Pollution of Sedimentary Ponds at an Industrial Plant in Janikowo (Poland)
Abstract
1. Introduction
2. Materials and Methods
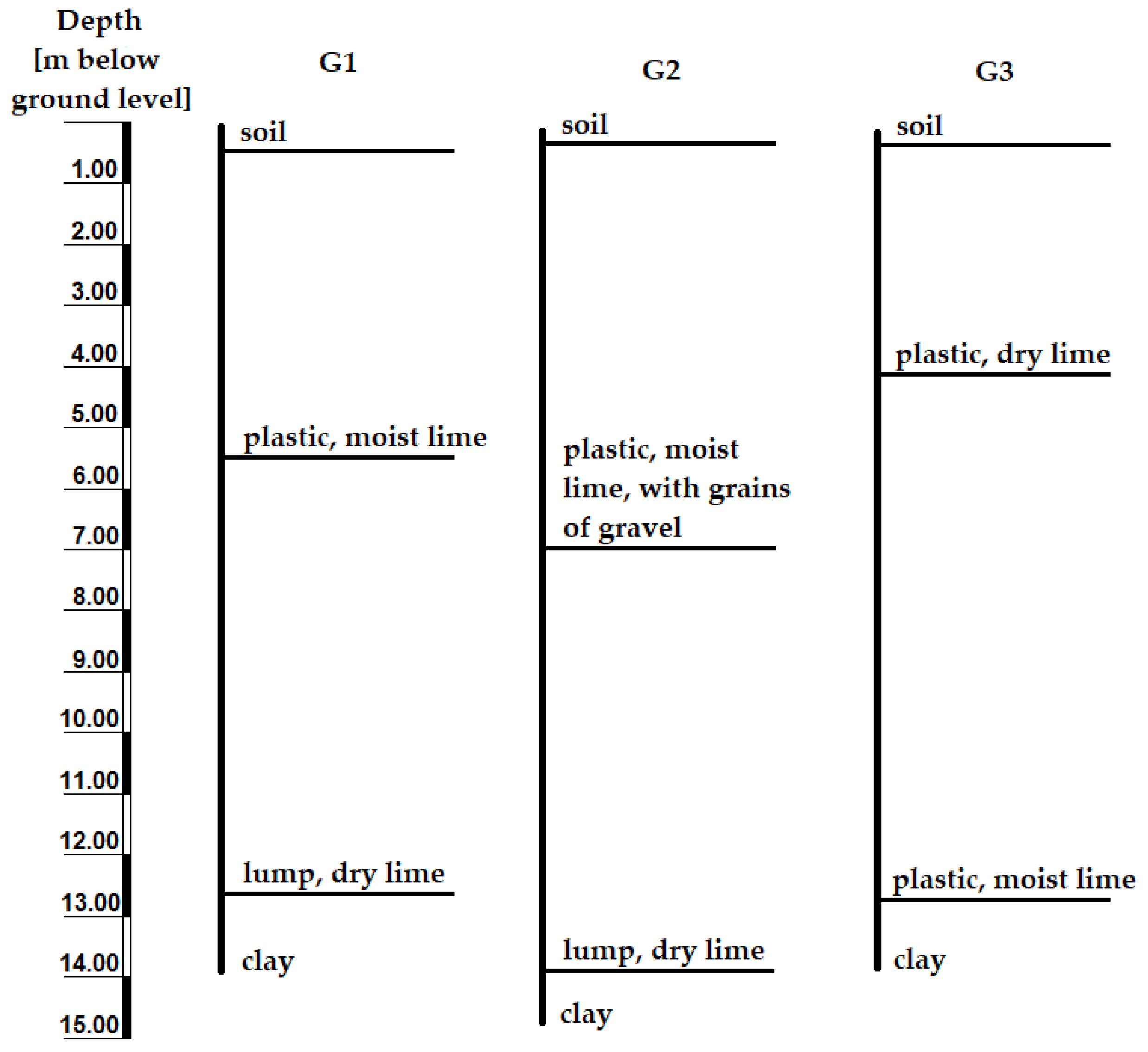
2.1. Characteristics of the Research Area
2.2. Examination of Waste and Groundwater Samples
3. Results and Discussion
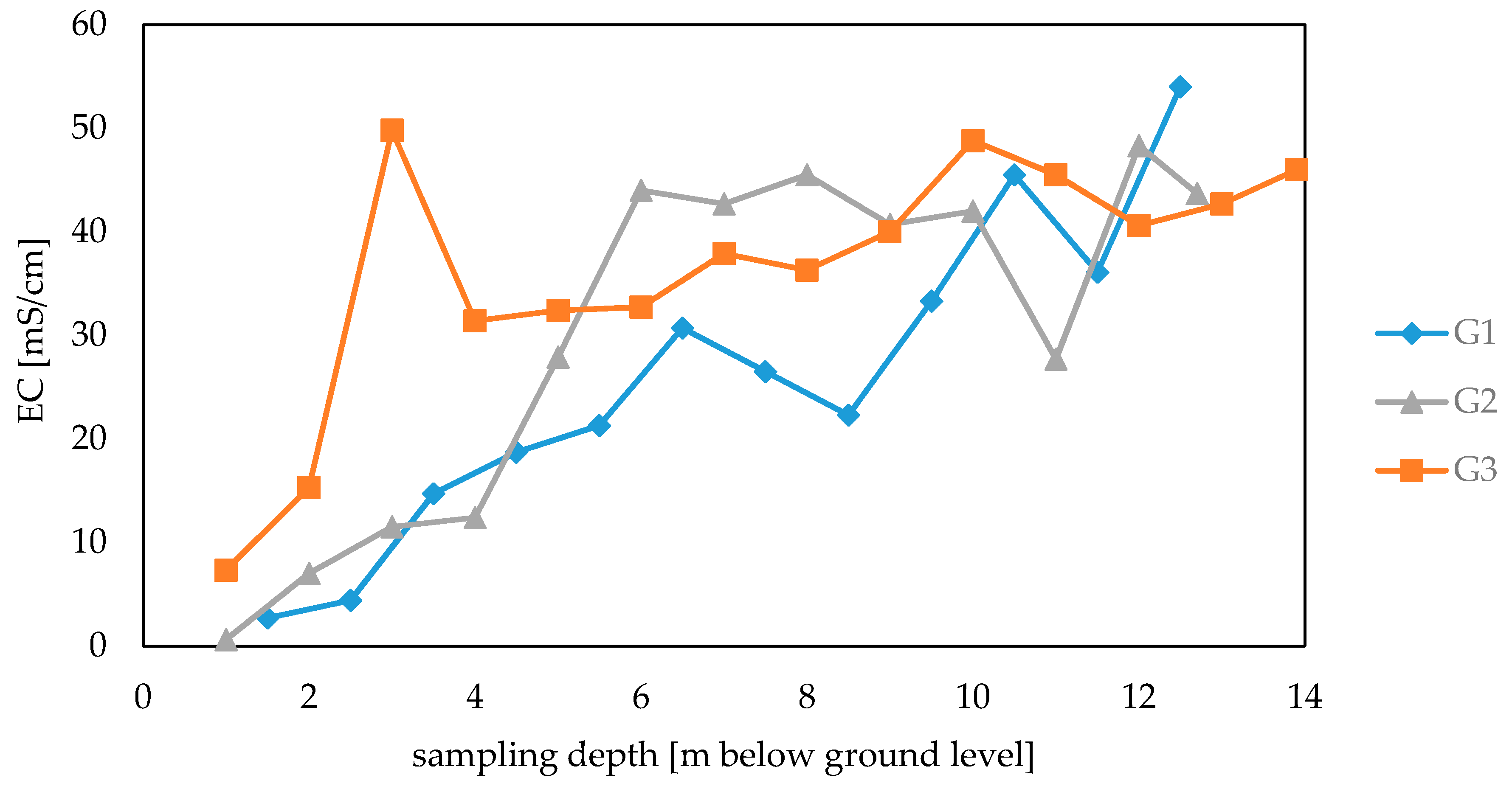
3.1. Electrolytic Conductivity of Waste Samples
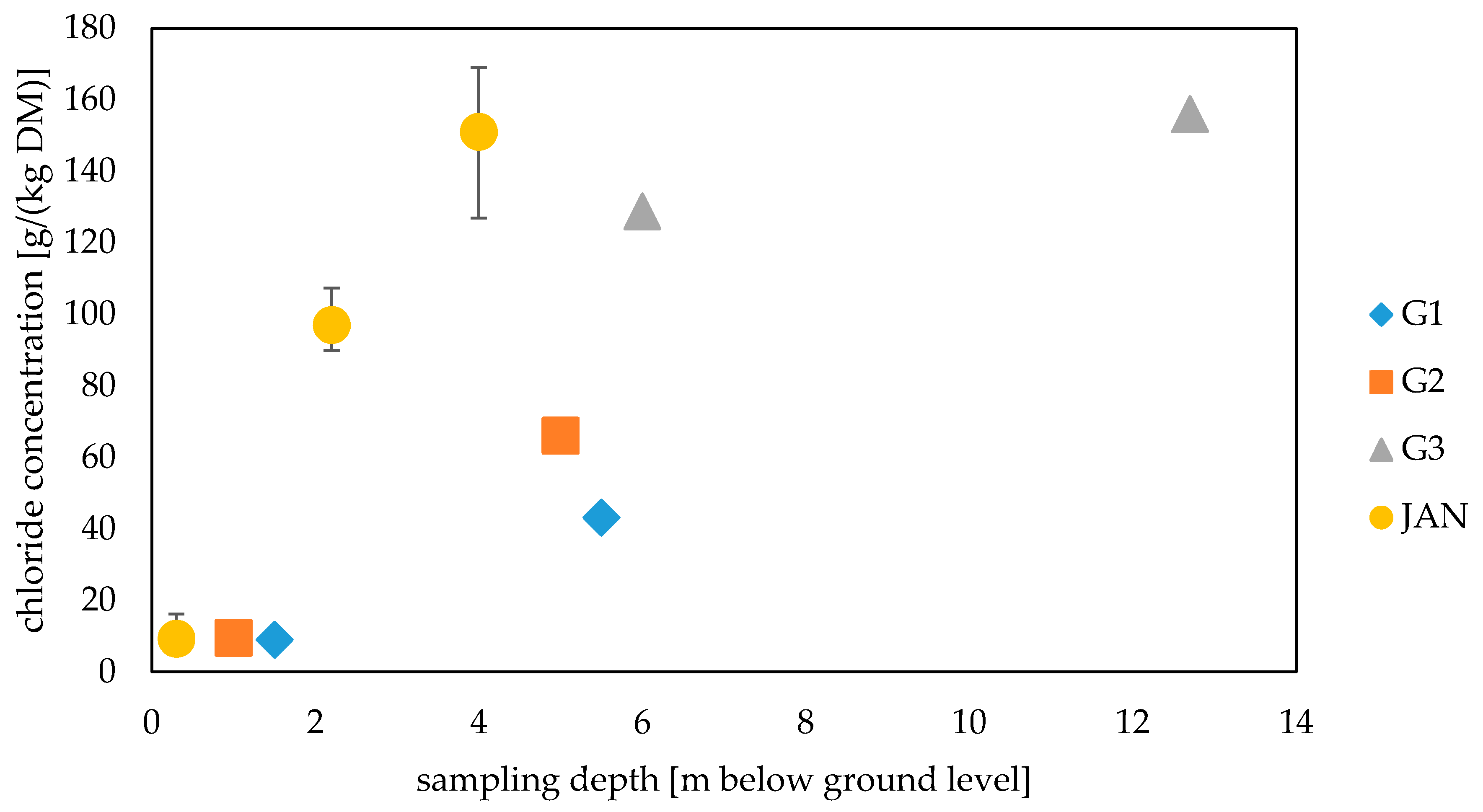
3.2. Chloride Ion Concentration in Waste Samples
3.3. Chloride Ion Concentration in Groundwater
4. Conclusions
- Waste deposits accumulated in the former sedimentary ponds are characterized by high electrolytic conductivity and high concentration of chloride ions, which excludes their agricultural use;
- Chlorides are leached from the sediments deep into the soil profile by rainwater and, with high probability, also by water seeping from the adjacent slag pond;
- Sedimentary ponds are still an important source of pollution released into soils and groundwater around the plant;
- Chloride concentration in waste has decreased in the last 20 years;
- Quality of groundwater in the area of sedimentary ponds has improved in the last 30 years in terms of chloride ion content, but still is not proper for human consumption.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wiater, J. Influence of waste disposal sites on the ground water quality and soil properties. Inż. Ekol. 2011, 26, 133–146. [Google Scholar]
- Urbańska, J.; Urbański, K. Selected aspects of reclamation of soda waste landfill sites. Geomat. Environ. Eng. 2012, 6, 83–90. [Google Scholar] [CrossRef]
- Kasikowski, T.; Buczkowski, R.; Lemanowska, E. Cleaner production in the ammonia–soda industry: An ecological and economic study. J. Environ. Manag. 2004, 73, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.A.; Effler, S.W. Decreases in pollutant loading from residual soda ash production waste. Water Air Soil Pollut. 2003, 146, 55–73. [Google Scholar] [CrossRef]
- Sutkowska, K.; Teper, L.; Stania, M. Tracing potential soil contamination in the historical Solvay soda ash plant area, Jaworzno, Southern Poland. Environ. Monit. Assess. 2015, 187, 704. [Google Scholar] [CrossRef] [PubMed]
- Gromiec, M.; Sadurski, A.; Zalewski, M.; Rowiński, P. Hazards related to water quality. Nauka 2014, 1, 99–122. [Google Scholar]
- Sroczyński, W. Prospects of biale morza (the “white seas”) management on the area after former solvay krakow soda works. Zarządzanie Krajobrazem Kulturowym Prace Komisji Krajobrazu Kulturowego 2008, 10, 423–430. [Google Scholar]
- Gliniak, M.; Betlej, M.; Mitura, A. The application of the PAN reclamation model on the example of former landfills Cracow soda works Solvay. Inz. Miner. 2015, 16, 131–136. [Google Scholar]
- Zając, E.; Zarzycki, J. Revegetation of reclaimed soda waste dumps: Effects of topsoil parameters. J. Elem. 2012, 17, 525–536. [Google Scholar] [CrossRef]
- Strzelecka, J.; Dąbrowski, M.; Hulisz, P.; Piernik, A. Changes in soil properties and plant biomass under the influence of soda waste ponds in Inowrocław, Poland. Ecol. Quest. 2011, 14, 69–71. [Google Scholar] [CrossRef][Green Version]
- Siuta, J. Reclamation efficiency of sewage sludge at the post-sodium waste landfill in Janikowo. Inż. Ekol. 2014, 36, 98–119. [Google Scholar] [CrossRef]
- Hulisz, P.; Piernik, A. Soils affected by soda industry in Inowrocław. In Technogenic Soils of Poland; Charzyński, P., Hulisz, P., Bednarek, R., Eds.; Polish Society of Soil Science: Toruń, Poland, 2013; pp. 125–140. [Google Scholar]
- Gur, N.; Aktas, Y.; Oztekin, E. Utilization of soda ash plant solid wastes in manufacture of cement. Cement. Concr. Comp. 2012, 47, 8866–8873. [Google Scholar]
- Çalban, T.; Kavci, E. Removal of calcium from soda liquid waste containing calcium chloride. Energy Sources Part A 2010, 32, 407–418. [Google Scholar] [CrossRef]
- Hulisz, P.; Krawiec, A.; Pindral, S.; Mendyk, Ł.; Pawlikowska, K. The impact of environmental conditions on water salinity in the area of the city of Inowrocław (north-central Poland). Bull. Geogr. Phys. Geogr. Ser. 2017, 13, 5–15. [Google Scholar] [CrossRef][Green Version]
- Kaczor-Kurzawa, D. Tectonically conditioned brine leakage into usable freshwater aquifers—Implications for the quality of groundwater exploited in central Poland. In Proceedings of the E3S Web of Conferences, Gdańsk, Poland, 17–22 June 2018; Volume 54, pp. 1–5. [Google Scholar] [CrossRef]
- Piernik, A.; Hulisz, P.; Rokicka, A. Micropattern of halophytic vegetation on technogenic soils affected by the soda industry. Soil Sci. Plant Nutr. 2015, 61, 98–112. [Google Scholar] [CrossRef]
- Raghunathan, C.; Tewari, A.; Khambhaty, Y.; Jothinayagam, J.T. Effect of soda ash industry effluent on seaweed epiphytic and ambient fauna in in situ condition in northwest coast of India. Nat. Environ. Pollut. Technol. 2007, 6, 1–14. [Google Scholar] [CrossRef]
- Kaszubkiewicz, J.; Musiał, A.; Waścińska, A.; Ochman, D. Changes of the physical and physico-chemical properties of selected soils during the processes of salinization and desalinization. Rocz. Glebozn. 2003, 54, 5–25. [Google Scholar]
- Sieczka, A.; Koda, E. Evaluation of chlorides transport parameters in natural soils based on laboratory studies. In MendelNet 2017 Proceedings of 24th International PhD Students Conference; Cerkal, R., Brezinova Belcredi, N., Eds.; Mendel University in Brno: Brno, Czech Republic, 2017; pp. 921–926. [Google Scholar]
- Sapek, A. Contemporary chloride sources in environment of inland waters. Ochr. Śr. Zas. Nat. 2009, 40, 455–464. [Google Scholar]
- Bojarska, K.; Bzowski, Z. Leaching test results of mining wastes from Upper Silesia Coal Basin Mines in aspect of influence on the environment. Gór. Geol. 2012, 7, 101–113. [Google Scholar]
- Effler, S.W.; Matthews, D.A. Impacts of a Soda Ash Facility on Onondaga Lake and the Seneca River, NY. Lake Reserv. Manag. 2003, 19, 285–306. [Google Scholar] [CrossRef][Green Version]
- EN 12457-2:2002. Characterisation of Waste-Leaching-Compliance Test for Leaching of Granular Waste Materials and Sludges—One Stage Batch Test at a Liquid to Solid Ratio of 10 L/kg for Materials with Particle Size below 4 mm (without or with Size Reduction). Available online: https://infostore.saiglobal.com/en-us/Standards/EN-12457-2-2002-332867_SAIG_CEN_CEN_765046/ (accessed on 12 February 2020).
- ISO 9297:1989. Water Quality—Determination of Chloride—Silver Nitrate Titration with Chromate Indicator (Mohr’s Method). Available online: https://www.iso.org/standard/16952.html (accessed on 12 February 2020).
- ISO 5667-20:2008. Water Quality—Sampling—Part 20: Guidance on the Use of Sampling Data for Decision Making—Compliance with Thresholds and Classification Systems. Available online: https://www.iso.org/standard/40003.html (accessed on 12 February 2020).
- Krasowska, M.; Banaszuk, P. Leaching of dissolved components from a small agricultural catchment during snowmelt induced high flow event. Woda Środowisko Obszary Wiejskie 2011, 11, 139–157. [Google Scholar]
- Krasowska, M. Effect of intense precipitation on the leaching of biogenic compounds from agricultural catchment. Inż. Ekol. 2016, 47, 1–9. [Google Scholar] [CrossRef]
- Fajer, M.; Rzetala, M.A. Mill pond sediments as the indicator of the environment of the drainage area (an example of Liswarta River, Odra basin, Poland). Environ. Sci. Pollut. Res. 2018, 25, 5832–5847. [Google Scholar] [CrossRef]
- Wiatrowska, K.M.; Komisarek, J.; Marcinek, J.; Kozłowski, M. Evaluation of the soil quality in the vicinity of Inowrocław soda plants. Arch. Environ. Prot. 2018, 44, 58–67. [Google Scholar] [CrossRef]
- Vangelas, K.M.; Looney, B.B.; Early, T.O.; Gilmore, T.; Chapelle, F.H.; Adams, K.M.; Sink, C.H. Monitored natural Attenuation of Clorinated Solvents—Moving beyond reductive dechlorination. Remediation 2006, 16, 5–23. [Google Scholar] [CrossRef]
- Gliniak, M.; Pawul, M.; Sobczyk, W. Impact of the transport and postindustrial landfills of Cracow Soda Works “Solvay” on the status and quality of water in Wilga river in Krakow. Logistyka 2014, 4, 4295–4302. [Google Scholar]
- Wiercik, P.; Szymańska-Pulikowska, A. The influence of municipal waste dumping ground in Wojczyce on groundwater quality. Infrastruktura i Ekologia Terenów Wiejskich 2010, 2010, 151–162. [Google Scholar]
- Janas, M.; Zawadzka, A. The impact of industrial waste landfill on the environment. Inż. Ekol. 2017, 18, 64–73. [Google Scholar]
- Gałko, G. Influence of landfill leachate on physical and chemical properties of loamy soil. Arch. Waste Manag. Environ. Prot. 2015, 17, 83–92. [Google Scholar]
- Ma, W.; Rotter, S.; Hoffmann, G.; Lehmann, A. Origins of chlorine in MSW and RDF: Species and analytical methods. Wit Trans. Ecol. Environ. 2008, 109, 551–558. [Google Scholar] [CrossRef]
- Jakubiak, M.; Śliwka, M. Management and reclamation of lands with raised soil salinity degraded by activity of mining industry. Gospod. Surowcami Miner. 2008, 24, 129–138. [Google Scholar]
- Małuszyńska, I.; Małuszyński, M.J. Study of soil salinity on growth and advancementof selected plants species. Inż. Ekol. 2009, 21, 32–39. [Google Scholar]
- Wójcik, P. Balanced Fertilization of Horticultural Plants; Instytut Ogrodnictwa: Skierniewice, Poland, 2014. [Google Scholar]
- Saraswathy, R.; Muralidhar, M.; Sanjoy, D.; Kumararaja, P.; Suvana, S.; Lalitha, N.; Katneni, V.K.; Nagavel, A.; Vijayan, K.K. Changes in soil and water quality at sediment-water interface of Penaeus vannamei culture pond at varying salinities. Aquac. Res. 2019, 50, 1096–1106. [Google Scholar] [CrossRef]
- Małecki, Z.J.; Wira, J.; Moshynsky, V.; Małecka, I. The threat of worsening the quality of surface and underground waters caused by an unrecultivated waste dumping site in Tłokinia Kościelna near Kalisz. Inż. Ekol. 2016, 46, 77–87. [Google Scholar] [CrossRef][Green Version]
- Wychowaniak, D.; Koda, E. Assessment of the soil-water system quality in the vicinity of old landfill with a vertical barrier. In Remediation, Reclamation and Revitalization; PZITS Oddział Wielkopolski: Poznań, Poland, 2014. [Google Scholar]
- Dz.U. 2017 poz. 2294. Order of the Minister of Environment of 7 December 2017 on the Quality of Water Intended for Human Consumption. Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20170002294 (accessed on 12 February 2020).



| Piezometer | Date of Establishment | Depth [m below Ground Level] | Depth of the Filter [m below Ground Level] |
|---|---|---|---|
| W1 | 1982 | 13 | 11.5–12.5 |
| W2 | 1989 | 20.1 | 19–20 |
| W3 | 1982 | 13 | 11.5–12.5 |
| W4 | 1982 | 13 | 11.5–12.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gołub, A.; Piekutin, J. Pollution of Sedimentary Ponds at an Industrial Plant in Janikowo (Poland). Water 2020, 12, 536. https://doi.org/10.3390/w12020536
Gołub A, Piekutin J. Pollution of Sedimentary Ponds at an Industrial Plant in Janikowo (Poland). Water. 2020; 12(2):536. https://doi.org/10.3390/w12020536
Chicago/Turabian StyleGołub, Adam, and Janina Piekutin. 2020. "Pollution of Sedimentary Ponds at an Industrial Plant in Janikowo (Poland)" Water 12, no. 2: 536. https://doi.org/10.3390/w12020536
APA StyleGołub, A., & Piekutin, J. (2020). Pollution of Sedimentary Ponds at an Industrial Plant in Janikowo (Poland). Water, 12(2), 536. https://doi.org/10.3390/w12020536





