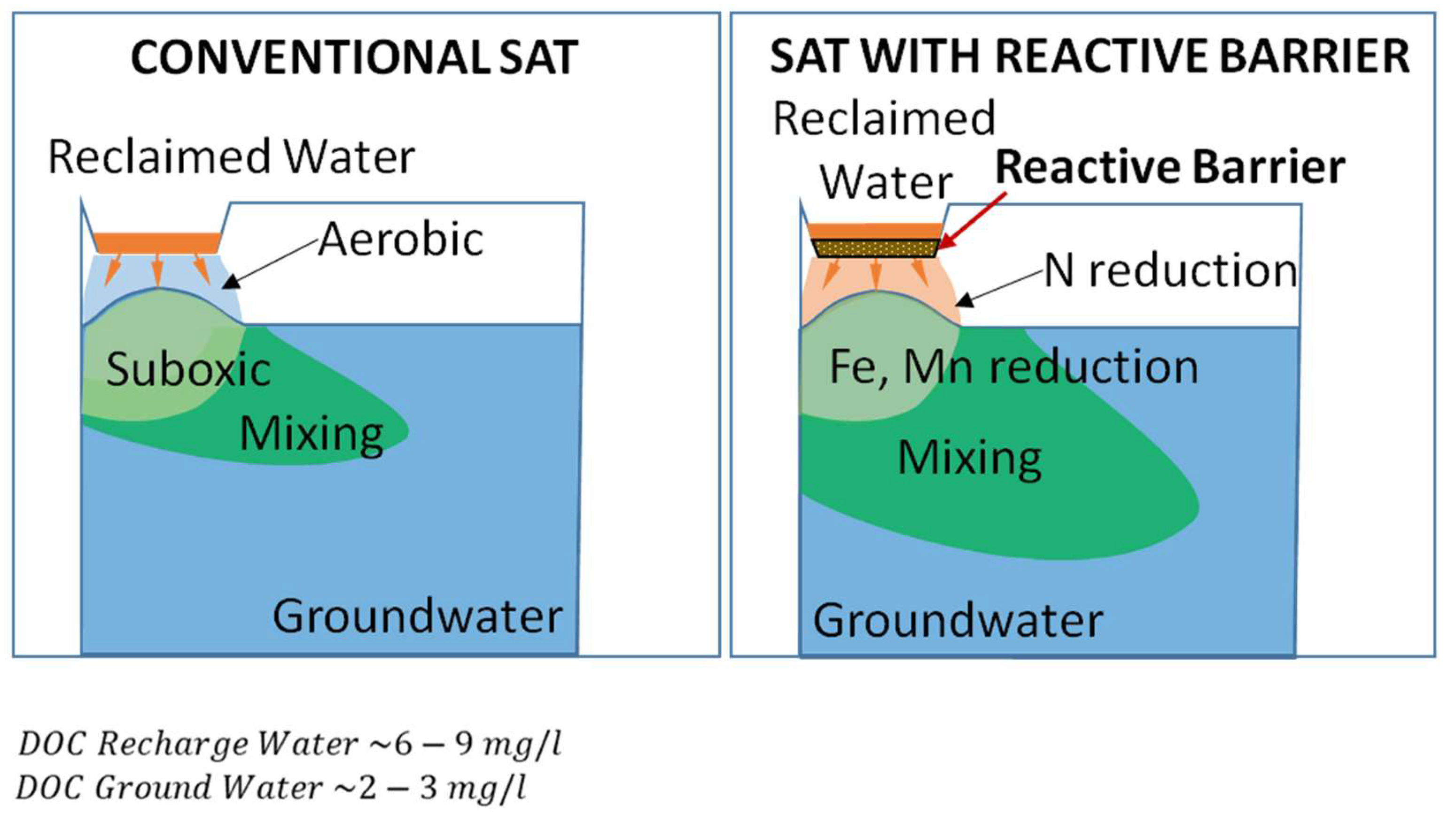
2.1. The Concept of the Reactive Barrier
2.2. Site Description
2.2.1. Sant Vicenç Dels Horts
2.2.2. Palamós Site
2.3. Analytical Methods
2.4. Assesing the Reactive Barrier Efficiency
3. Results and Discussion
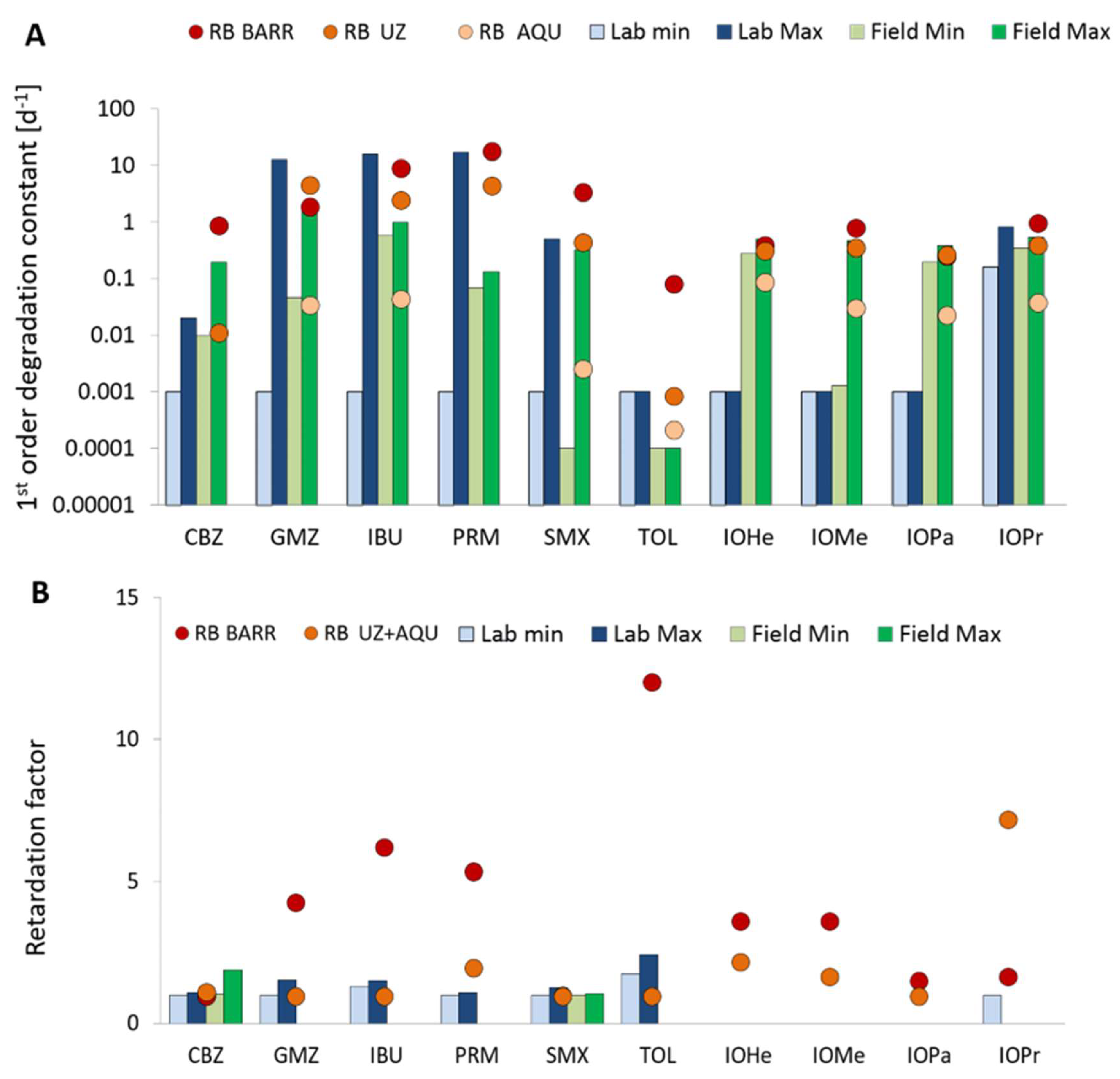
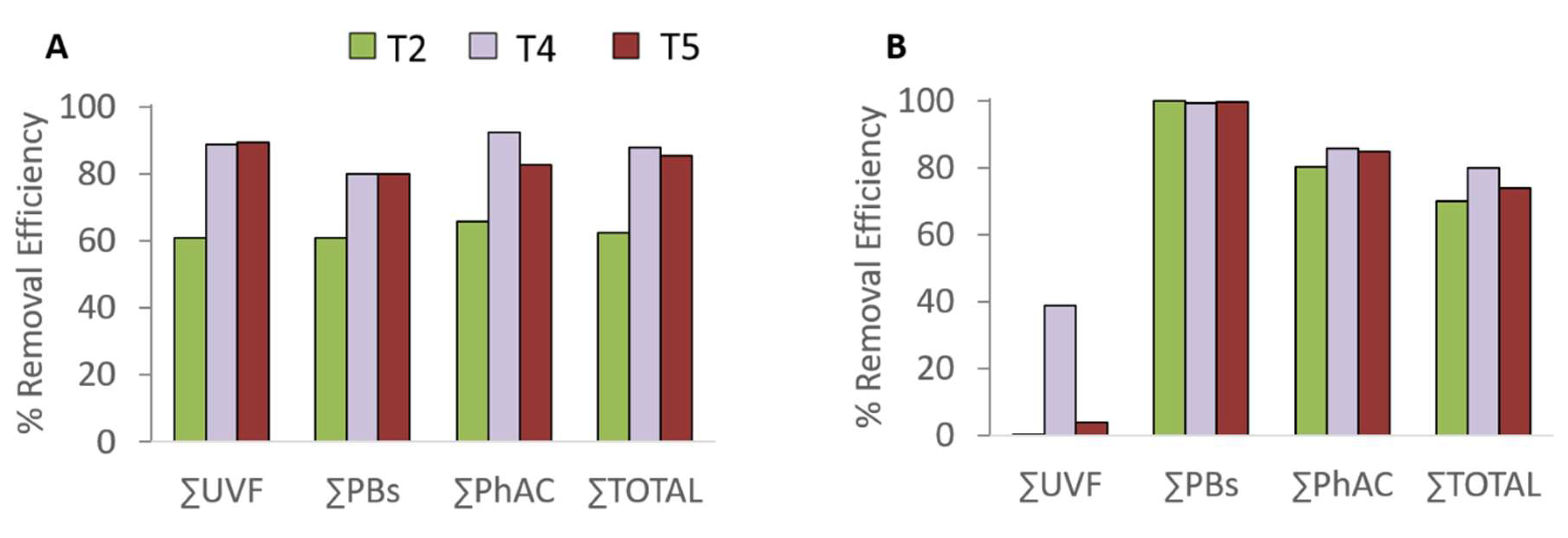
3.1. CECs Behavior
3.2. Pathogen Removal
3.3. Antibiotic-Resistant Bacteria (ARB) and Antibiotic-Resistant Genes (ARGs)
3.4. Public Acceptance of MAR
- MAR (re)naturalizes water in that water quality improvement processes make it hard to distinguish from natural water;
- Infiltration basins are beautiful, especially when covered with vegetation (Figure 2). This, together with the relatively large surface area of infiltration basins, suggests integrating them as part of landscape and territorial planning.
4. Conclusions and Current/Future Challenges in MAR
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wheeler, T.; Von Braun, J. Climate change impacts on global food security. Science 2013, 341, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Bouwer, H. Artificial recharge of groundwater: Hydrogeology and engineering. Hydrogeol. J. 2002, 10, 121–142. [Google Scholar] [CrossRef] [Green Version]
- Dillon, P.; Stuyfzand, P.; Grischek, T.; Lluria, M.; Pyne, R.D.G.; Jain, R.C.; Bear, J.; Schwarz, J.; Wang, W.; Fernandez, E.; et al. Sixty years of global progress in managed aquifer recharge. Hydrogeology 2018. [Google Scholar] [CrossRef] [Green Version]
- Rauch-Williams, T.; Hoppe-Jones, C.; Drewes, J.E. The role of organic matter in the removal of emerging trace organic chemicals during managed aquifer recharge. Water Res. 2010, 44, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Patterson, B.M.; Shackleton, M.; Furness, A.J.; Bekele, E.; Pearce, J.; Linge, K.L.; Busetti, F.; Spadek, T.; Toze, S. Behaviour and fate of nine recycled water trace organics during managed aquifer recharge in an aerobic aquifer. J. Contam. Hydrol. 2011, 122, 53–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valhondo, C.; Carrera, J.; Ayora, C.; Barbieri, M.; Nödler, K.; Licha, T.; Huerta, M. Behavior of nine selected emerging trace organic contaminants in an artificial recharge system supplemented with a reactive barrier. Environ. Sci. Pollut. Res 2014, 1–12. [Google Scholar] [CrossRef]
- Valhondo, C.; Carrera, J.; Ayora, C.; Tubau, I.; Martinez-Landa, L.; Nödler, K.; Licha, T. Characterizing redox conditions and monitoring attenuation of selected pharmaceuticals during artificial recharge through a reactive layer. Sci. Total Environ. 2015, 512, 240–250. [Google Scholar] [CrossRef]
- Stevik, T.K.; Aa, K.; Ausland, G.; Hanssen, J.F. Retention and removal of pathogenic bacteria in wastewater percolating through porous media: A review. Water Res. 2004, 38, 1355–1367. [Google Scholar] [CrossRef]
- Hrudey, S.E.; Payment, P.; Huck, P.M.; Gillham, R.W.; Hrudey, E.J. A fatal waterborne disease epidemic in Walkerton, Ontario: Comparison with other waterborne outbreaks in the developed world. Water Sci. Technol. 2003, 47, 7–14. [Google Scholar] [CrossRef]
- Craun, M.F.; Craun, G.F.; Calderon, R.L.; Beach, M.J. Waterborne outbreaks reported in the United States. J. Water Health 2006, 4, 19–30. [Google Scholar] [CrossRef]
- Pedley, S.; Howard, G. The public health implications of microbiological contamination of groundwater. Q. J. Eng. Geol. 1997, 30, 179–188. [Google Scholar] [CrossRef]
- Pandey, P.K.; Kass, P.H.; Soupir, M.L.; Biswas, S.; Singh, V.P. Contamination of water resources by pathogenic bacteria. AMB Express 2014, 4, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzouk, Y.; Goyal, S.M.; Gerba, C.P. Relationship of viruses and indicator bacteria in water and wastewater of Israel. Water Res. 1980, 14, 1585–1590. [Google Scholar] [CrossRef]
- Boe, M.; Real, D. De 7 de diciembre, por el que se establece el régimen jurídico de la reutilización de las aguas depuradas. boe 7 DICIEMBRE 2007, 1620, 50639–50661. [Google Scholar]
- Preston, S.H.; Van De Walle, E. Urban French Mortality in the Nineteenth Century; Taylor & Francis Ltd.: Abingdon, UK, 2016. [Google Scholar]
- Preston, S.H.; Van De Walle, E. Urban French Mortality in the Nineteenth Century. Popul. Stud. 1978, 32, 275–297. [Google Scholar] [CrossRef]
- Asano, T.; Cotruvo, J.A. Groundwater recharge with reclaimed municipal wastewater: Health and regulatory considerations. Water Res. 2004, 38, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Alcalde-Sanz, L.; Gawlik, B.M. Minimum Quality Requirements for Water Reuse in Agricultural Irrigation and Aquifer Recharge. Available online: https://publications.jrc.ec.europa.eu/repository/bitstream/JRC109291/jrc109291_online_08022018.pdf (accessed on 1 April 2020).
- Dillon, P. Future management of aquifer recharge. Hydrogeol. J. 2015, 13, 313–316. [Google Scholar] [CrossRef]
- EPA Contaminants of Emerging Concern including Pharmaceuticals and Personal Care Products. Available online: https://www.epa.gov/wqc/contaminants-emerging-concern-including-pharmaceuticals-andpersonal-care-products (accessed on 19 January 2019).
- Kinney, C.A.; Furlong, E.T.; Werner, S.L.; Cahill, J.D. Presence and distribution of wastewater-derived pharmaceuticals in soil irrigated with reclaimed water. Environ. Toxicol. Chem. 2006, 25, 317–326. [Google Scholar] [CrossRef]
- Urtiaga, A.M.; Pérez, G.; Ibáñez, R.; Ortiz, I. Removal of pharmaceuticals from a WWTP secondary effluent by ultrafiltration/reverse osmosis followed by electrochemical oxidation of the RO concentrate. Desalination 2013, 331, 26–34. [Google Scholar] [CrossRef]
- Esplugas, S.; Bila, D.M.; Krause, L.G.T.; Dezotti, M. Ozonation and advanced oxidation technologies to remove endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) in water effluents. J. Hazard. Mater. 2007, 149, 631–642. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Antimicrobial Effects of Antipyretics. Antimicrob. Agents Chemother. 2017, 61, e02268–e02316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauvé, S.; Desrosiers, M. A review of what is an emerging contaminant. Chem. Cent. J. 2014, 8, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Gunsch, C.K. Effects of selected pharmaceutically active compounds on the ammonia oxidizing bacterium Nitrosomonas europaea. Chemosphere 2011, 82, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Pennington, M.J.; Rothman, J.A.; Dudley, S.L.; Jones, M.B.; McFrederick, Q.S.; Gan, J.; Trumble, J.T. Contaminants of emerging concern affect Trichoplusia ni growth and development on artificial diets and a key host plant. Proc. Natl. Acad. Sci. USA 2017, 114, E9923–E9931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pennington, M.J.; Rothman, J.A.; Jones, M.B.; McFrederick, Q.S.; Gan, J.; Trumble, J.T. Effects of contaminants of emerging concern on Myzus persicae (Sulzer, Hemiptera: Aphididae) biology and on their host plant Capsicum annuum. Environ. Monit. Assess. 2018, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amariei, G.; Boltes, K.; Rosal, R.; Letón, P. Toxicological interactions of ibuprofen and triclosan on biological activity of activated sludge. J. Hazard. Mater. 2017, 334, 193–200. [Google Scholar] [CrossRef]
- McMahon, P.B.; Chapelle, F.H. Microbial production of organic acids in aquitard sediments and its role in aquifer geochemistry. Nature 1991, 349, 233–235. [Google Scholar] [CrossRef]
- Maeng, S.K.; Sharma, S.K.; Abel, C.D.T.; Magic-Knezev, A.; Amy, G.L. Role of biodegradation in the removal of pharmaceutically active compounds with different bulk organic matter characteristics through managed aquifer recharge: Batch and column studies. Water Res. 2011, 45, 4722–4736. [Google Scholar] [CrossRef]
- Greskowiak, J.; Prommer, H.; Massmann, G.; Nützmann, G. Modeling seasonal redox dynamics and the corresponding fate of the pharmaceutical residue phenazone during artificial recharge of groundwater. Environ. Sci. Technol. 2006, 40, 6615–6621. [Google Scholar] [CrossRef]
- Alidina, M.; Li, D.; Drewes, J.E. Investigating the role for adaptation of the microbial community to transform trace organic chemicals during managed aquifer recharge. Water Res. 2014, 56, 172–180. [Google Scholar] [CrossRef]
- Li, D.; Sharp, J.O.; Saikaly, P.E.; Ali, S.; Alidina, M.; Alarawi, M.S.; Keller, S.; Hoppe-Jones, C.; Drewes, J.E. Dissolved Organic Carbon Influences Microbial Community Composition and Diversity in Managed Aquifer Recharge Systems. Appl. Environ. Microbiol. 2012, 78, 6819–6828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alidina, M.; Shewchuk, J.; Drewes, J.E. Effect of temperature on removal of trace organic chemicals in managed aquifer recharge systems. Chemosphere 2015, 122, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Urase, T.; Ngo, H.H.; Hu, J.; Ong, S.L. Insight into metabolic and cometabolic activities of autotrophic and heterotrophic microorganisms in the biodegradation of emerging trace organic contaminants. Bioresour. Technol. 2013, 146, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Alidina, M.; Ouf, M.; Sharp, J.O.; Saikaly, P.; Drewes, J.E. Microbial community evolution during simulated managed aquifer recharge in response to different biodegradable dissolved organic carbon (BDOC) concentrations. Water Res. 2013, 47, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Chefetz, B.; Mualem, T.; Ben-Ari, J. Sorption and mobility of pharmaceutical compounds in soil irrigated with reclaimed wastewater. Chemosphere 2008, 73, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M.; Sims, J.T. Influence of surface and subsoil properties on herbigide sorption by Atlantic Coastal Plain soils. Soil Sci. 1993, 155, 339–348. [Google Scholar] [CrossRef]
- Xu, J.; Wu, L.; Chen, W.; Chang, A.C. Adsorption and degradation of ketoprofen in soils. J. Environ. Qual. 2009, 38, 1177–1182. [Google Scholar] [CrossRef]
- Valhondo, C.; Martinez-Landa, L.; Carrera, J.; Ayora, C.; Nödler, K.; Licha, T. Evaluation of EOC removal processes during artificial recharge through a reactive barrier. Sci. Total Environ. 2018, 612, 985–994. [Google Scholar] [CrossRef]
- Valhondo, C.; Martínez-Landa, L.; Carrera, J.; Díaz-Cruz, S.M.; Amalfitano, S.; Levantesi, C. Six artificial recharge pilot replicates to gain insight into water quality enhancement processes. Chemosphere 2020, 240, 124826. [Google Scholar] [CrossRef]
- Barbieri, M.; Carrera, J.; Sanchez-Vila, X.; Ayora, C.; Cama, J.; Köck-Schulmeyer, M.; de Alda, M.; Barceló, D.; Tobella Brunet, J.; Hernández García, M. Microcosm experiments to control anaerobic redox conditions when studying the fate of organic micropollutants in aquifer material. J. Contam. Hydrol. 2011, 126, 330–345. [Google Scholar] [CrossRef]
- Köck-Schulmeyer, M.; Ginebreda, A.; Postigo, C.; López-Serna, R.; Pérez, S.; Brix, R.; Llorca, M.; de Alda, M.L.; Petrovic, M.; Munnì, A.; et al. Wastewater reuse in Mediterranean semi-arid areas: The impact of discharges of tertiary treated sewage on the load of polar micro pollutants in the Llobregat river (NE Spain). Chemosphere 2011, 82, 670–678. [Google Scholar] [CrossRef]
- Alidina, M.; Li, D.; Ouf, M.; Drewes, J.E. Role of primary substrate composition and concentration on attenuation of trace organic chemicals in managed aquifer recharge systems. J. Environ. Manag. 2014, 144, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Nödler, K.; Licha, T.; Bester, K.; Sauter, M. Development of a multi-residue analytical method, based on liquid chromatography-tandem mass spectrometry, for the simultaneous determination of 46 micro-contaminants in aqueous samples. J. Chromatogr. A 2010, 1217, 6511–6521. [Google Scholar] [CrossRef] [PubMed]
- Gago-Ferrero, P.; Mastroianni, N.; Díaz-Cruz, M.S.; Barceló, D. Fully automated determination of nine ultraviolet filters and transformation products in natural waters and wastewaters by on-line solid phase extraction-liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2013, 1294, 106–116. [Google Scholar] [CrossRef]
- European Parliament and the Council of the European Union. In Proceedings of the 96/23/EC Commission Decision of Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results (Notified under Document Number C (2002) 3044) (Text withEEA Relevance) (2002/657/EC); Off J Eur communities 8–36; European Parliament and the Council of the European Union, Brussels, Belgium, 12 August 2002; 2002. [CrossRef]
- Valhondo, C.; Martinez-Landa, L.; Hidalgo, J.; Tubau, I.; De Pourcq, K.; Grau-Martinez, A.; Ayora, C. Tracer test Modeling for Local scale Residence Time Distribution characterization in an artificial recharge site. Hydrol. Earth Syst. Sci. 2016, 20, 4209–4221. [Google Scholar] [CrossRef] [Green Version]
- Stuyfzand, P.J.; Segers, W.; van Rooijen, N. Behavior of Pharmaceuticals and Other Emerging Pollutants in Various Artificial Recharge Systems in The Netherlands; ISMAR: Arizona, AZ, USA; ACACIA: Arizona, AZ, USA, 2007; Volume 3, pp. 231–245. [Google Scholar]
- Hidalgo, J.J.; Slooten, L.J.; Medina, A.; Carrera, J. A Newton-Raphson Based Code for Seawater Intrusion Modelling and Parameter Estimation. Available online: http://www.swim-site.nl/pdf/swim18_abstracts/Hidalgo.pdf (accessed on 1 April 2020).
- Medina, A.; Carrera, J. Coupled estimation of flow and solute transport parameters. Water Resour. Res. 1996, 32, 3063–3076. [Google Scholar] [CrossRef]
- Medina, A.; Carrera, J. Geostatistical inversion of coupled problems: Dealing with computational burden and different types of data. J. Hydrol. 2003, 281, 251–264. [Google Scholar] [CrossRef]
- Bertelkamp, C.; Schoutteten, K.; Vanhaecke, L.; Bussche, J.V.; Callewaert, C.; Boon, N.; Singhal, N.; van der Hoek, J.P.; Verliefde, A.R.D. A laboratory-scale column study comparing organic micropollutant removal and microbial diversity for two soil types. Sci. Total Environ. 2015, 536, 632–638. [Google Scholar] [CrossRef]
- Regnery, J.; Wing, A.D.; Alidina, M.; Drewes, J.E. Biotransformation of trace organic chemicals during groundwater recharge: How useful are first-order rate constants? J. Contam. Hydrol. 2015, 179, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Schaffer, M.; Kröger, K.F.; Nödler, K.; Ayora, C.; Carrera, J.; Hernández, M.; Licha, T. Influence of a compost layer on the attenuation of 28 selected organic micropollutants under realistic soil aquifer treatment conditions: Insights from a large scale column experiment. Water Res. 2015, 74, 110–121. [Google Scholar] [CrossRef]
- Grünheid, S.; Amy, G.; Jekel, M. Removal of bulk dissolved organic carbon (DOC) and trace organic compounds by bank filtration and artificial recharge. Water Res. 2005, 39, 3219–3228. [Google Scholar] [CrossRef] [PubMed]
- Henzler, A.F.; Greskowiak, J.; Massmann, G. Modeling the fate of organic micropollutants during river bank filtration (Berlin, Germany). J. Contam. Hydrol. 2014, 156, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Laws, B.V.; Dickenson, E.R.V.; Johnson, T.A.; Snyder, S.A.; Drewes, J.E. Attenuation of contaminants of emerging concern during surface-spreading aquifer recharge. Sci. Total Environ. 2011, 409, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Nham, H.T.T.; Greskowiak, J.; Nödler, K.; Rahman, M.A.; Spachos, T.; Rusteberg, B.; Massmann, G.; Sauter, M.; Licha, T. Modeling the transport behavior of 16 emerging organic contaminants during soil aquifer treatment. Sci. Total Environ. 2015, 514, 450–458. [Google Scholar] [CrossRef]
- Ranieri, E.; Verlicchi, P.; Young, T.M. Paracetamol removal in subsurface flow constructed wetlands. J. Hydrol. 2011, 404, 130–135. [Google Scholar] [CrossRef]
- Wiese, B.; Massmann, G.; Jekel, M.; Heberer, T.; Dünnbier, U.; Orlikowski, D.; Grützmacher, G. Removal kinetics of organic compounds and sum parameters under field conditions for managed aquifer recharge. Water Res. 2011, 45, 4939–4950. [Google Scholar] [CrossRef] [Green Version]
- Greskowiak, J.; Hamann, E.; Burke, V.; Massmann, G. The uncertainty of biodegradation rate constants of emerging organic compounds in soil and groundwater: A compilation of literature values for 82 substances. Water Res. 2017, 126, 122–133. [Google Scholar] [CrossRef]
- Bertelkamp, C.; Reungoat, J.; Cornelissen, E.R.; Singhal, N.; Reynisson, J.; Cabo, A.J.; van der Hoek, J.P.; Verliefde, A.R.D. Sorption and biodegradation of organic micropollutants during river bank filtration: A laboratory column study. Water Res. 2014, 52, 231–241. [Google Scholar] [CrossRef]
- Massmann, G.; Dünnbier, U.; Heberer, T.; Taute, T. Behaviour and redox sensitivity of pharmaceutical residues during bank filtration—Investigation of residues of phenazone-type analgesics. Chemosphere 2008, 71, 1476–1485. [Google Scholar] [CrossRef]
- Barbieri, M.; Licha, T.; Nödler, K.; Carrera, J.; Ayora, C.; Sanchez-Vila, X. Fate of β-blockers in aquifer material under nitrate reducing conditions: Batch experiments. Chemosphere 2012, 89, 1272–1277. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Ying, G.-G.; Shareef, A.; Kookana, R.S. Biodegradation of three selected benzotriazoles in aquifer materials under aerobic and anaerobic conditions. J. Contam. Hydrol. 2013, 151, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Maeng, S.K.; Sharma, S.K.; Lekkerkerker-Teunissen, K.; Amy, G.L. Occurrence and fate of bulk organic matter and pharmaceutically active compounds in managed aquifer recharge: A review. Water Res. 2011, 45, 3015–3033. [Google Scholar] [CrossRef] [PubMed]
- Regnery, J.; Wing, A.D.; Kautz, J.; Drewes, J.E. Introducing sequential managed aquifer recharge technology (SMART) From laboratory to full-scale application. Chemosphere 2016, 154, 8–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellauer, K.; Mergel, D.; Ruhl, A.S.; Filter, J.; Hübner, U.; Jekel, M.; Drewes, J.E. Advancing sequential managed aquifer recharge technology (SMART) using different intermediate oxidation processes. Water (Switzerland) 2017, 9, 221. [Google Scholar] [CrossRef] [Green Version]
- Guchi, E. Review on Slow Sand Filtration in Removing Microbial Contamination and Particles from Drinking Water. Am. J. Food Nutr. 2015, 3, 47–55. [Google Scholar] [CrossRef]
- Verma, S.; Daverey, A.; Sharma, A. Slow sand filtration for water and wastewater treatment—A review. Environ. Technol. Rev. 2017, 6, 47–58. [Google Scholar] [CrossRef]
- Perez-Mercado, L.F.; Lalander, C.; Joel, A.; Ottoson, J.; Dalahmeh, S.; Vinnerås, B. Biochar filters as an on-farm treatment to reduce pathogens when irrigating with wastewater-polluted sources. J. Environ. Manag. 2019, 248, 109295. [Google Scholar] [CrossRef]
- Matthess, G.; Pekdeger, A.; Schroeter, J. Persistence and transport of bacteria and viruses in groundwater—A concenptual evaluation. J. Contam. Hydrol. 1988, 2, 171–188. [Google Scholar] [CrossRef]
- Kim, H.N.; Bradford, S.A.; Walker, S.L. Escherichia coli O157:H7 Transport in Saturated Porous Media: Role of Solution Chemistry and Surface Macromolecules. Environ. Sci. Technol. 2009, 43, 4340–4347. [Google Scholar] [CrossRef]
- Park, Y.; Atwill, E.R.; Hou, L.; Packman, A.I.; Harter, T. Deposition of Cryptosporidium parvum Oocysts in porous media: A synthesis of attachment efficiencies measured under varying environmental conditions. Environ. Sci. Technol. 2012, 46, 9491–9500. [Google Scholar] [CrossRef]
- Bichai, F.; Barbeau, B.; Dullemont, Y.; Hijnen, W. Role of predation by zooplankton in transport and fate of protozoan (oo)cysts in granular activated carbon filtration. Water Res. 2010, 44, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Bradford, S.A.; Šimunek, J.; Torkzaban, S. Minimizing virus transport in porous media by optimizing solid phase inactivation. J. Environ. Qual. 2018, 47, 1058–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urfer, D. Use of bauxite for enhanced removal of bacteria in slow sand filters. Water Sci. Technol. Water Supply 2017, 17, 1007–1015. [Google Scholar] [CrossRef]
- Weiss, W.J.; Bouwer, E.J.; Aboytes, R.; LeChevallier, M.W.; O’Melia, C.R.; Le, B.T.; Schwab, K.J. Riverbank filtration for control of microorganisms: Results from field monitoring. Water Res. 2005, 39, 1990–2001. [Google Scholar] [CrossRef] [PubMed]
- Bekele, E.; Toze, S.; Patterson, B.; Higginson, S. Managed aquifer recharge of treated wastewater: Water quality changes resulting from infiltration through the vadose zone. Water Res. 2011, 45, 5764–5772. [Google Scholar] [CrossRef]
- Betancourt, W.Q.; Kitajima, M.; Wing, A.D.; Regnery, J.; Drewes, J.E.; Pepper, I.L.; Gerba, C.P. Assessment of virus removal by managed aquifer recharge at three full-scale operations. J. Environ. Sci. Heal. Part A Toxic Hazard. Subst. Environ. Eng. 2014, 49, 1685–1692. [Google Scholar] [CrossRef]
- Elkayam, R.; Aharoni, A.; Vaizel-Ohayon, D.; Katz, Y.; Negev, I.; Marano, R.B.; Cytryn, E.; Shtrasler, L.; Lev, O. Viral and Microbial Pathogens, Indicator Microorganisms, Microbial Source Tracking Indicators, and Antibiotic Resistance Genes in a Confined Managed Effluent Recharge System. J. Environ. Eng. 2018, 144, 05017011. [Google Scholar] [CrossRef]
- Schijven, J.F.; Hassanizadeh, S.M. Removal of viruses by soil passage: Overview of modeling, processes, and parameters. Crit. Rev. Environ. Sci. Technol. 2000, 30, 49–127. [Google Scholar] [CrossRef]
- Voigt, A.M.; Zacharias, N.; Timm, C.; Wasser, F.; Sib, E.; Skutlarek, D.; Parcina, M.; Schmithausen, R.M.; Schwartz, T.; Hembach, N.; et al. Association between antibiotic residues, antibiotic resistant bacteria and antibiotic resistance genes in anthropogenic wastewater—An evaluation of clinical influences. Chemosphere 2020, 241, 125032. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J.; Milakovic, M.; Švecová, H.; Ganjto, M.; Jonsson, V.; Grabic, R.; Udikovic-Kolic, N. Industrial wastewater treatment plant enriches antibiotic resistance genes and alters the structure of microbial communities. Water Res. 2019, 162, 437–445. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J.; Hammarén, R.; Pal, C.; Östman, M.; Björlenius, B.; Flach, C.F.; Fick, J.; Kristiansson, E.; Tysklind, M.; Larsson, D.G.J. Elucidating selection processes for antibiotic resistance in sewage treatment plants using metagenomics. Sci. Total Environ. 2016. [Google Scholar] [CrossRef] [PubMed]
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Bürgmann, H.; Sørum, H.; Norström, M.; Pons, M.N.; et al. Tackling antibiotic resistance: The environmental framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Frieden, T. Antibiotic resistance threats in the United States. Cent. Dis. Control Prev. 2019, 1–114. [Google Scholar] [CrossRef] [Green Version]
- Larsson, D.G.J.; Andremont, A.; Bengtsson-Palme, J.; Brandt, K.K.; de Roda Husman, A.M.; Fagerstedt, P.; Fick, J.; Flach, C.F.; Gaze, W.H.; Kuroda, M.; et al. Critical knowledge gaps and research needs related to the environmental dimensions of antibiotic resistance. Environ. Int. 2018, 117, 132–138. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation (WHO). Global Action Plan on Antimicrobial Resistance; WHO: Geneva, Switzerland, 2017; pp. 1–28. [Google Scholar]
- European Commission. A European One Health Action Plan against Antimicrobial Resistance; Commun FROM Comm TO Counc Eur Parliam; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- EU Decision 2018/840. Commission Implementing Decision (EU) 2018/840 of 5 June 2018. Off. J. Eur. Union. 2018, 141, 9–12. [Google Scholar]
- Sharma, V.K.; Johnson, N.; Cizmas, L.; McDonald, T.J.; Kim, H. A review of the influence of treatment strategies on antibiotic resistant bacteria and antibiotic resistance genes. Chemosphere 2016. [Google Scholar] [CrossRef]
- Gao, P.; Munir, M.; Xagoraraki, I. Correlation of tetracycline and sulfonamide antibiotics with corresponding resistance genes and resistant bacteria in a conventional municipal wastewater treatment plant. Sci. Total Environ. 2012. [Google Scholar] [CrossRef]
- Li, J.; Cheng, W.; Xu, L.; Strong, P.J.; Chen, H. Antibiotic-resistant genes and antibiotic-resistant bacteria in the effluent of urban residential areas, hospitals, and a municipal wastewater treatment plant system. Environ. Sci. Pollut. Res. 2015, 22, 4587–4596. [Google Scholar] [CrossRef]
- Pärnänen, K.M.M.; Narciso-da-Rocha, C.; Kneis, D.; Berendonk, T.U.; Cacace, D.; Do, T.T.; Elpers, C.; Fatta-Kassinos, D.; Henriques, I.; Jaeger, T.; et al. Antibiotic resistance in European wastewater treatment plants mirrors the pattern of clinical antibiotic resistance prevalence. Sci. Adv. 2019, 5, eaau9124. [Google Scholar] [CrossRef] [Green Version]
- Ju, F.; Beck, K.; Yin, X.; Maccagnan, A.; McArdell, C.S.; Singer, H.P.; Johnson, D.R.; Zhang, T.; Bürgmann, H. Wastewater treatment plant resistomes are shaped by bacterial composition, genetic exchange, and upregulated expression in the effluent microbiomes. ISME J. 2019, 13, 346–360. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Geng, J.; Ren, H.; Ding, L.; Xu, K.; Zhang, Y. Variation of antibiotic resistance genes in municipal wastewater treatment plant with A2O-MBR system. Environ. Sci. Pollut. Res. 2015, 22, 3715–3726. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Zhang, Q.; Nie, X.; Chen, B.; Xiao, Y.; Zhou, Q.; Liao, W.; Liang, X. Occurrence and elimination of antibiotic resistance genes in a long-term operation integrated surface flow constructed wetland. Chemosphere 2017. [Google Scholar] [CrossRef] [PubMed]
- Cui, E.; Wu, Y.; Zuo, Y.; Chen, H. Effect of different biochars on antibiotic resistance genes and bacterial community during chicken manure composting. Bioresour. Technol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.N.; Kummer, K.M.; Durmus, N.G.; Leuba, K.; Tarquinio, K.M.; Webster, T.J. Superparamagnetic iron oxide nanoparticles (SPION) for the treatment of antibiotic-resistant biofilms. Small 2012, 8, 3016–3027. [Google Scholar] [CrossRef]
- Nijhawan, A.; Labhasetwar, P.; Jain, P.; Rahate, M. Public consultation on artificial aquifer recharge using treated municipal wastewater. Resour. Conserv. Recycl. 2013. [Google Scholar] [CrossRef]
- Smith, H.M.; Brouwer, S.; Jeffrey, P.; Frijns, J. Public responses to water reuse—Understanding the evidence. J. Environ. Manag. 2018, 207, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Lazarova, V.; Asano, T.; Bahri, A.; Anderson, J. Milestones in Water Reuse: The Best Success Stories; IWA Publishing: London, UK, 2013. [Google Scholar]
- Hartley, T.W. Public perception and participation in water reuse. Desalination 2006, 187, 115–126. [Google Scholar] [CrossRef]
- Hurlimann, A.; Dolnicar, S. When public opposition defeats alternative water projects—The case of Toowoomba Australia. Water Res. 2010, 44, 287–297. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valhondo, C.; Carrera, J.; Martínez-Landa, L.; Wang, J.; Amalfitano, S.; Levantesi, C.; Diaz-Cruz, M.S. Reactive Barriers for Renaturalization of Reclaimed Water during Soil Aquifer Treatment. Water 2020, 12, 1012. https://doi.org/10.3390/w12041012
Valhondo C, Carrera J, Martínez-Landa L, Wang J, Amalfitano S, Levantesi C, Diaz-Cruz MS. Reactive Barriers for Renaturalization of Reclaimed Water during Soil Aquifer Treatment. Water. 2020; 12(4):1012. https://doi.org/10.3390/w12041012
Chicago/Turabian StyleValhondo, Cristina, Jesús Carrera, Lurdes Martínez-Landa, Jingjing Wang, Stefano Amalfitano, Caterina Levantesi, and M. Silvia Diaz-Cruz. 2020. "Reactive Barriers for Renaturalization of Reclaimed Water during Soil Aquifer Treatment" Water 12, no. 4: 1012. https://doi.org/10.3390/w12041012
APA StyleValhondo, C., Carrera, J., Martínez-Landa, L., Wang, J., Amalfitano, S., Levantesi, C., & Diaz-Cruz, M. S. (2020). Reactive Barriers for Renaturalization of Reclaimed Water during Soil Aquifer Treatment. Water, 12(4), 1012. https://doi.org/10.3390/w12041012







